Influences of Extreme Weather Conditions on the Carbon Cycles of Bamboo and Tea Ecosystems
Abstract
1. Introduction
2. Materials and Methods
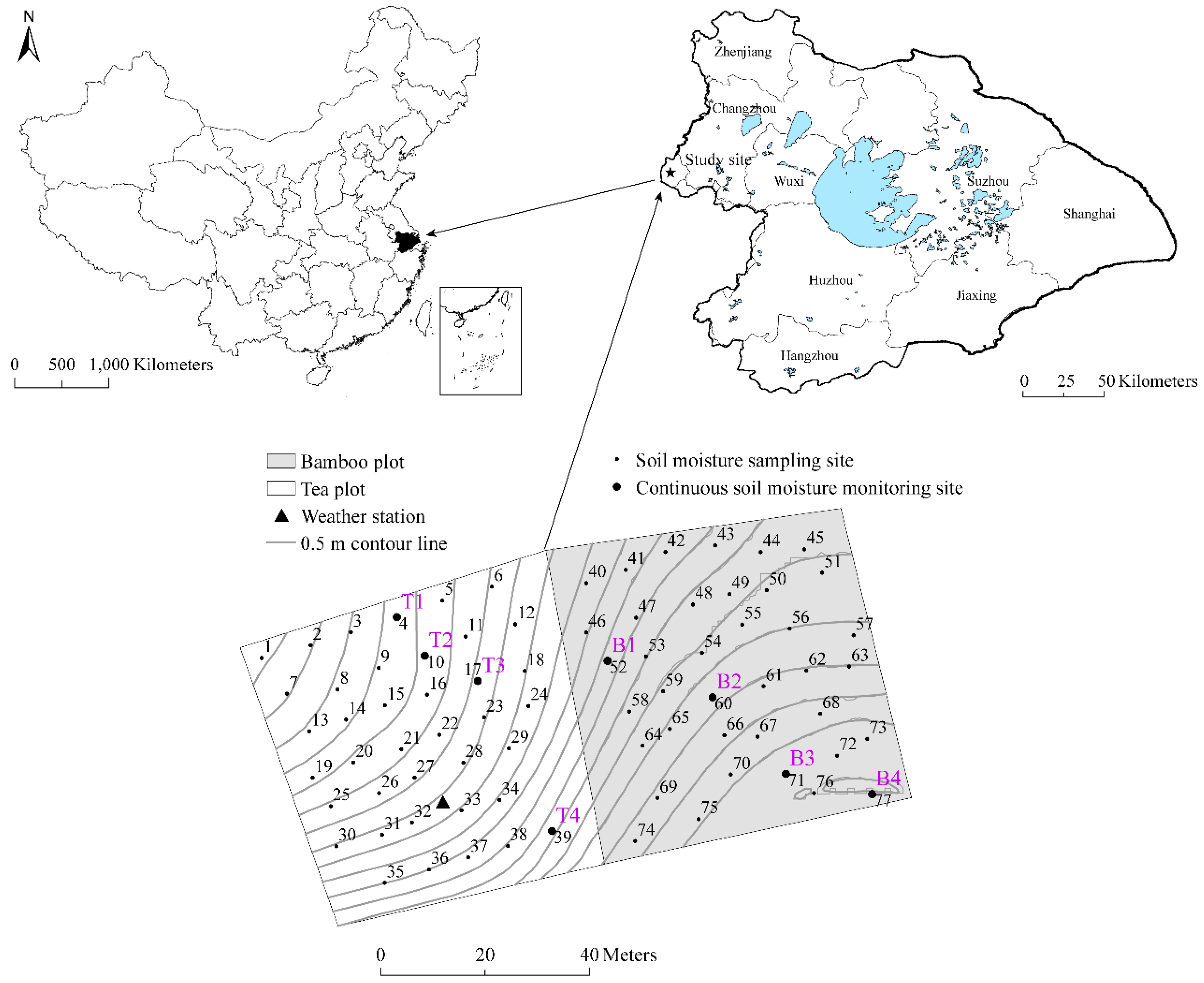
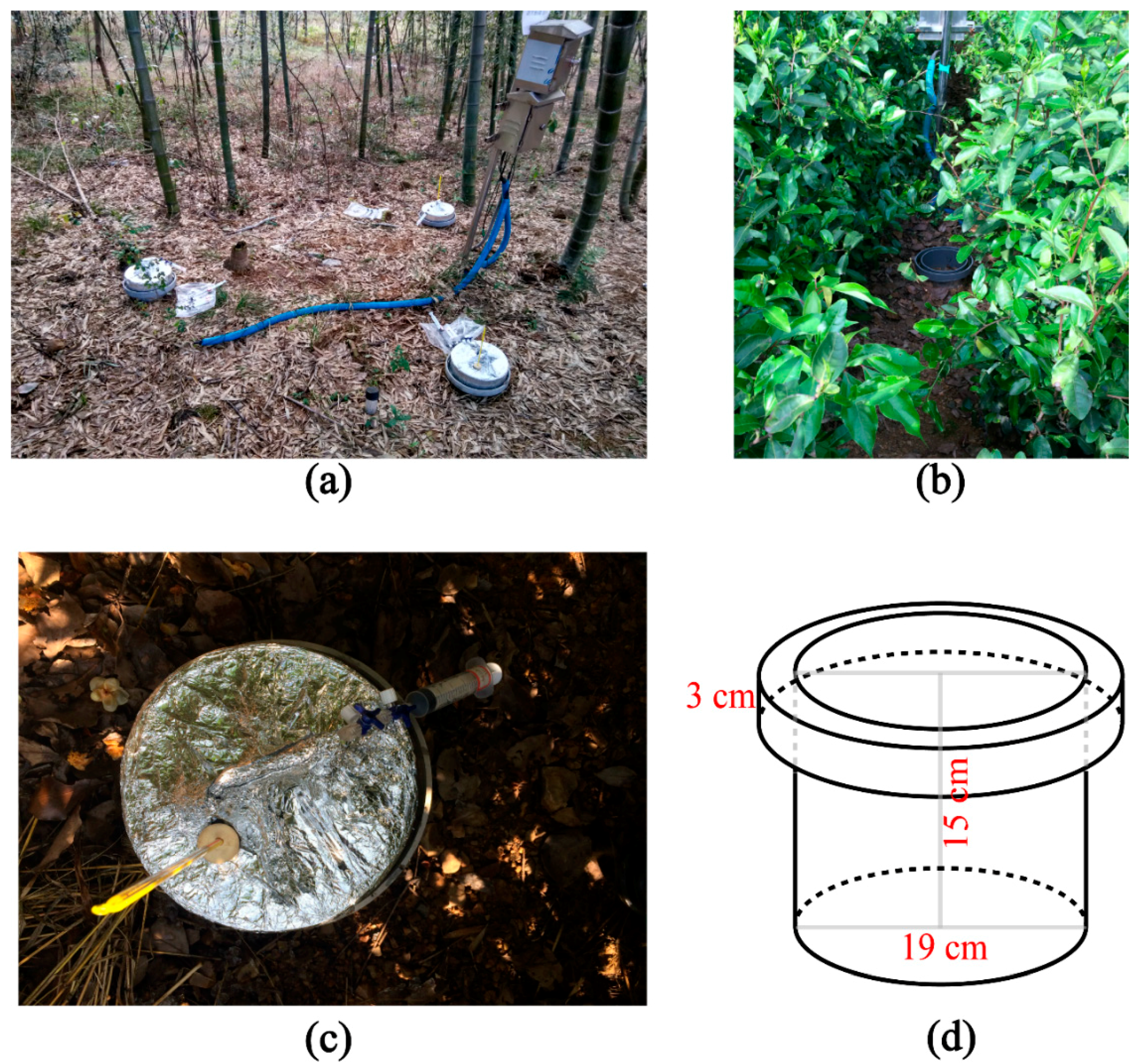
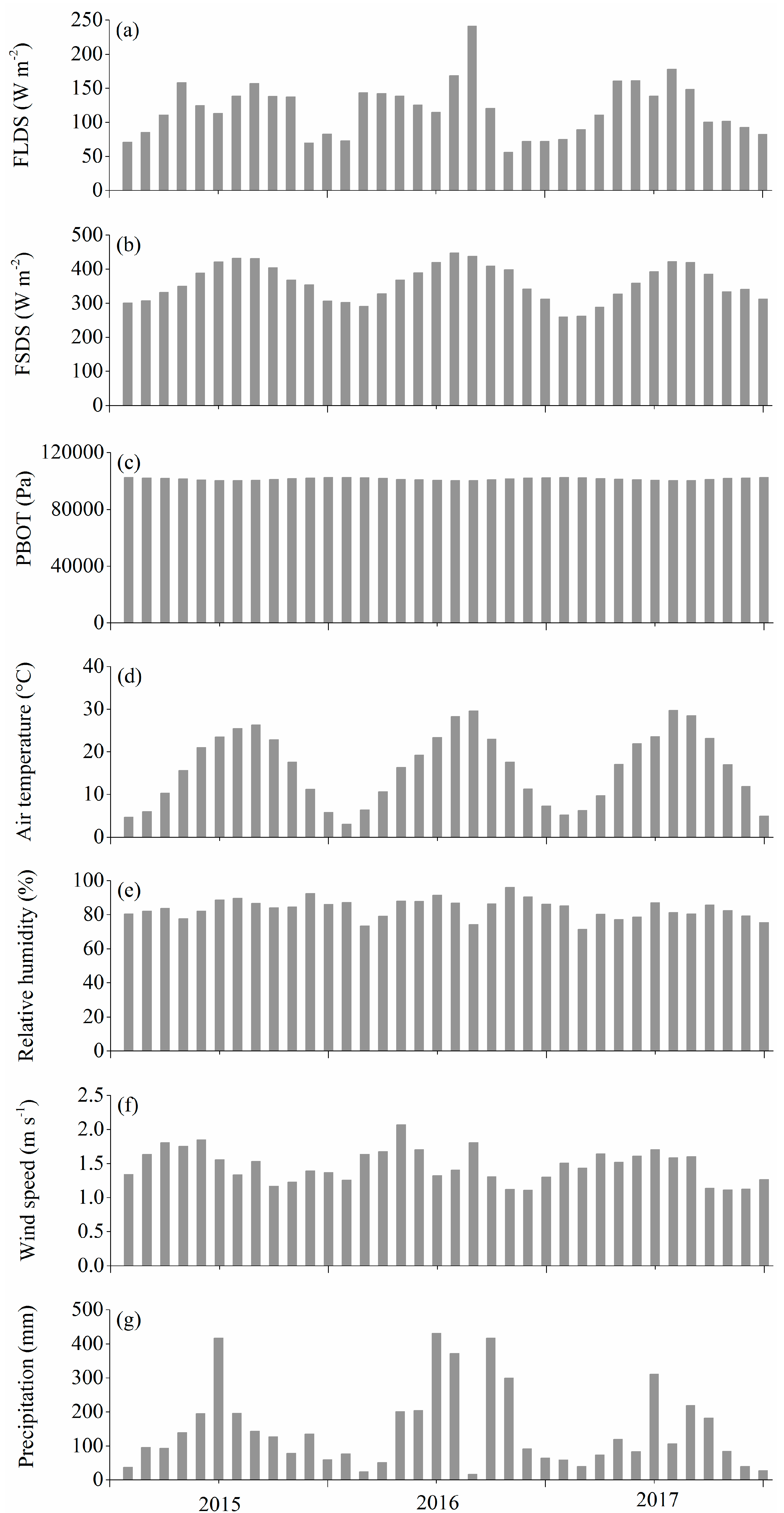
2.1. Study Sites and Data
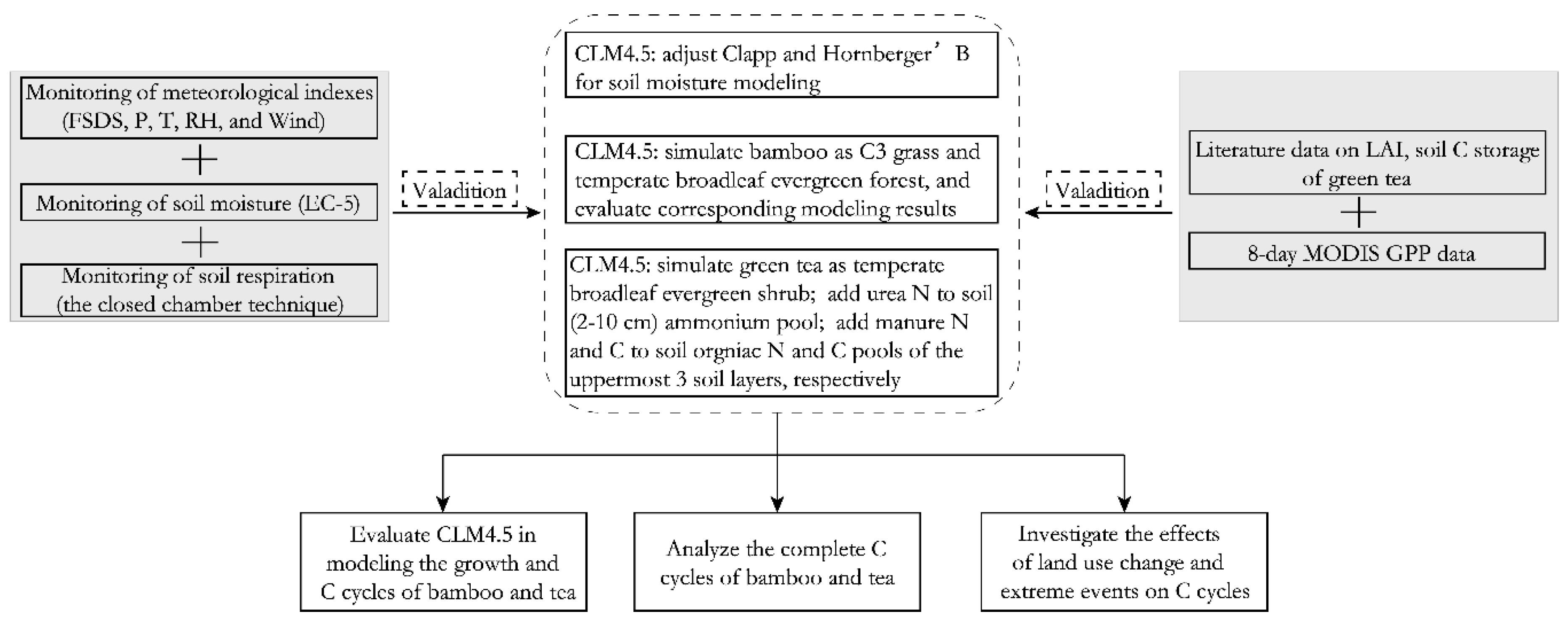
2.2. Land Surface Model
3. Results and Discussion
3.1. Observed and Modeled Soil Water Content
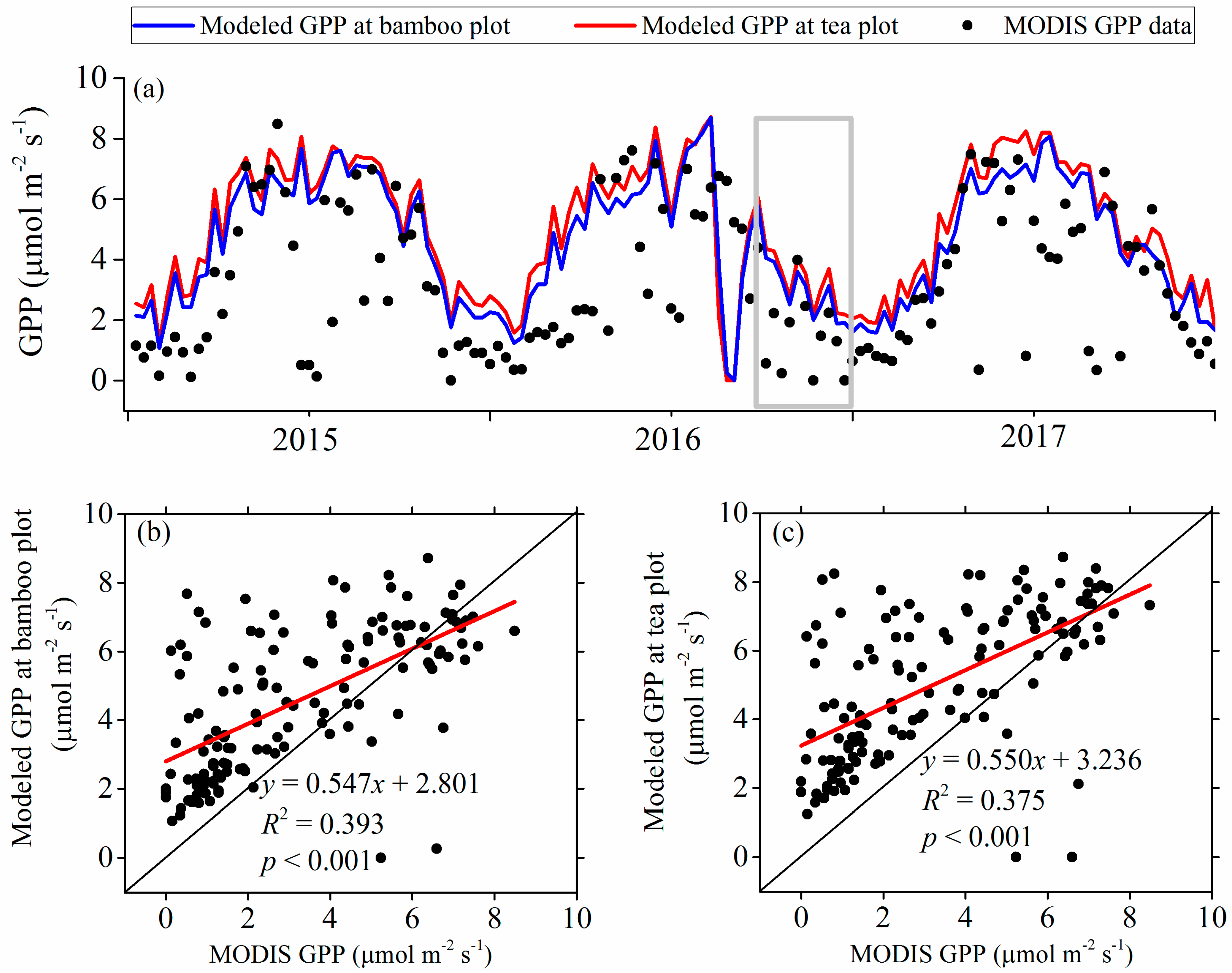
3.2. Modeled Plant Growth
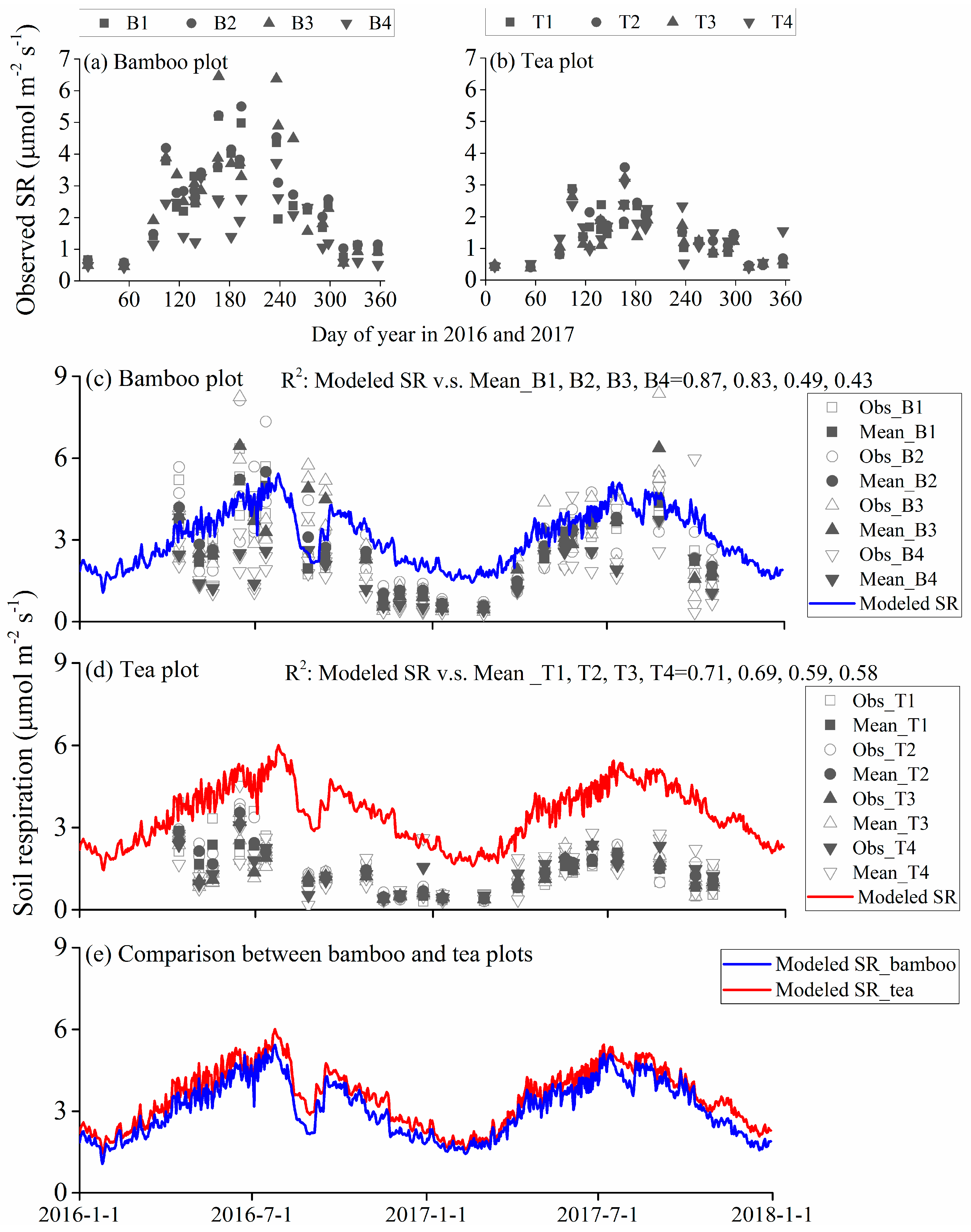
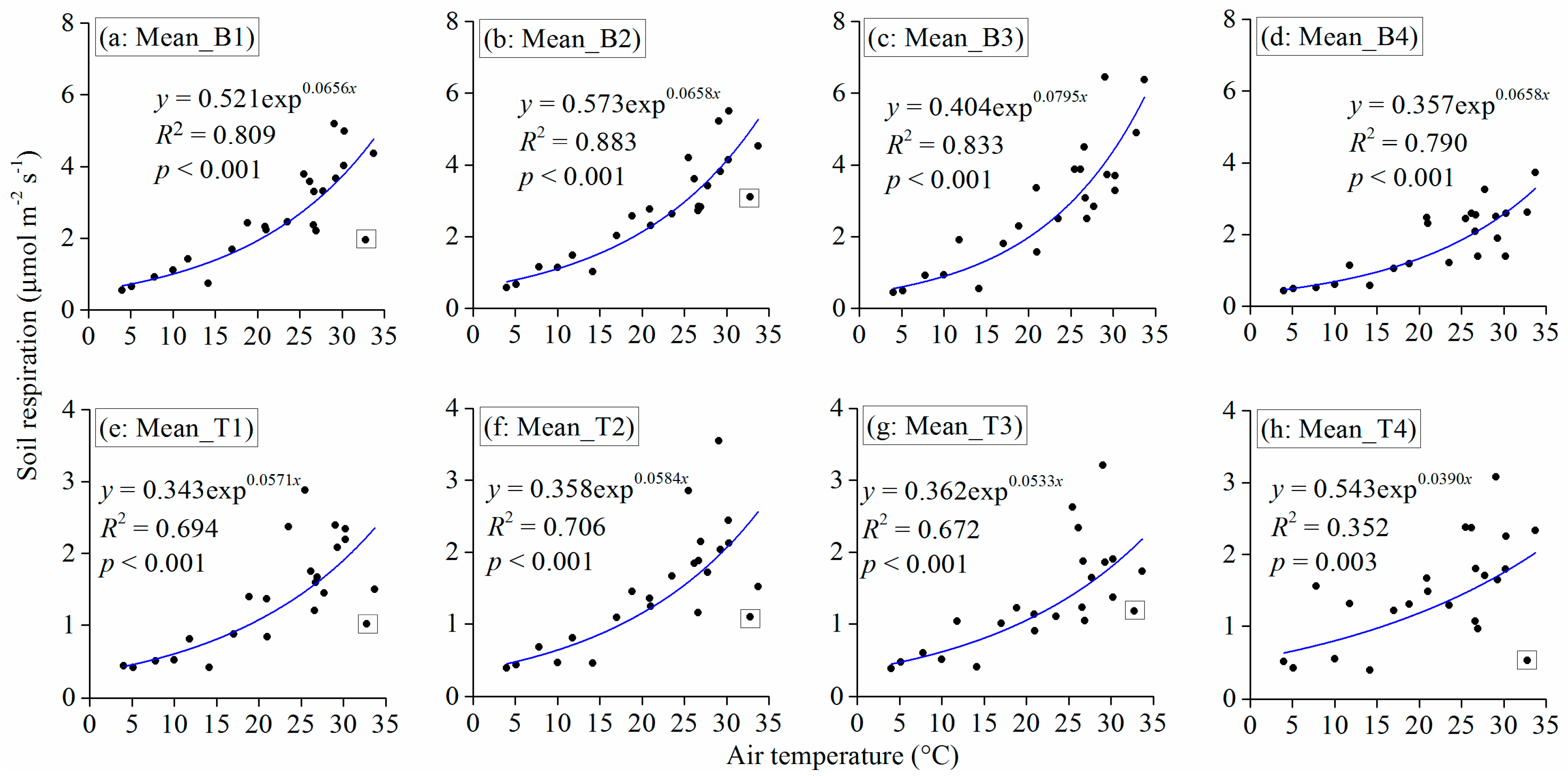
3.3. Observed and Modeled Total Soil Respiration and Heterotrophic Respiration
3.4. Influences of Climate and Land Use on the Carbon Cycle
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, M.; Feng, T.; Cai, J.; Zhou, G. Report on the World Tea Industry Development; Social Sciences Academic Press: Beijing, China, 2017; pp. 1–383. ISBN 978-7-5201-0325-1. [Google Scholar]
- Food and Agriculture Organization of the United Nations. World Tea Production and Trade: Current and Future Development; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Lu, E.; Liu, S.; Luo, Y.; Zhao, W.; Li, H.; Chen, H.; Zeng, Y.; Liu, P.; Wang, X.; Higgins, R.W.; et al. The atmospheric anomalies associated with the drought over the Yangtze River basin during spring 2011. J. Geophys. Res. Atmos. 2014, 119, 5881–5894. [Google Scholar] [CrossRef]
- China Meteorological Administration (CMA). China Climate Bulletin; CMA: Beijing, China, 2016. [Google Scholar]
- Herring, S.; Christidis, N.; Hoell, A.; Kossin, J.; Schreck, I.I.I.C.; Stott, P. Explaining Extreme Events of 2016 from a Climate Perspective. Bull. Am. Meteorol. Soc. 2018, 99, S1–S157. [Google Scholar] [CrossRef]
- Allan, R.; Soden, B. Atmospheric warming and amplification of precipitation extremes. Science 2008, 321, 1481–1484. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, N.; Seto, S.; Kanae, S.; Maeda, E.E.; Oki, T. Does higher surface temperature intensify extreme precipitation? Geophys. Res. Lett. 2011, 38, L16708. [Google Scholar] [CrossRef]
- Fischer, E.M.; Kutti, R. Observed heavy precipitation increase confirms theory and early models. Nat. Clim. Chang. 2016, 6, 986–991. [Google Scholar] [CrossRef]
- Wang, G.; Wang, D.; Trenberth, K.E.; Erfanian, A.; Yu, M.; Bosilovich, M.G.; Parr, D.T. The peak structure and future changes of the relationships between extreme precipitation and temperature. Nat. Clim. Chang. 2017, 7, 268–275. [Google Scholar] [CrossRef]
- Sterl, A.; Severijns, C.; Dijkstra, H.; Hazeleger, W.; van Oldenborgh, G.J.; van den Broeke, M.; Burgers, G.; van den Hurk, B.; van Leeuwen, P.J.; van Velthoven, P. When can we expect extremely high surface temperatures? Geophys. Res. Lett. 2008, 35, L14703. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; Mcdowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; 1535p. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Q.; Singh, V.P.; Shi, P.; Huang, Q.; Sun, P. Nonparametric integrated agrometeorological drought monitoring: Model development and application. J. Geophys. Res. Atmos. 2018, 123, 73–88. [Google Scholar] [CrossRef]
- Yang, J.; Li, M.; Xiao, L.; Zhou, H.; Guan, X.; Li, X.; Su, Z.; Xie, X.; Zong, Q.; Yang, Q. Annual Reports on China’s Tea Industry; Social Sciences Academic Press: Beijing, China, 2017; ISBN 978-7-5201-1707-4. [Google Scholar]
- SFAPRC. Forest Resources in China—The 8th National Forest Inventory; State Forestry Administration: Beijing, China, 2015. [Google Scholar]
- Song, X.; Zhou, G.; Jiang, H.; Yu, S.; Fu, J.; Li, W.; Wang, W.; Ma, Z.; Peng, C. Carbon sequestration by Chinese bamboo forests, and their ecological benefits: Assessment of potential, problems, and future challenges. Environ. Rev. 2011, 19, 418–428. [Google Scholar] [CrossRef]
- Nath, A.J.; Lal, R.; Das, A.K. Managing woody bamboos for carbon farming and carbon trading. Glob. Ecol. Conserv. 2015, 3, 654–663. [Google Scholar] [CrossRef]
- McClure, F.A. The Bamboos: A Fresh Perspective; Harvard University Press: Cambridge, MA, USA, 1966; p. 345. [Google Scholar]
- Xu, X.; Yang, G.; Tan, Y.; Tang, X.; Jiang, H.; Sun, X.; Zhuang, Q.; Li, H. Impacts of land use changes on net ecosystem production in the Taihu Lake Basin of China from 1985 to 2010. J. Geophys. Res. Biogeosci. 2017, 122, 690–707. [Google Scholar] [CrossRef]
- Liao, K.; Lv, L.; Yang, G.; Zhu, Q. Sensitivity of simulated hillslope subsurface flow to rainfall patterns, soil texture and land use. Soil Use Manag. 2016, 32, 422–432. [Google Scholar] [CrossRef]
- Liao, K.; Zhou, Z.; Lai, X.; Zhu, Q.; Feng, H. Evaluation of different approaches for identifying optimal sites to predict mean hillslope soil moisture content. J. Hydrol. 2017, 547, 10–20. [Google Scholar] [CrossRef]
- Lai, X.; Zhou, Z.; Zhu, Q.; Liao, K. Comparing the spatio-temporal variations of soil water content and soil free water content at the hillslope scale. Catena 2018, 160, 366–375. [Google Scholar] [CrossRef]
- Lai, X.; Zhu, Q.; Zhou, Z.; Liao, K. Influences of sampling size and pattern on the uncertainty of correlation estimation between soil water content and its influencing factors. J. Hydrol. 2017, 555, 41–50. [Google Scholar] [CrossRef]
- Hutchinson, G.L.; Livingston, G.P. Use of chamber systems to measure trace gas fluxes. In Agricultural Ecosystem Effects on Trace Gases and Global Climate; Harper, L.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1993; pp. 79–93. [Google Scholar]
- Oleson, K.; Lawrence, D.M.; Bonan, G.B.; Drewniak, B.; Huang, M.; Koven, C.D.; Levis, S.; Li, F.; Riley, W.J.; Subin, Z.M.; et al. Technical Description of Version 4.5 of the Community Land Model (CLM); NCAR Technical Note NCAR/TN-503CSTR; NCAR: Boulder, CO, USA, 2013; 420p. [Google Scholar]
- Running, S.W.; Hunt, E.R., Jr. Generalization of a forest ecosystem process model for other biomes, BIOME-BGC, and an applicationfor global-scale models. In Scaling Physiological Processes: Leaf to Globe; Ehleringer, J.R., Field, C., Eds.; Academic Press: San Diego, CA, USA, 1993; pp. 141–158. [Google Scholar]
- White, M.A.; Thornton, P.E.; Running, S.W.; Nemani, R.R. Parameterization and sensitivity analysis of the Biome-BGC terrestrial ecosystem model: Net primary production controls. Earth Interact. 2000, 4, 1–85. [Google Scholar] [CrossRef]
- Thornton, P.E.; Law, B.E.; Gholz, H.L.; Clark, K.L.; Falge, E.; Ellsworth, D.S.; Goldstein, A.H.; Monson, R.K.; Hollinger, D.; Falk, M.; et al. Modeling and measuring the effects of disturbance history and climate on carbon and water budgets in evergreen needleleaf forests. Agric. For. Meteorol. 2002, 113, 185–222. [Google Scholar] [CrossRef]
- Parton, W.; Stewart, J.; Cole, C. Dynamics of C, N, P and S in Grassland Soils—A Model. Biogeochemistry 1988, 5, 109–131. [Google Scholar] [CrossRef]
- Fu, C.; Lee, X.; Griffis, T.J.; Wang, G.; Wei, Z. Influences of root hydraulic redistribution on N2O emissions at AmeriFlux sites. Geophys. Res. Lett. 2018, 45, 5135–5143. [Google Scholar] [CrossRef]
- Fu, C.; Wang, G.; Bible, K.; Goulden, M.L.; Saleska, S.R.; Scott, R.L.; Cardon, Z.G. Hydraulic redistribution affects modeled carbon cycling via soil microbial activity and suppressed fire. Glob. Chang. Biol. 2018, 24, 3472–3485. [Google Scholar] [CrossRef] [PubMed]
- Thornton, P.E.; Rosenbloom, N.A. Ecosystem model spin-up: Estimating steady state conditions in a coupled terrestrial carbon and nitrogen cycle model. Ecol. Model. 2005, 189, 25–48. [Google Scholar] [CrossRef]
- Fu, C.; Wang, G.; Goulden, M.L.; Scott, R.L.; Bible, K.; Cardon, Z.G. Combined measurement and modeling of the hydrological impact of hydraulic redistribution using CLM4.5 at eight AmeriFlux sites. Hydrol. Earth Syst. Sci. 2016, 20, 2001–2018. [Google Scholar] [CrossRef]
- Zhu, Q.; Castellano, M.J.; Yang, G.S. Coupling soil water processes and nitrogen cycle across spatial scales: Potentials, bottlenecks and solutions. Earth-Sci. Rev. 2018. [Google Scholar] [CrossRef]
- Clapp, R.B.; Hornberger, G.M. Empirical equations for some soil hydraulic properties. Water Resour. Res. 1978, 14, 601–604. [Google Scholar] [CrossRef]
- Li, X.; Mao, F.; Du, H.; Zhou, G.; Xu, X.; Han, N.; Sun, S.; Gao, G.; Chen, L. Assimilating leaf area index of three typical types of subtropical forest in China from MODIS time series data based on the integrated ensemble Kalman filter and PROSAIL model. ISPRS J. Photogramm. Remote Sens. 2017, 126, 68–78. [Google Scholar] [CrossRef]
- Dutta, R. Impact of age and management factors on tea yield and modelling the influence of leaf area index on yield variations. Sci. Asia 2011, 37, 83–87. [Google Scholar] [CrossRef]
- Wei, Z.; Yoshimura, K.; Wang, L.; Miralles, D.; Jasechko, S.; Lee, X. Revisiting the contribution of transpiration to global terrestrial evapotranspiration. Geophys. Res. Lett. 2017, 44, 2792–2801. [Google Scholar] [CrossRef]
- Kutsch, W.L.; Staack, A.; Wötzel, J.; Middelhoff, U.; Kappen, L. Field measurements of root respiration and total soil respiration in an alder forest. New Phytol. 2001, 150, 157–168. [Google Scholar] [CrossRef]
- Vicca, S.; Balzarolo, M.; Filella, I.; Granier, A.; Herbst, M.; Knohl, A.; Longdoz, B.; Mund, M.; Nagy, Z.; Pintér, K.; et al. Remotely-sensed detection of effects of extreme droughts on gross primary production. Sci. Rep. 2016, 6, 28269. [Google Scholar] [CrossRef] [PubMed]
- Reichstein, M.; Bahn, M.; Ciais, P.; Frank, D.; Mahecha, M.D.; Seneviratne, S.I.; Zscheischler, J.; Beer, C.; Buchmann, N.; Frank, D.C.; et al. Climate extremes and the carbon cycle. Nature 2013, 500, 287–295. [Google Scholar] [CrossRef] [PubMed]



| Bamboo Plot | 4–7 cm | 7–12 cm | 21–34 cm | |
|---|---|---|---|---|
| B1 | 5 cm | 0.58 (0.61, 0.65) | − | − |
| 10 cm_1 | − | 0.56 (0.63, 0.65) | − | |
| 10 cm_2 | − | 0.53 (0.57, 0.70) | − | |
| 10 cm_3 | − | 0.56 (0.58, 0.54) | − | |
| 30 cm | − | − | 0.36 (0.40, 0.49) | |
| B2 | 5 cm | 0.61 (0.64, 0.62) | − | − |
| 10 cm_1 | − | 0.63 (0.64, 0.60) | − | |
| 10 cm_2 | − | 0.53 (0.54, 0.47) | − | |
| 10 cm_3 | − | 0.54 (0.60, 0.49) | − | |
| 30 cm | − | − | 0.55 (0.52, 0.53) | |
| B4 | 5 cm | 0.59 (0.61, 0.62) | − | − |
| 10 cm | − | 0.50 (0.55, 0.50) | − | |
| 30 cm | − | − | 0.51 (0.52, 0.51) | |
| 40 cm | − | − | 0.47 (0.48, 0.47) | |
| Tea Plot | 4–6 cm | 6–11 cm | 29–49 cm | |
| T2 | 5 cm_1 | 0.22 (0.21, 0.32) | − | − |
| 5 cm_2 | 0.64 (0.64, 0.41) | − | − | |
| 10 cm_1 | − | 0.09 (0.09, −) | − | |
| 10 cm_2 | − | 0.43 (0.44, 0.28) | − | |
| 30 cm | − | − | 0.52 (0.54, 0.74) | |
| T3 | 5 cm_1 | 0.60 (0.63, 0.74) | − | − |
| 5 cm_2 | 0.24 (0.23, 0.22) | − | − | |
| 10 cm | − | 0.60 (0.65, 0.72) | − | |
| 30 cm | − | − | 0.36 (0.44, 0.55) | |
| T4 | 5 cm_1 | 0.62 (0.67, 0.75) | − | − |
| 5 cm_2 | 0.39 (0.39, 0.42) | − | − | |
| 10 cm_1 | − | 0.65 (0.68, 0.80) | − | |
| 10 cm_2 | − | 0.40 (0.41, −) | − | |
| 30 cm | − | − | 0.47 (0.55, 0.61) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, C.; Zhu, Q.; Yang, G.; Xiao, Q.; Wei, Z.; Xiao, W. Influences of Extreme Weather Conditions on the Carbon Cycles of Bamboo and Tea Ecosystems. Forests 2018, 9, 629. https://doi.org/10.3390/f9100629
Fu C, Zhu Q, Yang G, Xiao Q, Wei Z, Xiao W. Influences of Extreme Weather Conditions on the Carbon Cycles of Bamboo and Tea Ecosystems. Forests. 2018; 9(10):629. https://doi.org/10.3390/f9100629
Chicago/Turabian StyleFu, Congsheng, Qing Zhu, Guishan Yang, Qitao Xiao, Zhongwang Wei, and Wei Xiao. 2018. "Influences of Extreme Weather Conditions on the Carbon Cycles of Bamboo and Tea Ecosystems" Forests 9, no. 10: 629. https://doi.org/10.3390/f9100629
APA StyleFu, C., Zhu, Q., Yang, G., Xiao, Q., Wei, Z., & Xiao, W. (2018). Influences of Extreme Weather Conditions on the Carbon Cycles of Bamboo and Tea Ecosystems. Forests, 9(10), 629. https://doi.org/10.3390/f9100629







