Productivity and Oil Content in Relation to Jatropha Fruit Ripening under Tropical Dry-Forest Conditions
Abstract
1. Introduction
2. Materials and Methods
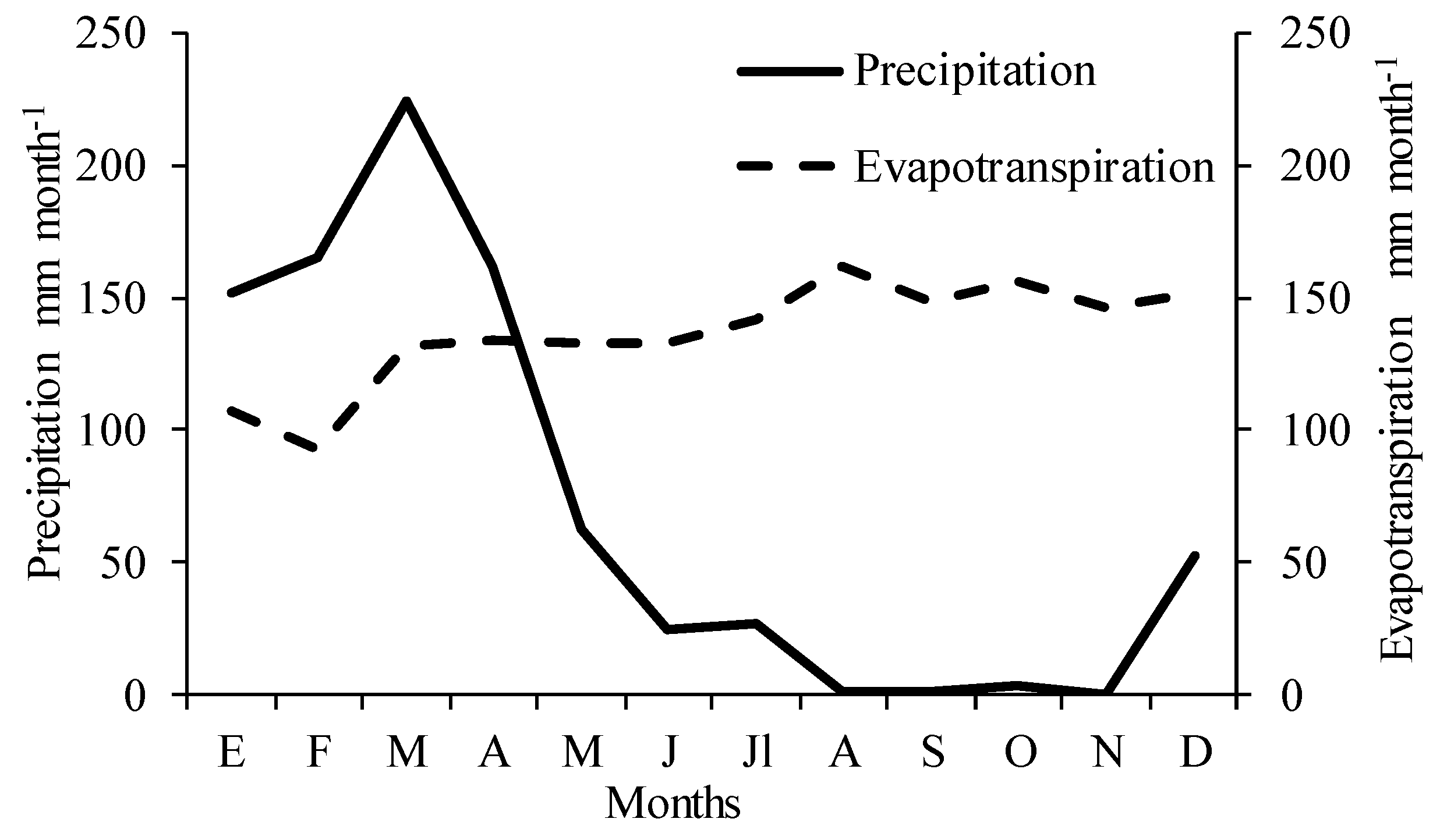
2.1. Study Area
2.2. Soil Conditions
2.3. Preparation of Experimental Site
2.4. Plot Sizes and Determination of Jatropha Productivity
2.5. Determination of Seed Weight, Moisture, and Oil Content at Different Fruit Ripening Stages
2.6. Statistical Analyses
3. Results
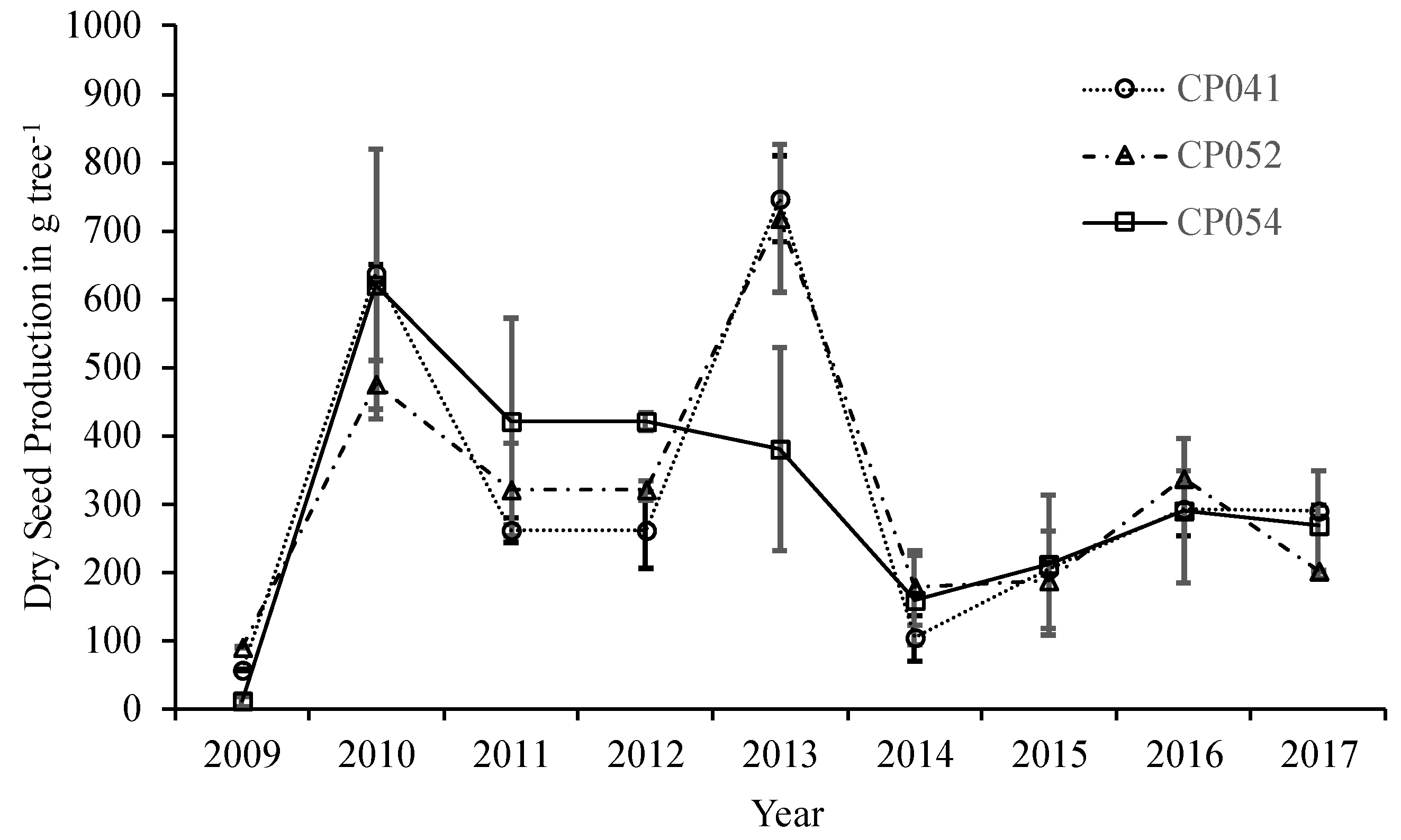
3.1. Jatropha Seed Productivity
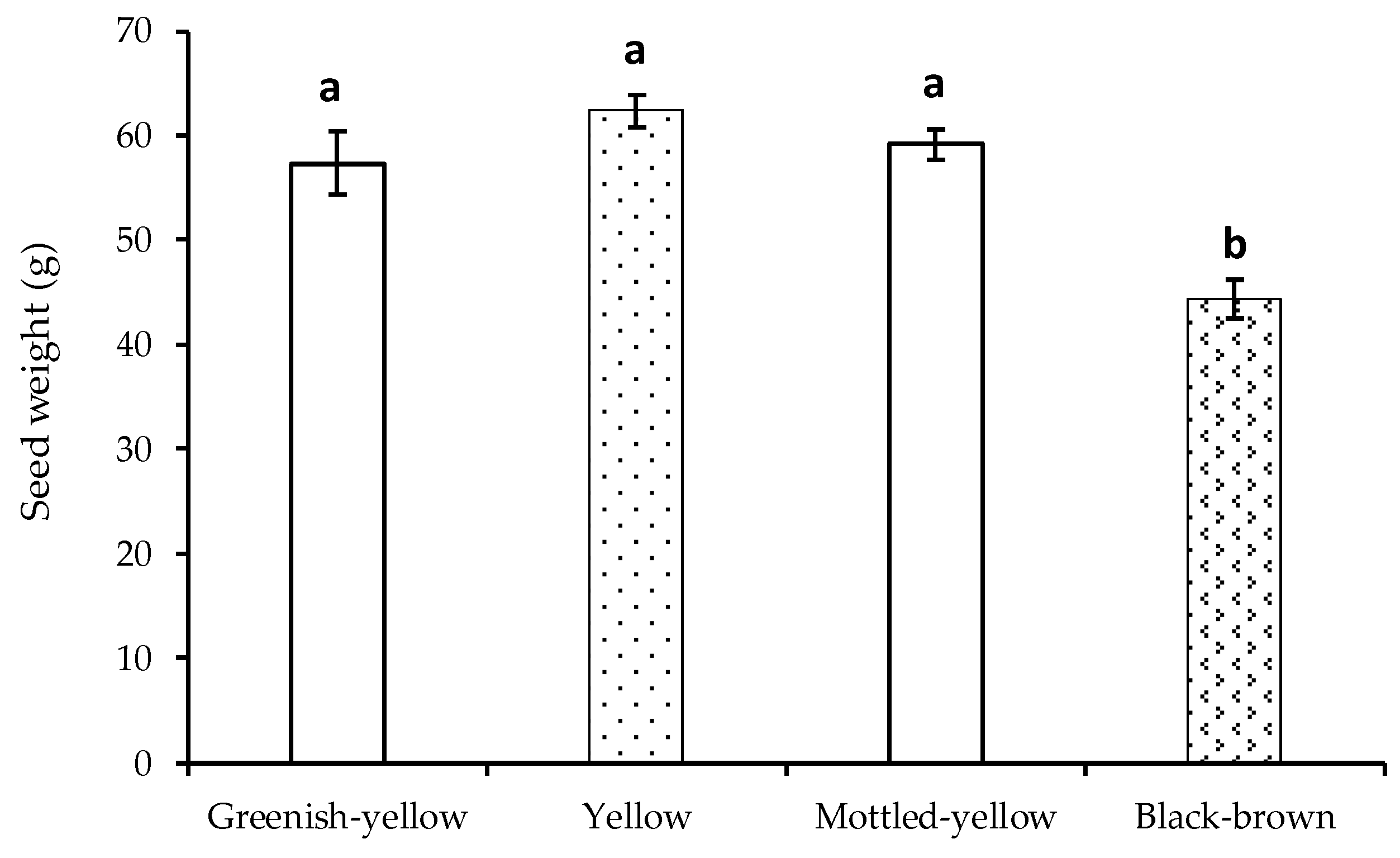
3.2. Seed Dry Weight and Fruit Ripening Stage
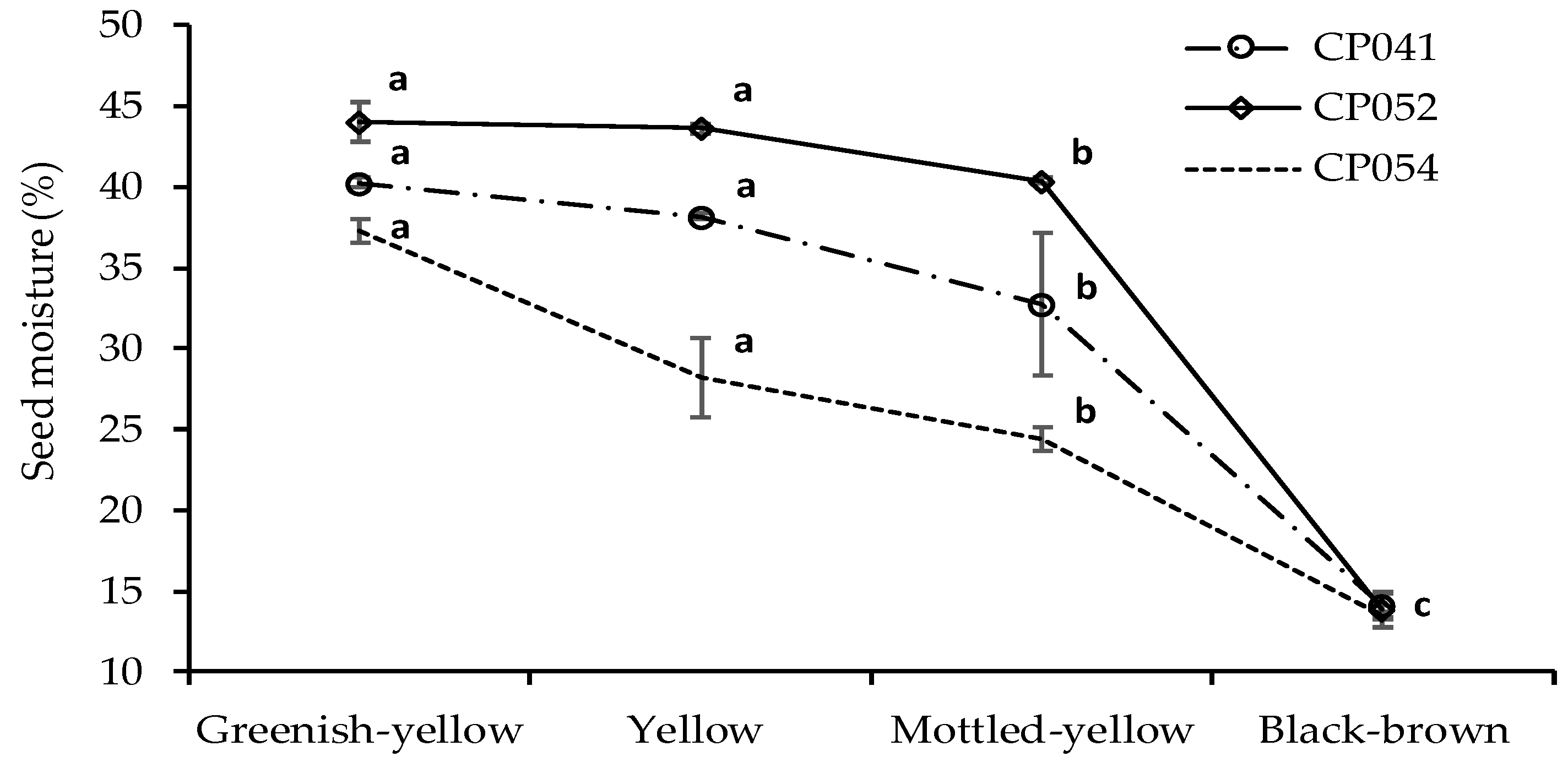
3.3. Seed Moisture Content and Fruit Ripening Stage
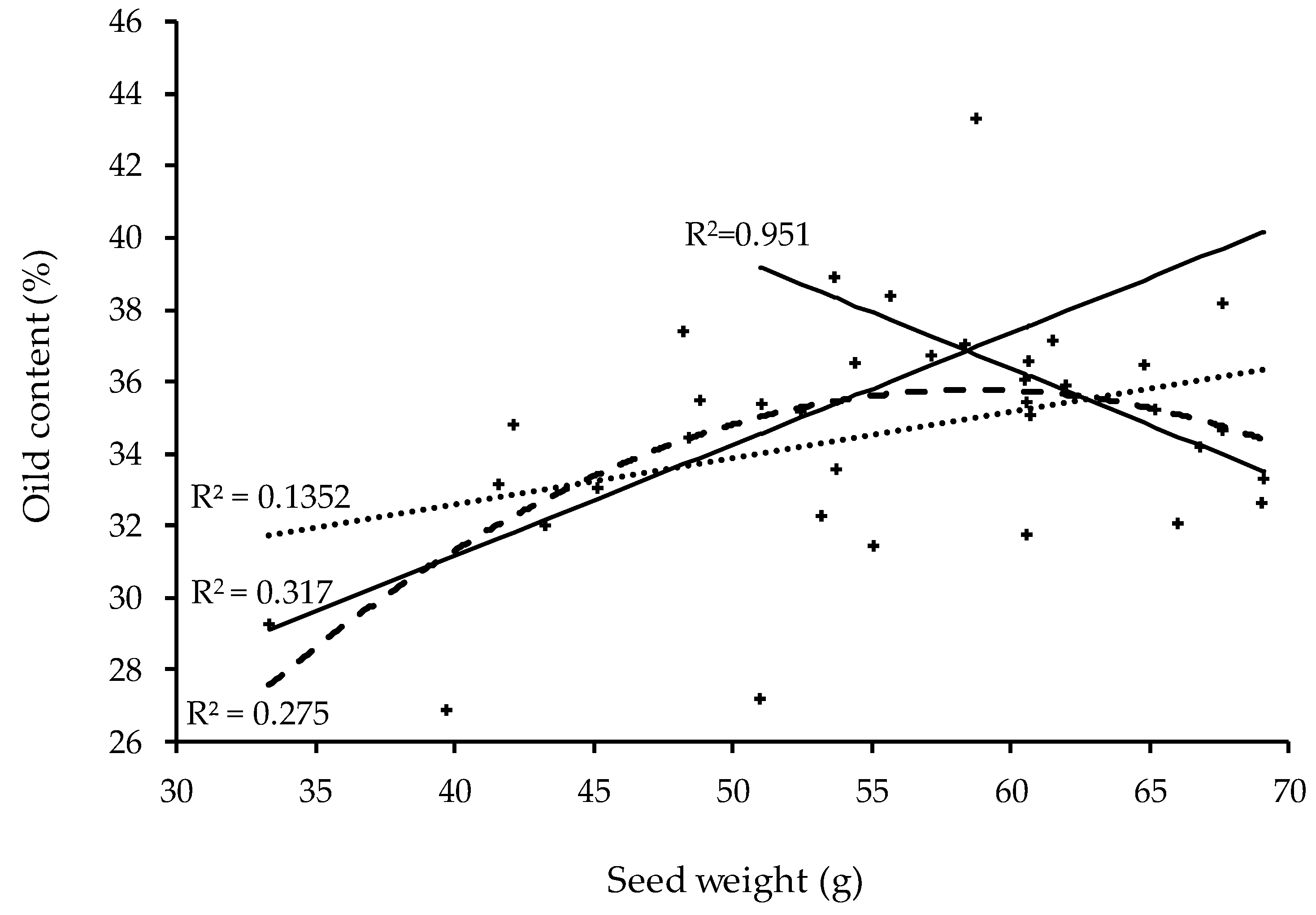
3.4. Oil Content and Fruit Ripening Stage
4. Discussion
4.1. Jatropha Maturity and Productivity
4.2. Seed Dry Weight
4.3. Seed Moisture Content
4.4. Oil Content
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cañadas-López, Á.; Rade-Loor, D.; Domínguez-Andrade, J.M.; Vargas-Hernández, J.J.; Molina-Hidrovo, C.; Macías-Loor, C.; Wehenkel, C. Variation in seed production of Jatropha curcas L. accessions under tropical dry forest conditions in Ecuador. New For. 2017, 48, 785–799. [Google Scholar] [CrossRef]
- Rade, D.; Cañadas, Á.; Zambrano, C. Silvopastoral system economical and financial feasibility with Jatropha curcas L. in Manabí, Ecuador. MVZ Cordoba 2017, 22, 6241–6255. [Google Scholar] [CrossRef]
- Afiff, S.A. Engineering the jatropha hype in Indonesia. Sustainability 2014, 6, 1686–1704. [Google Scholar] [CrossRef]
- Hunsberger, C.; Bolwig, S.; Corbera, E.; Creutzig, F. Livelihood impacts of biofuel crop production: Implications for governance. Geoforum 2014, 54, 248–260. [Google Scholar] [CrossRef]
- Everson, C.S.; Mengistu, M.G.; Gush, M.B. A field assessment of the agronomic performance and water use of Jatropha curcas in South Africa. Biomass Bioenergy 2013, 59, 59–69. [Google Scholar] [CrossRef]
- Edrisi, S.A.; Dubey, R.K.; Tripathi, V.; Bakshi, M.; Srivastava, P.; Jamil, S.; Singh, H.B.; Singh, N.; Abhilash, P.C. Jatropha curcas L.: A crucified plant waiting for resurgence. Renew. Sustain. Energy Rev. 2015, 41, 855–862. [Google Scholar] [CrossRef]
- Walmsley, D.; Bailis, R.; Klein, A.M. A global synthesis of jatropha cultivation: Insights into land use change and management practices. Environ. Sci. Technol. 2016, 50, 8993–9002. [Google Scholar] [CrossRef] [PubMed]
- Makungwa, S.D.; Chittock, A.; Skole, D.L.; Kanyama-Phiri, G.Y.; Woodhouse, I.H. Allometry for biomass estimation in jatropha trees planted as boundary hedge in farmers’ fields. Forests 2013, 4, 218–233. [Google Scholar] [CrossRef]
- Cañadas, L. El mapa Ecológico y Bioclimático del Ecuador; Editores Asociados Cia. Ltd.: Quito, Ecuador, 1983; pp. 50–120. [Google Scholar]
- Van Eijck, J.; Romijn, H.; Balkema, A.; Faaij, A. Global experience with jatropha cultivation for bioenergy: An assessment of socio-economic and environmental aspect. Renew. Sustain. Energ. Rev. 2014, 32, 869–889. [Google Scholar] [CrossRef]
- GTZ. Jatropha Reality Check: A Field Assessment of the Agronomic and Economic Viability of Jatropha and Other Oilseed Crops in Kenya; Study conducted by Endelevu Energy in collaboration with World Agroforestry Centre and Kenya Forestry Research Institute; GTZ: Nairobi, Kenya, 2009; 158p. [Google Scholar]
- Samsuri, A.; Zoveidaviampoor, M. Does the maturity of Jatropha curcas L. affect the quality and quantity of the yield of oil for biodiesel production? Int. J. Green Energy 2014, 11, 193–205. [Google Scholar] [CrossRef]
- Wassner, D.; Borrás, M.; Vaca-García, C.; Ploschuk, E. Harvest date modifies seed quality and oil composition of Jatropha curcas growth under subtropical conditions in Argentina. Ind. Crops Prod. 2016, 9, 318–326. [Google Scholar] [CrossRef]
- Silva, L.J.; Dias, D.C.F.S.; Dias, L.A.S.; Hilst, P.C. Physiological quality of Jatropha curcas L. seeds harvested at different development stage. Seed Sci. Technol. 2011, 39, 572–580. [Google Scholar] [CrossRef]
- Silip, J.J.; Tambunan, A.; Hambali, H.; Sutrisno, S.; Surahman, M. Lifecycle duration and maturity heterogeneity of Jatropha curcas Linn. J. Sustain. Dev. 2010, 3, 291–295. [Google Scholar] [CrossRef]
- Rao, G.R.; Korwar, G.R.; Shanker, A.K.; Ramakrishna, Y.S. Genetic associations, variability and diversity in seed characters, growth, reproductive phenology and yield in Jatropha curcas (L.) accessions. Trees 2008, 22, 697–709. [Google Scholar] [CrossRef]
- Kartika, I.A.; Yuliani, S.; Kailaku, S.I.; Rigal, L. Moisture sorption behavior of jatropha seed (Jatropha curcas) as a source of vegetable oil for biodiesel production. Biomass Bioenergy 2012, 36, 226–233. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S. An evaluation of multipurpose oil seed crop for industrial uses (Jatropha curcas L.): A review. Ind. Crops Prod. 2008, 28, 1–10. [Google Scholar] [CrossRef]
- Pradhan, R.C.; Naik, S.N.; Bhatnagar, N.; Vijay, V.K. Moisture-dependent physical properties of jatropha fruit. Ind. Crops Prod. 2009, 29, 341–347. [Google Scholar] [CrossRef]
- Jongschaap, R.E.E.; Corré, W.J.; Bindraban, P.S.; Brandenburg, W.A. Claims and Facts on Jatropha curcas L.: Global Jatropha curcas Evaluation; Breeding and propagation program (No. 158); Plant Research International: Wageningen, The Netherlands, 2007; 42p. [Google Scholar]
- Lal, S.B.; Mehere, B.; Chandra, R.; Larkin, A. Performance evaluation of Jatropha curcas in different districts of Uttar Pradesh. New Agric. 2004, 15, 141–144. [Google Scholar]
- Euler, H.; Gorriz, D. Case Study “Jatropha curcas”; Global Facilitation Unit for Underutilized Species (GFU) and Deutsche Gesellschaft für Technische Zusammenarbeit (GTZ): Frankfurt, Germany, 2004; 63p. [Google Scholar]
- Achten, W.M.J.; Maes, W.H.; Reubens, B.; Mathijs, E.; Singh, V.P.; Muys, B. Biomass production and allocation in Jatropha curcas L. seedlings under different levels of drought stress. Biomass Bionergy 2010, 34, 667–676. [Google Scholar] [CrossRef]
- Saturnino, H.M.; Pacheco, D.D.; Kakida, J.; Tominaga, N.; Gonçalves, N.O. Cultura do pinhão—Manso (Jatropha curcas L.). Informe Agropecuário 2005, 26, 44–78. [Google Scholar]
- Ouwens, K.D.; Francis, G.; Franken, Y.J.; Rijssenbeek, W.; Riedacker, A.; Foidl, N.; Jongschaap, R.; Bindraban, P. Position Paper on Jatropha curcas. State of the Art, Small and Large Scale Project Development; Wageningen University: Wageningen, The Netherlands, 2007; pp. 1–5. [Google Scholar]
- Manurung, R. Valorisation of jatropha curcas using the bio refinery concept. In Proceedings of the Expert Seminar on Jatropha curcas L. Agronomy and Genetics, Wageningen, The Netherland, 26–28 March 2007. [Google Scholar]
- Achten, W.M.J.; Verchot, L.; Frunken, Y.J.; Mathuijs, E.; Singh, V.P.; Aerts, R.; Muys, B. Jatropha bio-diesel production and use. Biomass Bioenergy 2008, 32, 1063–1084. [Google Scholar] [CrossRef]
- Ghosh, A.; Patolia, J.S.; Chaudhary, D.R.; Rao, S.N.; Kumar, D.; Boricha, G.N.; Zala, A. Response of Jatropha curcas under different spacing to jatropha de-oiled cake. In Proceedings of the Expert Seminar on Jatropha curcas L. Agronomy and Genetics, Wageningen, The Netherland, 26–28 March 2007. [Google Scholar]
- Iiyama, M.; Newman, D.; Munster, C.; Nyabenge, M.; Sileshi, G.; Moraa, V.; Onchieku, J.; Gaper-Mowo, J.; Jamnadass, R. Productivity of Jatropha curcas under smallholder farm conditions in Kenya. Agrofor. Syst. 2013, 87, 729–746. [Google Scholar] [CrossRef]
- Messemaker, L. The Green Myth? Assessment of the Jatropha Value Chain and Its Potential for Pro-Poor Biofuel Development in Northern Tanzania; Utrecht University: Utrecht, The Netherlands, 2008; 97p. [Google Scholar]
- Heller, J. Physic nut. Jatropha curcas L. Promoting the Conservation and Use of Underutilized and Neglected Crops; International Plant Genetic Resources Institute: Rome, Italy, 1996; 66p. [Google Scholar]
- Ginwal, H.S.; Phartyal, S.S.; Rawat, P.S.; Srivastava, R.L. Seed sources variation in morphology, germination and seedling growth of Jatropha curcas Linn. In Central India Silvae Genet 2005, 54, 76–80. [Google Scholar] [CrossRef]
- Kaushik, N. Effect of Capsule Maturity on Germination and Seedling Vigor in Jatropha curcas; Seed Science and Technology: Zürich, Switzerland, 2003; pp. 449–454. [Google Scholar]
- Rondanini, D.P.; Castro, D.N.; Searles, P.S.; Rousseaux, M.C. Contrasting patterns of fatty acid composition and oil accumulation during fruit growth in several olive varieties and locations in a non-mediterranean region. Eur. J. Agrom. 2014, 83, 79–90. [Google Scholar] [CrossRef]
- Subramanyam, K.; Dowlathabad, M.R.; Devanna, N.; Subramanyam, K. Eco-geographical variation of seed traits, oil content and categorization of Jatropha curcas (L) accessions Ecology. Eco-Environ. Cons 2010, 16, 29–33. [Google Scholar]
- Rathbauer, J.; Sonnleitner, A.; Pirot, A.; Zeller, R.; Bacovsky, R. Characterization of Jatropha curcas seeds and oil from Mali. Biomass Bioenergy 2012, 47, 201–210. [Google Scholar] [CrossRef]
- Wani, T.A.; Kitchulu, S.; Ram, G. Genetic variability studies for morphological and qualitative attributes among Jatropha curcas L. accessions grown under subtropical condition on North India. S. Afr. J. Bot. 2012, 79, 102–105. [Google Scholar] [CrossRef]
- Dias, L.D.S.; Missio, R.F.; Dias, D.C.F.S. Antiquity, botany, origin and domestication of Jatropha curcas (Euphorbiaceae), a plant species with potential for biodiesel production. Gen Mol. Res. 2012, 11, 2719–2728. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.C.F.S.; Ribeiro, F.P.; Dias, L.A.S.; Silva, D.J.H.; Vidigal, D.S. Tomato seed quality in relation to fruit maturation and port-harvest storage. Seed Sci. Technol. 2006, 34, 691–699. [Google Scholar] [CrossRef]
- Silva, L.B.; Martins, C.C.; Machado, C.G.; Nakagawa, J. Estádios de colheita e repouso pós-colheita dos frutos na qualidade de sementes de mamoneira. Rev. Bras. Sementes 2009, 31, 50–59. [Google Scholar] [CrossRef]
- Osorio, L.R.M.; Salvador, A.F.T.; Jongschaap, R.E.E.; Perez, C.A.A.; Sandoval, J.E.B.; Trindade, L.M.; Visser, R.G.F.; van Loo, E.N. High level of molecular and phenotypic biodiversity in Jatropha curcas from Central America compared to Africa, Asia and South America. BMC Plant Biol. 2014, 14, 77. [Google Scholar]
- Kaushik, N.; Kumar, K.; Kumar, S.; Kaushik, N.; Roy, S. Genetic variability and divergence studies in seed traits and oil content of Jatropha (Jatropha curcas L.) accessions. Biomass Bioenergy 2007, 31, 437–592. [Google Scholar] [CrossRef]
- Singer, S.D.; Zou, J.; Weselake, R.J. Abiotic factors influence plan storage lipid accumulation and composition. Plant Sci 2016, 243, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Aminul, M.I.; Negi, M.S.; Tripathi, S.B. Changes in oil content and fatty acid composition in Jatropha curcas during seed development. Ind. Crops Prod. 2015, 77, 508–510. [Google Scholar] [CrossRef]
- Srivastava, P.; Behera, S.; Gupta, J.; Jamil, S.; Singh, N.; Kumar, Y. Growth performance, variability in yield traits and oil content of selected accessions of Jatropha curcas L. growing in a large-scale planation site. Biomass Bioenergy 2011, 35, 3936–3942. [Google Scholar] [CrossRef]
- Wen, H.; Tang, M.; Sun, D.; Zhu, H.; Wei, J.; Chen, F.; Tang, L. Influence of Climatic Factors and Soil Types on Seed Weight and Oil Content of Jatropha curcas in Guangxi, China. Procedia Environ. Sci. 2012, 12, 439–444. [Google Scholar] [CrossRef]
- Emil, A.; Yaakob, Z.; Kumar, M.N.S.; Jahim, J.M.; Salimon, J. Comparative evaluation of physicochemical properties of jatropha seed oil from Malaysia, Indonesia and Thailand. J. Am. Oil Chem. Soc. 2010, 87, 689–695. [Google Scholar] [CrossRef]
- Pedraza, E.A.; Cayón, D.G. Caracterización morfo fisiológica de Jatropha curcas L. variedad Brasil cultivada en dos zonas de Colombia. Acta Agron. 2010, 51, 30–36. [Google Scholar] [CrossRef]
- Karaj, S.; Müller, J. Determination of physical, mechanical and chemical properties of seeds and kernels of Jatropha curcas L. Ind. Crops Prod. 2010, 32, 129–138. [Google Scholar] [CrossRef]
| pH | NH4 | P | Zn | Cu | Fe | Mn | Zn | B | K | Ca | Mg |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (in ppm) | (in meq 100 mL−1) | ||||||||||
| 7.3 | 17.12 | 23.01 | 1.82 | 7.14 | 12.11 | 23.75 | 1.82 | 0.92 | 2.07 | 20.90 | 4.02 |
| Effects | Source of Variation (p-Value) | ||
|---|---|---|---|
| Seed Weight | Seed Moisture | Oil Content | |
| Accession | 0.9688 | <0.0001 | 0.1497 |
| Fruit ripening stage | 0.0002 | <0.0001 | 0.3239 |
| Accession × fruit ripening stage | 0.9206 | <0.0001 | 0.2575 |
| Model | Equation | R2 | CV (%) | MSE | p-Value | b0 ± (se) | b1 ± (se) | b2 ± (se) |
|---|---|---|---|---|---|---|---|---|
| Linear | y = b0 + b1x | 0.135 | 8.695 | 3.012 | 0.02 | 27.441 (3.164) | 0.129 (0.056) | n.a. |
| Quadratic | y = b0 + b1x + b2x2 | 0.275 | 8.082 | 2.800 | 0.01 | −8.218 (14.444) | 1.499 (0.546) | −0.013 (0.005) |
| Piecewise § | y = b0 + b1x + b2(x − t)(x2) | 0.317 | 7.845 | 2.718 | 0.001 | 18.797 (4.082) | 0.309 (0.079) | −0.617 (0.208) |
| Country, Region | Age of Jatropha Plantation in Years | Productivity (in kg tree−1) | References |
|---|---|---|---|
| Brazil | 1 | 0.4 | Saturnino et al. [24] |
| Guatemala | 1 | 0.8 | Ouwens et al. [25] |
| Indonesia | 1 | 1.6–2.0 | Ouwens et al. [25] |
| Indonesia | 1 | 1.8 | Manurung [26] |
| India, Pradesh | 1 | 2.4 | Lal et al. [21] |
| India | 1.25 | 1.7 | Achten et al. [27] |
| India | 2 | 1.5 | Ouwens et al. [25] |
| Mali, Digini | 2 | 0.3 | Achten et al. [27] |
| India | 2.5 | 0.8 | Ghosh et al. [28] |
| India, Rajasthan | 2.5 | 0.1–0.5 | Achten et al. [27] |
| Kenya | 2–3 | <1.0 | Iiyama et al. [29] |
| Tanzania, Arusha | 3 | 0.5–2.0 | Messemaker [30] |
| Nicaragua | 5 | 4.5 | Heller [31] |
| Ecuador, CP052, 041, 054 | 6 | 0.2–0.3 | Cañadas et al. [1] |
| Ecuador, CP041 | 7 | 0.2 | Rade et al. [2] |
| Ecuador, CP041, 052, 054 | 8 | 0.1–0.3 | Present study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cañadas-López, Á.; Rade-Loor, D.Y.; Siegmund-Schultze, M.; Iriarte-Vera, M.; Domínguez-Andrade, J.M.; Vargas-Hernández, J.; Wehenkel, C. Productivity and Oil Content in Relation to Jatropha Fruit Ripening under Tropical Dry-Forest Conditions. Forests 2018, 9, 611. https://doi.org/10.3390/f9100611
Cañadas-López Á, Rade-Loor DY, Siegmund-Schultze M, Iriarte-Vera M, Domínguez-Andrade JM, Vargas-Hernández J, Wehenkel C. Productivity and Oil Content in Relation to Jatropha Fruit Ripening under Tropical Dry-Forest Conditions. Forests. 2018; 9(10):611. https://doi.org/10.3390/f9100611
Chicago/Turabian StyleCañadas-López, Álvaro, Diana Yasbhet Rade-Loor, Marianna Siegmund-Schultze, Marys Iriarte-Vera, Juan Manuel Domínguez-Andrade, Jesús Vargas-Hernández, and Christian Wehenkel. 2018. "Productivity and Oil Content in Relation to Jatropha Fruit Ripening under Tropical Dry-Forest Conditions" Forests 9, no. 10: 611. https://doi.org/10.3390/f9100611
APA StyleCañadas-López, Á., Rade-Loor, D. Y., Siegmund-Schultze, M., Iriarte-Vera, M., Domínguez-Andrade, J. M., Vargas-Hernández, J., & Wehenkel, C. (2018). Productivity and Oil Content in Relation to Jatropha Fruit Ripening under Tropical Dry-Forest Conditions. Forests, 9(10), 611. https://doi.org/10.3390/f9100611





