Leaf Phenology Variation within the Canopy and Its Relationship with the Transpiration of Populus tomentosa under Plantation Conditions
Abstract
1. Introduction
2. Materials and Methods
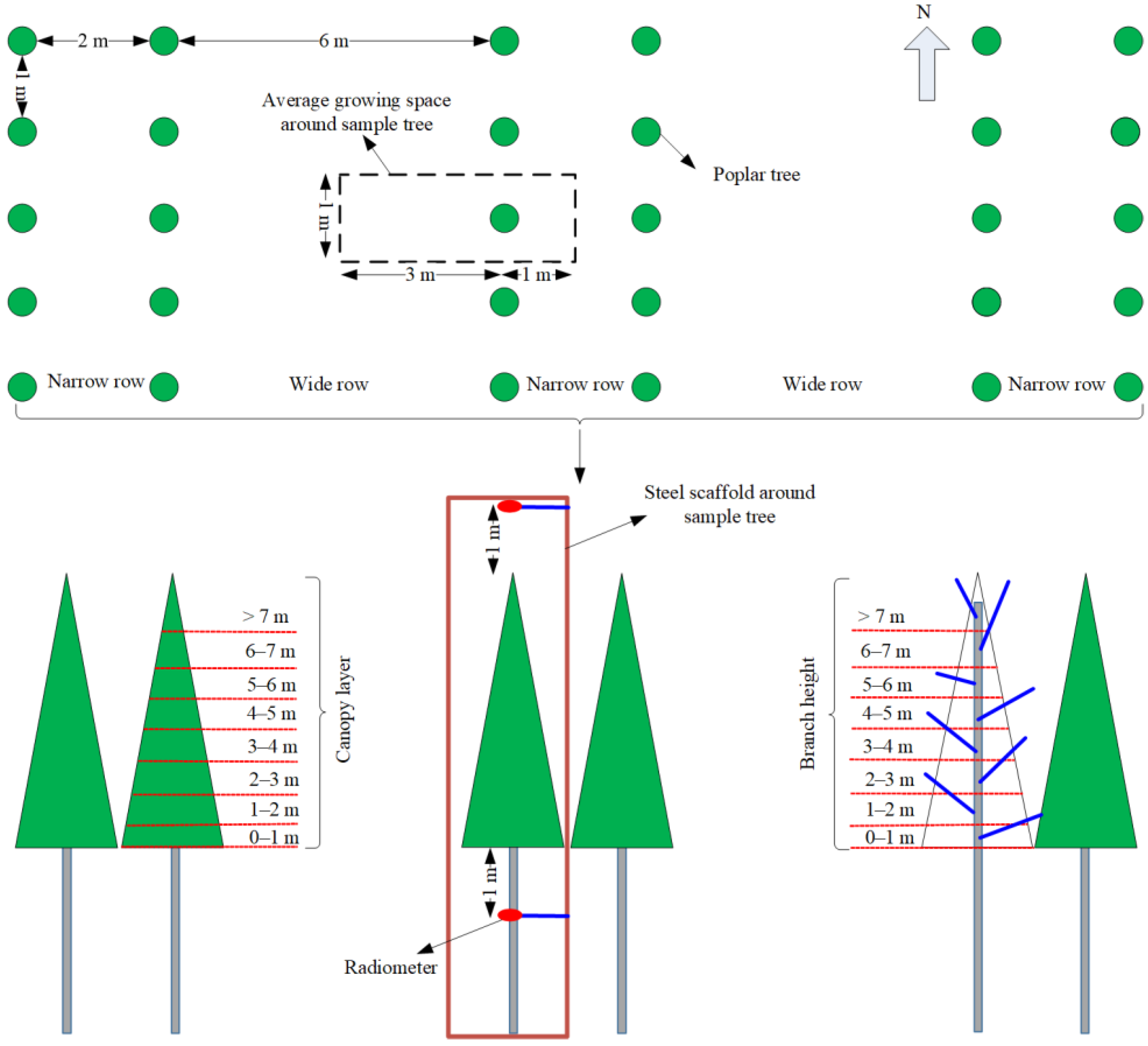
2.1. Experimental Site Description and Experimental Plantation
2.2. Field Experiment Measurements
2.2.1. Leaf Number and Leaf Area
2.2.2. Trunk Sap Flux
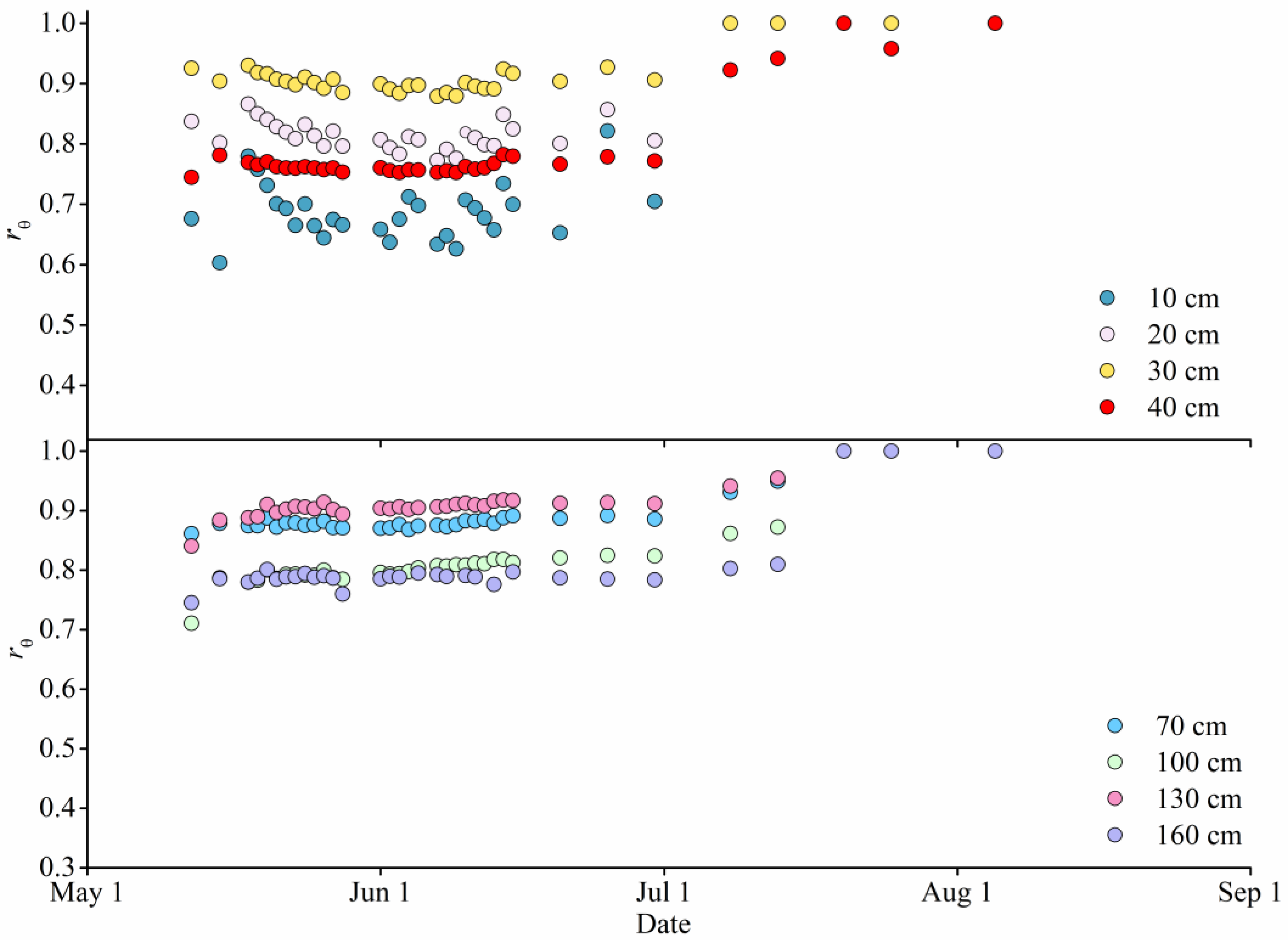
2.2.3. Soil Water Content
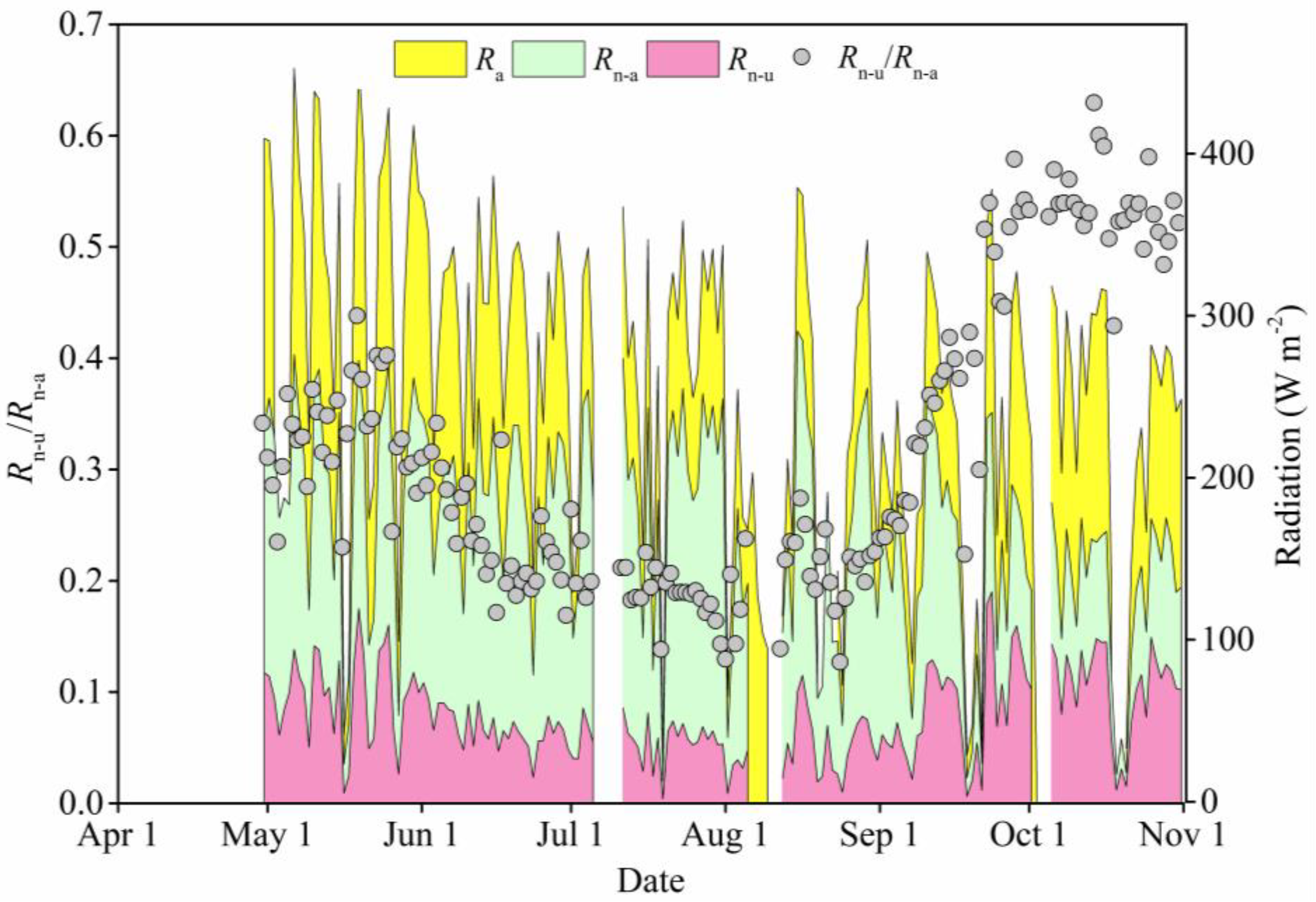
2.2.4. Net Solar Radiation Above and under the Sample Tree Canopy
2.2.5. Leaf Physiological Parameters and Meteorological Factors
2.3. Statistical Analysis
3. Results
3.1. Variation of Soil Water Availability
3.2. Dynamics of Light Interception by the Canopy
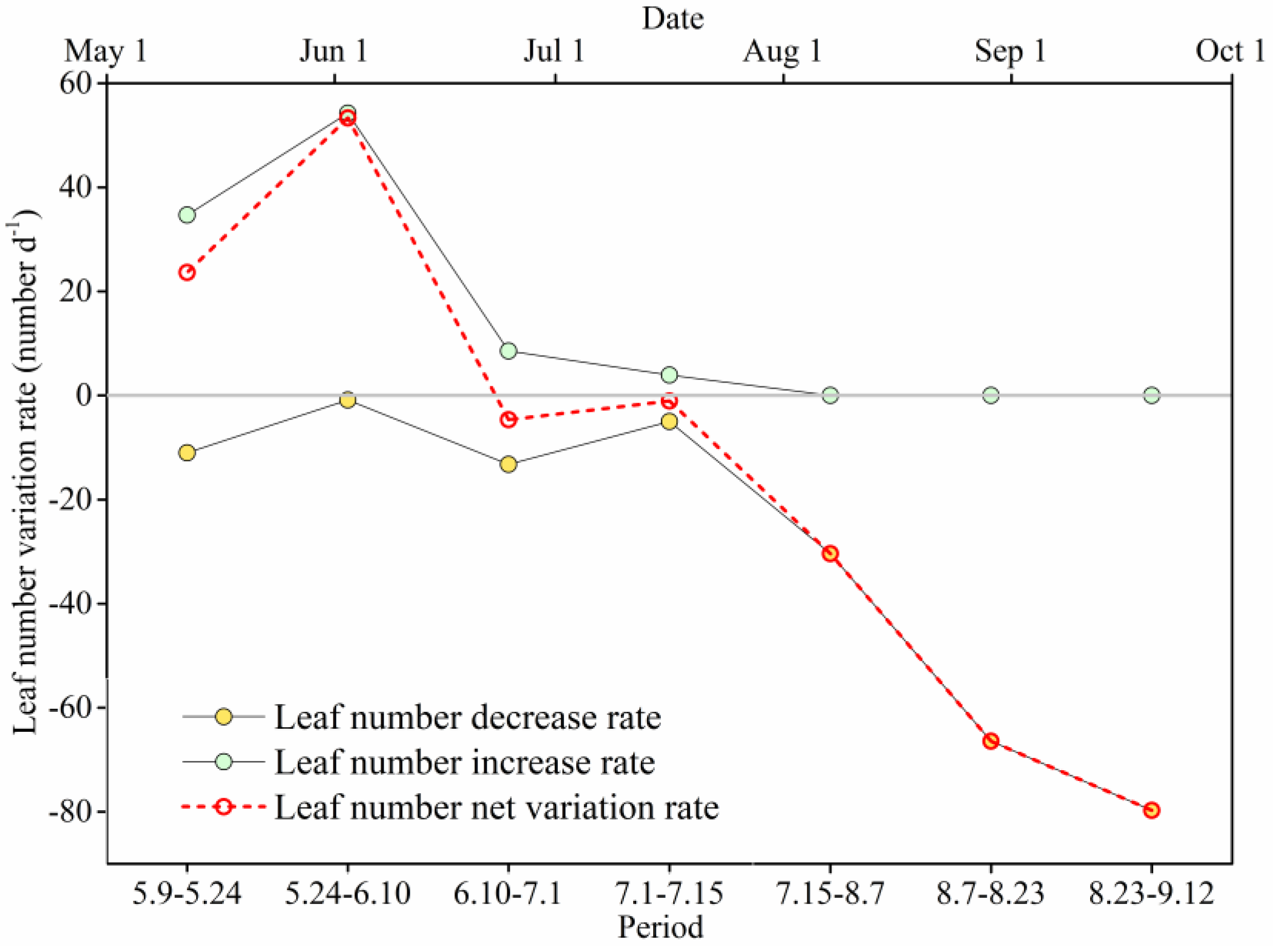
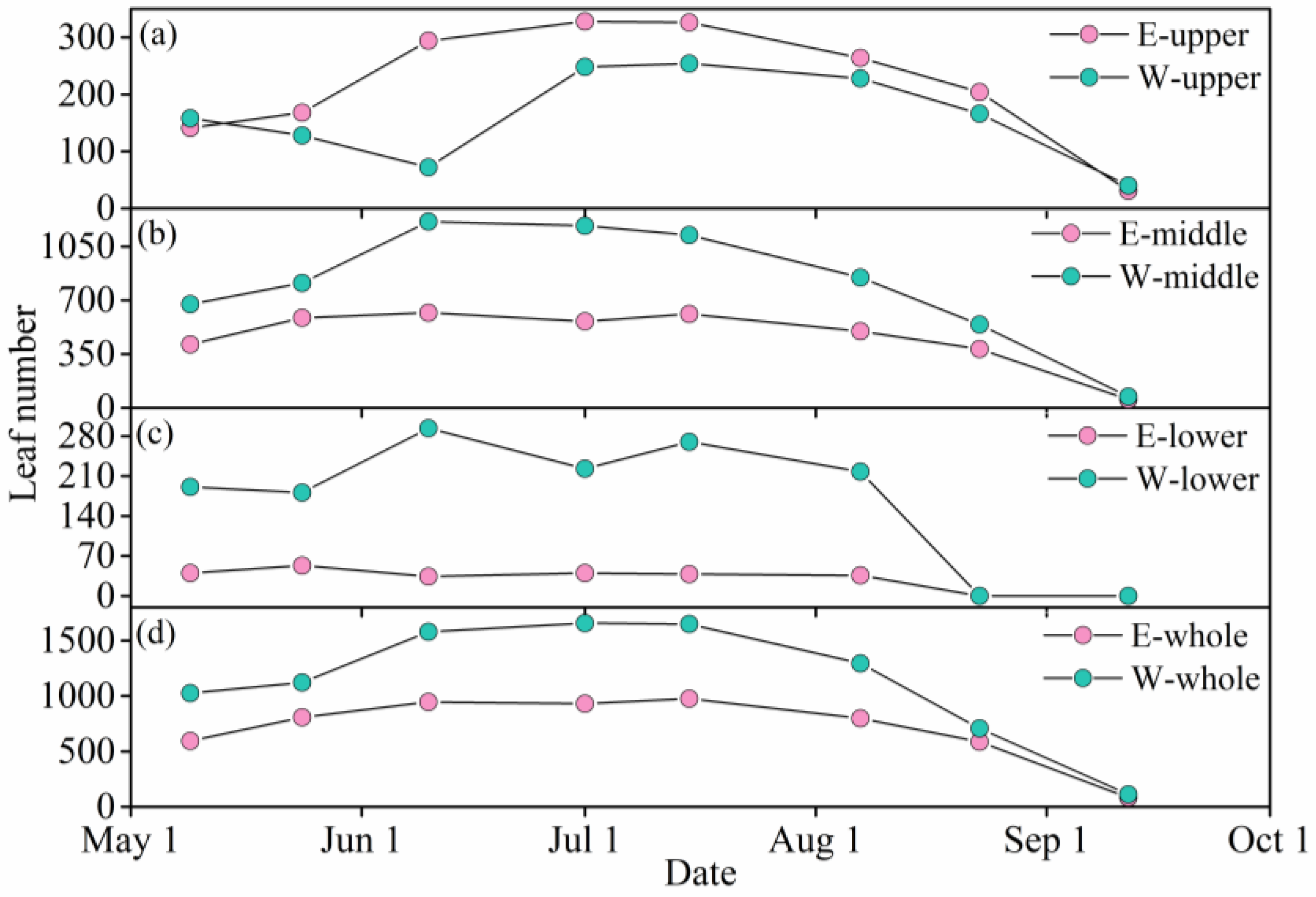
3.3. Leaf Number Variation in Different Branch Heights
3.4. Leaf Number Variation in Different Canopy Layers
3.5. Leaf Number Variation in Different Azimuthal Sides
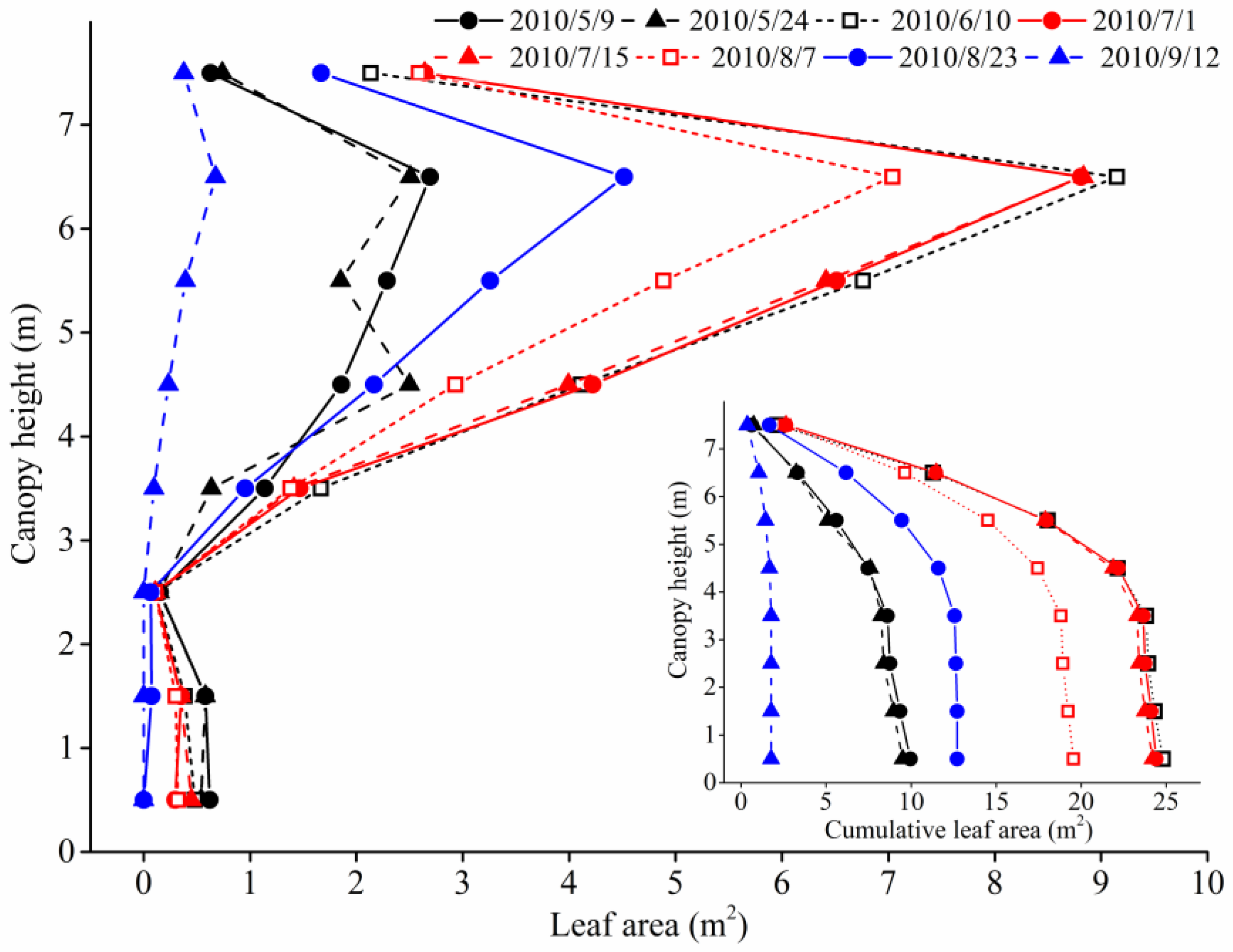
3.6. Leaf Area Dynamics and Vertical Distribution
3.7. Leaf Physiological Parameter Variation within the Canopy
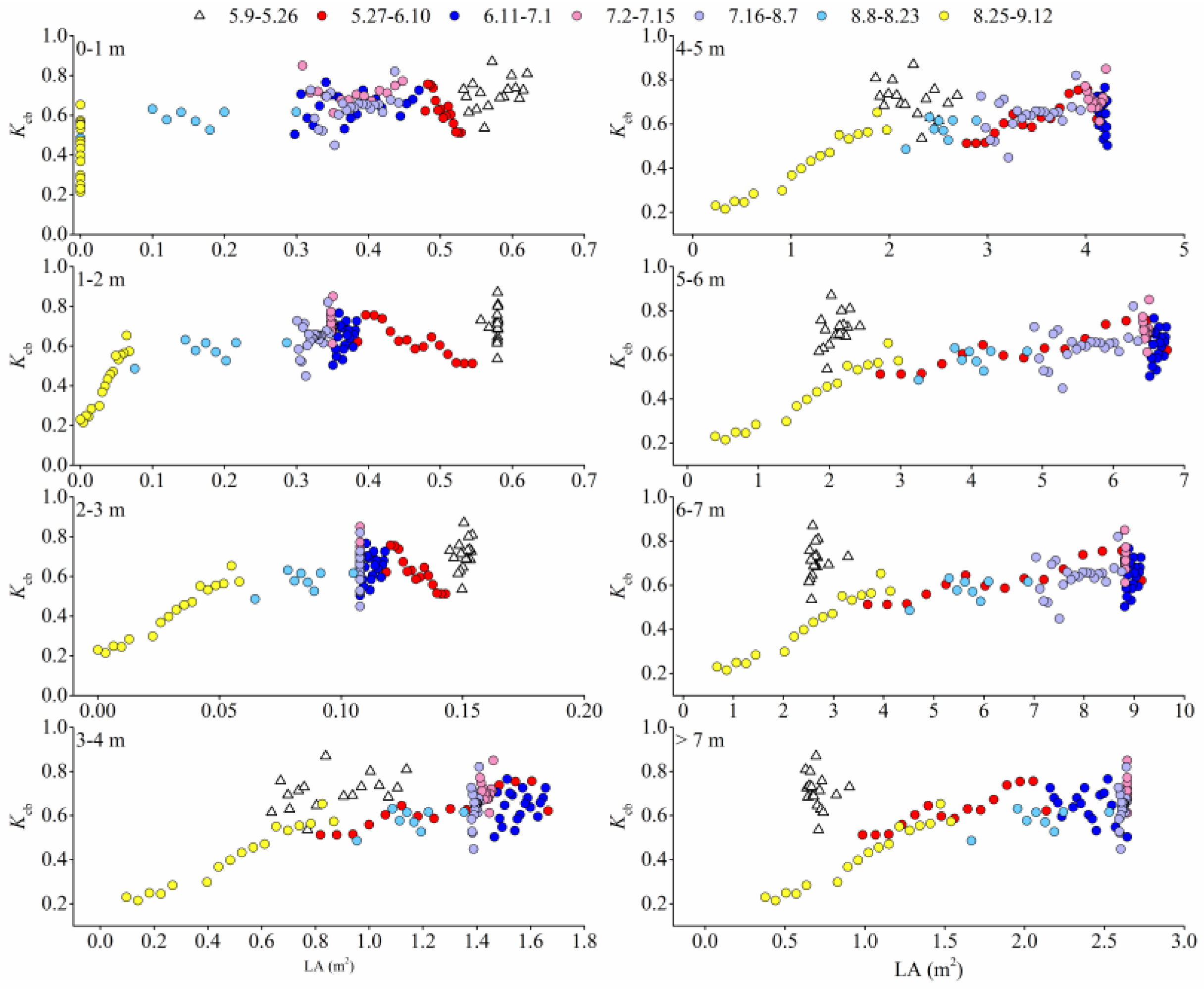
3.8. Response of Transpiration to Leaf Areas in Different Canopy Layers
4. Discussion
4.1. Leaf Phenology Variation within the Canopy
4.2. Leaf Area Vertical Distribution and Its Relationship with Canopy Light Interception
4.3. The Relationship between Transpiration and Leaf Area
4.4. Contribution of Leaves in Different Canopy Layers to Tree Transpiration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- SFA. Report of Forest Resources in China (2009–2013); China Forestry Press: Beijing, China, 2014. (In Chinese) [Google Scholar]
- Zheng, S.K. High Yield Cultivation of Poplar; Golden Shield Press: Beijing, China, 2006. (In Chinese) [Google Scholar]
- Fang, S.Z. Silviculture of poplar plantation in China: A review. Chin. J. Appl. Ecol. 2008, 19, 2308–2316, (In Chinese with English abstract). [Google Scholar]
- Liu, S.R.; Yang, Y.J.; Wang, H. Development strategy and management countermeasures of planted forests in China: Transforming from timber-centered single objective management towards multi-purpose management for enhancing quality and benefits of ecosystem services. Acta Ecol. Sin. 2018, 38, 1–10, (In Chinese with English abstract). [Google Scholar]
- Xi, B.Y.; Li, G.D.; Bloomberg, M.; Jia, L.M. The effects of subsurface irrigation at different soil water potential thresholds on the growth and transpiration of Populus tomentosa in the North China Plain. Aust. For. 2014, 77, 159–167. [Google Scholar] [CrossRef]
- Wang, Y. Research on Effects of Nitrogen Fertigation on Tree-Growth and Its Mechanisms of Action in Populus tomentosa Plantation. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2015. (In Chinese with English abstract). [Google Scholar]
- Kikuzawa, K. Leaf phenology as an optimal strategy for carbon gain in plants. Can. J. Bot. 1995, 73, 158–163. [Google Scholar] [CrossRef]
- Koike, T.; Kitao, M.; Maruyama, Y.; Mori, S.; Lei, T.T. Leaf morphology and photosynthetic adjustments among deciduous broad-leaved trees within the vertical canopy profile. Tree Physiol. 2001, 21, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Osada, N.; Takeda, H.; Okuda, T.; Awang, M. Within-crown variation in the timing of leaf emergence and fall of Malaysian trees in association with crown development patterns. Am. J. Bot. 2005, 92, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Oren, R.; Hinckley, T.M. Actual and potential transpiration and carbon assimilation in an irrigated poplar plantation. Tree Physiol. 2008, 28, 559–577. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, L. Influences of phenological differences on leaf-level carbon budget between the upper and lower crown of Lyonia ovalifolia. Botany 2013, 91, 25–33. [Google Scholar] [CrossRef]
- Osada, O.; Takeda, H.; Furukawa, A.; Awang, M. Leaf dynamics and maintenance of tree crowns in a Malaysian rain forest stand. J. Ecol. 2001, 89, 774–782. [Google Scholar] [CrossRef]
- Gong, D.Z.; Kang, S.Z.; Yao, L.M.; Zhang, L. Estimation of evapotranspiration and its components from an apple orchard in northwest China using sap flow and water balance methods. Hydrol. Process 2007, 21, 931–938. [Google Scholar] [CrossRef]
- Tie, Q.; Hu, H.C.; Tian, F.Q.; Guan, H.D.; Lin, H. Environmental and physiological controls on sap flow in a subhumid mountainous catchment in North China. Agric. For. Meteorol. 2017, 240, 46–57. [Google Scholar] [CrossRef]
- Xi, B.Y.; Di, N.; Wang, Y.; Duan, J.; Jia, L.M. Modeling stand water use response to soil water availability and groundwater level for a mature Populus tomentosa plantation located on the North China Plain. For. Ecol. Manag. 2017, 391, 63–74. [Google Scholar] [CrossRef]
- Gressler, E.; Jochner, S.; Capdevielle-Vargas, R.M.; Morellato, L.P.C.; Menzel, A. Vertical variation in autumn leaf phenology of Fagus sylvatica L. in southern Germany. Agric. For. Meteorol. 2015, 201, 176–186. [Google Scholar] [CrossRef]
- Coble, A.P.; VanderWall, B.; Mau, A.; Cavaleri, M.A. How vertical patterns in leaf traits shift seasonally and the implications for modeling canopy photosynthesis in a temperate deciduous forest. Tree Physiol. 2016, 36, 1077–1091. [Google Scholar] [CrossRef] [PubMed]
- Oláh, V.; Szőllősi, E.; Lakatos, Á.; Kanalas, P.; Nyitrai, B.; Mészáros, I. Springtime leaf development of mature sessile oak trees as based on multi-seasonal monitoring data. Acta Silv. Lignaria Hung. 2012, 8, 21–30. [Google Scholar]
- Niinemets, Ü.; Keenan, T.F.; Hallik, L. A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytol. 2015, 205, 973–993. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Q.; Feng, M.; Li, Z.J. Investigation of bud burst, shoot growth and leaf expansion in Populus euphratica of different ages. Acta Ecol. Sin. 2015, 35, 1198–1207, (In Chinese with English abstract). [Google Scholar]
- Al Yamani, W.; Green, S.; Pangilinan, R.; Dixon, S.; Shahid, S.A.; Kemp, P.; Clothier, B. Water use of Al Ghaf (Prosopis cineraria) and Al Sidr (Ziziphus spina-christi) forests irrigated with saline groundwater in the hyper-arid deserts of Abu Dhabi. Agric. Water Manag. 2018, 203, 105–114. [Google Scholar] [CrossRef]
- Watt, M.S.; Whitehead, D.; Richardson, B.; Mason, E.G.; Leckie, A.C. Modelling the influence of weed competition on the growth of young Pinus radiate at a dry land site. For. Ecol. Manag. 2003, 178, 271–286. [Google Scholar] [CrossRef]
- Xi, B.Y.; Bloomberg, M.; Watt, M.S.; Wang, Y.; Jia, L.M. Modeling growth response to soil water availability simulated by HYDRUS for a mature triploid Populus tomentosa plantation located on the North China Plain. Agric. Water Manag. 2016, 176, 243–254. [Google Scholar] [CrossRef]
- Du, S.Q.; Kang, S.Z.; Li, F.S.; Du, T.S. Water use efficiency is improved by alternate partial root-zone irrigation of apple in arid northwest China. Agric. Water Manag. 2017, 179, 184–192. [Google Scholar] [CrossRef]
- Wei, Z.W.; Yoshimura, K.; Wang, L.X.; Miralles, D.G.; Jasechko, S.; Lee, X.H. Revisiting the contribution of transpiration to global terrestrial evapotranspiration. Geophys. Res. Lett. 2017, 44, 2792–2801. [Google Scholar] [CrossRef]
- Zhang, P.D.; Wu, F.; Kang, X.Y. Genotypic variation in wood properties and growth traits of triploid hybrid clones of Populus tomentosa at three clonal traits. Tree Genet. Genomes 2012, 8, 1041–1050. [Google Scholar] [CrossRef]
- Zhu, Z.T.; Lin, H.B.; Kang, X.Y. Studies on allotriploid breeding of Populus tomentosa B301 clones. Sci. Silvae Sin. 1995, 31, 499–505, (In Chinese with English abstract). [Google Scholar]
- Granier, A. Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol. 1987, 3, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Requirements; FAO Irrigation and Drainage Paper No. 56; FAO: Rome, Italy, 1998. [Google Scholar]
- Hillel, D. Introduction to Environmental Soil Physics; Elsevier Academic Press: San Diego, CA, USA, 2004. [Google Scholar]
- Zhang, Y.X.; Equiza, M.A.; Zheng, Q.S.; Tyree, M.T. Factors controlling plasticity of leaf morphology in Robinia pseudoacacia L. II: The impact of water stress on leaf morphology of seedling grown in a controlled environment chamber. Ann. For. Sci. 2012, 69, 39–47. [Google Scholar] [CrossRef]
- Vitasse, Y. Ontogenic changes rather than difference in temperature cause understory trees to leaf out earlier. New Phytol. 2013, 198, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Laube, J.; Sparks, T.H.; Estrella, N.; Menzel, A. Does humidity trigger tree phenology? Proposal for an air humidity based framework for bud development in spring. New Phytol. 2014, 202, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.Z. Studies on Photosynthetic Characteristics and Canopy Management of Triploid Chinese White Poplar Pulpwood. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2010. (In Chinese with English abstract). [Google Scholar]
- Sellin, A.; Kupper, P. Effects of light availability versus hydraulic constraints on stomatal responses within a crown of silver birch. Oecologia 2005, 142, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Reeves, I.; Emery, R.J.N. Seasonal patterns of cytokinins and microclimate and the mediation of gas exchange among canopy layers of mature Acer saccharum trees. Tree Physiol. 2007, 27, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.E.; Ayars, J.E. Grapevine water use and the crop coefficient are linear functions of the shaded area measured beneath the canopy. Agric. For. Meteorol. 2005, 132, 201–211. [Google Scholar] [CrossRef]
- De Medeiros, G.A.; Arruda, F.B.; Sakai, E. Crop coefficient for irrigated beans derived using three reference evaporation methods. Agric. For. Meteorol. 2005, 135, 135–143. [Google Scholar] [CrossRef]
- Duchemin, B.; Hadria, R.; Erraki, S.; Boulet, G.; Maisongrande, P.; Chehbouni, A.; Escadafal, R.; Ezzahar, J.; Hoedjes, J.C.B.; Kharrou, M.H.; et al. Monitoring wheat phenology and irrigation in Central Morocco: On the use of relationships between evapotranspiration, crops coefficients, leaf area index and remotely-sensed vegetation indices. Agric. Water Manag. 2006, 79, 1–27. [Google Scholar] [CrossRef]



| First-Order Branch Number | Branch Height Range (m) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0–1 | 1–2 | 2–3 | 3–4 | 4–5 | 5–6 | 6–7 | >7 | |
| 1 | 2.50 a (NW) | 1.34 (NW) b | 2.30 (N) | 1.15 (NE) | 0.80 (W) | 2.00 (E) | - c (NW) | - c (N) |
| 2 | 1.01 (NE) | 1.00 (E) | 1.30 (NE) | 1.20 (NE) | 1.30 (SW) | - c (SE) | - c (NW) | |
| 3 | 2.40 (E) | 1.30 (N) | 1.55 (SW) | 0.80 (E) | - c (E) | - c (SE) | ||
| 4 | 2.10 (N) | 1.05 (E) | 0.50 (N) | - c (S) | ||||
| 5 | 1.00 (E) | 1.00 (N) | - c (NE) | |||||
| 6 | 0.70 (E) | 2.10 (NE) | - c (W) | |||||
| 7 | 1.60 (NW) | - c (E) | ||||||
| 8 | 1.20 (S) | - c (NW) | ||||||
| 9 | 0.60 (NE) | - c (SW) | ||||||
| 10 | 2.50 (N) | - c (NE) | ||||||
| 11 | 2.60 (W) | - c (NE) | ||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, G.; Di, N.; Clothier, B.; Duan, J.; Li, D.; Jia, L.; Xi, B.; Ma, F. Leaf Phenology Variation within the Canopy and Its Relationship with the Transpiration of Populus tomentosa under Plantation Conditions. Forests 2018, 9, 603. https://doi.org/10.3390/f9100603
Wang Y, Li G, Di N, Clothier B, Duan J, Li D, Jia L, Xi B, Ma F. Leaf Phenology Variation within the Canopy and Its Relationship with the Transpiration of Populus tomentosa under Plantation Conditions. Forests. 2018; 9(10):603. https://doi.org/10.3390/f9100603
Chicago/Turabian StyleWang, Ye, Guangde Li, Nan Di, Brent Clothier, Jie Duan, Doudou Li, Liming Jia, Benye Xi, and Fengfeng Ma. 2018. "Leaf Phenology Variation within the Canopy and Its Relationship with the Transpiration of Populus tomentosa under Plantation Conditions" Forests 9, no. 10: 603. https://doi.org/10.3390/f9100603
APA StyleWang, Y., Li, G., Di, N., Clothier, B., Duan, J., Li, D., Jia, L., Xi, B., & Ma, F. (2018). Leaf Phenology Variation within the Canopy and Its Relationship with the Transpiration of Populus tomentosa under Plantation Conditions. Forests, 9(10), 603. https://doi.org/10.3390/f9100603








