Stem Radius Variation in Response to Hydro-Thermal Factors in Larch
Abstract
1. Introduction
2. Materials and Methods
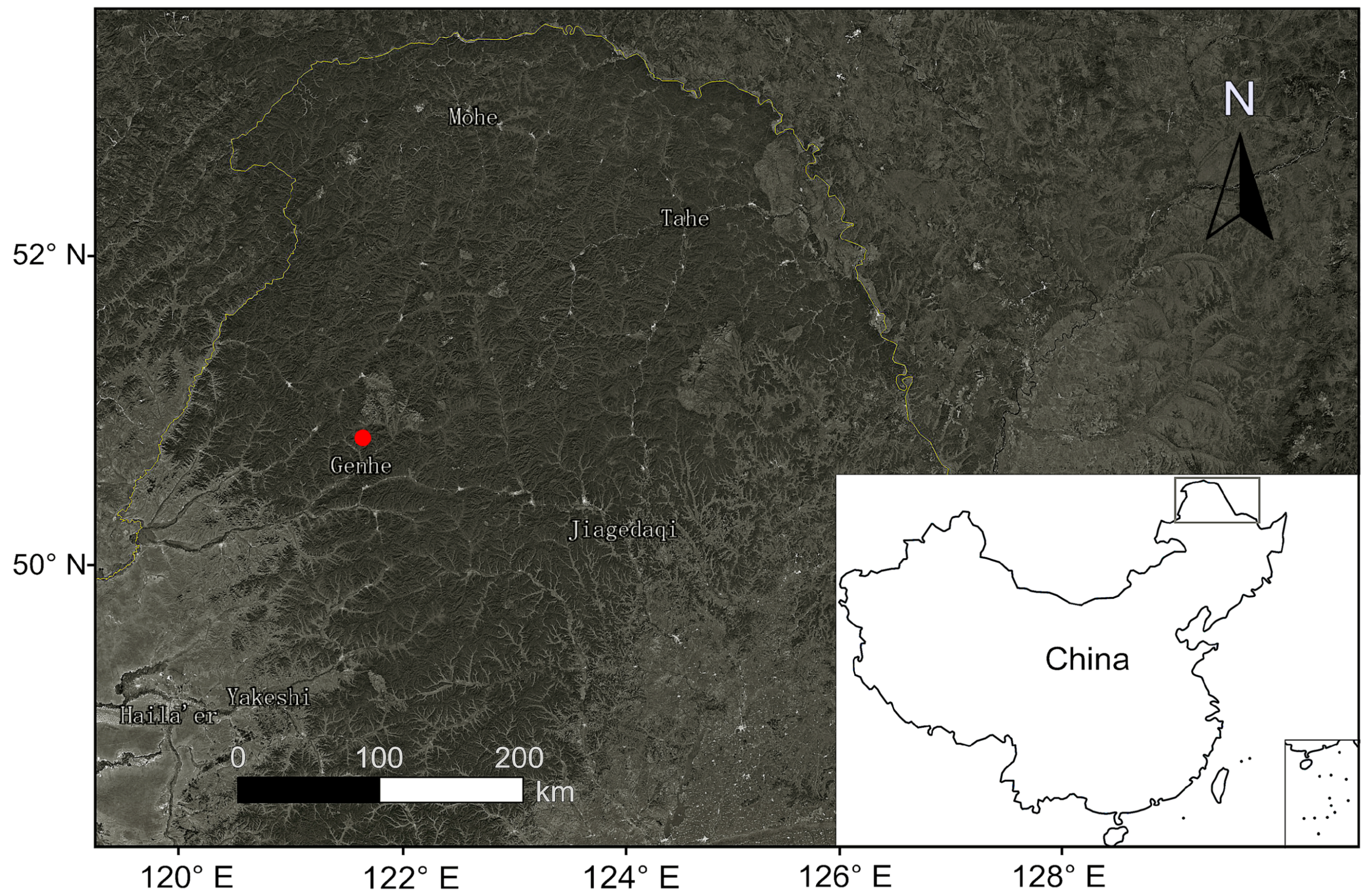
2.1. Study Area
2.2. Measurement of Larch Stem Radius Variation
2.3. Measurement of Related Hydro-Thermal Factors
2.4. Data Analysis
3. Results
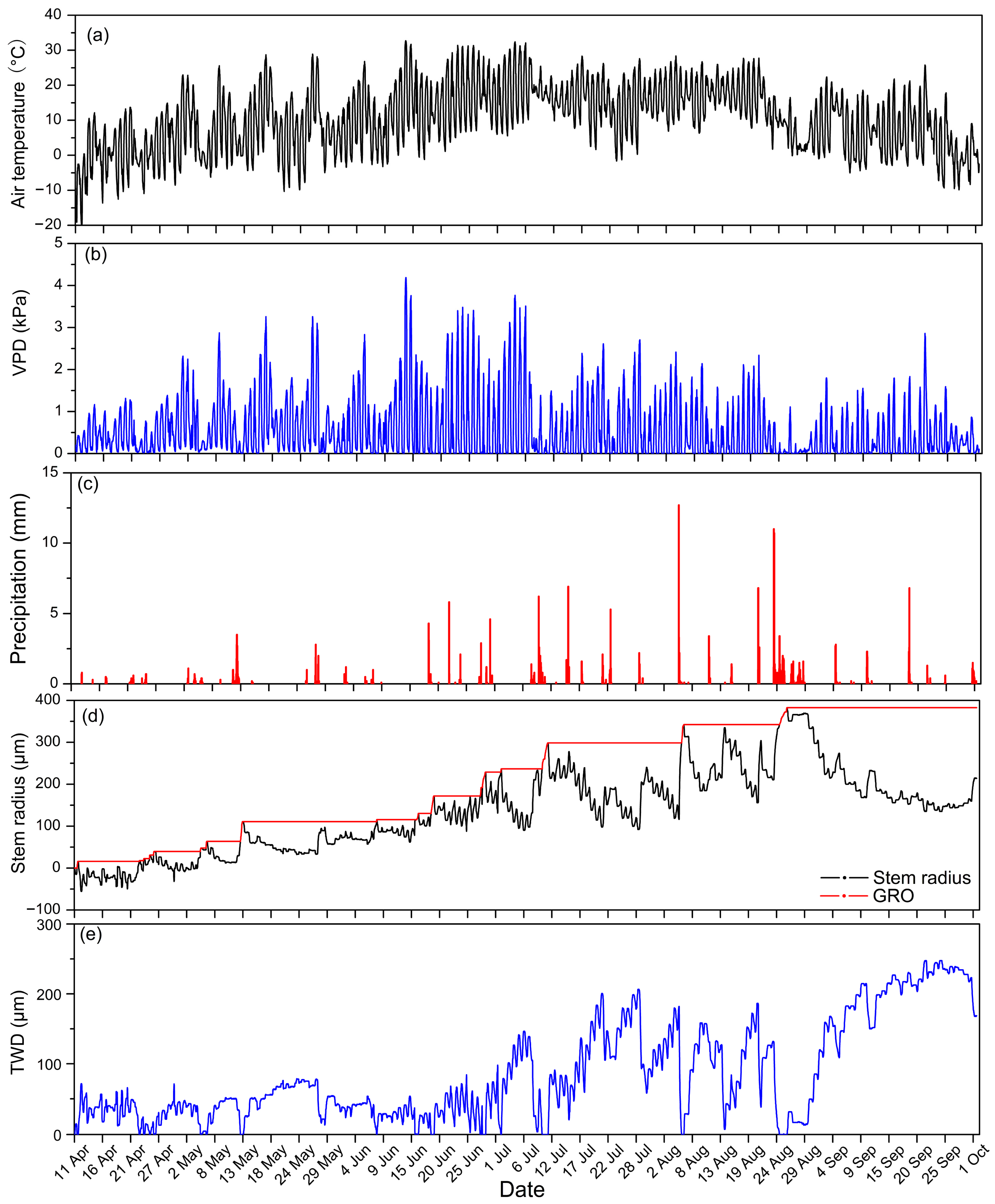
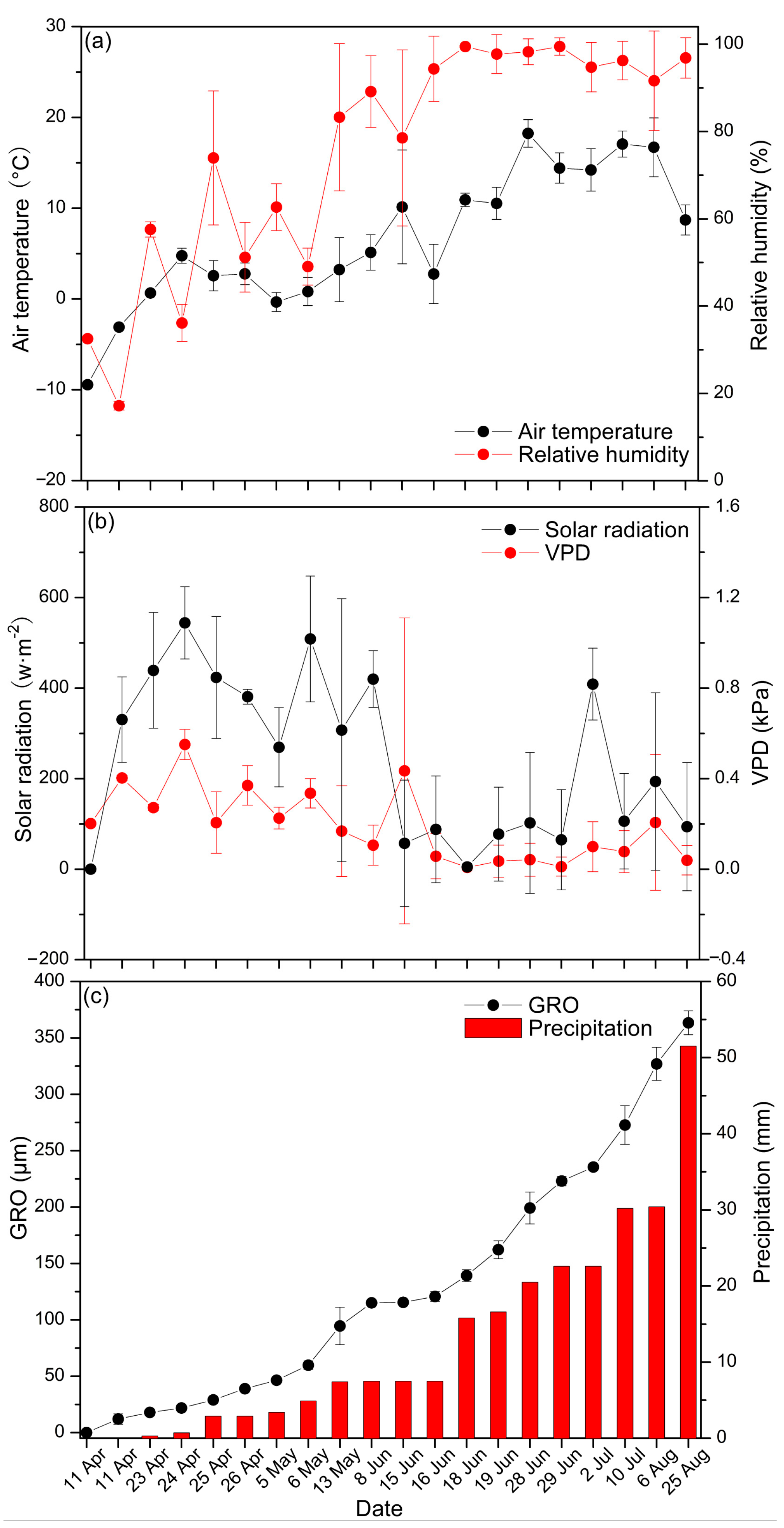
3.1. Environmental Conditions for Growth of Larch
3.2. Change Trends of Larch Stem Radius Variation and Hydro-Thermal Factors
3.3. Larch Stem Radius Variations and Its Response to Hydro-Thermal Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Drew, D.M.; Downes, G.M. The use of precision dendrometers in research on daily stem size and wood property variation: A review. Dendrochronologia 2009, 27, 159–172. [Google Scholar] [CrossRef]
- Cocozza, C.; Tognetti, R.; Giovannelli, A.; Giovannelli, A. High-Resolution Analytical Approach to Describe the Sensitivity of Tree–Environment Dependences through Stem Radial Variation. Forests 2018, 9, 134. [Google Scholar] [CrossRef]
- De Swaef, T.; De Schepper, V.; Vandegehuchte, M.W.; Steppe, K. Stem diameter variations as a versatile research tool in ecophysiology. Tree Physiol. 2015, 35, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Deslauriers, A.; Rossi, S.; Anfodillo, T. Dendrometer and intra-annual tree growth: What kind of information can be inferred? Dendrochronologia 2008, 25, 113–124. [Google Scholar] [CrossRef]
- Araki, M.G.; Kajimoto, T.; Han, Q.; Kawasaki, T.; Utsugi, H.; Gyokusen, K.; Chiba, Y. Effect of stem radial growth on seasonal and spatial variations in stem CO2 efflux of Chamaecyparis obtusa. Trees 2015, 29, 499–514. [Google Scholar] [CrossRef]
- Turcotte, A.; Rossi, S.; Deslauriers, A.; Krause, C.; Morin, H. Dynamics of depletion and replenishment of water storage in stem and roots of black spruce measured by dendrometers. Front. Plant Sci. 2011, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.; Hölttä, T.; Berninger, F.; Mäkinen, H.; Nöjd, P.; Mencuccini, M.; Nikinmaa, E. Separating water-potential induced swelling and shrinking from measured radial stem variations reveals a cambial growth and osmotic concentration signal. Plant Cell Environ. 2016, 39, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, R. Radial stem variations—A source of tree physiological information not fully exploited yet. Plant Cell Environ. 2016, 39, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Granier, A. Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol. 1987, 3, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Y.; Tian, A.; Yu, P.; Xiong, W.; Xu, L.; Wang, Y.; Liu, Z.; Wang, Y.; Tian, A. Intra-Annual Variation of Stem Radius of Larix principis-rupprechtii and Its Response to Environmental Factors in Liupan Mountains of Northwest China. Forests 2017, 8, 382. [Google Scholar] [CrossRef]
- Bouriaud, O.; Leban, J.M.; Bert, D.; Deleuze, C. Intra-annual variations in climate influence growth and wood density of Norway spruce. Tree Physiol. 2005, 25, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Brito, P.; Wieser, G.; Oberhuber, W.; Gruber, A.; Lorenzo, J.R.; González-Rodríguez, Á.M.; Jiménez, M.S. Water availability drives stem growth and stem water deficit of Pinus canariensis in a drought-induced treeline in Tenerife. Plant Ecol. 2016, 218, 277–290. [Google Scholar] [CrossRef]
- Meng, Z.; Duan, A.; Chen, D.; Dassanayake, K.B.; Wang, X.; Liu, Z.; Liu, H.; Gao, S. Suitable indicators using stem diameter variation-derived indices to monitor the water status of greenhouse tomato plants. PLoS ONE 2017, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Deslauriers, A.; Anfodillo, T.; Rossi, S.; Carraro, V. Using simple causal modeling to understand how water and temperature affect daily stem radial variation in trees. Tree Physiol. 2007, 27, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Proseus, T.E.; Boyer, J.S. Turgor Pressure Moves Polysaccharides into Growing Cell Walls of Chara corallina. Ann. Bot. 2005, 95, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Cocozza, C.; Giovannelli, A.; Lasserre, B.; Cantini, C.; Lombardi, F.; Tognetti, R. A novel mathematical procedure to interpret the stem radius variation in olive trees. Agric. For. Meteorol. 2012, 161, 80–93. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B.; Qin, C.; He, M.; Feng, S.; Liu, J. Two phases of seasonal stem radius variations of Sabina przewalskii Kom. in northwestern China inferred from sub-diurnal shrinkage and expansion patterns. Trees 2012, 26, 1747–1757. [Google Scholar] [CrossRef]
- Zweifel, R.; Häsler, R. Frost-induced reversible shrinkage of bark of mature subalpine conifers. Agric. For. Meteorol. 2000, 102, 213–222. [Google Scholar] [CrossRef]
- Köcher, P.; Horna, V.; Leuschner, C. Environmental control of daily stem growth patterns in five temperate broad-leaved tree species. Tree Physiol. 2012, 32, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Meng, Z.; Chang, X.; Deng, Z.; Li, Y.; Lv, M. Determination of a suitable indicator of tomato water content based on stem diameter variation. Sci. Hortic. 2017, 215, 142–148. [Google Scholar] [CrossRef]
- Zweifel, R.; Item, H.; Häsler, R. Link between diurnal stem radius changes and tree water relations. Tree Physiol. 2001, 21, 869–877. [Google Scholar] [CrossRef] [PubMed]
- King, G.; Fonti, P.; Nievergelt, D.; Büntgen, U.; Frank, D. Climatic drivers of hourly to yearly tree radius variations along a 6 °C natural warming gradient. Agric. For. Meteorol. 2013, 168, 36–46. [Google Scholar] [CrossRef]
- Vaganov, E.A.; Hughes, M.K.; Kirdyanov, A.V.; Schweingruber, F.H.; Silkin, P.P. Influence of snowfall and melt timing on tree growth in subarctic Eurasia. Nature 1999, 400, 149–151. [Google Scholar] [CrossRef]
- Gruber, A.; Zimmermann, J.; Wieser, G.; Oberhuber, W. Effects of climate variables on intra-annual stem radial increment in Pinus cembra (L.) along the alpine treeline ecotone. Ann. For. Sci. 2009, 66, 503. [Google Scholar] [CrossRef] [PubMed]
- Körner, C.; Basler, D.; Hoch, G.; Kollas, C.; Lenz, A.; Randin, C.F.; Vitasse, Y.; Zimmermann, N.E. Where, why and how? Explaining the low–temperature range limits of temperate tree species. J. Ecol. 2016, 104, 1076–1088. [Google Scholar] [CrossRef]
- Hu, L.; Fan, Z. Stem radial growth in response to microclimate in an Asian tropical dry karst forest. Acta Ecol. Sin. 2016, 36, 401–409. [Google Scholar] [CrossRef]
- De Schepper, V.; Steppe, K.; Van Labeke, M.C.; Lemeur, R. Detailed analysis of double girdling effects on stem diameter variations and sap flow in young oak trees. Environ. Exp. Bot. 2010, 68, 149–156. [Google Scholar] [CrossRef]
- González-Rodríguez, Á.M.; Brito, P.; Lorenzo, J.R.; Gruber, A.; Oberhuber, W.; Wieser, G. Seasonal cycles of sap flow and stem radius variation of Spartocytisus supranubius in the alpine zone of Tenerife, Canary Islands. Alp. Bot. 2017, 1–12. [Google Scholar] [CrossRef]
- Zweifel, R.; Zimmermann, L.; Newbery, D.M. Modeling tree water deficit from microclimate: An approach to quantifying drought stress. Tree Physiol. 2005, 25, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Steppe, K.; De Pauw, D.J.; Lemeur, R.; Vanrolleghem, P.A. A mathematical model linking tree sap flow dynamics to daily stem diameter fluctuations and radial stem growth. Tree Physiol. 2006, 26, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, J.; Vergeynst, L.L.; Overlaet-Michiels, L.; Steppe, K. How important is woody tissue photosynthesis in poplar during drought stress? Trees 2016, 30, 63–72. [Google Scholar] [CrossRef]
- Vandegehuchte, M.W.; Bloemen, J.; Vergeynst, L.L.; Steppe, K. Woody tissue photosynthesis in trees: Salve on the wounds of drought? New Phytol. 2015, 208, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Maaten, E.V.D.; Bouriaud, O.; Maaten-Theunissen, M.V.D.; Mayer, H.; Spiecker, H. Meteorological forcing of day-to-day stem radius variations of beech is highly synchronic on opposing aspects of a valley. Agric. For. Meteorol. 2013, 181, 85–93. [Google Scholar] [CrossRef]
- Steduto, P.; Hsiao, T.C.; Fereres, E. On the conservative behavior of biomass water productivity. Irrig. Sci. 2007, 25, 189–207. [Google Scholar] [CrossRef]
- Zweifel, R.; Zimmermann, L.; Zeugin, F.; Newbery, D.M. Intra-annual radial growth and water relations of trees: Implications towards a growth mechanism. J. Exp. Bot. 2006, 57, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Perämäki, M.; Nikinmaa, E.; Sevanto, S.; Ilvesniemi, H.; Siivola, E.; Hari, P.; Vesala, T. Tree stem diameter variations and transpiration in Scots pine: An analysis using a dynamic sap flow model. Tree Physiol. 2001, 21, 889–897. [Google Scholar] [CrossRef] [PubMed]
| Stem Radius | Tree Water Deficit-Induced Stem Shrinkage | |||||
|---|---|---|---|---|---|---|
| Drought Season | Moist Season | Relative Growth Rate | Drought Season | Moist Season | Relative Growth Rate | |
| Mean | 37.798 | 198.141 | 524.21% | 37.737 | 117.554 | 311.51% |
| Standard deviation | 40.538 | 66.036 | 162.90% | 19.107 | 72.633 | 380.14% |
| n | 3264 | 5089 | - | 3264 | 5089 | - |
| Mean | Standard Deviation | Variation Coefficient | n | |
|---|---|---|---|---|
| Stem radius | 135.486 | 97.060 | 71.64% | 8353 |
| TWD | 86.365 | 69.814 | 80.84% | 8353 |
| GRO | 215.497 | 116.141 | 53.89% | 299 |
| SFD | 0.016 | 0.059 | 361.74% | 4465 |
| Relative humidity | 69.653 | 27.422 | 39.37% | 8353 |
| VPD | 0.543 | 0.698 | 128.53% | 8353 |
| Precipitation | 0.072 | 0.484 | 674.61% | 4177 |
| Solar radiation | 203.664 | 252.549 | 124.00% | 8353 |
| Air temperature | 10.389 | 9.263 | 89.16% | 8353 |
| Precipitation | SFD | Relative Humidity | VPD | Solar Radiation | Air Temperature | SFDclear days | |
|---|---|---|---|---|---|---|---|
| SR0.5 h | 0.068 *** | −0.040 ** | 0.478 *** | −0.189 *** | −0.079 *** | 0.282 *** | −0.041 |
| SR1 day | 0.235 ** | −0.102 | 0.805 *** | −0.367 *** | −0.362 *** | 0.39 *** | - |
| SR7 day | 0.556 ** | 0.588 ** | 0.939 *** | −0.443 * | −0.481 * | 0.493 * | - |
| SR21 day | 0.818 * | 0.801 * | 0.982 *** | −0.44 | −0.582 | 0.635 | - |
| TWD0.5 h | 0.01 | 0.093 *** | 0.094 *** | −0.016 | −0.095 *** | 0.005 | 0.209 *** |
| TWD1 day | −0.025 | 0.209 * | 0.194 * | −0.107 | −0.262 *** | −0.021 | - |
| TWD7 days | −0.056 | 0.647 *** | 0.384 | −0.427 * | −0.573 ** | −0.067 | - |
| TWD21 days | 0.146 | 0.839 ** | 0.591 | −0.559 | −0.723 * | 0.103 | - |
| GROperiod | 0.366 *** | 0.351 *** | 0.602 *** | −0.327 *** | −0.341 *** | 0.568 *** | 0.046 |
| GRO1 day | 0.158 * | 0.159 | 0.688 *** | −0.323 *** | −0.4 *** | 0.273 *** | - |
| GRO7 days | 0.357 | 0.716 *** | 0.833 *** | −0.51 ** | −0.606 ** | 0.309 | - |
| GRO21 days | 0.631 | 0.917 ** | 0.937 ** | −0.546 | −0.715 * | 0.222 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Zhang, Q.; Liu, X.; Meng, M. Stem Radius Variation in Response to Hydro-Thermal Factors in Larch. Forests 2018, 9, 602. https://doi.org/10.3390/f9100602
Tian Y, Zhang Q, Liu X, Meng M. Stem Radius Variation in Response to Hydro-Thermal Factors in Larch. Forests. 2018; 9(10):602. https://doi.org/10.3390/f9100602
Chicago/Turabian StyleTian, Yuan, Qiuliang Zhang, Xuan Liu, and Meng Meng. 2018. "Stem Radius Variation in Response to Hydro-Thermal Factors in Larch" Forests 9, no. 10: 602. https://doi.org/10.3390/f9100602
APA StyleTian, Y., Zhang, Q., Liu, X., & Meng, M. (2018). Stem Radius Variation in Response to Hydro-Thermal Factors in Larch. Forests, 9(10), 602. https://doi.org/10.3390/f9100602





