Drought-Induced Changes in Wood Density Are Not Prevented by Thinning in Scots Pine Stands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Sites
2.2. Experimental Design and Tree Sampling
2.3. Sample Processing
2.4. Statistical Analyses
3. Results
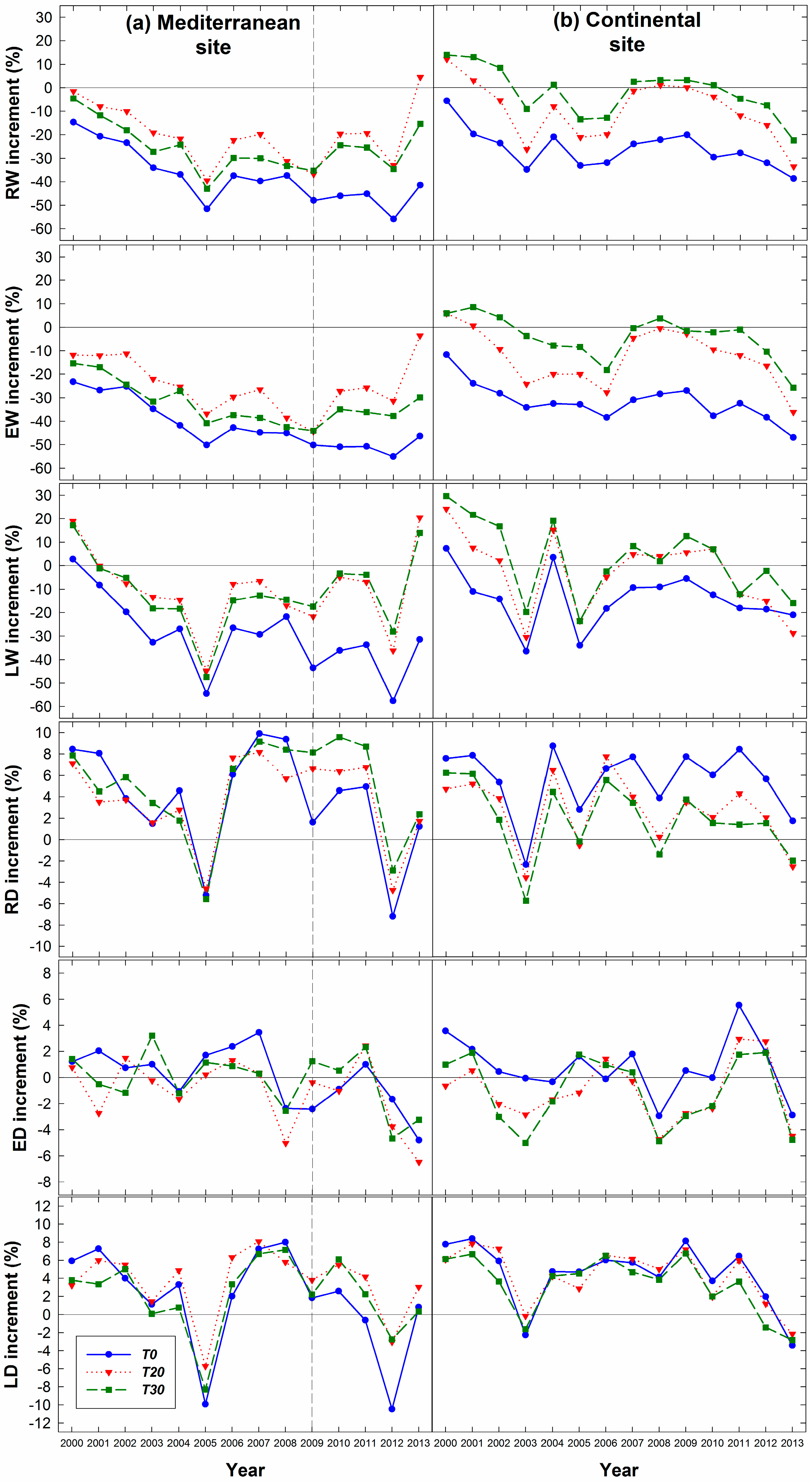
3.1. Influence of Thinning Intensity on Ring Width
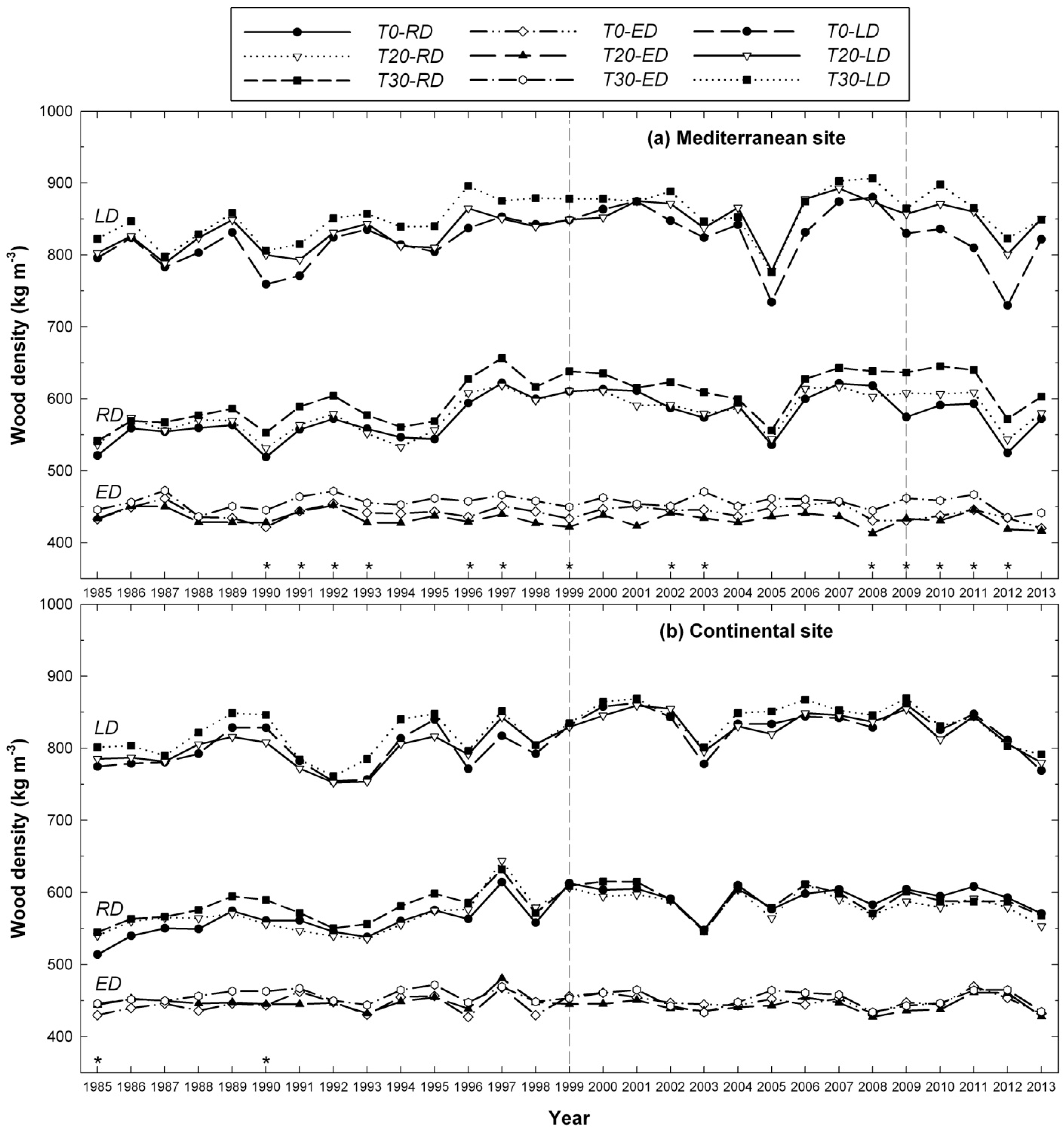
3.2. Influence of Thinning Intensity on Wood Density
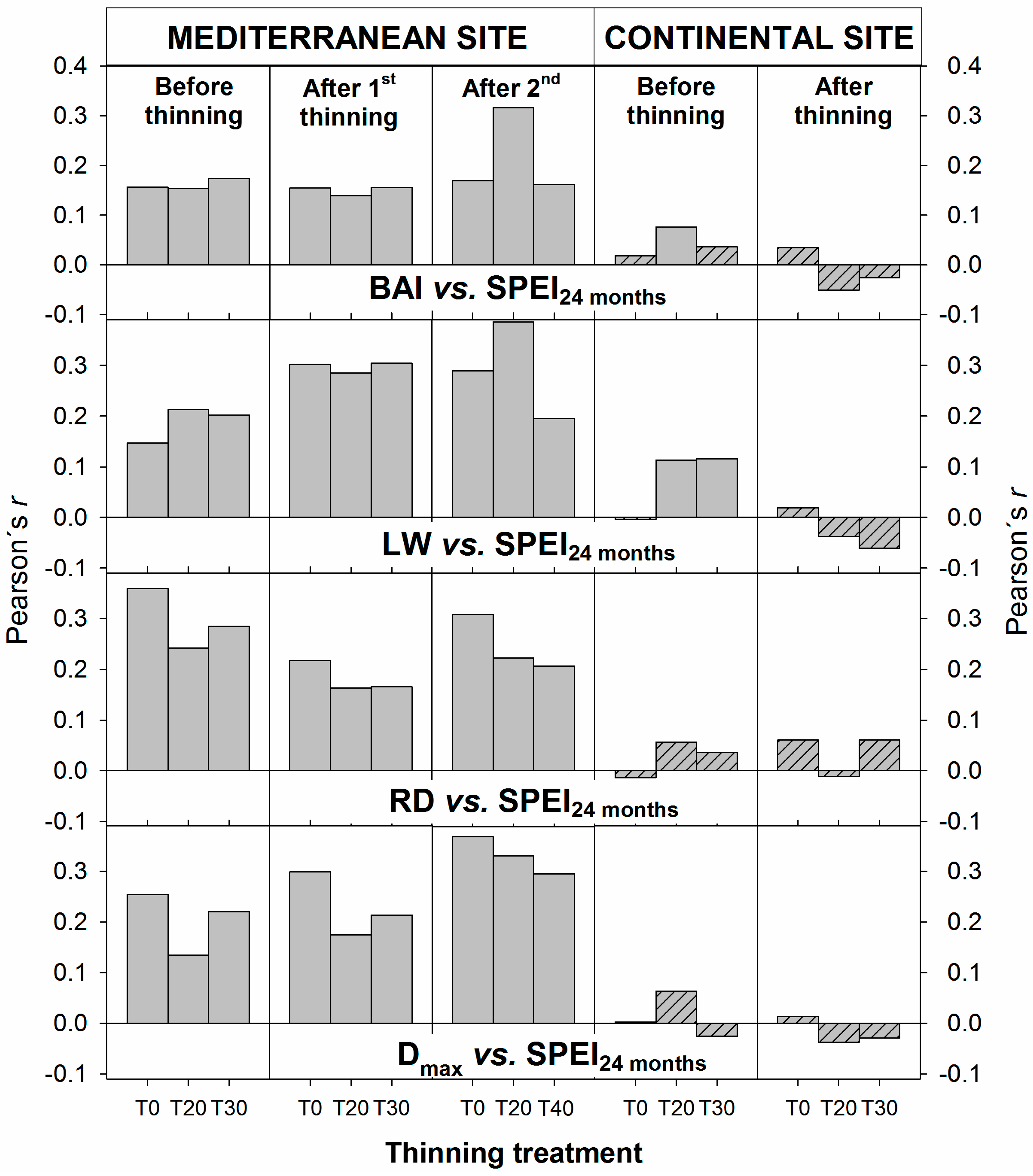
3.3. Interacting Effects between Climate and Thinning on Wood Features
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fritts, H.C. Tree Rings and Climate; Blackburn Press: Caldwell, NJ, USA, 2001. [Google Scholar]
- Martínez-Meier, A.; Sánchez, L.; Pastorino, M.; Gallo, L.; Rozenberg, P. What is hot in tree rings? The wood density of surviving Douglas-firs to the 2003 drought and heat wave. For. Ecol. Manag. 2008, 256, 837–843. [Google Scholar] [CrossRef]
- Vaganov, E.A.; Hughes, M.K.; Shashkin, A.V. Growth Dynamics of Conifer Tree Rings: Images of Past and Future Environments; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Downes, G.; Drew, D.M. Climate and growth influences on wood formation and utilisation. South. For. 2008, 70, 155–167. [Google Scholar] [CrossRef]
- Zobel, B.J.; van Buijtenen, J.P. Wood Variation: Its Causes and Control; Springer: Berlin, Germany, 1989. [Google Scholar]
- Camarero, J.J.; Rozas, V.; Olano, J.M. Minimum wood density of Juniperus thurifera is a robust proxy of spring water availability in a continental Mediterranean climate. J. Biogeogr. 2014, 41, 1105–1114. [Google Scholar] [CrossRef]
- Zeller, L.; Ammer, C.; Annighöfer, P.; Biber, P.; Marshall, J.; Schütze, G.; del Río, M.; Pretzsch, H. Tree ring wood density of Scots pine and European beech lower in mixed-species stands compared with monocultures. For. Ecol. Manag. 2017, 400, 363–374. [Google Scholar] [CrossRef]
- Auty, D.; Achim, A.; Macdonald, E.; Cameron, A.D.; Gardiner, B.A. Models for predicting wood density variation in Scots pine. Forestry 2014, 87, 449–458. [Google Scholar] [CrossRef]
- Piutti, E.; Cescatti, A. A quantitative analysis of the interactions between climatic response and intraspecific competition in European beech. Can. J. For. Res. 1997, 27, 277–284. [Google Scholar] [CrossRef]
- Carson, S.D.; Cown, D.J.; McKinley, R.B.; Moore, J.R. Effects of site, silviculture and seedlot on wood density and estimated wood stiffness in radiate pine at mid-rotation. N. Z. J. For. Sci. 2014, 44, 26. [Google Scholar] [CrossRef]
- Bontemps, J.D.; Gelhaye, P.; Nepveu, G.; Hervé, J.C. When tree rings behave like foam: moderate historical decrease in the mean ring density of common beech paralleling a strong historical growth increase. Ann. For. Sci. 2013, 70, 329–343. [Google Scholar] [CrossRef]
- Jozsa, L.A.; Middleton, G.R. A Discussion of Wood Quality Attributes and Their Practical Implications; Special Publication No. SP-34; Canada—British Columbia Partnership Agreement on Forest Resource Development: FRDA II.; Forintek Canada Corporation: Vancouver, BC, Canada, 1994. [Google Scholar]
- Mansfield, S.D.; Parish, R.; Goudie, J.; Kang, K.Y.; Ott, P. The effects of crown ratio on the transition from juvenile to mature wood production in lodge pole pine in western Canada. Can. J. For. Sci. 2007, 37, 1450–1459. [Google Scholar]
- Linares, J.C.; Delgado-Huertas, A.; Carreira, J.A. Climatic trends and different drought adaptive capacity and vulnerability in a mixed Abies pinsapo–Pinus halepensis forest. Clim. Chang. 2011, 105, 67–90. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Camarero, J.J.; Dobbertin, M.; Fernández-Cancio, A.; Vilà-Cabrera, A.; Manzanedo, R.D.; Navarro-Cerrillo, R.M. Contrasting vulnerability and resilience to drought-induced decline of densely planted vs. natural rear-edge Pinus nigra forests. For. Ecol. Manag. 2013, 310, 956–967. [Google Scholar] [CrossRef]
- Sohn, J.A.; Saha, S.; Bauhus, J. Potential of forest thinning to mitigate drought stress: A meta-analysis. For. Ecol. Manag. 2016, 380, 261–273. [Google Scholar] [CrossRef]
- Linares, J.C.; Camarero, J.J.; Carreira, J.A. Interacting effects of changes in climate and forest cover on mortality and growth of the southernmost European fir forests. Glob. Ecol. Biogeogr. 2009, 18, 485–497. [Google Scholar] [CrossRef]
- Sánchez-Miranda, A.; Jiménez, M.N.; Gálvez Garrido, C.R.; Navarro, F.B.; Ripoll, M.A.; Hevia, A.; Sánchez-Salguero, R. Thinning Modulates Climate-Growth Responses of Pinus Halepensis Mill. under Semiarid Mediterranean Conditions; Hevia, A., Sánchez-Salguero, R., Linares, J.C., Olano, J.M., Camarero, J.J., Gutiérrez, E., Helle, G., Gärtner, H., Eds.; TRACE—Tree Rings in Archaeology, Climatology and Ecology; Scientific Technical Report 16/04; GFZ German Research Centre for Geosciences: Potsdam, Germany, 2016; Volume 14, pp. 111–119. [Google Scholar]
- Peltola, H.; Kilpeläinen, A.; Sauvala, K.; Räisänen, T.; Ikonen, V.P. Effects of early thinning regime and tree status on the radial growth and wood density of Scots pine. Silva Fenn. 2007, 41, 489–505. [Google Scholar] [CrossRef]
- Blanco, J.A.; Zavala, M.A.; Imbert, J.B.; Castillo, F.J. Sustainability of forest management practices: Evaluation through a simulation model of nutrient cycling. For. Ecol. Manag. 2005, 213, 209–228. [Google Scholar] [CrossRef]
- Tang, J.; Qi, Y.; Xu, M.; Misson, L.; Goldstein, A.H. Forest thinning and soil respiration in a ponderosa pine plantation in the Sierra Nevada. Tree Physiol. 2005, 25, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Ericson, B. Effect of Thinning on the Basic Density and Content of Latewood and Heartwood in Scots Pine and Norway Spruce; Research Notes No. 10, Department of Forest Yield Research; Royal College of Forestry: Stockholm, Sweden, 1966. [Google Scholar]
- Lin, F.C.; Chung, C.H.; Zeng, J.L.; Yang, T.H.; Wang, S.Y.; Lin, C.J. Effect of thinning on the ring characteristics of Japanese cedar plantation trees. J. Wood Sci. 2012, 58, 104–112. [Google Scholar] [CrossRef]
- Filipescu, C.N.; Lowell, E.C.; Koppenaal, R.; Mitchell, A.K. Modeling regional and climatic variation of wood density and ring width in intensively managed Douglas-fir. Can. J. For. Res. 2014, 44, 220–229. [Google Scholar] [CrossRef]
- Kellomäki, S.; Koski, V.; Niemelä, P. Management of forest ecosystems. In Forest Resources and Sustainable Management; Kellomäki, S., Ed.; Gummerus Oy: Jyväskylä, Finland, 1998; pp. 219–309. [Google Scholar]
- Gardiner, B.A.; Leban, J.M.; Auty, D.; Simpson, H.L. Models for predicting the wood density of British-grown Sitka spruce. Forestry 2011, 84, 119–132. [Google Scholar] [CrossRef]
- Peltola, H.; Gort, J.; Pulkkinen, P.; Zubizarreta Gerendiain, A.; Karppinen, J.; Ikonen, V.P. Differences in growth and wood density traits in Scots pine (Pinus sylvestris L.) genetic entries grown at different spacing and sites. Silva Fenn. 2009, 43, 339–354. [Google Scholar] [CrossRef]
- Fajardo, A. Wood density is a poor predictor of competitive ability among individuals of the same species. For. Ecol. Manag. 2016, 372, 217–225. [Google Scholar] [CrossRef]
- Mörling, T. Evaluation of annual ring width and ring density development following fertilization and thinning of Scots pine. Ann. For. Sci. 2002, 59, 29–40. [Google Scholar] [CrossRef]
- Schneider, R.; Zhang, S.Y.; Swift, D.E.; Bégin, J.; Lussier, J.M. Predicting selected wood properties of jack pine following commercial thinning. Can. J. For. Res. 2008, 38, 2030–2043. [Google Scholar] [CrossRef]
- Jaakkola, T.; Mäkinen, H.; Saranpää, P. Wood density in Norway spruce: Changes with thinning intensity and tree age. Can. J. For. Res. 2005, 35, 1767–1778. [Google Scholar] [CrossRef]
- Megraw, R.A. Wood Quality Factors in Loblolly Pine. The Influence of Tree Age, Position in Tree, and Cultural Practice on Wood Specific Gravity, Fiber Length, and Fibril Angle; TAPPI Press: Atlanta, GE, USA, 1985. [Google Scholar]
- Paul, B.H. Specific gravity changes in southern pines after release. South. Lumberman 1958, 163, 122–124. [Google Scholar]
- Larson, P.R. Wood Formation and the Concept of Wood Quality; Bulletin No. 7; Yale University School of Forestry: New Haven, CT, USA, 1969. [Google Scholar]
- Primicia, I.; Camarero, J.J.; Imbert, J.B.; Castillo, F.J. Effects of thinning and canopy type on growth dynamics of Pinus sylvestris: Inter-annual variations and intra-annual interactions with microclimate. Eur. J. For. Res. 2013, 132, 121–135. [Google Scholar] [CrossRef]
- Peltola, H.; Miina, J.; Rouvinen, I.; Kellomäki, S. Effect of early thinning on the diameter growth distribution along the stem of Scots pine. Silva Fenn. 2002, 36, 813–825. [Google Scholar] [CrossRef]
- Splechtna, B.E.; Dobry, J.; Klinka, K. Tree-ring characteristics of subalpine fir (Abies lasiocarpa (Hook.) Nutt.) in relation to elevation and climatic fluctuations. Ann. For. Sci. 2001, 57, 89–100. [Google Scholar] [CrossRef]
- Koubaa, A.; Zhang, S.Y.T.; Makni, S. Defining the transition from earlywood to latewood in black spruce based on intra-ring wood density profiles from X-ray densitometry. Ann. For. Sci. 2002, 59, 511–518. [Google Scholar] [CrossRef]
- Papadakis, J. Climates of the World, Their Classification, Similitudes, Differences, and Geographic Distribution; Self-Edited: Buenos Aires, Argentina, 1970. [Google Scholar]
- Walter, H. Climatic diagrams as a means to comprehend the various climatic types for ecological and agriculture purposes. In The Water Relation of Plants, Proceedings of the Symposium of the British Ecological Society, London, UK, 28–30 March 1963; Rutter, A.J., Whitehead, F.H., Eds.; Blackwell Scientific Publications: London, UK, 1963; pp. 3–9. [Google Scholar]
- Running, S.W.; Nemani, R.R.; Hungerford, R.D. Extrapolation of synoptic meteorological data in mountainous terrain and its use for simulating forest evapotranspiration and photosynthesis. Can. J. For. Res. 1987, 17, 472–483. [Google Scholar] [CrossRef]
- Lo, Y.H.; Blanco, J.A.; Seely, B.; Welham, C.; Kimmins, J.P. Generating reliable meteorological data in mountainous areas with scarce presence of weather records: The performance of MTCLIM in interior British Columbia, Canada. Environ. Modell. Softw. 2011, 26, 644–657. [Google Scholar] [CrossRef]
- González de Andrés, E.; Seely, B.; Blanco, J.A.; Imbert, J.B.; Lo, Y.H.; Castillo, F.J. Increased complementarity in water-limited environments in Scots pine and European beech mixtures under climate change. Ecohydrology 2016. [Google Scholar] [CrossRef]
- Andrew, L. Simple Experimental Design for Forestry Trials; FRI Bulletin No. 71; Forest Research Institute: Rotorua, New Zealand, 1986. [Google Scholar]
- QMS. Tree Ring Analyzer Users Guide Model QTRS-01X; Quintek Measurement Systems: Knoxville, TN, USA, 1999. [Google Scholar]
- Polge, H. Fifteen years of wood radiation densitometry. Wood Sci. Technol. 1978, 12, 187–196. [Google Scholar] [CrossRef]
- Mäkinen, H.; Hynynen, J. Wood density and tracheid properties of Scots pine: Responses to repeated fertilization and timing of the first commercial thinning. Forestry 2014, 87, 437–447. [Google Scholar] [CrossRef]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measuring. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multi-scalar drought index sensitive to global warming: The Standardized Precipitation Evapotranspiration Index—SPEI. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Gutiérrez, E. Dendroclimatological study of Pinus sylvestris L. in southern Catalonia (Spain). Tree-Ring Bull. 1989, 49, 1–9. [Google Scholar]
- Bogino, S.; Fernández Nieto, M.J.; Bravo, F. Climate effect on radial growth of Pinus sylvestris at its southern and western distribution limits. Silva Fenn. 2009, 43, 609–623. [Google Scholar] [CrossRef]
- Del Río, M.; Bravo-Oviedo, A.; Pretzsch, H.; Löf, M.; Ruíz-Peinado, R. A review of thinning effects on Scots pine stands: From growth and yield to new challenges under global change. For. Syst. 2017, 26, eR03S. [Google Scholar] [CrossRef]
- Blanco, J.A.; Imbert, J.B.; Castillo, F.J. Thinning affects nutrient resorption and nutrient-use efficiency in two Pinus sylvestris stands in the Pyrenees. Ecol. Appl. 2009, 19, 682–698. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.A.; Imbert, J.B.; Castillo, F.J. Thinning affects Pinus sylvestris needle decomposition rates and chemistry differently depending on site conditions. Biogeochemistry 2011, 106, 397–414. [Google Scholar] [CrossRef]
- Guller, B. The effects of thinning treatments on density, MOE, MOR and maximum crushing strength of Pinus brutia Ten. wood. Ann. For. Sci. 2007, 64, 467–475. [Google Scholar] [CrossRef]
- Tasissa, G.; Burkhart, H.E. Modeling thinning effects on ring width distribution in loblolly pine (Pinus taeda). Can. J. For. Res. 1997, 27, 1291–1301. [Google Scholar] [CrossRef]
- Moschler, W.W.; Dougal, E.F.; McRae, D.D. Density and growth ring characteristics of Pinus taeda L. following thinning. Wood Fiber Sci. 1989, 21, 313–319. [Google Scholar]
- Pape, R. Influence of thinning and tree diameter class on the development of basic density and annual ring width in Picea abies. Scand. J. For. Res. 1999, 14, 27–37. [Google Scholar] [CrossRef]
- Pukkala, T.; Miina, J.; Kellomäki, S. Response to different thinning intensities in young Pinus sylvestris. Scand. J. For. Res. 1998, 13, 141–150. [Google Scholar] [CrossRef]
- Sohn, J.A.; Hatig, F.; Kohler, M.; Huss, J.; Bauhus, J. Heavy and frequent thinning promotes drought adaptation in Pinus sylvestris forests. Ecol. Appl. 2016, 26, 2190–2205. [Google Scholar] [CrossRef] [PubMed]
- Koga, S.; Zhang, S.Y.; Bégin, J. Effects of precommercial thinning on annual radial growth and wood density in balsam fir (Abies balsamea). Wood Fiber Sci. 2002, 34, 625–642. [Google Scholar]
- Jones, P.D.; Fox, T.R. Wood density in Pinus taeda x Pinus rigida and response 10 years after thinning in Virginia. For. Prod. J. 2007, 57, 70–73. [Google Scholar]
- MacPeak, M.D.; Burkart, L.F.; Weldon, D. Comparison of grade, yield, and mechanical properties of lumber produced from young fast-grown and older slow-grown planted Slash pine. For. Prod. J. 1990, 40, 11–14. [Google Scholar]
- Wodzicki, T.J. Natural factors affecting wood structure. Wood Sci. Technol. 2001, 3, 5–26. [Google Scholar] [CrossRef]
- Rozenberg, P.; Van Loo, J.; Hannrup, B.; Grabner, M. Clonal variation of wood density record of cambium reaction to water deficit in Picea abies (L.) Karst. Ann. For. Sci. 2002, 59, 533–540. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Piñol, J. Drought-induced mortality and hydraulic architecture in pine populations of the NE Iberian Peninsula. For. Ecol. Manag. 2002, 161, 247–256. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Navarro-Cerillo, R.M.; Camarero, J.J.; Fernández-Cancio, A. Selective drought-induced decline of pine species in southeastern Spain. Clim. Chang. 2012, 113, 767–785. [Google Scholar] [CrossRef]
- Chuine, I.; Yiou, P.; Viovy, N.; Seguin, B.; Daux, V.; Le Roy Ladurie, E. Historical phenology: grape ripening as a past climate indicator. Nature 2004, 432, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, S.M. To die or not to die: early-warning signals of dieback in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef]
- González de Andrés, E.; Camarero, J.J.; Blanco, J.A.; Imbert, J.B.; Lo, Y.H.; Sangüesa-Barreda, G.; Castillo, F.J. Tree-to-tree competition in mixed European beech–Scots pine forests has different impacts on growth and water-use efficiency depending on site conditions. J. Ecol. 2017. [Google Scholar] [CrossRef]
- Eilmann, B.; Zweifel, R.; Buchmann, N.; Pannatier, E.G.; Rigling, A. Drought alters timing, quantity, and quality of wood formation in Scots pine. J. Exp. Bot. 2011, 62, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, R.; Item, H.; Häsler, R. Link between diurnal stem radius changes and tree water relations. Tree Physiol. 2001, 21, 869–877. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Sánchez-Salguero, R.; Camarero, J.J.; Gutiérrez, E.; González Rouco, F.; Gazol, A.; Sangüesa-Barreda, G.; Andreu-Hayles, L.; Linares, J.C.; Seftigen, K. Assessing forest vulnerability to climate warming using a process-based model of tree growth: Bad prospects for rear-edges. Glob. Chang. Biol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Taylor, F.W.; Burton, J.D. Growth ring characteristics, specific gravity, and fiber length of rapidly grown loblolly pine. Wood Fiber 1982, 14, 204–210. [Google Scholar]
- Kärenlampi, P.P.; Riekkinen, M. Maturity and growth rate effects on Scots pine basic density. Wood Sci. Technol. 2004, 38, 465–473. [Google Scholar] [CrossRef]
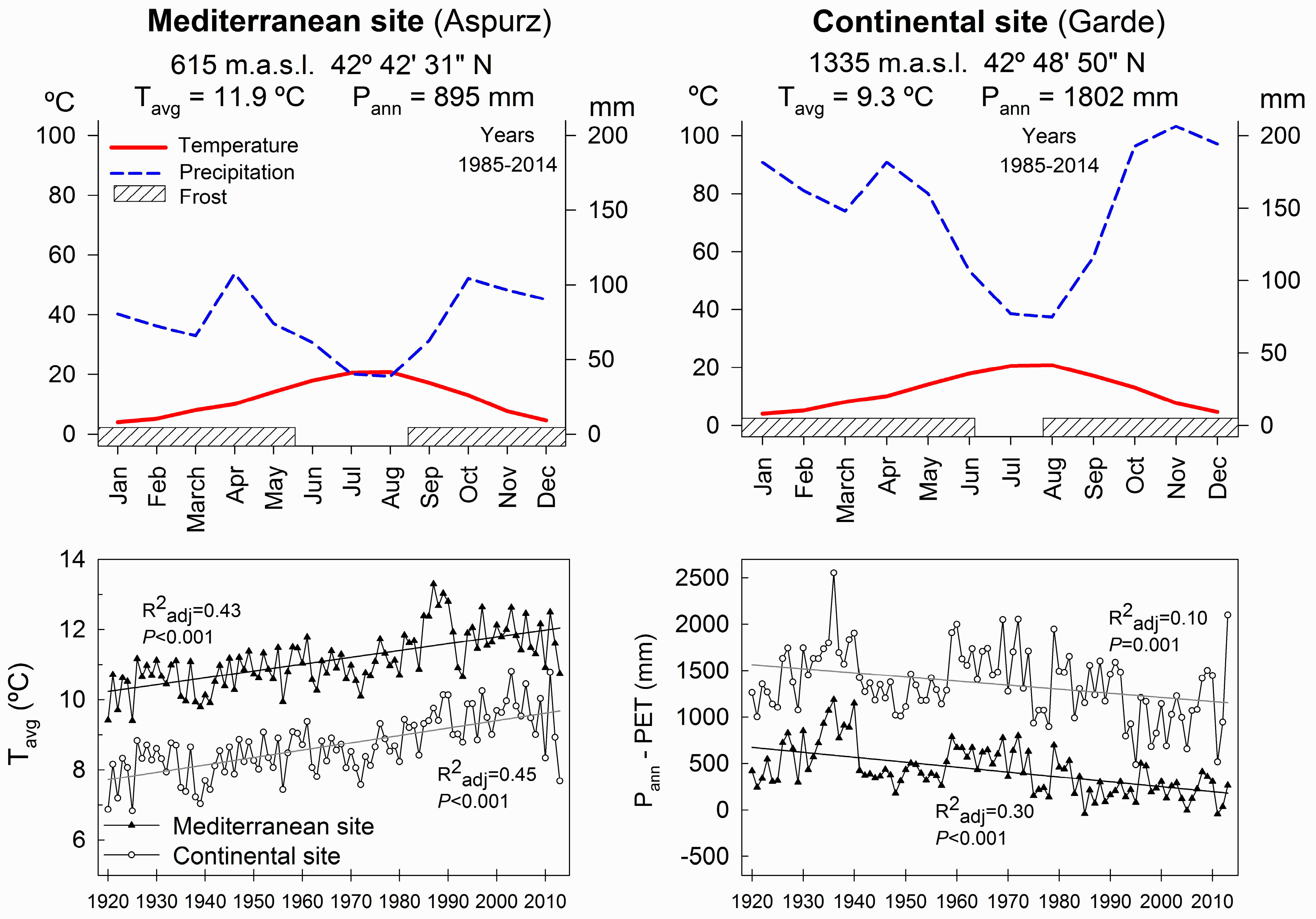
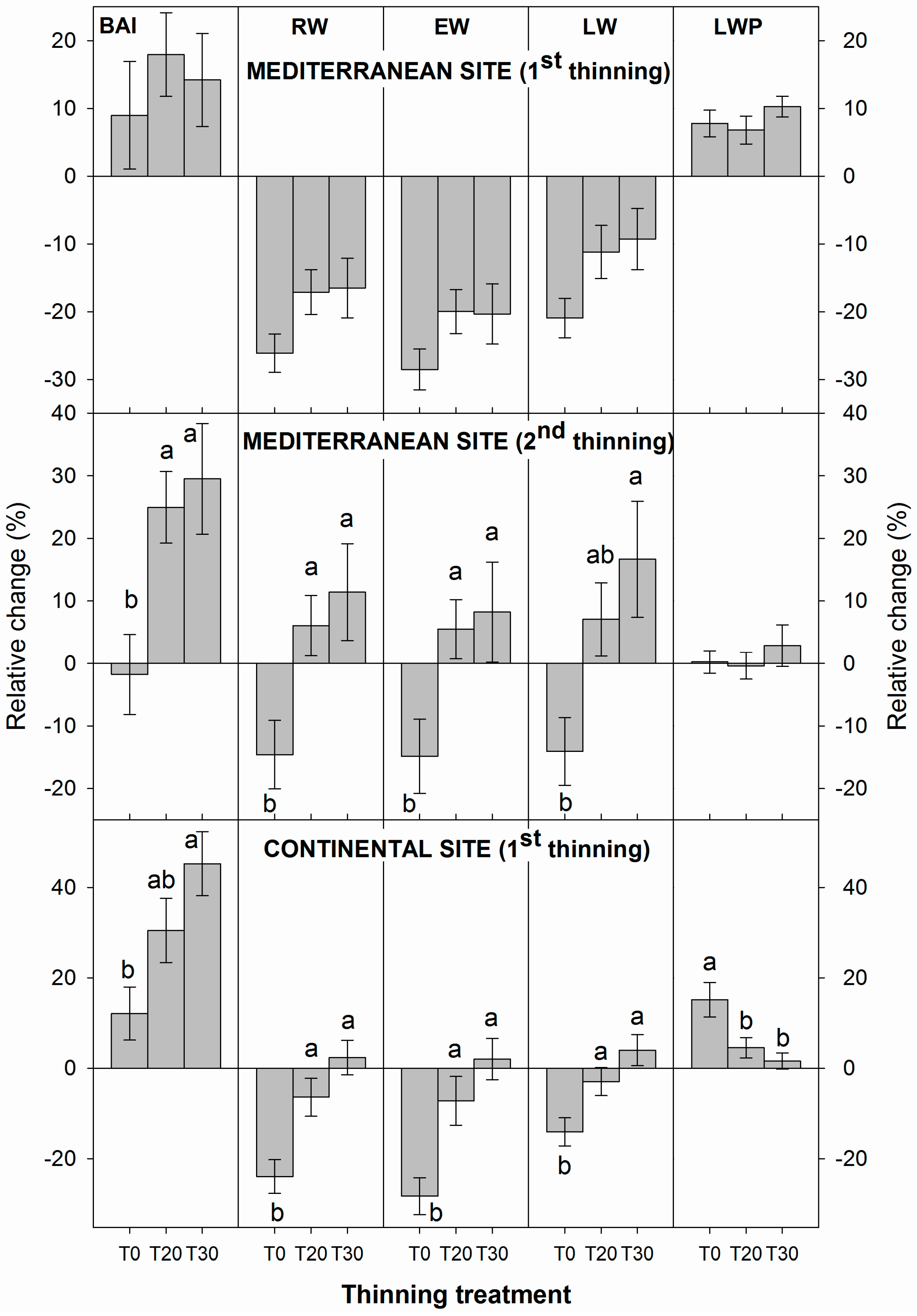
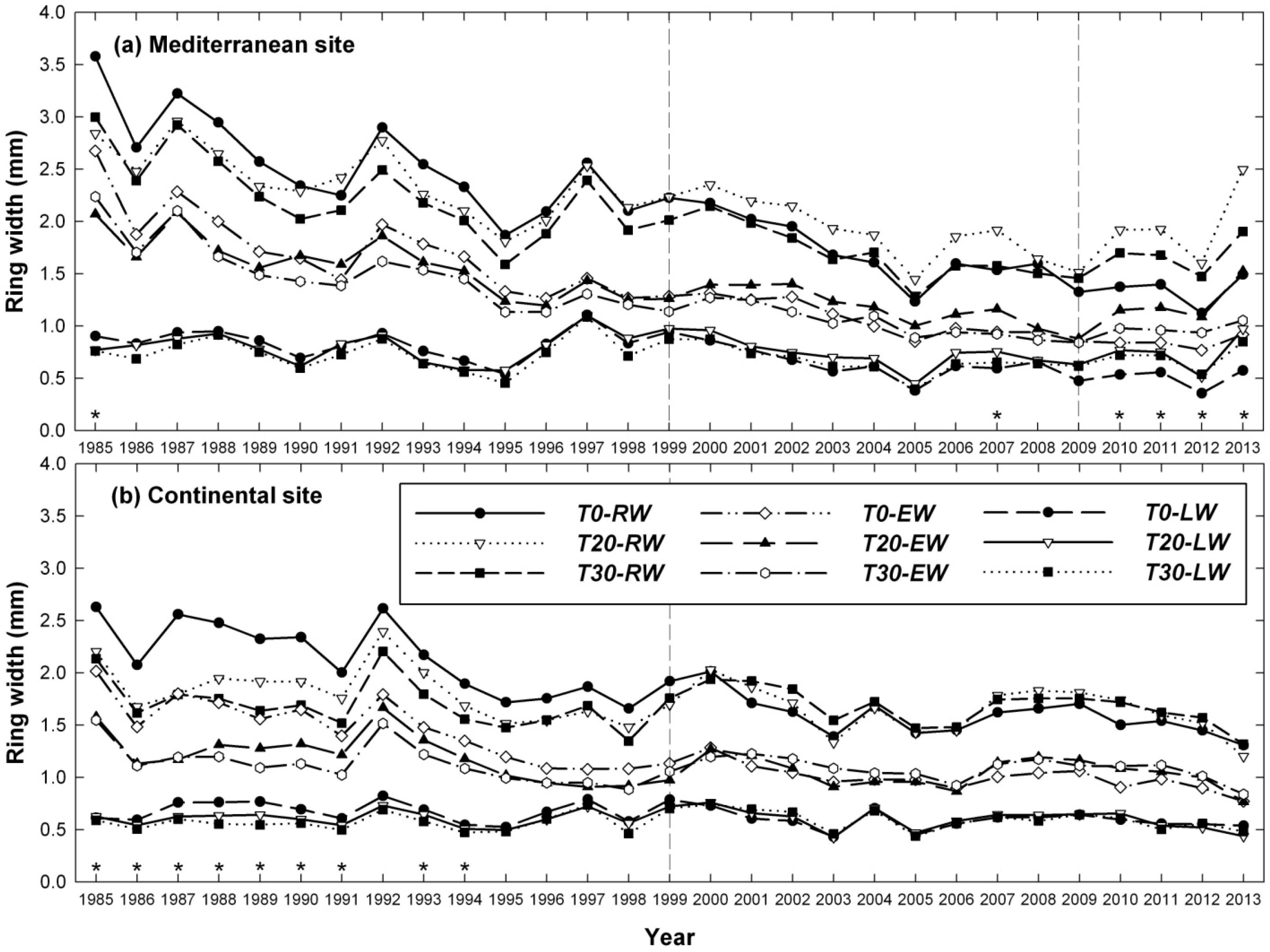
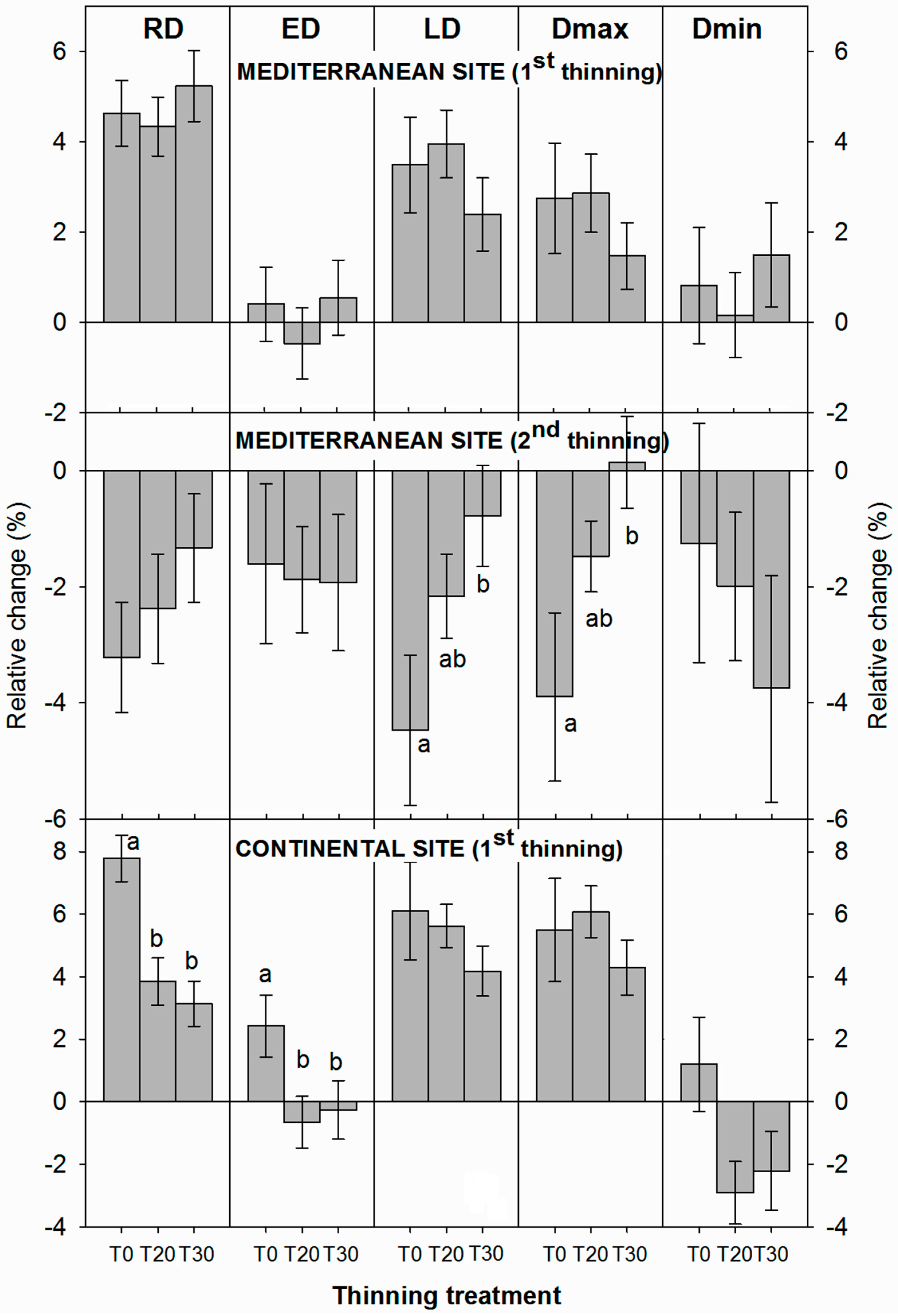
| Variable | Cold Wet Mediterranean Site 1 | Cold wet Continental Site 1 |
|---|---|---|
| Name of nearest town | Aspurz | Garde |
| Latitude N | 42°42′31″ | 42°48′50″ |
| Longitude W | 1°08′40″ | 0°52′30″ |
| Altitude a.s.l. (m) | 625 | 1335 |
| Mean slope (%) | 7 | 40 |
| Aspect | N | NE |
| Mean annual precipitation (mm) 2 | 895 | 1802 |
| Mean temperature (°C) 2 | 11.9 | 9.3 |
| Soil type (FAO) | Haplic Alisol | Dystric Cambisol |
| Texture (FAO) | Sandy loam | Clay loam |
| Maximum rooting depth | 45 cm | 35 cm |
| Saturated water content | 42.6% | 51.3% |
| Nitrogen content (%; soil horizons A/B) | 0.31/0.15 | 0.27/0.23 |
| Organic Matter (%; soil horizons A/B) | 11.69/1.14 | 9.88/3.05 |
| C/N ratio (soil horizons A/B) | 22.60/4.40 | 22.00/7.70 |
| Variable | Mediterranean Site | Continental Site | ||||
|---|---|---|---|---|---|---|
| Site index (m) 2 | 29 | 23 | ||||
| In 1999 (before 1st thinning) | ||||||
| Mean stand age (years) | 32 ± 4.3 | 37 ± 3.8 | ||||
| Treatment | T0 | T20 | T30 | T0 | T20 | T30 |
| Tree density (trees ha−1) | 3517 ± 681 | 3811 ± 340 | 4820 ± 491 | 3555 ± 358 | 2933 ± 268 | 3202 ± 135 |
| Mean height (m) 1 | 12.9 ± 0.5 | 12.9 ± 0.4 | 12.5 ± 0.5 | 10.9 ± 0.4 | 12.8 ± 0.4 | 13.3 ± 0.1 |
| Dbh (cm) 2 | 12.7 ± 1.2 | 11.6 ± 0.4 | 10.6 ± 0.7 | 13.3 ± 0.7 | 14.4 ± 0.6 | 13.7 ± 0.12 |
| Mean basal area (m2 ha−1) | 42.3 ± 2.0 | 40.1 ± 2.0 | 41.6 ± 1.2 | 49.0 ± 5.1 | 47.4 ± 0.7 | 47.0 ± 1.4 |
| In 2013 (tree coring) | ||||||
| Mean stand age (years) | 46 ± 2.3 | 51 ± 2.1 | ||||
| Treatment | T0 | T20 | T30 | T0 | T20 | T30/40 |
| Tree density (trees ha−1) | 1456 ± 156 | 1125 ± 83 | 1078 ± 147 | 1552 ± 280 | 1286 ± 93 | 1164 ± 49 |
| Mean height (m) | 17.8 ± 1.3 | 18.1 ± 0 .8 | 17.8 ± 1.2 | 14.7 ± 0.7 | 15.7 ± 0.3 | 15.2 ± 0.2 |
| Dbh (cm) | 18.9 ± 0 .8 | 19.9 ± 1.3 | 18.9 ± 0.9 | 14.9 ± 0.9 | 18.2 ± 0.4 | 18.7 ± 0.2 |
| Mean basal area (m2 ha−1) | 40.9 ± 1.4 | 35.0 ± 2.4 | 30.4 ± 1.5 | 55.4 ± 6.5 | 53.3 ± 1.3 | 48.7 ± 0.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candel-Pérez, D.; Lo, Y.-H.; Blanco, J.A.; Chiu, C.-M.; Camarero, J.J.; González de Andrés, E.; Imbert, J.B.; Castillo, F.J. Drought-Induced Changes in Wood Density Are Not Prevented by Thinning in Scots Pine Stands. Forests 2018, 9, 4. https://doi.org/10.3390/f9010004
Candel-Pérez D, Lo Y-H, Blanco JA, Chiu C-M, Camarero JJ, González de Andrés E, Imbert JB, Castillo FJ. Drought-Induced Changes in Wood Density Are Not Prevented by Thinning in Scots Pine Stands. Forests. 2018; 9(1):4. https://doi.org/10.3390/f9010004
Chicago/Turabian StyleCandel-Pérez, David, Yueh-Hsin Lo, Juan A. Blanco, Chih-Ming Chiu, J. Julio Camarero, Ester González de Andrés, J. Bosco Imbert, and Federico J. Castillo. 2018. "Drought-Induced Changes in Wood Density Are Not Prevented by Thinning in Scots Pine Stands" Forests 9, no. 1: 4. https://doi.org/10.3390/f9010004
APA StyleCandel-Pérez, D., Lo, Y.-H., Blanco, J. A., Chiu, C.-M., Camarero, J. J., González de Andrés, E., Imbert, J. B., & Castillo, F. J. (2018). Drought-Induced Changes in Wood Density Are Not Prevented by Thinning in Scots Pine Stands. Forests, 9(1), 4. https://doi.org/10.3390/f9010004









