Patterns of Early Postfire Succession of Alpine, Subalpine and Lichen-Woodland Vegetation: 21 Years of Monitoring from Permanent Plots
Abstract
:1. Introduction
2. Methods
2.1. Study Area
2.2. Selection of Sampling Sites
2.3. Sampling
2.4. Statistical Analyses
3. Results
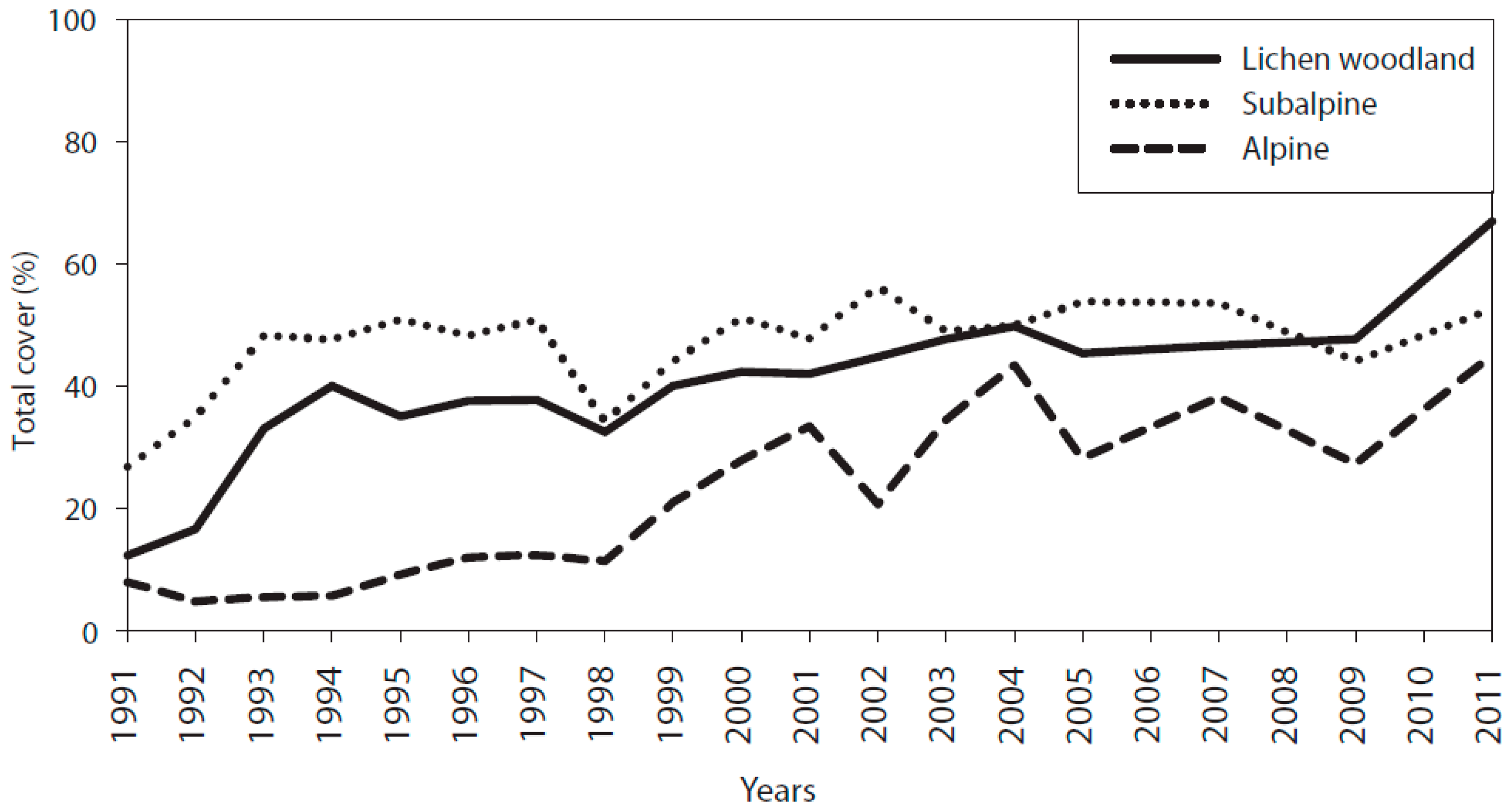
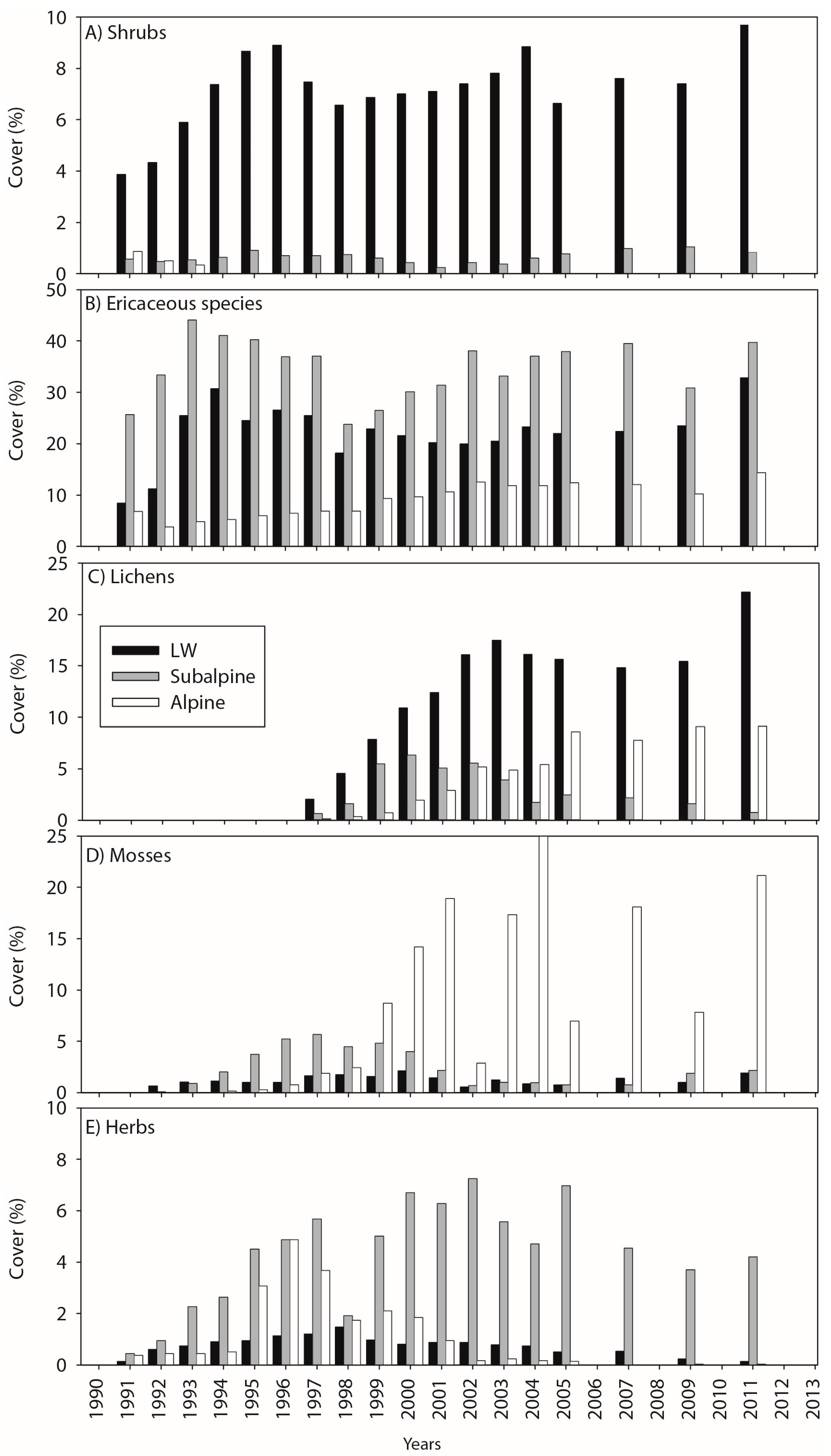
3.1. Cover
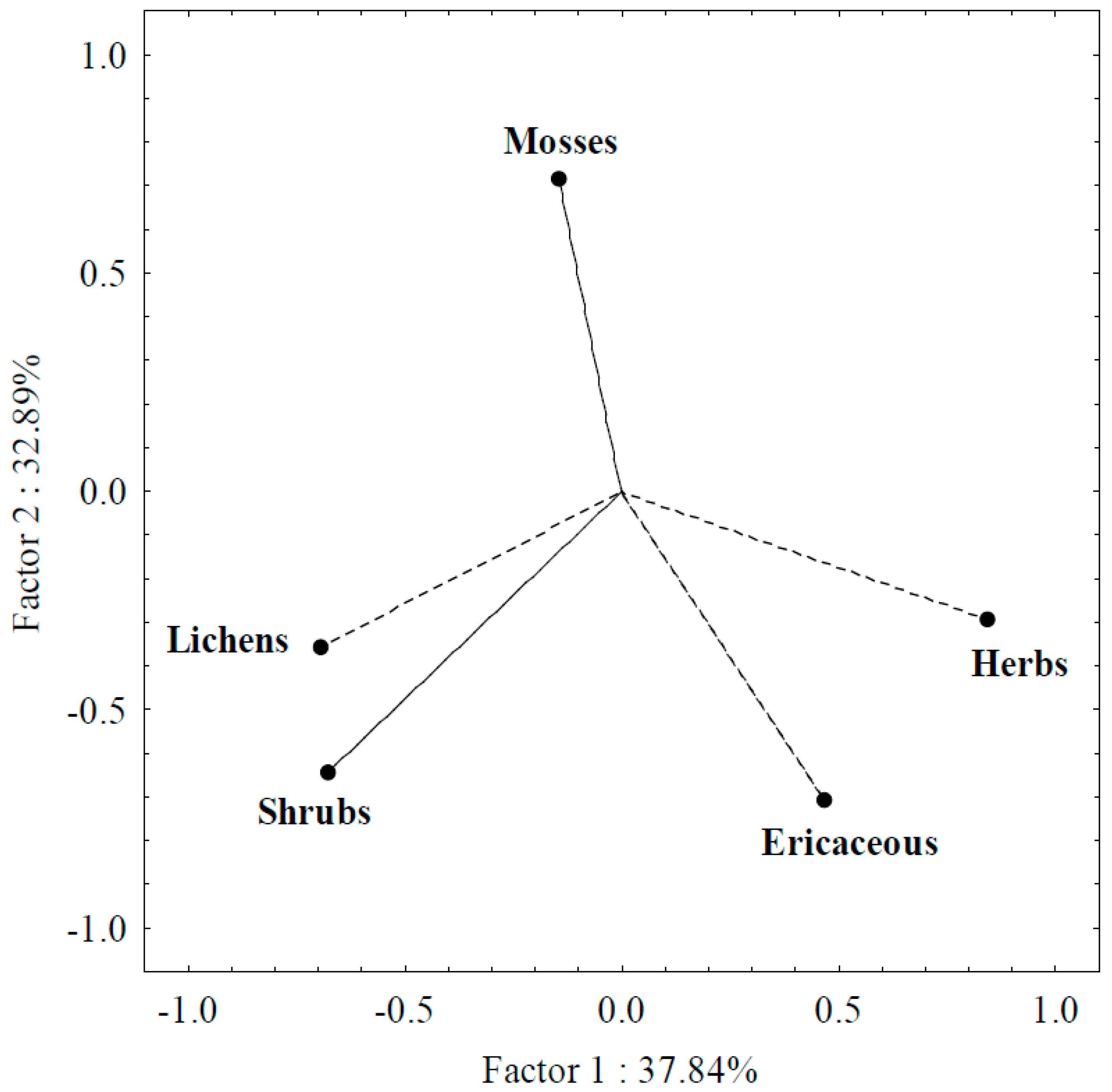
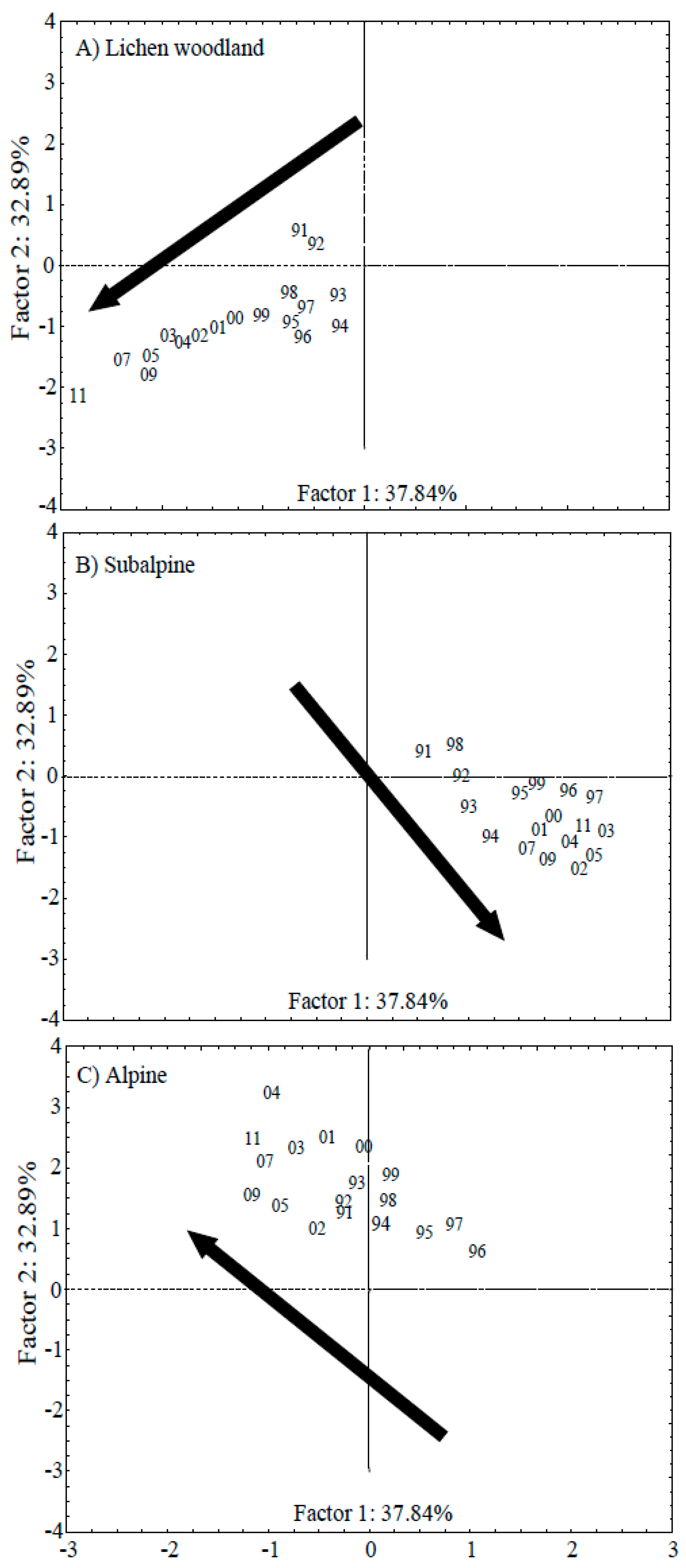
3.2. PCA Analysis
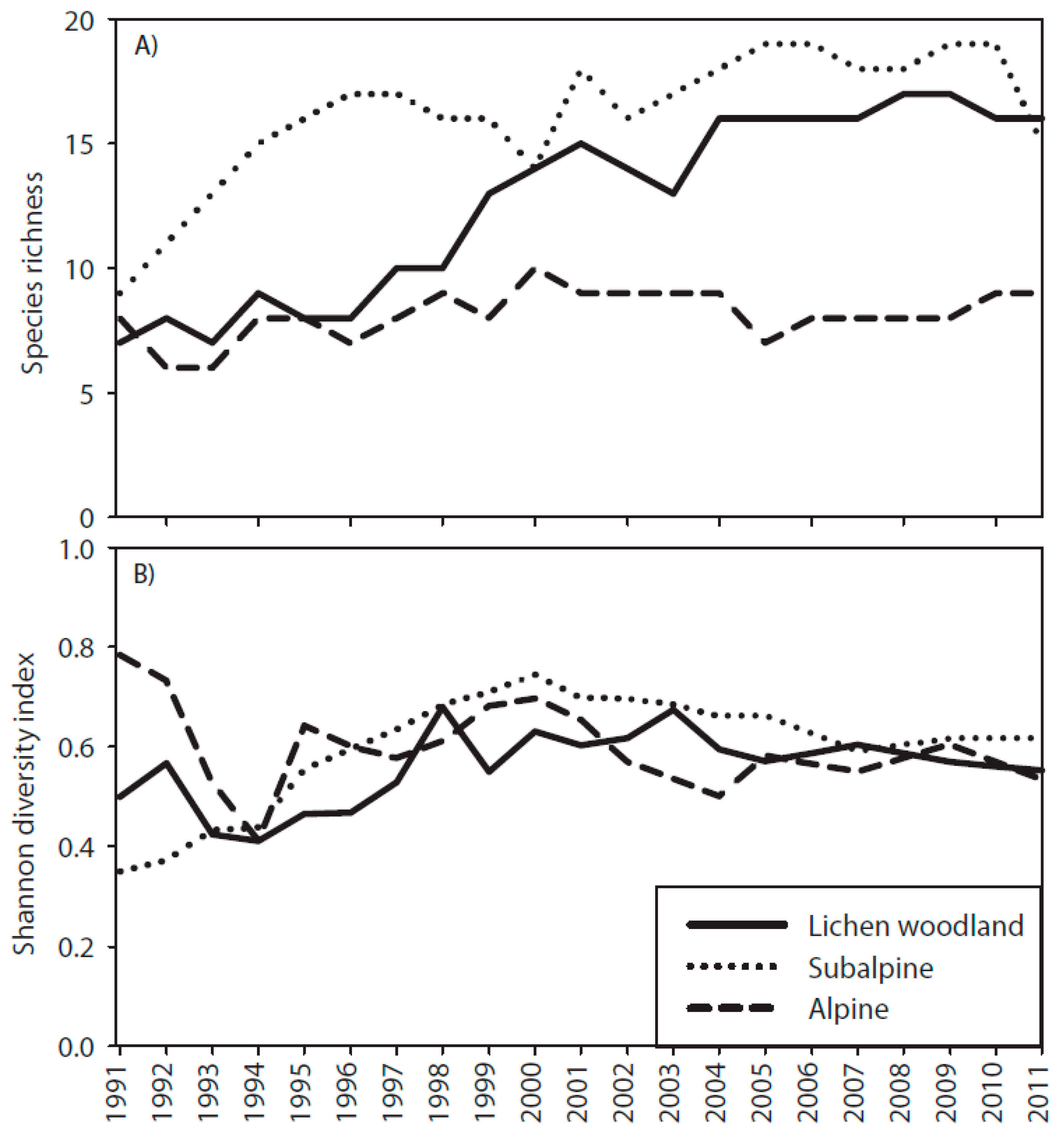
3.3. Species Richness and Shannon Diversity Index
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Note
- Pickett, S.T. Space-for-time substitution as an alternative to long-term studies. In Long-Term Studies in Ecology; Springer: New York, NY, USA, 1989; pp. 110–135. [Google Scholar]
- Foster, B.L.; Tilman, D. Dynamic and static views of succession: Testing the descriptive power of the chronosequence approach. Plant Ecol. 2000, 146, 1–10. [Google Scholar] [CrossRef]
- Munroe, J.S. Estimates of little ice age climate inferred through historical rephotography, northern uinta mountains, USA. Arct. Antarct. Alp. Res. 2003, 35, 489–498. [Google Scholar] [CrossRef]
- Buell, M.F.; Buell, H.F.; Small, J.A. Invasion of trees in secondary succession on the new jersey piedmont. Bull. Torrey Bot. Club 1971, 98, 67–74. [Google Scholar] [CrossRef]
- Beeftink, W. Vegetation responses to changes in tidal inundation of salt marshes. In Disturbance in Grasslands; Springer: Berlin, Germany, 1987; pp. 97–117. [Google Scholar]
- Peet, R.K.; Christensen, N.L. Competition and tree death. Bioscience 1987, 37, 586–595. [Google Scholar] [CrossRef]
- Glenn-Lewin, D.C.; van der Maarel, E. Patterns and processes of vegetation dynamics. In Plant Succession: Theory and Prediction; Glenn-Lewin, D.C., Peet, R.K., Veblen, T.T., Eds.; Chapman & Hall: London, UK, 1992; pp. 11–59. [Google Scholar]
- Payette, S.; Delwaide, A.; Caccianiga, M.; Beauchemin, M. Accelerated thawing of subarctic peatland permafrost over the last 50 years. Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef]
- Hustich, I. The boreal limits of conifers. Arctic 1953, 6, 149–162. [Google Scholar] [CrossRef]
- Rowe, J.S.; Scotter, G.W. Fire in the boreal forest. Quat. Res. 1973, 3, 444–464. [Google Scholar] [CrossRef]
- Bonan, G.B.; Shugart, H.H. Environmental factors and ecological processes in boreal forests. Annu. Rev. Ecol. Syst. 1989, 20, 1–28. [Google Scholar] [CrossRef]
- Boucher, Y.; Perrault-Hébert, M.; Fournier, R.; Drapeau, P.; Auger, I. Cumulative patterns of logging and fire (1940–2009): Consequences on the structure of the eastern canadian boreal forest. Landsc. Ecol. 2016, 32, 361–375. [Google Scholar] [CrossRef]
- Heinselman, M.L. Fire intensity and frequency as factors in the distribution and structure of the northern ecosystems. In Fire Regimes and Ecosystem Properties; Mooney, H.H., Bonnicksen, J.M., Christensen, N.L., Lotan, J.E., Reiners, W.A., Eds.; US Forest Service: Washington, DC, USA, 1981; Volume WO–26, pp. 7–57. [Google Scholar]
- Johnson, E. Fire and Vegetation Dynamics: Studies from the North American Boreal Forest; Cambridge University Press: Cambridge, UK, 1992; p. 92. [Google Scholar]
- Rowe, J.S. Forest Regions of Canada; Canadian Forestry Service: Victoria, BC, Canada, 1972; p. 172.
- Payette, S. Fire as a controlling process in the north american boreal forest. In A systems Analysis of the Global Boreal Forest; Shugart, H., Leemans, R., Bonan, G., Eds.; Cambridge University Press: Cambridge, UK, 1992; pp. 144–169. [Google Scholar]
- Johnson, E.; Miyanishi, K. Subarctic lichen woodlands. In Savanna, Barren and Rock Outcrop Plant Communities of North America; Anderson, R., Fralish, J., Baskin, J., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 421–436. [Google Scholar]
- Girard, F.; Payette, S.; Gagnon, R. Origin of the lichen-spruce woodland in the closed-crown forest zone of eastern canada. Glob. Ecol. Biogeogr. 2009, 18, 291–303. [Google Scholar] [CrossRef]
- Morneau, C.; Payette, S. A dendroecological method to evaluate past caribou (Rangifer tarandus L.) activity. Ecoscience 1998, 5, 64–76. [Google Scholar] [CrossRef]
- Ahti, T. Taxonomic Studies on Reindeer Lichens (Cladonia, Subgenus Cladina); Suomalaisen Kirjallisuuden Kirjapaino: Helsinki, Finland, 1961. [Google Scholar]
- Payette, S.; Morneau, C.; Sirois, L.; Desponts, M. Recent fire history of the northern quebec biomes. Ecology 1989, 70, 656–673. [Google Scholar] [CrossRef]
- Bergerud, A. Abundance of forage on the winter range of newfoundland caribou. Can. Field Nat. 1971, 85, 39–52. [Google Scholar]
- Coxson, D.S.; Marsh, J. Lichen chronosequences (postfire and postharvest) in lodgepole pine (pinus contorta) forests of northern interior british columbia. Can. J. Bot. 2001, 79, 1449–1464. [Google Scholar]
- Vézeau, C.; Payette, S. Gap expansion in old-growth subarctic forests: The climate–pathogen connection. New Phytol. 2016, 212, 1044–1056. [Google Scholar] [CrossRef] [PubMed]
- Payette, S.; Bhiry, N.; Delwaide, A.; Simard, M. Origin of the lichen woodland at its southern range limit in eastern canada: The catastrophic impact of insect defoliators and fire on the spruce-moss forest. Can. J. For. Res. 2000, 30, 288–305. [Google Scholar] [CrossRef]
- Jasinski, J.P.P.; Payette, S. The creation of alternative stable states in the southern boreal forest, Québec, Canada. Ecol. Monogr. 2005, 75, 561–583. [Google Scholar] [CrossRef]
- Girard, F.; Payette, S.; Gagnon, R. Rapid expansion of lichen woodlands within the closed-crown boreal forest zone over the last 50 years caused by stand disturbances in Eastern Canada. J. Biogeogr. 2008, 35, 529–537. [Google Scholar] [CrossRef]
- Boisclair, J. Parc des Grands-Jardins. Le Plan Directeur; Ministère du Loisir, de la Chasse et de la Pêche, Direction du Plein Air et des Parcs: Québec, QC, Canada, 1990; p. 255.
- Dussart, E.; Payette, S. Ecological impact of clear-cutting on black spruce-moss forests in Southern Québec. Ecoscience 2002, 9, 533–543. [Google Scholar] [CrossRef]
- Blais, J. Trends in the frequency, extent, and severity of spruce budworm outbreaks in Eastern Canada. Can. J. For. Res. 1983, 13, 539–547. [Google Scholar] [CrossRef]
- Simard, M.; Payette, S. Accurate dating of spruce budworm infestation using tree growth anomalies. Ecoscience 2003, 10, 204–216. [Google Scholar] [CrossRef]
- Dion, L. La Dynamique Forestière des Hauts Sommets de Saint-Urbain (Charlevoix); Université Laval: Québec, QC, Canada, 1988. [Google Scholar]
- Bussières, B.; Payette, S.; Filion, L. Déboisement et entourbement des hauts sommets de charlevoix à l’holocène supérieur: Origine des étages alpin et subalpin. Géographie Physique et Quaternaire 1996, 50, 258–269. [Google Scholar] [CrossRef]
- Mueller-Dombois, D.; Ellenberg, H. Aims and Methods of Vegetation Ecology; John Wiley & Sons: New York, NY, USA, 1974; p. 547. [Google Scholar]
- Sokal, R.; Rohlf, F. Biometry. The Principles and Practice of Statistics in Biological Research, 3rd ed.; W.H. Freeman & Co.: New York, NY, USA, 1995. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community ecology package. 2011. [Google Scholar]
- Foster, D. Vegetation development following fire in picea-mariana (black spruce) pleurozium forests of southeastern labrador, Canada. J. Ecol. 1985, 73, 517–534. [Google Scholar] [CrossRef]
- Schimmel, J.; Granstrom, A. Fire severity and vegetation response in the boreal swedish forest. Ecology 1996, 77, 1436–1450. [Google Scholar] [CrossRef]
- Hollingsworth, T.N.; Johnstone, J.F.; Bernhardt, E.L.; Chapin, F.S., 3rd. Fire severity filters regeneration traits to shape community assembly in alaska’s boreal forest. PLoS ONE 2013, 8, e56033. [Google Scholar] [CrossRef] [PubMed]
- Auclair, A.N. Postfire regeneration of plant and soil organic pools in a picea mariana-cladonia stellaris ecosystem. Can. J. For. Res. 1985, 15, 279–291. [Google Scholar] [CrossRef]
- Flinn, M.A.; Wein, R.W. Depth of underground plant organs and theoretical survival during fire. Can. J. Bot. 1977, 55, 2550–2554. [Google Scholar] [CrossRef]
- Flinn, M.A.; Pringle, J.K. Heat tolerance of rhizomes of several understory species. Can. J. Bot. 1983, 61, 452–457. [Google Scholar] [CrossRef]
- Wiensczyk, A.; Swift, K.; Morneault, A.; Thiffault, N.; Szuba, K.; Bell, F.W. An overview of the efficacy of vegetation management alternatives for conifer regeneration in boreal forests. For. Chron. 2011, 87, 175–200. [Google Scholar] [CrossRef]
- Granström, A.; Schimmel, J. Heat effects on seeds and rhizomes of a selection of boreal forest plants and potential reaction to fire. Oecologia 1993, 94, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Frego, K.A.; Carleton, T.J. Microsite tolerance of four bryophytes in a mature black spruce stand: Reciprocal transplants. Bryologist 1995, 98, 452–458. [Google Scholar] [CrossRef]
- Morneau, C.; Payette, S. Postfire lichen spruce woodland recovery at the limit of the boreal forest in northern quebec. Can. J. Bot. 1989, 67, 2770–2782. [Google Scholar] [CrossRef]
- Rouse, W.R. Microclimatic changes accompanying burning in subarctic lichen woodland. Arct. Antarct. Alp. Res. 1976, 357–376. [Google Scholar] [CrossRef]
- Connell, J.H.; Slatyer, R.O. Mechanisms of succession in natural communities and their role in community stability and organization. Am. Nat. 1977, 111, 1119–1144. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girard, F.; Payette, S.; Delwaide, A. Patterns of Early Postfire Succession of Alpine, Subalpine and Lichen-Woodland Vegetation: 21 Years of Monitoring from Permanent Plots. Forests 2017, 8, 346. https://doi.org/10.3390/f8090346
Girard F, Payette S, Delwaide A. Patterns of Early Postfire Succession of Alpine, Subalpine and Lichen-Woodland Vegetation: 21 Years of Monitoring from Permanent Plots. Forests. 2017; 8(9):346. https://doi.org/10.3390/f8090346
Chicago/Turabian StyleGirard, François, Serge Payette, and Ann Delwaide. 2017. "Patterns of Early Postfire Succession of Alpine, Subalpine and Lichen-Woodland Vegetation: 21 Years of Monitoring from Permanent Plots" Forests 8, no. 9: 346. https://doi.org/10.3390/f8090346
APA StyleGirard, F., Payette, S., & Delwaide, A. (2017). Patterns of Early Postfire Succession of Alpine, Subalpine and Lichen-Woodland Vegetation: 21 Years of Monitoring from Permanent Plots. Forests, 8(9), 346. https://doi.org/10.3390/f8090346




