Forest Structure and Composition Affect Bats in a Tropical Evergreen Broadleaf Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Bat Monitoring
2.3. Forest Characteristics
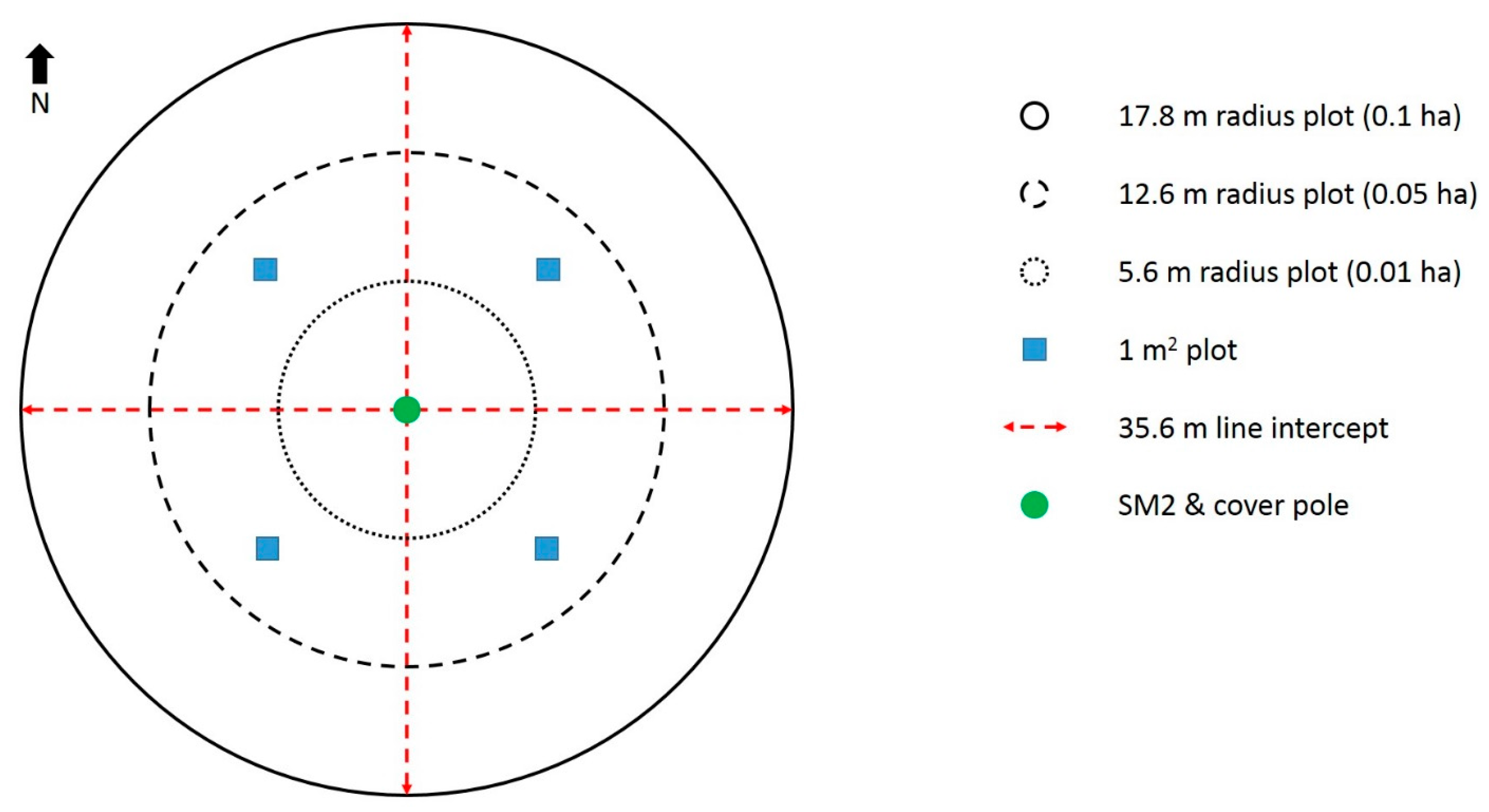
2.3.1. Ground Strata
2.3.2. Shrub Strata
2.3.3. Ground-Shrub Strata
2.3.4. Understory Strata
2.3.5. Midstory Strata
2.3.6. Overstory Strata
2.3.7. Other
2.3.8. Topography
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Simmons, N.B.; Voss, R.S. The mammals of Paracou, French Guiana: A Neotropical lowland rainforest fauna: Part 1 Bats. In Bulletin of the American Museum of Natural History; American Museum of Natural History: New York, NY, USA, 1998; Volume 237, pp. 1–219. [Google Scholar]
- Voss, R.S.; Emmons, L.H. Mammalian diversity in Neotropical lowland rainforests: A preliminary assessment. In Bulletin of the American Museum of Natural History; American Museum of Natural History: New York, NY, USA, 1996; Volume 230, pp. 1–115. [Google Scholar]
- O’Shea, T.J.; Cryan, P.M.; Hayman, D.T.S.; Plowright, R.K.; Streicker, D.G. Multiple mortality events in bats: A global review. Mammal Rev. 2016, 46, 173–190. [Google Scholar] [CrossRef]
- Voigt, C.C.; Kingston, T. (Eds.) Bats in the Anthropocene: Conservation of Bats in a Changing World; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Bader, E.; Jung, K.; Kalko, E.K.V.; Page, R.A.; Rodriguez, R.; Sattler, T. Mobility explains the response of aerial insectivorous bats to anthropogenic habitat change in the Neotropics. Biol. Conserv. 2015, 186, 97–106. [Google Scholar] [CrossRef]
- Kunz, T.H.; Fenton, M.B. Bat Ecology; The University of Chicago Press: Chicago, IL, USA, 2003. [Google Scholar]
- Patterson, B.D.; Willig, M.R.; Stevens, R.D. Trophic strategies, niche portioning, and patterns of ecological organization. In Bat Ecology; Kunz, T.H., Fenton, M.B., Eds.; University of Chicago Press: Chicago, IL, USA, 2003; pp. 536–579. [Google Scholar]
- Kalka, M.B.; Smith, A.R.; Kalko, E.K.V. Bats limit arthropods and herbivory in a tropical forest. Science 2008, 320, 71. [Google Scholar] [CrossRef] [PubMed]
- Kunz, T.H.; Braun de Torrez, E.; Bauer, D.; Lobova, T.; Fleming, T.H. Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. 2011, 1223, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Lobova, T.A.; Mori, S.A.; Blanchard, F.; Peckham, H.; Charles-Dominique, P. Cercopia as a food resource for bats in French Guiana and the significance of fruit structure in seed dispersal and longevity. Am. J. Bot. 2003, 90, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Miller, B. Beyond mist-nets: What the rest of the bats can tell us about forests. In The Cutting Edge; Fimbel, R.A., Grajal, A., Robinson, J.G., Eds.; Columbia University Press: New York, NY, USA, 2001; pp. 154–156. [Google Scholar]
- Muscarella, R.; Fleming, T.H. The role of frugivorous bats in tropical forest succession. Biol. Rev. 2007, 82, 573–590. [Google Scholar] [CrossRef] [PubMed]
- Fenton, M.B.; Acharya, L.; Audet, D.; Hickey, M.B.C.; Merriman, C.; Obrist, M.K.; Syme, D.M.; Adkins, B. Phyllostomid bats (Chiroptera: Phyllostomidae) as indicators of habitat disruption in the Neotropics. Biotropica 1992, 24, 440–446. [Google Scholar] [CrossRef]
- Jones, G.; Jacobs, D.S.; Kunz, T.H.; Willig, M.R.; Racey, P.A. Carpe noctem: The importance of bats as bioindicators. Endanger. Spec. Res. 2009, 8, 93–115. [Google Scholar] [CrossRef]
- Medellín, R.A.; Equihua, M.; Amin, M.A. Bat diversity and abundance as indicators of disturbance in Neotropical rainforests. Conserv. Biol. 2000, 14, 1666–1675. [Google Scholar] [CrossRef]
- Bridgewater, S.A. Natural History of Belize: Inside the Maya Forest; University of Texas Press: Austin, TX, USA, 2012. [Google Scholar]
- Bawa, K.S.; Seidler, R. Natural forest management and conservation of biodiversity in tropical forests. Conserv. Biol. 1998, 12, 46–55. [Google Scholar] [CrossRef]
- Laurence, W.F. A crisis in the making: Responses of Amazonian forests to land use and climate change. Trends Ecol. Evol. 1998, 13, 411–415. [Google Scholar] [CrossRef]
- Burslem, D.F.R.P.; Whitmore, T.C.; Brown, G.C. Short-term effects of cyclone impact and long-term recovery of tropical forest on Kolobangara, Solomon Islands. J. Ecol. 2000, 88, 1063–1078. [Google Scholar] [CrossRef]
- Shiels, A.B.; González, G.; Lodge, D.J.; Willig, M.R.; Zimmerman, J.K. Cascading effects of canopy opening and debris deposition from a large scale hurricane experiment in a tropical rainforest. Bioscience 2015, 65, 871–881. [Google Scholar] [CrossRef]
- Brigham, R.M.; Grindall, S.D.; Firman, M.C.; Morissette, J.L. The influence of structural clutter on activity patterns of insectivorous bats. Can. J. Zool. 1997, 75, 131–136. [Google Scholar] [CrossRef]
- Estrada, A.; Coates-Estrada, R.; Meritt, D., Jr. Bats, species richness, and abundance in tropical rain forest fragments and in agricultural habitats at Los Tuxtlas, Mexico. Ecography 1993, 16, 309–318. [Google Scholar] [CrossRef]
- Marques, J.T.; Pereira, M.J.R.; Palmeirim, J.M. Patterns in the use of rainforest vertical space by Neotropical aerial insectivorous bats: All the action is up in the canopy. Ecography 2016, 39, 476–486. [Google Scholar] [CrossRef]
- Presley, S.J.; Willig, M.R.; Wunderle, J.M., Jr.; Saldanha, L.N. Effects of reduced-impact logging and forest physiognomy on bat populations of lowland Amazonian forest. J. Appl. Ecol. 2008, 45, 14–25. [Google Scholar] [CrossRef]
- Rainho, A.; Augusto, A.M.; Palmeirim, J.M. Influence of vegetation clutter on the capacity of ground foraging bats to capture prey. J. Appl. Ecol. 2010, 47, 850–858. [Google Scholar] [CrossRef]
- Soriano, P.J.; Ochoa, J. The consequences of timber exploitation for bat communities in tropical America. In The Cutting Edge: Conserving Wildlife in Logged Tropical Forest; Fimbel, R.A., Grajal, A., Robinson, J.G., Eds.; Columbia University Press: New York, NY, USA, 2001; pp. 153–166. [Google Scholar] [CrossRef]
- Jung, K.; Kaiser, S.; Bohm, S.; Nieschulze, J.; Kalko, K.V. Moving in three dimensions: Effects of structural complexity on occurrence and activity of insectivorous bats in managed forest stands. J. Appl. Ecol. 2012, 49, 523–531. [Google Scholar] [CrossRef]
- Kalko, E.K.V.; Villegas, S.E.; Schmidt, M.; Wegmann, M.; Meyer, C.F.J. Flying high—Assessing the use of the aerosphere by bats. Integr. Comp. Biol. 2008, 48, 60–73. [Google Scholar]
- Norberg, U.M.; Rayner, J.M.V. Ecological morphology and flight in bats (Mammalia; Chiroptera): Wing adaptations, flight performance, foraging strategy and echolocation. Philos. Trans. R. Soc. Bull. 1987, 316, 335–427. [Google Scholar] [CrossRef]
- Peters, S.L.; Malcolm, J.R.; Zimmerman, B.L. Effects of selective logging on bat communities in the southeastern Amazon. Conserv. Biol. 2006, 20, 1410–1421. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, H.U.; Kalko, E.K.V. Echolocation by insect-eating bats. Bioscience 2001, 51, 557–569. [Google Scholar] [CrossRef]
- Castro-Arellano, I.; Presley, S.J.; Saldanha, L.N.; Willig, M.R.; Wunderle, J.M., Jr. Effects of reduced impact logging on bat biodiversity in terra firme forest of lowland Amazonia. Biol. Conserv. 2007, 138, 269–285. [Google Scholar] [CrossRef]
- Castro-Arellano, I.; Presley, S.J.; Willig, M.R.; Wunderle, J.M.; Saldanha, L.N. Reduced-impact logging and temporal activity of understory bats in lowland Amazonia. Biol. Conserv. 2009, 142, 2131–2139. [Google Scholar] [CrossRef]
- Clarke, F.M.; Rostant, L.V.; Racey, P.A. A comparison of logging systems and bat diversity in the Neotropics. Conserv. Biol. 2005, 19, 1194–1204. [Google Scholar]
- Clarke, F.M.; Rostant, L.V.; Racey, P.A. Life after logging: Post-logging recovery of a Neotropical bat community. J. Appl. Ecol. 2005, 42, 409–420. [Google Scholar] [CrossRef]
- Bullridge Co., Ltd. Sustainable Forest Management Plan for the Chiquibul Forest Reserve 2012–2016; Bullridge Co., Ltd.: Cayo, Belize, 2006. [Google Scholar]
- Hayes, J.P. Assumptions and practical considerations in the design and interpretation of echolocation monitoring studies. Acta Chiropterol. 2000, 2, 225–236. [Google Scholar]
- Ahlén, I.; Baagøe, H.J. Use of ultrasound detectors for bat studies in Europe: Experiences from field identification, surveys, and monitoring. Acta Chiropterol. 1999, 1, 137–150. [Google Scholar]
- Fenton, M.B. Choosing the ‘correct’ bat detector. Acta Chiropterol. 2000, 2, 215–224. [Google Scholar]
- Lemen, C.; Freeman, P.W.; White, J.A.; Andersen, B.R. The problem of low agreement among automated identification programs for acoustical surveys of bats. Western N. Am. Nat. 2015, 75, 218–225. [Google Scholar] [CrossRef]
- Agranat, I. Detecting Bats with Ultrasonic Microphones: Understanding He Effects of Microphone Variance and Placement on Detection Rates; Wildlife Acoustics, Inc.: Maynard, MA, USA, 2014. [Google Scholar]
- Wildlife Acoustics, Inc. Kaleidoscope Pro 3; Wildlife Acoustics, Inc.: Maynard, MA, USA, 2015. [Google Scholar]
- Gomes, G.A.; Reid, F.A.; Tuttle, M.D. Bats of Trinidad and Tobago: A Field Guide and Natural History; West Indies: Trinidad, Trinidad and Tobago, 2015. [Google Scholar]
- Jakobsen, L.; Kalko, E.K.V.; Surlykke, A. Echolocation beam shape in emballonurid bats, Saccopteryx bilineata and Cormura brevirostris. Behav. Ecol. Sociobiol. 2012, 66, 1493–1502. [Google Scholar] [CrossRef]
- Jung, K.; Kalko, E.K.V.; von Helverson, O. Echolocation calls in Central American emballonurid bats: Signal design and call frequency alternation. J. Zool. 2007, 272, 125–137. [Google Scholar] [CrossRef]
- Jung, K.; Molinari, J.; Kalko, E.K.V. Driving Factors for the evolution of species-specific echolocation call design in New World free-tailed bats (Molossidae). PLoS ONE 2014, 9, e85279. [Google Scholar] [CrossRef] [PubMed]
- Mora, E.C.; Macías, S.; Hechavarría, J.; Vater, M.; Kössl, M. Evolution of the heteroharmonic strategy for target-range computation in the echolocation of Mormoopidae. Front. Phys. 2013, 4, 141. [Google Scholar] [CrossRef] [PubMed]
- Rydell, J.; Arita, H.T.; Santos, M.; Granados, J. Acoustic identification of insectivorous bats (order Chiroptera) of Yucatan, Mexico. J. Zool. 2002, 257, 27–36. [Google Scholar] [CrossRef]
- Betts, B.J. Effect of inter-individual variations in echolocation calls on identification of big-brown and silver-haired bats. J. Wildl. Manag. 1998, 62, 1003–1010. [Google Scholar] [CrossRef]
- Loeb, S.C.; O’Keefe, J.M. Habitat use by forest bats of South Carolina in relation to local, stand, and landscape characteristics. J. Wildl. Manag. 2006, 70, 1210–1218. [Google Scholar] [CrossRef]
- Russo, D.; Voigt, C.C. The use of automated identification of bat echolocation calls in acoustic monitoring: A cautionary note for a sound analysis. Ecol. Indic. 2016, 66, 598–602. [Google Scholar] [CrossRef]
- Rydell, J.; Nyman, S.; Eklof, J.; Jones, G.; Russo, D. Testing the performances of automated identification of bat echolocation calls: A request for prudence. Ecol. Indic. 2017, 78, 416–420. [Google Scholar] [CrossRef]
- Higgins, K.F.; Jenkins, K.J.; Clambey, G.K.; Uresk, D.W.; Naugle, D.E.; Norland, J.E.; Barker, W.T. Vegetation sampling and monitoring. In Techniques for Wildlife Investigations and Management; Braun, C.E., Ed.; The Wildlife Society and Port City Press: Baltimore, MD, USA, 2005; pp. 524–553. [Google Scholar]
- Hays, R.L.; Summers, C.; Seitz, W. Estimating Wildlife Habitat Variables; FWS/OBS-81/47; U.S. Fish and Wildlife Service: Washington, DC, USA, 1981.
- Griffith, B.; Youtie, B.A. Two devices for estimating foliage density and deer hiding cover. Wildl. Soc. Bull. 1988, 16, 206–210. [Google Scholar]
- United States Forest Service. Volume 1: Field Data Collection Procedures for Phase 2 Plots. In Forest Inventory and Analysis National Core Field Guide; Version 7.0; United States Forest Service: Newtown Square, PA, USA, 2015. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- Tirpak, J.M.; Giuliano, W.M.; Allen, T.J.; Bittner, S.; Edwards, J.W.; Friedhof, S.; Harper, C.A.; Igo, W.K.; Stauffer, D.F.; Norman, G.W. Ruffed grouse-habitat preference in the central and southern Appalachians. For. Ecol. Manag. 2010, 260, 1525–1538. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; John Wiley and Sons, Inc.: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- McGarigal, K.; Cushman, S.A.; Stafford, S.G. Multivariate Statistics for Wildlife and Ecology Research; Springer: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- SYSTAT. Statistics II; Version 12; SYSTAT Software: San Jose, CA, USA, 2007. [Google Scholar]
- Conover, W.J. Practical Nonparametric Statistics, 3rd ed.; John Wiley and Sons, Inc.: New York, NY, USA, 1999. [Google Scholar]
- International Union for the Conservation of Nature. The IUCN Red List of Threatened Species. Available online: http://www.iucnredlist.org/ (accessed on 12 September 2016).
- Reid, F.A. A Field Guide to the Mammals of Central America and Southeast Mexico, 2nd ed.; Oxford University Press: New York, NY, USA, 2009. [Google Scholar]
- Lim, B.K.; Engstrom, M.D.; Patton, J.C.; Bickham, J.W. Systematic review of small fruit-eating bats (Artibeus) from the Guianas, and a re-evaluation of A. glaucus bogotensis. Acta Chiropterol. 2008, 10, 243–256. [Google Scholar] [CrossRef]
- Estrada-Villegas, S.; McGill, B.J.; Kalko, E.K.V. Climate, habitat, and species interactions at different scales determine the structure of a Neotropical bat community. Ecology 2012, 93, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Kalko, E.K.V. Echolocation signal design, foraging habitats and guild structure in six Neotropical sheath-tailed bats (Emballonuridae). Symp. Zool. Soc. Lond. 1995, 67, 259–273. [Google Scholar]
- Russo, D.; Cistrone, L.; Jones, G. Emergence time in forest bats: The influence of canopy closure. Acta Oecol. 2007, 31, 119–126. [Google Scholar] [CrossRef]
- Mikula, P.; Morelli, F.; Lucan, R.K.; Jones, D.N.; Tryjanowski, P. Bats as prey of diurnal birds: A global perspective. Mammal Rev. 2016, 46, 160–174. [Google Scholar] [CrossRef]
- Meyer, C.F.J.; Fründ, J.; Lizano, W.P.; Kalko, E.K.V. Ecological correlates of vulnerability to fragmentation in Neotropical bats. J. Appl. Ecol. 2008, 45, 381–391. [Google Scholar] [CrossRef]
- Malcolm, J.R.; Ray, J.C. Influence of timber extraction routes on central African small-mammal communities, forest structure, and tree diversity. Conserv. Biol. 2000, 14, 1623–1638. [Google Scholar] [CrossRef]
- Adis, J. Terrestrial invertebrates: Survival strategies, group spectrum, dominance and activity patterns. In The Central Amazon Floodplain—Ecology of a Pulsing System; Junk, W.J., Ed.; Springer: Berlin, Germany, 1997; pp. 299–317. [Google Scholar] [CrossRef]
- Basset, T. Vertical stratification of arthropod assemblages. In Arthropods of Tropical Forests—Spatio-Temporal Dynamics and Resource Use in the Canopy; Basset, Y., Novotony, V., Miller, S.E., Kitching, R.L., Eds.; Cambridge University Press: Cambridge, UK, 2003; pp. 17–27. [Google Scholar]
- Basset, Y. Stratification and diel activity of arthropods in a lowland rainforest in Gabon. Biol. J. Linn. Soc. 2011, 72, 585–607. [Google Scholar] [CrossRef]
- Barrios, H. Insect herbivores feeding on conspecific seedlings and trees. In Arthropods of Tropical Forests—Spatio-Temporal Dynamics and Resource Use in the Canopy; Basset, Y., Novotony, V., Miller, S.E., Kitching, R.L., Eds.; Cambridge University Press: Cambridge, UK, 2003; pp. 282–290. [Google Scholar]
- Parker, G.G. Structure and microclimate of forest canopies. In Forest Canopies: A Review of Research on a Biological Frontier; Lowman, M., Nadkarni, N., Eds.; Academic Press: Cambridge, MA, USA, 1995; pp. 73–106. [Google Scholar]
- Cox, M.R.; Willcox, E.V.; Keyser, P.D.; VanderYacht, A.L. Bat response to prescribed fire and overstory thinning in hardwood forest on the Cumberland Plateau, Tennessee. For. Ecol. Manag. 2016, 359, 221–231. [Google Scholar] [CrossRef]
- Humes, M.L.; Hayes, J.P.; Collopy, M.W. Bat activity in thinned, unthinned, and old-growth forests in western Oregon. J. Wildl. Manag. 1999, 63, 553–561. [Google Scholar] [CrossRef]
- Ellis, A.M.; Patton, L.L.; Castleberry, S.B. Bat activity in upland and riparian habitats in the Georgia Piedmont. In Proceedings of the Annual Conference of the Southeast Association of Fish and Wildlife Agencies; Southeastern Association of Fish and Wildlife Agencies: Jackson, MS, USA, 2002; Volume 56, pp. 210–218. [Google Scholar]
- Loeb, S.C.; Waldrop, T.A. Bat activity in relation to fire and fire surrogate treatments in southern pine stands. For. Ecol. Manag. 2008, 255, 3185–3192. [Google Scholar] [CrossRef]
- Menzel, J.M.; Menzel, M.A.; Kilgo, J.C.; Ford, M.; Edwards, J.W.; McCracken, G.F. Effect of habitat and foraging height on bat activity in the coastal plain of South Carolina. J. Wildl. Manag. 2005, 69, 235–245. [Google Scholar] [CrossRef]
- Titchenell, M.A.; Williams, R.A.; Gehrt, S.D. Bat response the shelterwood harvests and forest structure in oak-hickory forests. For. Ecol. Manag. 2011, 262, 980–988. [Google Scholar] [CrossRef]
- Rodríguez-Durán, A.; Kunz, T.H. Biogeography of West Indian bats: An ecological perspective. In Biogeography of the West Indies: Patterns and Perspectives; Woods, C.A., Sergile, F.E., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 355–368. [Google Scholar]
| Strata | Characteristic | Abbreviation | Mean ± SE a |
|---|---|---|---|
| Topography | Elevation (m) | Elev | 636.5 ± 10.9 |
| Slope (%) | Slope | 12.4 ± 3.4 | |
| Ground b | Total cover (%) | GTotCov | 97.6 ± 0.7 |
| Hardwood cover (%) | GHdCov | 5.7 ± 0.7 | |
| Palm cover (%) | GPaCov | 4.3 ± 0.8 | |
| Graminoid cover (%) | GGrCov | 0.6 ± 0.4 | |
| Forb cover (%) | GFoCov | 5.4 ± 0.8 | |
| Litter cover (%) | GLiCov | 84.5 ± 2.5 | |
| Litter depth (mm) | GLd | 31.1 ± 2.8 | |
| Bare soil (%) | GBs | 1.8 ± 0.6 | |
| Exposed rock (%) | GEr | 0.6 ± 0.3 | |
| Shrub c | Canopy cover (%) | SCc | 10.6 ± 0.9 |
| Ground-shrub d | Vertical obstruction from 5.6 m (%) | Vo56 | 64.2 ± 3.9 |
| Vertical obstruction from 17.8 m (%) | Vo178 | 95.3 ± 1.0 | |
| Understory e | Total density (no./ha) | UTotDen | 4729.2 ± 374.3 |
| Hardwood density (no./ha) | UVarTotDen | 4016.7 ± 349.9 | |
| Palm density (no./ha) | UPaDen | 2841.7 ± 304.4 | |
| Vine density (no./ha) | UViDen | 1325 ± 167.2 | |
| Total dbh (cm) | UTotDbh | 3.1 ± 0.1 | |
| Hardwood dbh (cm) | UHdDbh | 2.8 ± 0.1 | |
| Palm dbh (cm) | UPaDbh | 4.2 ± 0.5 | |
| Palm height (m) | UPaHt | 1.4 ± 0.1 | |
| Vine basal diameter (cm) | UViBd | 2.9 ± 0.1 | |
| Midstory f | Total density (no./ha) | MTotDen | 575.8 ± 44.1 |
| Hardwood density (no./ha) | MHdDen | 557.5 ± 44.6 | |
| Palm density (no./ha) | MPaDen | 18.3 ± 5.4 | |
| Total dbh (cm) | MTotDbh | 19.1 ± 0.4 | |
| Hardwood dbh (cm) | MHdDbh | 19.1 ± 0.4 | |
| Palm dbh (cm) | MPaDbh | 19.0 ± 1.5 | |
| Overstory g | Total density (no./ha) | OTotDen | 31.3 ± 3.3 |
| Hardwood density (no./ha) | OHdDen | 30.8 ± 3.2 | |
| Palm density (no./ha) | OPaDen | 0.4 ± 0.4 | |
| Total dbh (cm) | OTotDbh | 56.1 ± 3.0 | |
| Hardwood dbh (cm) | OHdDbh | 56.1 ± 3.0 | |
| Palm dbh (cm) | OPaDbh | 43.5 ± 0.0 | |
| Total canopy cover (%) | OTotCc | 93.4 ± 1.0 | |
| Harwood canopy cover (%) | OHdCc | 64.3 ± 3.3 | |
| Palm canopy cover (%) | OPaCc | 29.4 ± 3.6 | |
| Canopy height (m) | OCanHt | 19.8 ± 0.7 | |
| Canopy base (m) | OCanBa | 10.6 ± 0.4 | |
| Canopy depth (m) | OCanDp | 9.2 ± 0.4 | |
| Other | Log density (no./ha) | LogDen | 72.0 ± 10.0 |
| Log midpoint diameter (cm) | LogDia | 21.6 ± 1.3 | |
| Log length (m) | LogLen | 7.2 ± 0.8 | |
| Stump density (no./ha) | StumpDen | 17.1 ± 3.2 | |
| Stump basal diameter (cm) | StumpBd | 30.0 ± 4.1 | |
| Stump height (m) | StumpHt | 0.7 ± 0.1 | |
| Snag density (no./ha) | SnagDen | 34.6 ± 5.5 | |
| Snag dbh (cm) | SnagDbh | 20.7 ± 1.6 | |
| Snag height (m) | SnagHt | 6.6 ± 0.4 | |
| Snag decay class (1–4) | SnagDecC | 3.0 ± 0.2 |
| Group | Bat Activity (No. of passes) | Relative Bat Activity |
|---|---|---|
| Emballonuridae | 108 | 0.011 |
| Balantiopteryx spp. | 30 | 0.003 |
| Diclidurus spp. | 70 | 0.007 |
| Peropteryx spp. | 2 | 0.000 |
| Rhynchonycteris spp. | 1 | 0.000 |
| Saccopteryx spp. | 5 | 0.000 |
| Molossidae | 5071 | 0.518 |
| Cynomops spp. | 35 | 0.004 |
| Eumops spp. | 4655 | 0.475 |
| Molossus spp. | 20 | 0.002 |
| Promops spp. | 361 | 0.037 |
| Mormoopidae | 1203 | 0.123 |
| Mormoops spp. | 49 | 0.005 |
| Pteronotus spp. | 1154 | 0.118 |
| Noctilionidae | 53 | 0.005 |
| Noctilio spp. | 53 | 0.005 |
| Vespertilionidae | 3356 | 0.343 |
| Eptesicus spp. | 3 | 0.000 |
| Lasiurus spp. | 1286 | 0.131 |
| Myotis spp. | 2053 | 0.210 |
| Perimyotis spp. | 14 | 0.001 |
| Dependent Variable | Independent Variable (Forest Characteristic b) | Relationship a | ||
|---|---|---|---|---|
| β | p | r2 | ||
| Genus richness (No. of species) | GPaCov | 0.424 | 0.037 | 0.182 |
| OTotDen | −0.489 | 0.017 | 0.232 | |
| OHdDen | −0.534 | 0.009 | 0.271 | |
| Total bat activity c (No. of bat passes) | GFoCov | −0.522 | 0.016 | 0.235 |
| Dependent Variable | Independent Variable (Forest Characteristic b) | Relationship a | ||
|---|---|---|---|---|
| β | p | r2 | ||
| Molossidae | ||||
| Promops spp. | GTotCov | −0.523 | 0.012 | 0.253 |
| GPaCov | 0.479 | 0.019 | 0.226 | |
| GLiCov | 0.412 | 0.047 | 0.167 | |
| UTotDbh | −0.485 | 0.016 | 0.236 | |
| MTotDen | −0.423 | 0.039 | 0.179 | |
| MHdDen | −0.474 | 0.019 | 0.225 | |
| OCanDp | 0.504 | 0.012 | 0.253 | |
| Mormoopidae | GHdCov | 0.438 | 0.041 | 0.176 |
| Vo178 | −0.445 | 0.032 | 0.192 | |
| SnagDbh | 0.603 | 0.011 | 0.294 | |
| Pteronotus spp. | Vo178 | −0.472 | 0.022 | 0.217 |
| SnagDbh | 0.541 | 0.030 | 0.226 | |
| StumpHt | 0.790 | 0.045 | 0.258 | |
| Vespertilionidae | ||||
| Lasiurus spp. | UPaHt | 0.564 | 0.004 | 0.318 |
| OTotDen | −0.497 | 0.017 | 0.232 | |
| OHdDen | −0.484 | 0.022 | 0.216 | |
| StumpBd | −0.681 | 0.023 | 0.301 | |
| Myotis spp. | Vo56 | −0.407 | 0.048 | 0.166 |
| Vo178 | −0.469 | 0.023 | 0.213 | |
| MTotDbh | 0.421 | 0.041 | 0.177 | |
| SnagDbh | 0.613 | 0.012 | 0.288 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willcox, E.V.; Giuliano, W.M.; Watine, L.N.; Mills, D.J.; Andreu, M.G. Forest Structure and Composition Affect Bats in a Tropical Evergreen Broadleaf Forest. Forests 2017, 8, 317. https://doi.org/10.3390/f8090317
Willcox EV, Giuliano WM, Watine LN, Mills DJ, Andreu MG. Forest Structure and Composition Affect Bats in a Tropical Evergreen Broadleaf Forest. Forests. 2017; 8(9):317. https://doi.org/10.3390/f8090317
Chicago/Turabian StyleWillcox, Emma V., William M. Giuliano, Lauren N. Watine, Daniel J. Mills, and Michael G. Andreu. 2017. "Forest Structure and Composition Affect Bats in a Tropical Evergreen Broadleaf Forest" Forests 8, no. 9: 317. https://doi.org/10.3390/f8090317
APA StyleWillcox, E. V., Giuliano, W. M., Watine, L. N., Mills, D. J., & Andreu, M. G. (2017). Forest Structure and Composition Affect Bats in a Tropical Evergreen Broadleaf Forest. Forests, 8(9), 317. https://doi.org/10.3390/f8090317





