The Risks Associated with Glyphosate-Based Herbicide Use in Planted Forests
Abstract
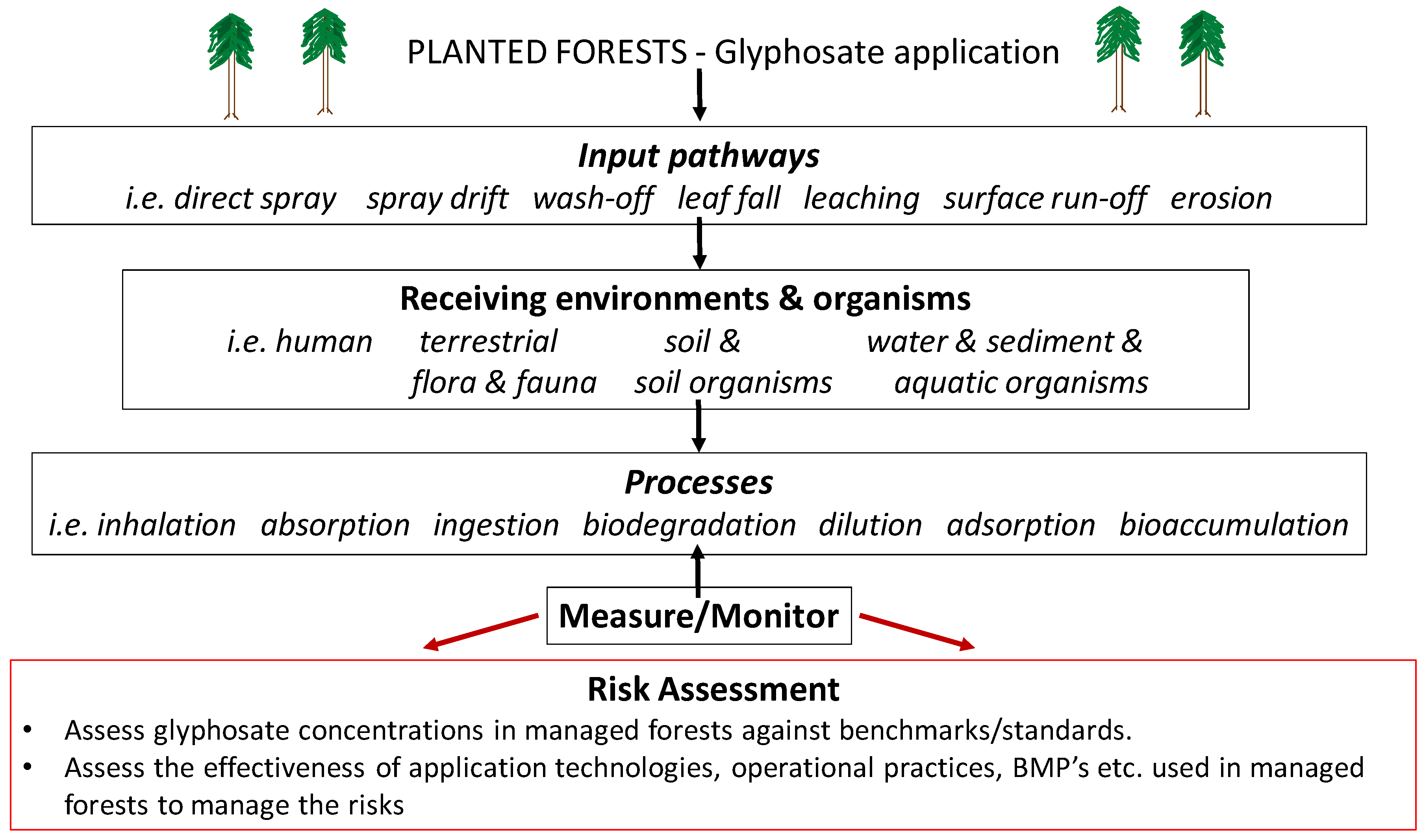
:1. Introduction
2. Glyphosate-Based Herbicide Use in Planted Forest Management Internationally
2.1. Australasia
2.2. South Africa
2.3. The United States of America
2.4. Canada
2.5. Other Regions
3. Environmental Fate of Glyphosate-Based Herbicides in Forests
3.1. Initial Deposition and Fate in Terrestrial Vegetation
3.2. Fate in Litter and Soils
3.3. Fate in the Aquatic Environment
4. Risk of Glyphosate-Based Herbicides to the Forest Environment
4.1. Direct Effects
4.2. Indirect Effects
5. Risk of Toxicological Effects on Humans
6. Best Management Practices and Mitigation of Risks
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| Product | Concentration of Glyphosate and Form in which it occurs in the Herbicide Product | Country | Manufacturer |
|---|---|---|---|
| Glymac™ Dry 700 | 700 g/kg glyphosate (monoammoium salt) | Australia | Macspread Pty Ltd. |
| Glymount 450 | 450 g/L glyphosate (isopropylamine salt) | Australia | Growchoice Pty Ltd. |
| Glyphosate 450 CT | 450 g/L (isopropylamine salt) | Australia | Nufarm |
| MacPhersons Bi Dri 700 | 700 g/kg glyphosate (mono-ammonium salt) | Australia | Mac Phersons |
| Redox 450 | 450 g/L glyphosate (isopropylamine salt) | Australia | Redox |
| Round-up Attack | 570 g/L glyphosate (potassium salt) | Australia | Nufarm |
| Roundup Biactive | 360 g/L glyphosate (isopropylamine salt) | Australia | Sinochem International |
| Weedmaster Duo | 360 g/L glyphosate (isopropylamine salt & monoammonium salt) | Australia | Nufarm |
| Panzer® Gold | 484 g/L glyphosate | Chile | Dow AgroSciences |
| Rango Full | 540 g/L glyphosate | Chile | |
| Mamba DMA 480 SL L8388 | 480 g/L glyphosate (dimethylamine salt) | South Africa | Dow AgroSciences |
| Glygran 710 SG L8449 | 710 g/kg glyphosate (ammonium salt) | South Africa | Villa Crop Protection |
| Roundup max L6790 | 680 g/kg glyphosate (ammonium salt) | South Africa | Monsanto SA |
| Glyphogan 360 SL L5393 | 360 g/L glyphosate (isopropylamine salt) | South Africa | Makhteshim-Agan |
| Sharda Glyphosate 360 SL L8901 | 360 g/L glyphosate (isopropylamine salt) | South Africa | Sharda International Africa cc. |
| Kalach 510 SL L8311 | 510 g/kg glyphosate (isopropylamine salt) | South Africa | ArystaLifeScience |
| Mamba Max 480 SL L7714 | 480 g /kg glyphosate (isopropylamine salt) | South Africa | Dow AgroSciences |
| Erase L6206 | 360 g/L glyphosate (isopropylaminesalt) | South Africa | Plaaskem |
| Tumbleweed L4781 | 240 g/L glyphosate (isopropylammonium salt) | South Africa | Enviro Weed Control Systems |
| Mamba 360 SL L4817 | 360 g/Lglyphosate (isopropylamine salt) | South Africa | Dow AgroSciences |
| Roundup L0407 | 360 g /Lglyphosate (isopropylamine salt) | South Africa | Monsanto SA |
| Springbok 360 L6719 | 360 g/L glyphosate (isopropylamine salt) | South Africa | ArystaLifeScience |
| Kilo Max 700 WSG L8310 | 700 g/L glyphosate (sodium salt) | South Africa | ArystaLifeScience |
| Bounty 500 WSG L6698 | 500 g/Kg glyphosate (sodium salt) | South Africa | Meridian Agritech |
| Erase Granule L7948 | 500 g/Kg glyphosate (sodium salt) | South Africa | Plaaskem |
| Muscle-Up 500 SG L7641 | 500 g/Kg glyphosate (sodium salt) | South Africa | Ag-Chem Africa |
| Glyphosate WSG L7119 | 500 g/L glyphosate (sodium salt) | South Africa | ArystaLifeScience |
| Slash Plus 540 SL L8819 | 540 g/L glyphosate (potassium salt) | South Africa | Universal Crop Protection |
| Roundup Turbo L7166 | 450 g/L glyphosate (potassium salt) | South Africa | Monsanto SA |
| Riverdale Razor Herbide | 356 g/L glyphosate(isopropylamine salt) | USA | Nufarm Americas Inc. |
| Razor pro | 356 g/L glyphosate (isopropylamine salt) | USA | Nufarm Americas Inc. |
| Glyphosate 4 Plus | 356 g /L glyphosate (isopropylamine salt) | USA | Alligare, LLC |
| Glyphosate 5.4 | 480 g/L glyphosate (isopropylamine salt) | USA | Alligare, LLC |
| Accord | 480 g /L glyphosate (isopropylamine salt) | USA | Dow AgroSciences |
| Accord XRT | 480 g/L glyphosate (isopropylamine salt) | USA | Dow AgroSciences |
| Accord XRT II | 480 g/L glyphosate (isopropylamine salt) | USA | Dow AgroSciences |
| Foresters | 480 g/L glyphosate (isopropylamine salt) | USA | NuFarmAmercica Inc. |
| One step | 76 g/L glyphosate (isopropylamine salt) | USA | BASF |
| Refuge | 599 g/L glyphosate (monopotasium salt) | USA | Syngenta |
| Rodeo | 480 g/L glyphosate (isopropylamine salt) | USA | Dow AgroSciences |
| AGPRO Glyphosate 360 | 360 g/L glyphosate (isopropylamine salt) | New Zealand | AGPRO |
| Deal 510 | 510 g/L glyphosate (isopropylamine salt) | New Zealand | Orion |
| AGPRO Glyphosate 360 | 360 g/L glyphosate (isopropylamine salt) | New Zealand | AGPRO |
| Weedmaster® Dry | 680 g/kg glyphosate (monoammonium salt) | New Zealand | Nufarm |
| AGPRO Green Glyphosate 510 | 510 g/L glyphosate (isopropylamine salt) | New Zealand | AGPRO |
| Vision Silvicultural Herbicide | 356 g/L glyphosate (isopropylamine salt) | Canada | Monsanto Canada Inc. |
| Vision Max | 540 g/L glyphosate (potassium salt) | Canada | Monsanto Canada Inc. |
| Forza™ | 360 g/L glyphosate (isopropylamine salt) | Canada | Cheminova Canada Inc. |
| Vantage Forestry Herbicide Solution | 410 g/L glyphosate (isopropylamine salt) | Canada | Dow AgroSciences |
References
- Grossbard, E.; Atkinson, D. The Herbicide Glyphosate; Butterworths: London, UK, 1985; Volume 2. [Google Scholar]
- Baylis, A.D. Why glyphosate is a global herbicide: Strengths, weaknesses and prospects. Pest Manag. Sci. 2000, 56, 299–308. [Google Scholar] [CrossRef]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest Manage. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Giesy, J.P.; Dobson, S.; Solomon, K.R. Ecotoxicological risk assessment for Roundup® herbicide. Rev. Environ. Contam. Toxicol. 2000, 167, 35–120. [Google Scholar]
- Durkin, P.R. Glyphosate Human Health and Ecological Risk Assessment; SERA TR 02_43-09-04a; Syracuse Environmental Research Associates, Inc.: Fayetteville, NY, USA, 2003. [Google Scholar]
- Tatum, V.L. Toxicity, transport, and fate of forest herbicides. Wildl. Soc. Bull. 2004, 32, 1042–1048. [Google Scholar] [CrossRef]
- Thompson, D.G. Ecological impacts of major forest-use pesticides. In Ecological Impact of Toxic Chemicals; Sanchez-Bayo, F., van den Brink, P.J., Mann, R.M., Eds.; Bentham Science Publishers Ltd.: Sharjah, UAE, 2011; pp. 88–110. [Google Scholar]
- Durkin, P.R. Glyphosate Human Health and Ecological Risk Assessment. Final report; SERA TR 02_43-09-04a; Syracuse Environmental Research Associates, Inc.: Manlius, NY, USA, 2011; p. 313. [Google Scholar]
- Duke, S.O.; Powles, S.B. Glyphosate-resistant weeds and crops. Pest Manage. Sci. 2008, 64, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Powles, S.B. Evolved glyphosate-resistant weeds around the world: Lessons to be learnt. Pest Manag. Sci. 2008, 64, 360–365. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Glyphosate. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2015; Volume 112. [Google Scholar]
- Friends of the Earth Europe. The Environmental Impacts of Glyphosate; Friends of the Earth Europe: Brussels, Belgium, 2013. [Google Scholar]
- Corporations Control Our Food. GREENPEACE, 2016. Available online: http://www.greenpeace.org/international/en/campaigns/agriculture/problem/Corporations-Control-Our-Food/ (accessed on 17 April 2017).
- Forest Conservation. Conservation Council of New Brunswick, 2017. Available online: http://www.conservationcouncil.ca/our-programs/forest-conservation/ (accessed on 17 April 2017).
- Bartell, S.M.; Gardner, R.H.; O’Neill, R.V. Ecological Risk Estimation; Lewis Publishers Incorporated: Chelsea, MI, USA, 1992. [Google Scholar]
- Roberts, S.M.; James, R.C.; Williams, P.L. Principles of Toxicology: Environmental and Industrial Applications, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Klaassen, C.D. Casarett & Doull’s Toxicology: The Basic Science of Poisons, 8th ed.; McGraw-Hill Education: New York, NY, USA, 2013. [Google Scholar]
- Williams, G.M.; Kroes, R.; Munro, I.C. Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul. Toxicol. Pharmacol. 2000, 31, 117–165. [Google Scholar] [CrossRef] [PubMed]
- Solomon, K.R.; Thompson, D.G. Ecological risk assessment for aquatic organisms from over-water uses of glyphosate. J. Toxicol. Environ. Health Part B Crit. Rev. 2003, 6, 289–324. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.P.; Sullivan, D.S. Vegetation management and ecosystem disturbance: impact of glyphosate herbicide on plant and animal diversity in terrestrial systems. Environ. Rev. 2003, 11, 37–59. [Google Scholar] [CrossRef]
- Couture, G.; Legris, J.; Langevin, R.; Laberge, L. Evaluation of the Impacts of Glyphosate as Used in Forests; Publ No RN95-3082; Ministere des Ressources Naturelles, Direction de l’environnement Forestier: Quebec, QC, Canada, 1995; Volume 187. [Google Scholar]
- Vereecken, H. Mobility and leaching of glyphosate: A review. Pest Manage. Sci. 2005, 61, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J. 2015, 13, 107. [Google Scholar]
- PMRA. Glyphosate; 1-800-267-6315 or 613-736-3799; Pest Management Regulatory Agency: Ontario, ON, Canada, 2015.
- United States Environmental Protection Agency. Re-registration Eligibility Decision (RED): Glyphosate; EPA-738-R-93-014; Office of Pesticide Programs and Toxic Substances, US Environmental Protection Agency: Washington, DC, USA, 1993.
- JMPR. Joint FAO/WHO Meeting on Pesticide Residues. Geneva, Switzerland, 9–13 May 2016. Summary Report; Food and Agriculture Organisation and World Health Organisation: Geneva, Switzerland, 2016; p. 6. [Google Scholar]
- Kogan, M.; Alister, C. Glyphosate use in forest plantations. Chil. J. Agric. Res. 2010, 70, 652–665. [Google Scholar] [CrossRef]
- Wagner, R.G.; Little, K.M.; Richardson, B.; Mcnabb, K. The role of vegetation management for enhancing productivity of the world’s forests. Forestry 2006, 79, 57–79. [Google Scholar] [CrossRef]
- Ellison, D.; Morris, C.E.; Locatelli, B.; Sheil, D.; Cohen, J.; Murdiyarso, D.; Gutierrez, V.; Noordwijk, M.V.; Creed, I.F.; Pokorny, J.; et al. Trees, forests and water: Cool insights for a hot world. Glob. Environ. Chang. 2017, 43, 51–61. [Google Scholar] [CrossRef]
- Thompson, D.G.; Pitt, D.G. A review of Canadian forest vegetation management research and practice. Ann. For. Sci. 2003, 60, 559–572. [Google Scholar] [CrossRef]
- Jenkin, B.M.; Tomkins, B. Pesticides in Plantations: The Use of Chemical Pesticides by the Australian Plantation Forest Industry; Forest and Wood Products Research and Development Corporation: Melbourne, Australia, 2006.
- Willoughby, I.; Balandier, P.; Scott Bensen, N.; McCarthy, N.; Claridge, J. Forest Vegetation Management in Europe: Current Practice and Future Requirements; Cost Action E47; COST Office: Brussels, Belgium, 2009. [Google Scholar]
- Little, K.M.; Rolando, C.A. General guidelines for vegetation management in South Africa plantations. In South African Forestry Handbook, 5th ed.; Bredenkamp, B.V., Ed.; South African Institute of Forestry: Pretoria, South Africa, 2012; pp. 107–121. [Google Scholar]
- Rolando, C.A.; Watt, M.S. Herbicides for use in management of certified Pinus radiata plantations in New Zealand. Aust. For. 2014, 77, 123–132. [Google Scholar] [CrossRef]
- Herbicide Use Patterns on Corporate Forest Lands in the United States. 2011. Available online: https://www.researchgate.net/publication/282986628_Herbicide_use_patterns_on_corporate_forest_lands_in_the_United_States_2011 (accessed on 24 May 2017).
- Silviculture. National Forestry Database, 2015. Available online: http://nfdp.ccfm.org/silviculture/quick_facts_e.php (accessed on 17 April 2017).
- Trends in Pesticide Use in New Zealand: 2004. Available online: https://www.researchgate.net/publication/297442070_Trends_in_Pesticide_Use_in_New_Zealand_2004 (accessed on 24 May 2017).
- NHMRC. Australian Drinking Water Guidelines 6; Version 3.2; National Resource Management Ministerial Council, National Health and Medical Research Council, Australian Givernment: Canberra, Australia, 2016.
- AGDA, A.G.D.o.A. Australia’s State of the Forests Report. Criterion 2: Maintenance of Productive Capacity of Forest Ecosystems; Department of Agriculture, Australian Government: Canberra, Australia, 2013.
- Boyd, D.R. The Water We Drink. An International Comparison of Drinking Water Quality Standards and Guidelines; David Suzuki Foundation: Vancouver, BC, Canada, 2006. [Google Scholar]
- National Forestry Database. 2017. Available online: http://nfdp.ccfm.org/data/compendium/html/comp_96e.html (accessed on 17 April 2017).
- Rolando, C.A.; Garrett, L.G.; Baillie, B.R.; Watt, M.S. A survey of herbicide use and a review of environmental fate in New Zealand planted forests. N. Z. J. For. Sci. 2013, 43, 17. [Google Scholar] [CrossRef]
- Hall, P. Logging Residue Distribution; Liro Forestry Solutions: Rotorua, New Zealand, 1999; Volume 24, p. 6. [Google Scholar]
- Ross Gillies, HVP Plantations, Churchill, Victoria, Australia.
- Little, K.M.; Rolando, C.A.; Morris, C.D. An integrated analysis of 33 Eucalyptus trials linking the onset of competition-induced tree growth suppression with management, physiographic and climatic factors. Ann. For. Sci. 2007, 64, 585–591. [Google Scholar] [CrossRef]
- Gous, M. Assessing the Value of Glyphosate in South African Agricultural Sector; Department of Agricultural Economics, Extension and Rural Development University of Pretoria: Pretoria, South Africa, 2014. [Google Scholar]
- Little, K.M.; Rolando, C.A. Regional vegetation management standards for commercial Eucalyptus plantations in South Africa. South. For. 2008, 70, 87–97. [Google Scholar]
- Rolando, C.A.; Little, K.M. Regional vegetation management standards for commercial pine plantations in South Africa. South. For. 2009, 71, 187–199. [Google Scholar] [CrossRef]
- Roberts, J.C.; Little, K.M.; Light, M.E. The use of glyphosate for the management of secondary coppice regrowth in a Eucalyptus grandis × E. urophylla coppice stand in Zululand, South Africa. South. For. 2016, 78, 217–223. [Google Scholar]
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28. [Google Scholar] [CrossRef] [PubMed]
- Newton, M.; Horner, L.M.; Cowell, J.E.; White, D.E.; Cole, E.C. Dissipation of glyphosate and aminomethylphosphonic acid in North American forests. J. Agric. Food Chem. 1994, 42, 1795–1802. [Google Scholar] [CrossRef]
- Newton, M.; Howard, K.M.; Kelpsas, B.R.; Danhaus, R.; Lottman, C.M.; Dubelman, S. Fate of glyphosate in an Oregon forest ecosystem. J. Agric. Food Chem. 1984, 32, 1144–1151. [Google Scholar] [CrossRef]
- Thompson, D.G.; Pitt, D.G.; Buscarini, T.; Staznik, B.; Thomas, D.R. Initial deposits and persistence of forest herbicide residues in sugar maple (Acer saccharum) foliage. Can. J. For. Res. 1994, 24, 2251–2262. [Google Scholar] [CrossRef]
- Legris, J.; Couture, G. Résidus de Glyphosate dans le Gibier (Lievre, Orignal et cerf de Virginie) Suite à des Pulvérisations en Milieu Forestier en 1988; Quebec Ministere des Forest, Direction de l’évaluation Environnementale: Charlesbourg, QC, Canada, 1991. [Google Scholar]
- Franz, J.E.; Mao, M.K.; Sikorski, J.A. Glyphosate: A Unique Global Herbicide; American Chemical Society: Washington, DC, USA, 1997. [Google Scholar]
- Feng, J.C.; Thompson, D.G.; Reynolds, P.E. Fate of glyphosate in a Canadian forest watershed. 1. Aquatic residues and off-target deposit assessment. J. Agric. Food Chem. 1990, 38, 1110–1118. [Google Scholar] [CrossRef]
- Thompson, D.G.; Pitt, D.G.; Buscarini, T.M.; Staznik, B.; Thomas, D.R. Comparative fate of glyphosate and triclopyr herbicides in the forest floor and mineral soil of an Acadian forest regeneration site. Can. J. For. Res. 2000, 30, 1808–1816. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Gimsing, A.L. Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: a review. Pest Manage. Sci. 2008, 64, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, A.; Celano, G.; Conte, P. Adsoprtion of glyphosate by humic substances. J. Agric. Food Chem. 1996, 44, 2442–2446. [Google Scholar] [CrossRef]
- Feng, J.C.; Thompson, D.G. Fate of glyphosate in a Canadian forest watershed. 2. Persistence in foliage and soils. J. Agric. Food Chem. 1990, 38, 1118–1125. [Google Scholar] [CrossRef]
- Roy, D.N.; Konar, S.K.; Banerjee, S.; Charles, D.A.; Thompson, D.G.; Prasad, R. Persistence, movement, and degradation of glyphosate in selected Canadian boreal forest soils. J. Agric. Food Chem. 1989, 37, 437–440. [Google Scholar] [CrossRef]
- Villarreal-Chiu, J.F.; Acosta-Cortés, A.G.; Kumar, S.; Kaushik, G. Biological Limitations on Glyphosate Biodegradation. Green Technol. Environ. Sustain. 2017, 179–201. [Google Scholar]
- Annett, R.; Habibi, H.R.; Hontela, A. Impact of glyphosate and glyphosate-based herbicides on the freshwater environment. J. Appl. Toxicol. 2014, 34, 458–479. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. The WHO Recommended Classifcation Pesticides by Hazard and Guidelines to Classification 2009; World Health Organisation: Geneva, Switzerland, 2010; p. 78. [Google Scholar]
- MacBean, C. The Pesticide Manual, 16th ed.; British Crop Protection Council: Hampshire, UK, 2012; p. 1439. [Google Scholar]
- Republic of South Africa Government Gazette Staatskoerant. Available online: http://www.gov.za/sites/www.gov.za/files/Act181of1993.pdf (accessed on 26 May 2017).
- WHO, W.H.O. Guidelines for Drinking-Water Quality; World Health Organisation: Geneva, Switzerland, 2011; p. 564. [Google Scholar]
- Ministry of Health. Drinking-Water Standards for New Zealand 2005 (Revised 2008); Ministry of Health: Wellington, New Zealand, 2008.
- Thompson, D.; Chartrand, D.; Staznik, B.; Leach, J.; Hodgins, P. Integrating advanced technologies for optimization of aerial herbicide applications. New For. 2009, 40, 45–66. [Google Scholar] [CrossRef]
- Thompson, D.G.; Wojtaszek, B.F.; Staznik, B.; Chartrand, D.T.; Stephenson, G.R. Chemical and biomonitoring to assess potential acute effects of Vision® herbicide on native amphibian larvae in forest wetlands. Environ. Toxicol. Chem. 2004, 23, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Louch, J.; Tatum, V.; Allen, G.; Hale, V.C.; McDonnell, J.; Danehy, R.J.; Ice, G. Potential risks to freshwater aquatic organisms following a silvicultural application of herbicides in Oregon’s Coast Range. Integr. Environ. Assess. Manag. 2017, 13, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.T.; Watts, R.G.; Moul, D.J. Effects of different dilution water types on the acute toxicity to juvenile Pacific salmonids and rainbow trout of glyphosate and its formulated products. Bull. Environ. Contam. Toxicol. 1989, 43, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Goldsborough, L.G.; Beck, A.E. Rapid dissipation of glyphosate in small forest ponds. Arch. Environ. Contam. Toxicol. 1989, 18, 537–544. [Google Scholar] [CrossRef]
- Gluns, D.R. Herbicide Residue in Surface Water Following an Application of Roundup in the Revelstoke Forest District; RR88001-NE; British Columbia Ministry of Forests: Nelson, British Columbia, Canada, 1989.
- Adams, G.W.; Smith, T.; Miller, J.D. The absence of glyphosate residues in wet soil and the adjacent watercourse after a forestry application in new Brunswick. Northern J. Appl. For. 2007, 24, 230–232. [Google Scholar]
- Wan, M.T.K. The Persistence of Glyposate and Its Metabolite Amino-methyl-phosphonic Acid in Some Coastal British Columbia Streams; Regional Program Report 85-01; Department of the Environment Conservation and Protection, Environmental Protection Service, Pacific and Yuokon Region: Vancouver, BC, Canada, 1986.
- Edge, C.B.; Thompson, D.G.; Hao, C.; Houlahan, J.E. A silviculture application of the glyphosate-based herbicide VisionMAX to wetlands has limited direct effects on amphibian larvae. Environ. Toxicol. Chem. 2012, 31, 2375–2383. [Google Scholar] [CrossRef] [PubMed]
- Wojtaszek, B.F.; Staznik, B.; Chartrand, D.T.; Stephenson, G.R.; Thompson, D.G. Effects of Vision® herbicide on mortality, avoidance response, and growth of amphibian larvae in two forest wetlands. Environ. Toxicol. Chem. 2004, 23, 832–842. [Google Scholar] [CrossRef] [PubMed]
- PPDB. Pesticide Properties Database; University of Hertfordshire: Hatfield, UK, 2016. [Google Scholar]
- Guiseppe, K.F.L.; Drummond, F.A.; Stubbs, C.; Woods, S. The Use of Glyphosate Herbicides in Managed Forest Ecosystems and their Effects on Non-Target Organisms with Particular Reference to Ants as Bioindicators; Maine Agriculrtual and Forest Experiment Station Technical Bulletine 192; Maine Agricultrual and Forest Experiment Station, University of Maine: Orono, ME, USA, 2006; p. 51. [Google Scholar]
- Mackay, D.; Fraser, A. Bioaccumulation of persistent organic chemicals: Mechanisms and models. Environ. Pollut. 2000, 110, 375–391. [Google Scholar] [CrossRef]
- Fletcher, K.; Freedman, B. Effects of the herbicides glyphosate, 2, 4, 5-trichlorophenoxyacetic acid, and 2, 4-dichlorophenoxyacetic acid on forest litter decomposition. Can. J. For. Res. 1986, 16, 6–9. [Google Scholar] [CrossRef]
- Castilho, A.F.; Viana, R.G.; da Silva Santos, R.T.; da Costa, Y.K.S.; Oliveira, M.F.; Pereira, K.D. The impact of glyphosate herbicides on soil microbial activity from the Carajás National Forest. Revista de Ciências Agrárias/Amaz. J. Agric. Environ. Sci. 2016, 59, 302–309. [Google Scholar] [CrossRef]
- Edwards, C.A.; Bohlen, P.J. Biology and Ecology of Earthworms, 3rd ed.; Chapman & Hall: London, UK, 1996. [Google Scholar]
- Houston, A.; Visser, S.; Lautenschlager, R. Response of microbial processes and fungal community structure to vegetation management in mixedwood forest soils. Can. J. Bot. 1998, 76, 2002–2010. [Google Scholar]
- Busse, M.D.; Ratcliff, A.W.; Shestak, C.J.; Powers, R.F. Glyphosate toxicity and the effects of long-term vegetation control on soil microbial communities. Soil Biol. Biochem. 2001, 33, 1777–1789. [Google Scholar] [CrossRef]
- Ohtonen, R.; Munsen, A.; Brand, D. Soil microbial community response to silvicultural intervention in coniferous plantation ecosystems. Ecol. Appl. 1992, 2, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Canadian Council of Ministers of the Environment. Canadian water quality guidelines for the protection of aquatic life: Glyphosate. In Canadian Environmental Quality Guidelines; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 2012. [Google Scholar]
- Folmar, L.C.; Sanders, H.O.; Julin, A.M. Toxicity of the herbicide glyphosate and several of its formulations to fish and aquatic invertebrates. Arch. Environ. Contam. Toxicol. 1979, 8, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Edge, C.B.; Gahl, M.K.; Pauli, B.D.; Thompson, D.G.; Houlahan, J.E. Exposure of juvenile green frogs (Lithobates clamitans) in littoral enclosures to a glyphosate-based herbicide. Ecotoxicol. Environ. Saf. 2011, 74, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Edge, C.B.; Gahl, M.K.; Thompson, D.G.; Houlahan, J.E. Laboratory and field exposure of two species of juvenile amphibians to a glyphosate-based herbicide and Batrachochytrium dendrobatidis. Sci. Total Environ. 2013, 444, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Cole, E.C.; McComb, W.C.; Newton, M.; Chambers, C.L.; Leeming, P.J. Response of amphibians to clearcutting, burning, and glyphosate application in the Oregon Coast Range. J. Wildl. Manag. 1997, 61, 656–664. [Google Scholar] [CrossRef]
- Effects of the Herbicide Roundup on Coho Salmon Fingerlings in an Over-Sprayed Tributary of Carnation Creek, British Columbia. Available online: http://agris.fao.org/agris-search/search.do?recordID=US201302679263 (accessed on 24 May 2017).
- Kreutzweiser, D.P.; Kingsbury, P.D.; Feng, J.C. Drift response of stream invertebrates to aerial applications of glyphosate. Bull. Environ. Contam. Toxicol. 1989, 42, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Magbanua, F.S.; Townsend, C.R.; Hageman, K.J.; Matthaei, C.D. Individual and combined effects of fine sediment and the herbicide glyphosate on benthic macroinvertebrates and stream ecosystem function. Freshwat. Biol. 2013, 58, 1729–1744. [Google Scholar] [CrossRef]
- Wang, N.; Besser, J.M.; Buckler, D.R.; Honegger, J.L.; Ingersoll, C.G.; Johnson, B.T.; Kurtzweil, M.L.; MacGregor, J.; McKee, M.J. Influence of sediment on the fate and toxicity of a polyethoxylated tallowamine surfactant system (MON 0818) in aquatic microcosms. Chemosphere 2005, 59, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Widenfalk, A.; Bertilsson, S.; Sundh, I.; Goedkoop, W. Effects of pesticides on community composition and activity of sediment microbes–responses at various levels of microbial community organization. Environ. Pollut. 2008, 152, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Tsui, M.T.K.; Chu, L.M. Aquatic toxicity of glyphosate-based formulations: Comparison between different organisms and the effects of environmental factors. Chemosphere 2003, 52, 1189–1197. [Google Scholar] [CrossRef]
- Baker, L.F.; Mudge, J.F.; Thompson, D.G.; Houlahan, J.E.; Kidd, K.A. The combined influence of two agricultural contaminants on natural communities of phytoplankton and zooplankton. Ecotoxicology 2016, 25, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Edginton, A.N.; Sheridan, P.M.; Stephenson, G.R.; Thompson, D.G.; Boermans, H.J. Comparative effects of pH and Vision® herbicide on two life stages of four anuran amphibian species. Environ. Toxicol. Chem. 2004, 23, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Howe, C.M.; Berrill, M.; Pauli, B.D.; Helbing, C.C.; Werry, K.; Veldhoen, N. Toxicity of glyphosate-based pesticides to four North American frog species. Environ. Toxicol. Chem. 2004, 23, 1928–1938. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Hathaway, K.M.; Folt, C.L. Multiple stress effects of Vision® herbicide, pH, and food on zooplankton and larval amphibian species from forest wetlands. Environ. Toxicol. Chem. 2004, 23, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Environmental Risk Management Authority. Environmental Risk Mangement Authority Decision: Application to Import and Manufacture for Release, Roundup TransorbTM, for Use as a Herbicide for the Control of Weeds in Non-Selective Situations; ERMA New Zealand Evaluation and Review Report: Application HSR03010; Environmental Risk Management Authority: Wellington, New Zealand, 2005; p. 22.
- ANZECC. Australian and New Zealand Guidelines for Fresh and Marine Water Quality; Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand: Auckland, New Zealand, 2000.
- Mensah, P.K.; Palmer, C.G.; Muller, W.J. Derivation of South African water quality guidelines for Roundup® using species sensitivity distribution. Ecotoxicol. Environ. Saf. 2013, 96, 24–31. [Google Scholar] [CrossRef] [PubMed]
- INERIS. Toxicological and Environmental Data Sheet—Glyphosate. 2008. Available online: http://www.ineris.fr/substances/fr/substance/1031 (accessed on 24 May 2017).
- Baker, L.F.; Mudge, J.F.; Houlahan, J.E.; Thompson, D.G.; Kidd, K.A. The direct and indirect effects of a glyphosate-based herbicide and nutrients on Chironomidase (Diptera) emerging from small wetlands. Environ. Toxicol. Chem. 2014, 33, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B. Controversy over the use of herbicides in forestry, with particular reference to glyphosate usage. J. Environ. Sci. Health 1991, C8, 277–286. [Google Scholar] [CrossRef]
- Freedman, B.; Morash, R.; MacKinnon, D. Short-term changes in vegetation after the silvicultural spraying of glyphosate herbicide onto regenerating clearcuts in Nova Scotia, Canada. Can. J. For. Res. 1993, 23, 2300–2311. [Google Scholar] [CrossRef]
- Lautenschlager, R.; Sullivan, T.P. Effects of herbicide treatments on biotic components in regenerating northern forests. For. Chron. 2002, 78, 695–731. [Google Scholar] [CrossRef]
- Anthony, R.G.; Morrison, M.L. Influence of glyphosate herbicide on small-mammal populations in western Oregon. Northwest Sci. 1985, 59, 159–168. [Google Scholar]
- D’Anieri, P.; Leslie, D., Jr.; McCormack, M., Jr. Small mammals in glyphosate-treated clearcuts in northern Maine. Can. Field-Nat. Ottawa ON 1987, 101, 547–550. [Google Scholar]
- Gagné, N.; Bélanger, L.; Huot, J. Comparative responses of small mammals, vegetation, and food sources to natural regeneration and conifer release treatments in boreal balsam fir stands of Quebec. Can. J. For. Res. 1999, 29, 1128–1140. [Google Scholar] [CrossRef]
- Santillo, D.J.; Leslie, D.M., Jr.; Brown, P.W. Responses of small mammals and habitat to glyphosate application on clearcuts. J. Wildl. Manag. 1989, 53, 164–172. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Sullivan, D.S.; Lautenschlager, R.; Wagner, R.G. Long-term influence of glyphosate herbicide on demography and diversity of small mammal communities in coastal coniferous forest. Northwest Sci. 1997, 71, 6–17. [Google Scholar]
- MacKinnon, D.; Freedman, B. Effects of silvicultural use of the herbicide glyphosate on breeding birds of regenerating clearcuts in Nova Scotia, Canada. J. Appl. Ecol. 1993, 30, 395–406. [Google Scholar] [CrossRef]
- Santillo, D.J.; Brown, P.W.; Leslie, D.M., Jr. Response of songbirds to glyphosate-induced habitat changes on clearcuts. J. Wildl. Manag. 1989, 53, 64–71. [Google Scholar] [CrossRef]
- Easton, W.E.; Martin, K. The effect of vegetation management on breeding bird communities in British Columbia. Ecol. Appl. 1998, 8, 1092–1103. [Google Scholar] [CrossRef]
- Guynn, D.C., Jr.; Guynn, S.T.; Wigley, T.B.; Miller, D.A. Herbicides and forest biodiversity-what do we know and where do we go from here? Wildl. Soc. Bull. 2004, 32, 1085–1092. [Google Scholar] [CrossRef]
- Guilherme, S.; Gaivao, I.; Santos, M.; Pacheco, M. European eel (Anguilla anguilla) genotoxic and pro-oxidant responses following short-term exposure to Roundup®—A glyphosate-based herbicide. Mutagenesis 2010, 25, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, S.; Gaivão, I.; Santos, M.; Pacheco, M. DNA damage in fish (Anguilla anguilla) exposed to a glyphosate-based herbicide–elucidation of organ-specificity and the role of oxidative stress. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2012, 743, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.W.; Poulin, R.; Tompkins, D.M.; Townsend, C.R. Synergistic effects of glyphosate formulation and parasite infection on fish malformations and survival. J. Appl. Ecol. 2010, 47, 498–504. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Glyphosate; Pesticide tolerances. In Federal Register; EPA-HQ-OPP-2-12-0132; Environmental Protection Agency: Washington, DC, USA, 2013; Volume 78, pp. 25397–25401. [Google Scholar]
- Tarazona, J.V.; Court-Marques, D.; Tiramani, M.; Reich, H.; Pfeil, R.; Istace, F.; Crivellente, F. Glyphosate toxicity and carcinogenicity: A review of the scientific basis of the European Union assessment and its differences with IARC. Arch. Toxicol. 2017, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.D.; Rimmer, D.A.; Garrod, A.N.I.; Helps, J.E.; Mawdsley, C. Operator exposure when applying amenity herbicides by all-terrain vehicles and controlled droplet applicators. Ann. Occup. Hyg. 2005, 49, 25–32. [Google Scholar] [PubMed]
- Lavy, T.L.; Cowell, J.E.; Steinmetze, J.R.; Massey, J.H. Conifer seedling nursery worker exposure to glyphosate. Arch. Environ. Contam. Toxicol. 1992, 22, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Dost, F.N. Toxicology and Potential Health Risk of Chemicals That May Be Encountered by Workers Using Forest Vegetation Management Options: Part IV: Risk to Workers Using Glyphosate Formulations; Ministry of Forests: Fort Fraser, BC, Canada, 2003.
- Dissmeyer, G.E. Drinking Water from Forests and Grasslands: A Synthesis of the Scientific Literature; United States Department of Agriculture Forest Service, Southern Research Station: Asheville, NC, Canada, 2000; p. 246.
- Dudley, N.; Stolton, S. Running Pure: The Importance of Forest Protected Areas to Drinking Water; World Bank and WWF Alliance for Forest Conservation and Sustainable Use: Geneva, Switzerland, 2003; p. 112. [Google Scholar]
- Close, M.E.; Skinner, A. Sixth national survey of pesticides in groundwater in New Zealand. N. Z. J. Mar. Freshw. Res. 2012, 46, 443–457. [Google Scholar] [CrossRef]
- Battaglin, W.A.; Meyer, M.T.; Kuivila, K.M.; Dietze, J.E. Glyphosate and its degradation product AMPA occur frequently and widely in US soils, surface water, groundwater, and precipitation. JAWRA J. Am. Water Resour. Assoc. 2014, 50, 275–290. [Google Scholar] [CrossRef]
- Giroux, I.; Pelletier, L. Présence de pesticides dans l’eau au Québec: Bilan dans quatre cours d’eau de zones en culture de maïs et de soya en 2008, 2009 et 2010; Ministère du Développement durable, de l’Environnement et des Parcs, Direction du suivi de l’état de l’environnement, Gouvernement du Québec: Québec, QC, Canada, 2012; p. 46. [Google Scholar]
- Health Canada. Guidelines for Canadian Drinking Water Quality—Summary Table; Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada: Ottawa, ON, Canada, 2014. [Google Scholar]
- Little, K.; Willoughby, I.; Wagner, R.; Adamas, P.; Frochet, H.; Gava, J.; Gous, S.; Lautenschlager, R.; Orlander, G.; Sankaran, K.; et al. Towards reduced herbicide use in forest vegetation management. S. Afr. For. J. 2006, 207, 63–79. [Google Scholar] [CrossRef]
- Thompson, D.; Leach, J.; Noel, M.; Odsen, S.; Mihajlovich, M. Aerial forest herbicide application: Comparative assessment of risk mitigation strategies in Canada. For. Chron. 2012, 88, 176–184. [Google Scholar] [CrossRef]
- Forest Stewardship Council. FSC Pesticide Policy: Guidance on Implementation; FSC-GUI-30-001 VERSION 2-0 EN; Forest Stewardship Council: Bonn, Germany, 2007. [Google Scholar]
- Wilson, P. UK Woodlands Assurance Standard. UK, 2012. Available online: www.ukwas.org.uk (accessed on 11 April 2017).
- Forest Stewardship Council. FSC Facts and Figures; FSC International: Bonn, Germany, 2017. [Google Scholar]
- PEFC Global Certification: Forest Management & Chain of Custody. Available online: https://pefc.org/resources/webinar/747-pefc-global-certification-forest-management-chain-of-custody (accessed on 26 May 2017).
- New Zealand Forest Owners Association. New Zealand Environmental Code of Practice for Plantation Forestry; Version 1; New Zealand Forest Owners Association: Wellington, New Zealand, 2007. [Google Scholar]
- Fertilizers, Farm Feeds, Agricultural Remedies and Stock Remedies Act No. 36 of 1947. Available online: https://www.environment.gov.za/sites/default/files/docs/remedies_stockremedies_act36_of1947.pdf (accessed on 26 May 2017).
- Forestry, P. Guidelines for Plantation Forestry in South Australia 2009; Primary Industries and Resources SA: South Australia, April 2009; p. 62. ISBN 978-1-921399-25-1IS.
- Standards New Zealand. Management of agrichemicals; Ministry of Business Innovation and Employment: Wellington, New Zealand, 2004.
- Payne, N.J. Spray dispersal from aerial silvicultural glyphosate applications. Crop Protect. 1993, 12, 463–469. [Google Scholar] [CrossRef]
- Engage Agro. Vision Max Silvicultural Herbicide; Engage Agro Corporation: Winnipeg, MB, Canada, 2015. [Google Scholar]
- Schilling, E. Compendium of Forestry Best Management Practices for Controlling Nonpoint Source Pollution in North America; National Council for Air and Stream Improvement, Southern Regional Centre: Newberry, FL, USA, 2009; p. 230. [Google Scholar]
| Country | Amount (Tonnes Annum−1) | Application Rate (kg a.i. ha−1) | Timing of Application | Method of Application (Manual/Aerial) | Reference |
|---|---|---|---|---|---|
| New Zealand | 175 *** | 3.0–3.5 | Pre-plant only | Aerial only | [37] |
| Canada | 275 * | 1.9–2.7 | Typically post plant | Aerial | [7] |
| Australia | 200–250 *** | 0.3–3.2 | Mainly pre-plant | Ground based mechanical and aerial | [31,38,39] |
| Chile | 200–295 *** | 2.0–2.5 ** | Pre-and post-plant | Manual only | Perscomm |
| USA | 185 *** | 1.0–3.0 | Mainly pre-plant | Aerial only | [35,40] |
| South Africa | 146–193 *** | 0.7–1.0 | Pre-plant and post-plant | Aerial (pre-plant application) and manual (post-plant) | [33] |
| Parameter | Unit | Glyphosate Isopropylammonium | EFSA 2015 Recommendations |
|---|---|---|---|
| Kow (log P) | <−5.4 (20 °C) | ||
| Solubility in water | g/L | 1050 (25 °C, pH 4.3) | |
| Koc | 1424 | ||
| pKa | 5.77 | ||
| ARfD | mg/kg bw | 0.5 | |
| NOAEL (2 years) rats | mg/kg bw | 31 | |
| AOEL | mg/kg bw | 0.1 | |
| Drinking water standard # | µg L−1 | Australia, 1000; USA, 700 | |
| Acute Oral LD50 (rats) | mg/kg bw | >5000 | |
| Inhalation LC50 (4 h) (rats) | mg/L air | 1.3 | |
| Acceptable daily intake (ADI) | mg/kg bw per day | 1 (2004) | 0.5 |
| WHO Toxicity class | 2B | ||
| WHO Hazard class | III (slightly hazardous) | ||
| LC50 Trout (96 h) | mg/L | >1000 | |
| LC50 Daphnia (48 h) | mg/L | 930 | |
| EC50 Algae (72 h) | mg/L | 72.9 | |
| LC50 Earthworm (14 day) | mg/kg soil | >5000 | |
| LD50 Bee | µg/bee | >100 | |
| DT50 soil | Days | 1–130 | |
| DT50 water | Days | <190 | |
| Major metabolite in soil and water | metabolised to aminomethylphosphonic acid (AMPA) |
| Water | Sediment | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Location | Waterbody | Application Method | SMZ Width (m) | Glyphosate kg a.i. ha−1 | Max. Conc. (µg L−1) | DL (µg L−1) | DT50 | Max. Conc. (µg L−1) | DL (µg L−1) |
| Oregon, USA [52] | stream | aerial | 0 | 3.3 | 270 b,# | ~≤1 day | ~550 | ||
| British Columbia, Canada [72] | stream | aerial | 0 | 3 | 23 b/100 c | 5 | 400 | 40–100 | |
| British Columbia, Canada [72] | stream | aerial | 60−100 | 3 | <DL b/25 c | 5 | 200 | 40–100 | |
| British Columbia, Canada [72] | stream | ground | 10 | 0.5−1.5 | <DL b/<DL c | 5 | 50 | 40–100 | |
| British Columbia, Canada [72] | stream | aerial * | 50–100 | 1.54 | <DL b/<DL c | 5 | <DL | 40–100 | |
| Manitoba, Canada [73] | ponds (n = 4) | aerial | 0 | 0.89 | 16–141 b | 0.25–0.50 | 1.5–3.5 days | ||
| British Columbia, Canada [74] | stream | aerial | 100 | 1.78 | <DL | 5 | |||
| British Columbia, Canada [74] | stream | ground | 10 | 1.4 | <DL | 5 | |||
| British Columbia, Canada [60] | ephemeral stream (750) | aerial | 0 | 2 | <1.5 b; ~143 c | 0.1 | 580 | 30 | |
| British Columbia, Canada [60] | stream (1600) | aerial | 0 | 2 | 162 b,#; ~130 c | 0.1 | 6800 | 30 | |
| British Columbia, Canada [60] | ephemeral stream | aerial | 10 | 2 | <DL | 0.1 | 30 | ||
| British Columbia, Canada [60] | ephemeral stream (1450) | aerial | 10 | 2 | 2.47 b | 0.1 | <DL | 30 | |
| Oregon, USA [51] | stream/pond | aerial | 0 | 4.12 a | 0.03/0.9 b | 0.001 | ≤10 h | 110/2360 | 50 |
| Michigan, USA [51] | stream/pond | aerial | 0 | 4.12 a | 1.24/1.7 b | 0.001 | ≤10 h | 690/1920 | 50 |
| Georgia, USA [51] | stream/pond | aerial | 0 | 4.12 a | 0.04/1.0 b | 0.001 | ≤10 h | 180/260 | 50 |
| New Brunswick, USA [75] | stream | ground-based | 65 | 1.67 | <DL | 25 | |||
| Ontario, Canada [70] | wetlands (n = 24) | aerial—over wetland | 0 | 1.07–2.14 | 1950 # (mean 330) b | 0.02 | |||
| Ontario, Canada [70] | wetlands (n = 11) | aerial—alongside wetland | 0 | 1.07–2.14 | (mean 180) b | 0.02 | |||
| Ontario, Canada [70] | wetlands (n = 16) | aerial—buffered wetland | 30–60 | 1.07–2.14 | 310 (mean 30) b | 0.02 | |||
| Reference | Glyphosate Guideline |
|---|---|
| New Zealand’s Environmental Risk Management Authority [103] | EEL a −0.37 mg a.i. L−1 |
| Australian and New Zealand guidelines for fresh and marine water quality [104] | Moderate reliability guideline figure (trigger value b) of 1200 µg a.i. L−1 for protection of 95% of freshwater species. |
| For slightly-moderately disturbed systems the 99% protection value of 370 µg a.i. L−1 is recommended | |
| Canadian Water Quality Guidelines for the Protection of Aquatic Life [88] | Long-term exposure c −800 µg a.i. L−1 |
| Short-term exposure d −27,000 µg a.i. L−1 | |
| South African water quality guidelines (SAWQGs) for Roundup (a.e. 360 g L−1) [105] | Short-term exposure e −0.250 (0.106–0.589) mg L−1 |
| Long-term exposure f −0.002 (0.000–0.021) mg L−1 | |
| French Water Quality Guidelines [106] | Threshold value (PNEC) g −28 µg L−1 |
| Max. acceptable concentration −70 µg L−1 | |
| Environmental quality guideline ‒0.1 µg L−1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rolando, C.A.; Baillie, B.R.; Thompson, D.G.; Little, K.M. The Risks Associated with Glyphosate-Based Herbicide Use in Planted Forests. Forests 2017, 8, 208. https://doi.org/10.3390/f8060208
Rolando CA, Baillie BR, Thompson DG, Little KM. The Risks Associated with Glyphosate-Based Herbicide Use in Planted Forests. Forests. 2017; 8(6):208. https://doi.org/10.3390/f8060208
Chicago/Turabian StyleRolando, Carol A., Brenda R. Baillie, Dean G. Thompson, and Keith M. Little. 2017. "The Risks Associated with Glyphosate-Based Herbicide Use in Planted Forests" Forests 8, no. 6: 208. https://doi.org/10.3390/f8060208
APA StyleRolando, C. A., Baillie, B. R., Thompson, D. G., & Little, K. M. (2017). The Risks Associated with Glyphosate-Based Herbicide Use in Planted Forests. Forests, 8(6), 208. https://doi.org/10.3390/f8060208





