Screening of Date Palm (Phoenix dactylifera L.) Cultivars for Salinity Tolerance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants, Seed Germination, and Growth Conditions
2.2. Plant Growth and Leaf Area Measurements
2.3. Gas Exchange and Chlorophyll Fluorescence Measurements
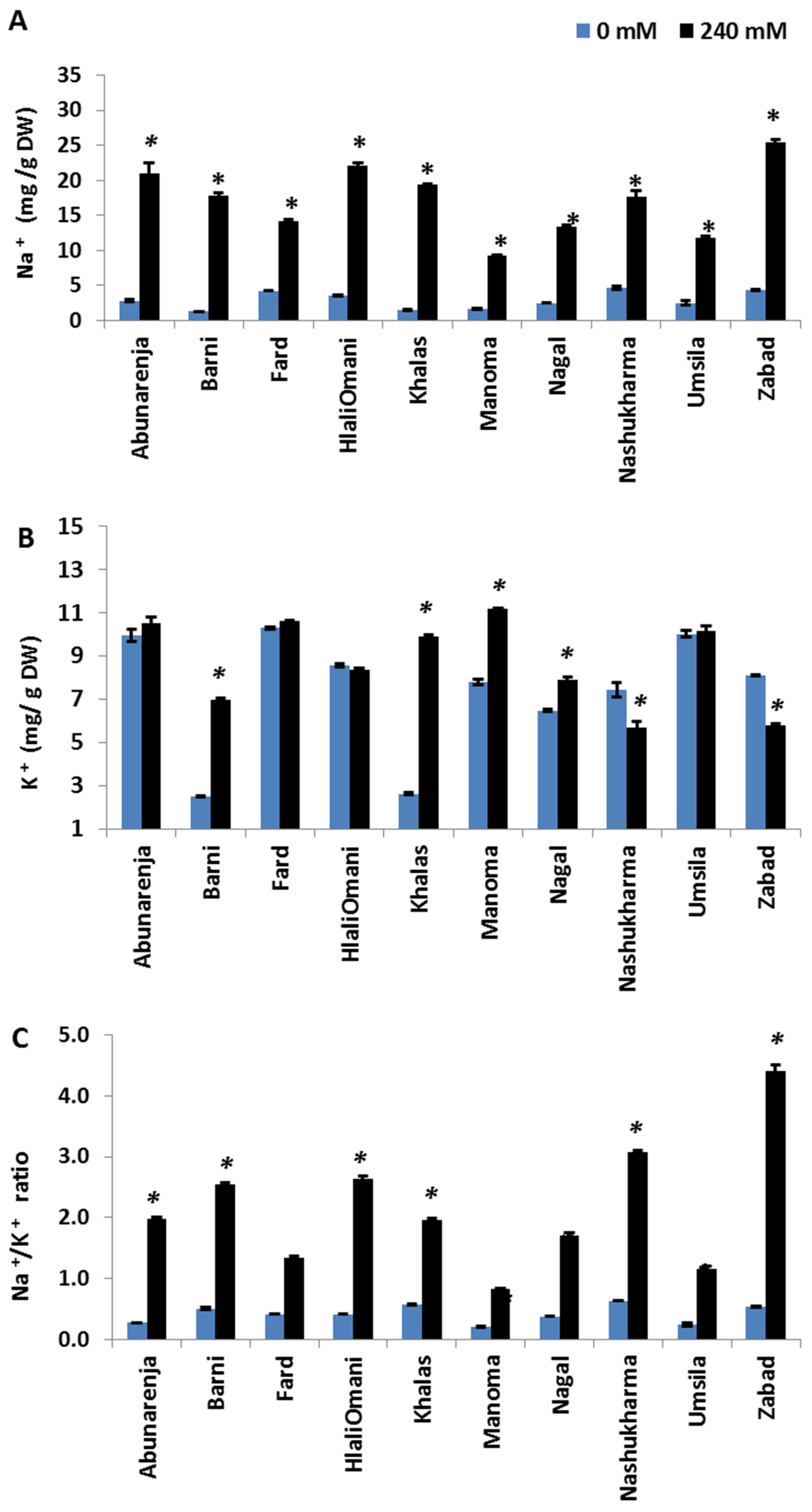
2.4. Measurement of Na+ and K+ Concentrations
2.5. Chlorophyll Concentration
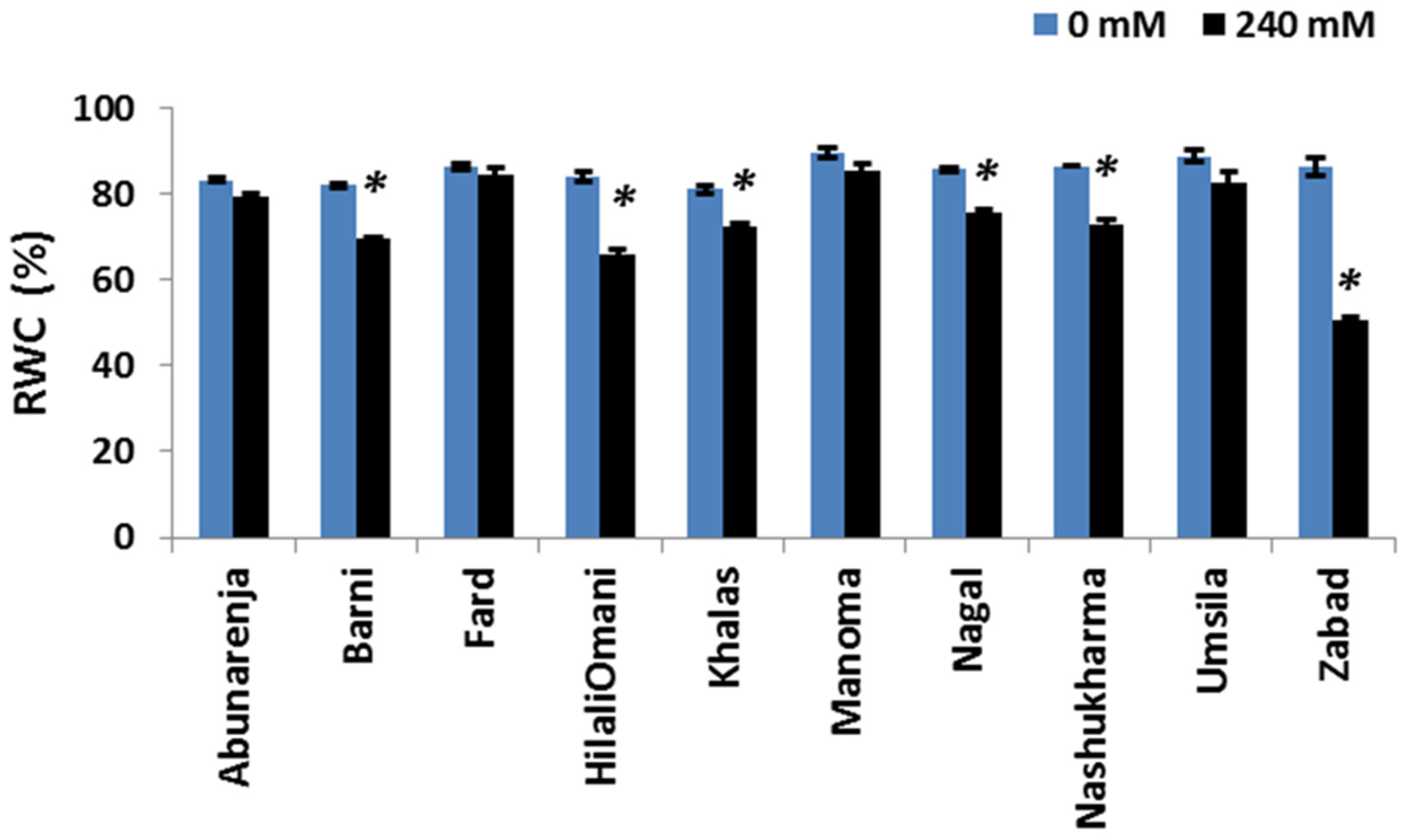
2.6. Relative Water Content
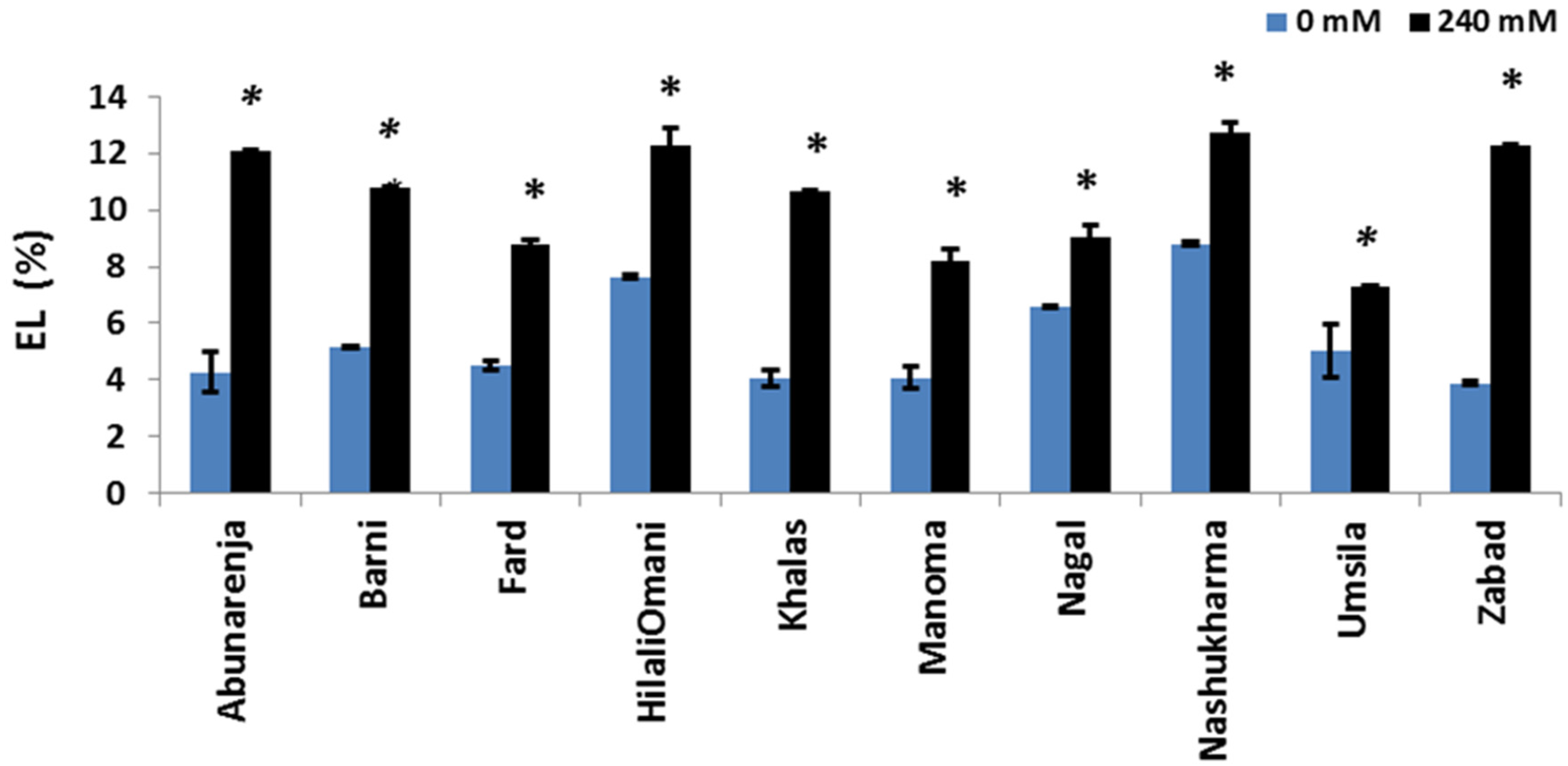
2.7. Electrolyte Leakage
2.8. Statistical Analysis
3. Results
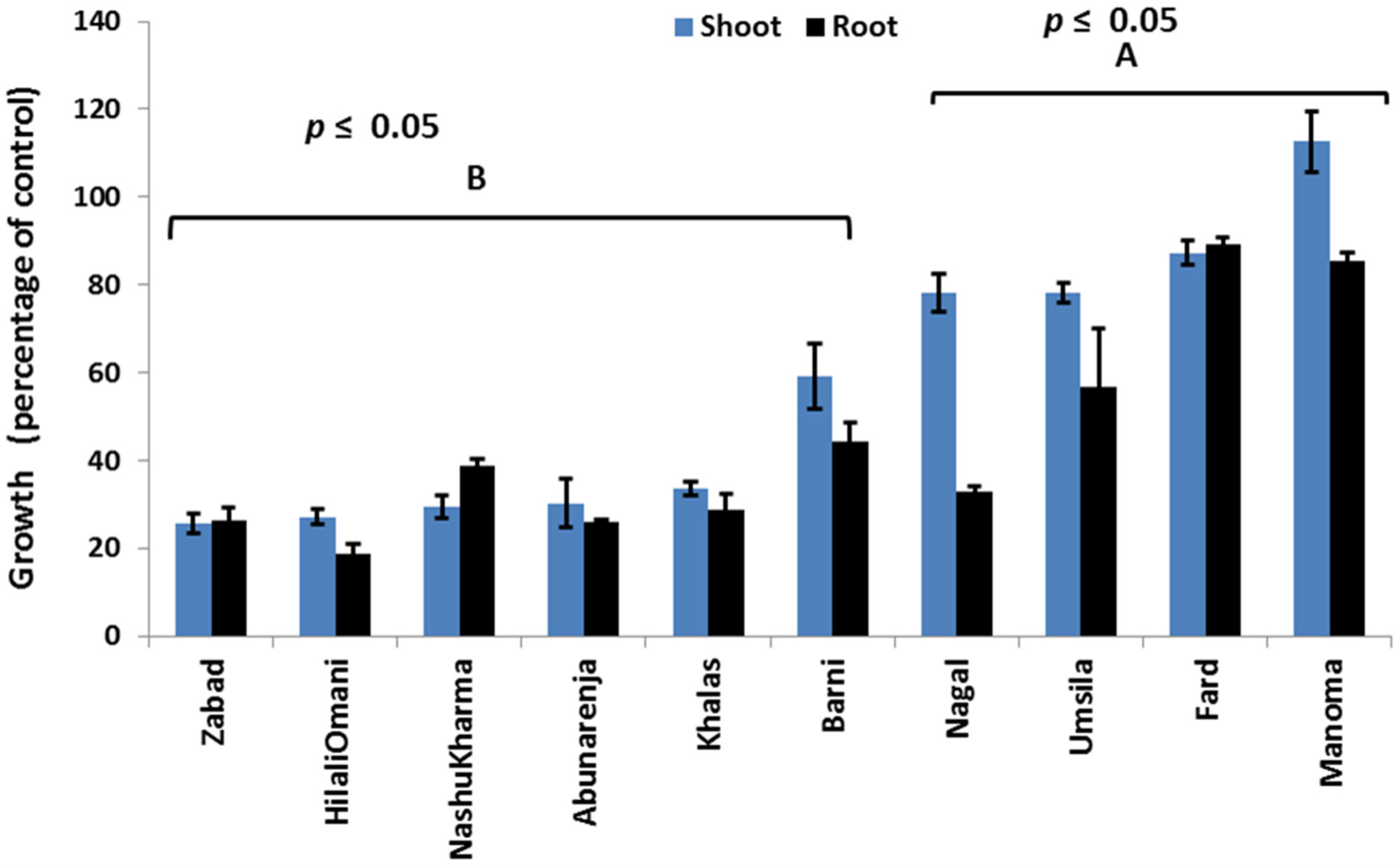
3.1. Effects of Salinity on Growth
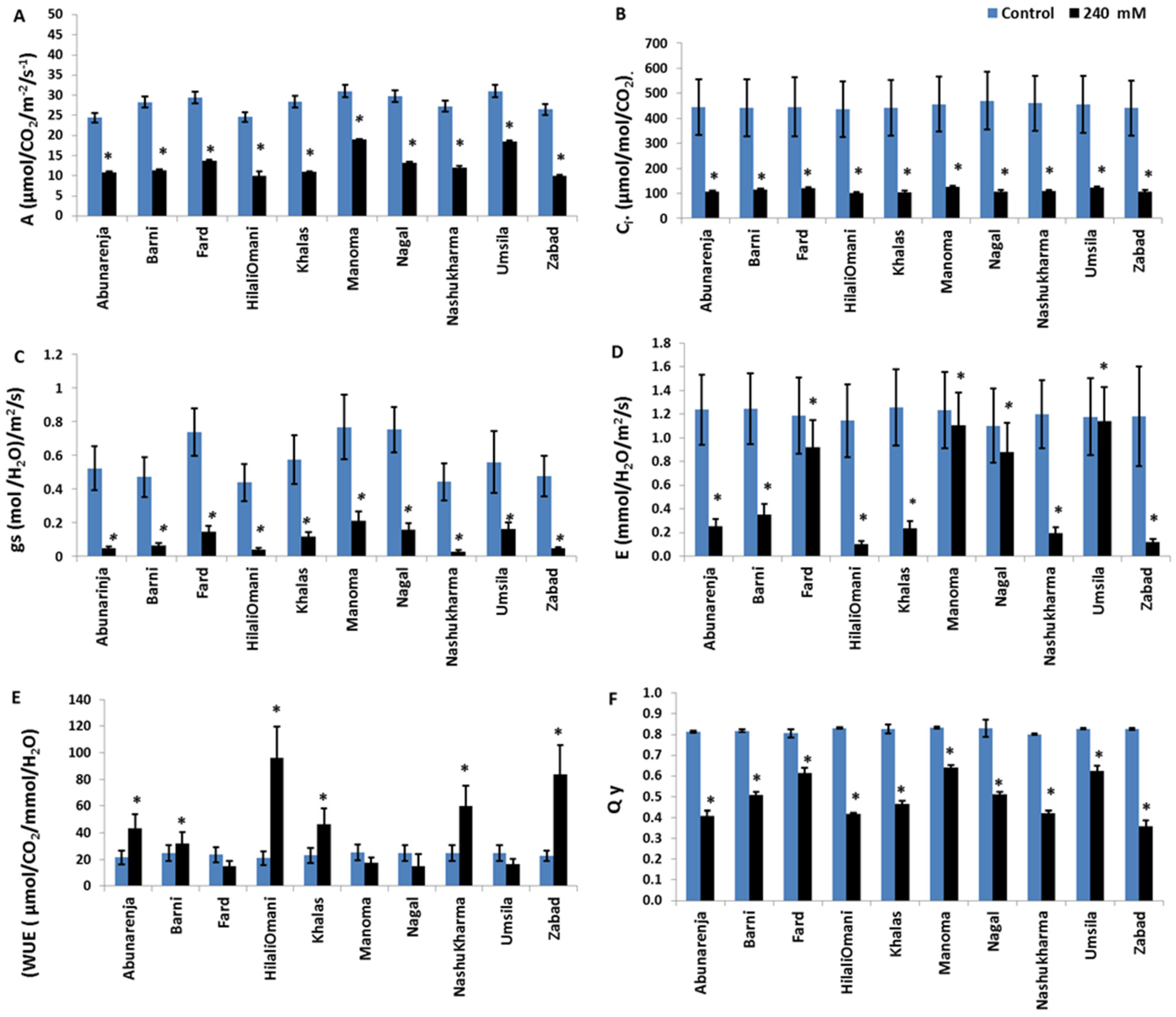
3.2. Photosynthesis and Quantum Yield Efficiency of PSII Activity
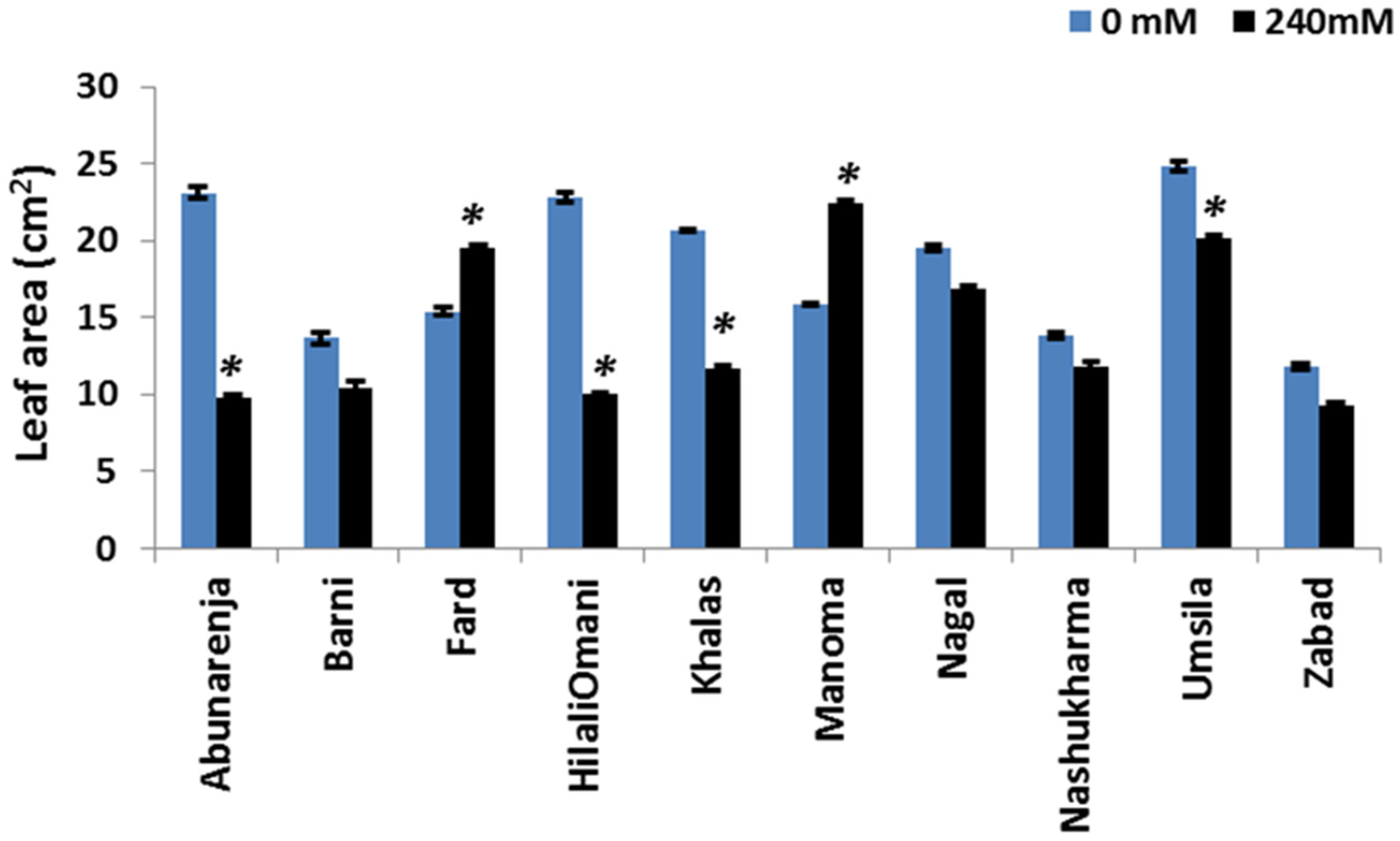
3.3. Effect of Salt Stress on Leaf Area
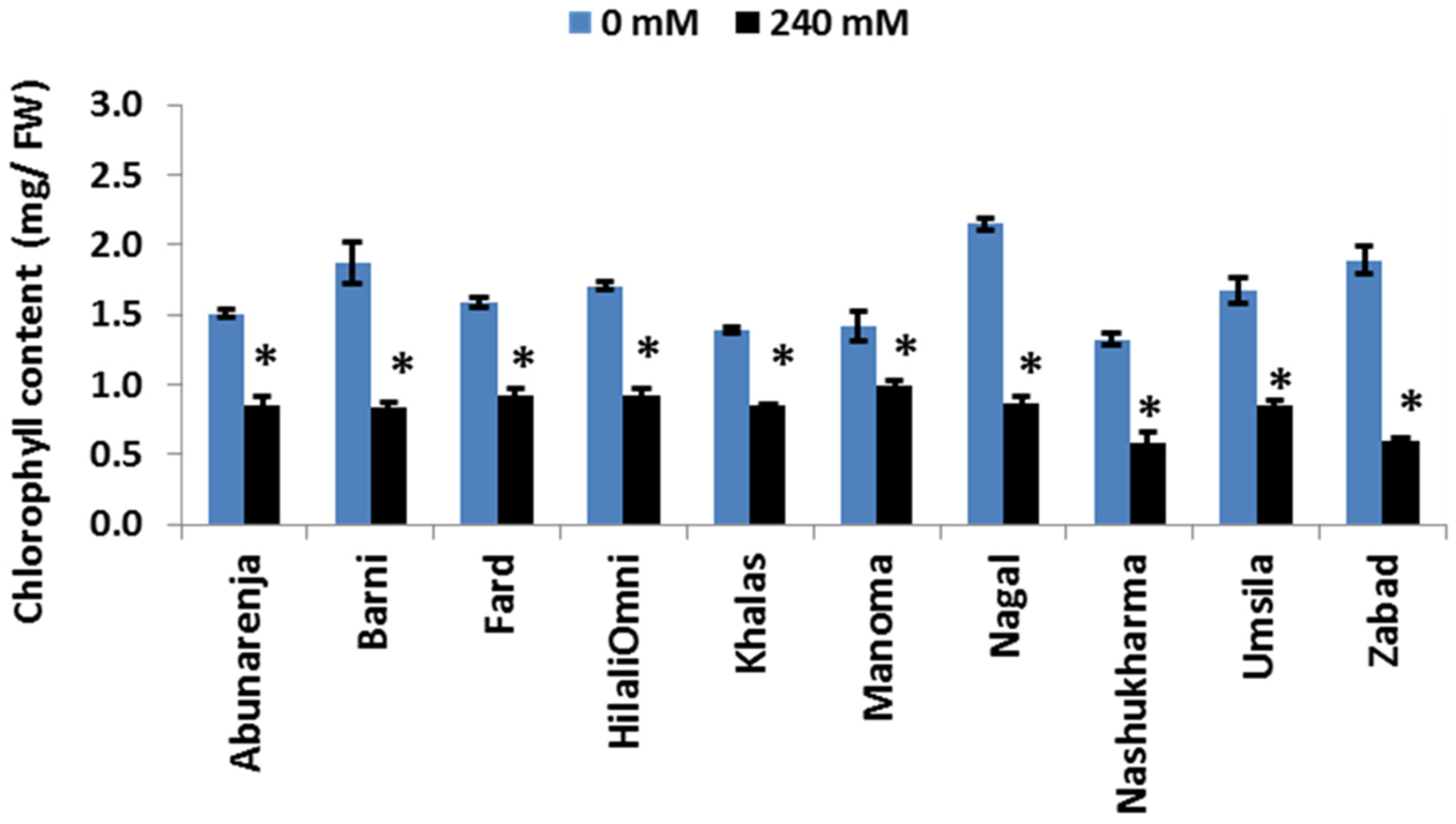
3.4. Effect of Salt Stress on Total Chlorophyll Concentration
3.5. Effect of Salt Stress on Relative Water Content (RWC)
3.6. Effect of Salt Stress on Na+ and K+ Concentrations
3.7. Effect of Salt Stress on Electrolyte Leakage (EL)
3.8. Interaction Effects between Cultivars and Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shabala, S. Learning from halophytes: Physiological basis and strategies to improve abiotic stress tolerance in crops. Ann. Bot. 2013, 112, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Tattini, M.; Melgar, J.; Traversi, M. Responses of olea europaea to high salinity: A brief-ecophysiological-review. Adv. Hortic. Sci. 2008, 22, 159–173. [Google Scholar]
- Garcı́a-Sánchez, F.; Jifon, J.L.; Carvajal, M.; Syvertsen, J.P. Gas exchange, chlorophyll and nutrient contents in relation to NA+ and Cl− accumulation in ‘sunburst’ mandarin grafted on different rootstocks. Plant Sci. 2002, 162, 705–712. [Google Scholar] [CrossRef]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hort. 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Anschütz, U.; Becker, D.; Shabala, S. Going beyond nutrition: Regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J. Plant Physiol. 2014, 171, 670–687. [Google Scholar] [CrossRef] [PubMed]
- Plett, D.C.; Møller, I. Na+ transport in glycophytic plants: What we know and would like to know. Plant Cell Environ. 2010, 33, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Assaha, D.V.; Mekawy, A.M.M.; Ueda, A.; Saneoka, H. Salinity-induced expression of hkt may be crucial for Na+ exclusion in the leaf blade of huckleberry (solanum scabrum mill.), but not of eggplant (Solanum melongena L.). Biochem. Biophys. Res. Commun. 2015, 460, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Cotsaftis, O.; Plett, D.; Shirley, N.; Tester, M.; Hrmova, M. A two-staged model of Na+ exclusion in rice explained by 3d modeling of hkt transporters and alternative splicing. PLoS ONE 2012, 7, e39865. [Google Scholar] [CrossRef] [PubMed]
- Fujimaki, S.; Maruyama, T.; Suzui, N.; Kawachi, N.; Miwa, E.; Higuchi, K. Base to tip and long-distance transport of sodium in the root of common reed (phragmites australis (cav.) trin. Ex steud.) at steady state under constant high-salt conditions. Plant Cell Physiol. 2015, 56, 943–950. [Google Scholar] [PubMed]
- Vinocur, B.; Altman, A. Recent advances in engineering plant tolerance to abiotic stress: Achievements and limitations. Curr. Opin. Biotechnol. 2005, 16, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Mekawy, A.M.M.; Assaha, D.V.; Yahagi, H.; Tada, Y.; Ueda, A.; Saneoka, H. Growth, physiological adaptation, and gene expression analysis of two egyptian rice cultivars under salt stress. Plant Physiol. Biochem. 2015, 87, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; James, R.A.; Xu, B.; Athman, A.; Conn, S.J.; Jordans, C.; Byrt, C.S.; Hare, R.A.; Tyerman, S.D.; Tester, M. Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nat. Biotechnol. 2012, 30, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Al-Kateeb, S.; Al-Kateeb, A.; Ali-Dinar, H. Photosynthesis Efficiency of Date Palm Varieties Grown in Saudi Arabia; The Final Technical Report of the Research Project Submitted by the Date Palm Research Centre to the Deanship of Scientific Research; King Faisal University: Hofuf, Saudi Arabia, 2002. [Google Scholar]
- Alhammadi, M.S.; Kurup, S.S. Impact of Salinity Stress on Date Palm (Phoenix Dactylifera L.)—A Review; INTECH Open Access Publisher: Vienna, Austria, 2012. [Google Scholar]
- Aljuburi, H.J.; Maroff, A. The growth and mineral composition of hatamy date palm seedlings as affected by sea water and growth regulators. In Proceedings of the III International Date Palm Conference, Abu Dhabi, UAE, 19–21 February 2006; pp. 161–164. [Google Scholar]
- Alrasbi, S.A.R.; Hussain, N.; Schmeisky, H. Evaluation of the growth of date palm seedlings irrigated with saline water in the sultanate of Oman. In Proceedings of the IV International Date Palm Conference, Abu Dhabi, UAE, 15–17 March 2010; pp. 233–246. [Google Scholar]
- Sperling, O.; Lazarovitch, N.; Schwartz, A.; Shapira, O. Effects of high salinity irrigation on growth, gas-exchange, and photoprotection in date palms (Phoenix dactylifera L., cv. Medjool). Environ. Exp. Bot. 2014, 99, 100–109. [Google Scholar] [CrossRef]
- Yaish, M.W.; Kumar, P.P. Salt tolerance research in date palm tree (Phoenix dactylifera L.), past, present, and future perspectives. Front. Plant Sci. 2015, 6, 348. [Google Scholar] [CrossRef] [PubMed]
- Youssef, T.; Awad, M.A. Mechanisms of enhancing photosynthetic gas exchange in date palm seedlings (Phoenix dactylifera L.) under salinity stress by a 5-aminolevulinic acid-based fertilizer. J. Plant Growth Regul. 2008, 27, 1–9. [Google Scholar] [CrossRef]
- Al-Yahyai, R.; Al-Kharusi, L. Physical and chemical quality attributes of freeze-stored dates. Int. J. Agric. Biol. 2012, 14, 97–100. [Google Scholar]
- Aljuburi, H.V. Effect of sodium chloride on seedling growth of four date palm varieties. Ann. Arid Zone 1993, 31, 259–262. [Google Scholar]
- Yaish, M. Proline accumulation is a general response to abiotic stress in the date palm tree (Phoenix dactylifera L.). Genet. Mol. Res. 2015, 14, 9943–9950. [Google Scholar] [CrossRef] [PubMed]
- Assaha, D.V.; Ueda, A.; Saneoka, H. Comparison of growth and mineral accumulation of two solanaceous species, Solanum scabrum mill.(huckleberry) and S. Melongena l.(eggplant), under salinity stress. Soil Sci. Plant Nutr. 2013, 59, 912–920. [Google Scholar]
- Porra, R.; Thompson, W.; Kriedemann, P. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Barr, H.; Weatherley, P. A re-examination of the relative turgidity technique for estimating water deficit in leaves. Aust. J. Biol. Sci. 1962, 15, 28. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Liu, L.; Ueda, A.; Nagaoka, T.; Saneoka, H. Effects of drought stress on growth, solute accumulation and membrane stability of leafy vegetable, huckleberry (Solanum scabrum mill.). J. Environ. Biol. 2016, 37, 107. [Google Scholar] [PubMed]
- Meziane, D.; Shipley, B. Direct and indirect relationships between specific leaf area, leaf nitrogen and leaf gas exchange. Effects of irradiance and nutrient supply. Ann. Bot. 2001, 88, 915–927. [Google Scholar] [CrossRef]
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis; John Wiley & Sons: Abingdon, Oxon, UK, 2013. [Google Scholar]
- Zhu, J.-K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Yeo, A.; Yeo, M.; Flowers, S.; Flowers, T. Screening of rice (Oryza sativa L.) genotypes for physiological characters contributing to salinity resistance, and their relationship to overall performance. TAG Theor. Appl. Genet. 1990, 79, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Colmer, T.; Munns, R.; Flowers, T. Improving salt tolerance of wheat and barley: Future prospects. Anim. Prod. Sci. 2006, 45, 1425–1443. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [PubMed]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; DIAZ-ESPEJO, A.; GalmES, J.; Kaldenhoff, R.; Medrano, H.; RIBAS-CARBO, M. Rapid variations of mesophyll conductance in response to changes in Co2 concentration around leaves. Plant Cell Environ. 2007, 30, 1284–1298. [Google Scholar] [CrossRef] [PubMed]
- Ghoulam, C.; Foursy, A.; Fares, K. Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ. Exper. Bot. 2002, 47, 39–50. [Google Scholar] [CrossRef]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Cramer, G.R.; Lynch, J.; Läuchli, A.; Epstein, E. Influx of Na+, K+, and Ca2+ into roots of salt-stressed cotton seedlings effects of supplemental Ca2+. Plant Physiol. 1987, 83, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J.; Amtmann, A. K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ ratios. Ann. Bot. 1999, 84, 123–133. [Google Scholar] [CrossRef]
- Shabala, S. Regulation of potassium transport in leaves: From molecular to tissue level. Ann. Bot. 2003, 92, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Cuin, T.A.; Svistunenko, D.; Smith, S.J.; Miller, A.J.; Shabala, S.; Sokolik, A.; Yurin, V. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: Single-channel properties, genetic basis and involvement in stress-induced cell death. J. Cell Sci. 2010, 123, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- Yaish, M.W.; Patankar, H.V.; Assaha, D.V.; Zheng, Y.; Al-Yahyai, R.; Sunkar, R. Genome-wide expression profiling in leaves and roots of date palm (Phoenix dactylifera L.) exposed to salinity. BMC Genom. 2017, 18, 246. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Kharusi, L.; Assaha, D.V.M.; Al-Yahyai, R.; Yaish, M.W. Screening of Date Palm (Phoenix dactylifera L.) Cultivars for Salinity Tolerance. Forests 2017, 8, 136. https://doi.org/10.3390/f8040136
Al Kharusi L, Assaha DVM, Al-Yahyai R, Yaish MW. Screening of Date Palm (Phoenix dactylifera L.) Cultivars for Salinity Tolerance. Forests. 2017; 8(4):136. https://doi.org/10.3390/f8040136
Chicago/Turabian StyleAl Kharusi, Latifa, Dekoum V. M. Assaha, Rashid Al-Yahyai, and Mahmoud W. Yaish. 2017. "Screening of Date Palm (Phoenix dactylifera L.) Cultivars for Salinity Tolerance" Forests 8, no. 4: 136. https://doi.org/10.3390/f8040136
APA StyleAl Kharusi, L., Assaha, D. V. M., Al-Yahyai, R., & Yaish, M. W. (2017). Screening of Date Palm (Phoenix dactylifera L.) Cultivars for Salinity Tolerance. Forests, 8(4), 136. https://doi.org/10.3390/f8040136







