Acute Oak Decline and Agrilus biguttatus: The Co-Occurrence of Stem Bleeding and D-Shaped Emergence Holes in Great Britain
Abstract
:1. Introduction
- Test the null hypothesis that the presence of stem bleeds and emergence holes on individual trees are independent.
- Examine whether stem bleeds are more likely to occur on hosts before or after exit holes appear.
- Assess the spatial relationship between symptom types (trees with emergence holes in relation to those with stem bleeds.
- Determine the within year variation in symptom expression, whether the number of stem bleeds correlates with the number of new emergence holes.
- Quantify the number of Agrilus species present within AOD affected woodland.
2. Materials and Methods
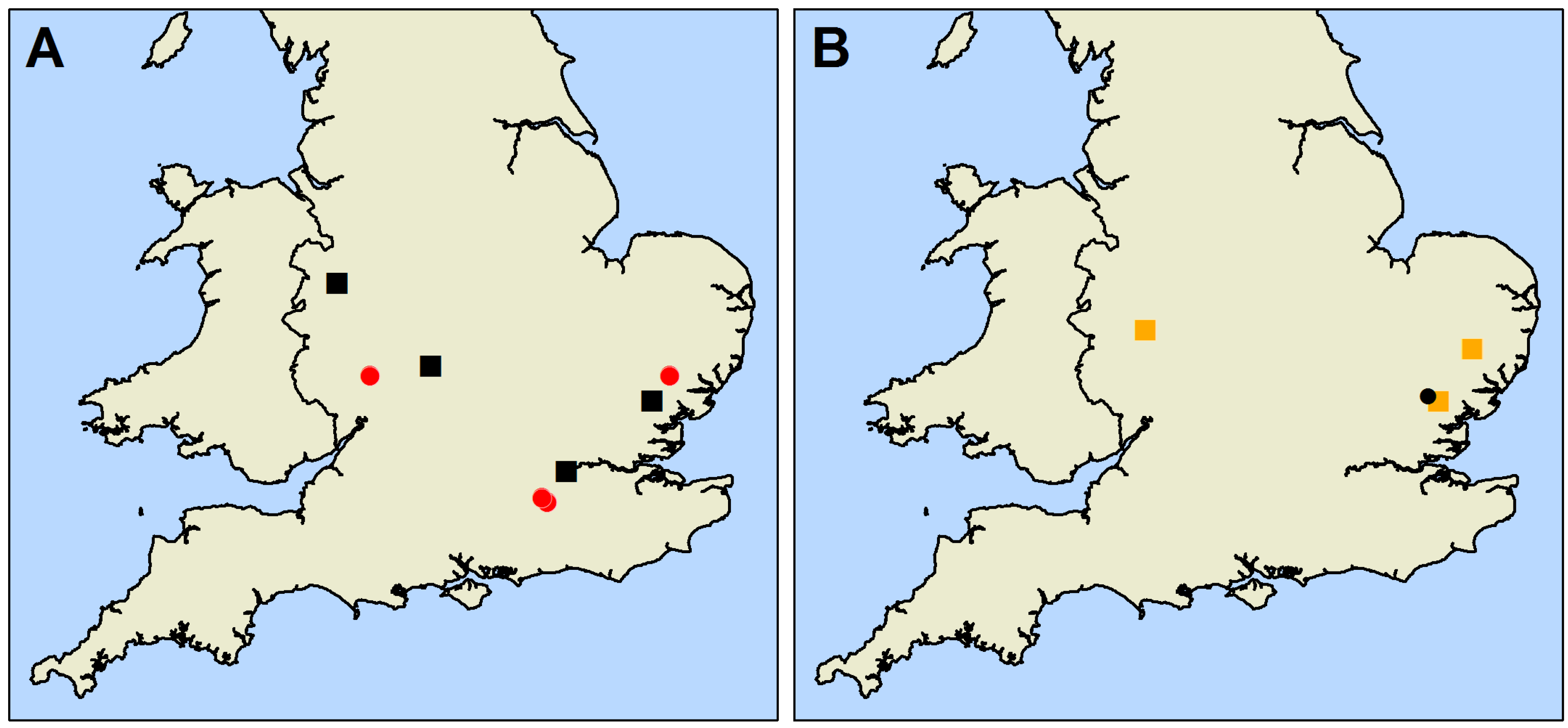
2.1. Mapping and Data Collection
2.2. Co-Occurrence of Stem Symptoms and Emergence Holes
2.3. Sequential Development of External Symptoms and Signs
2.4. Spatial Relationship between Emergence Holes and Stem Bleeds
2.5. Within-Year Symptom Development
2.6. Trapping Experiments
3. Results
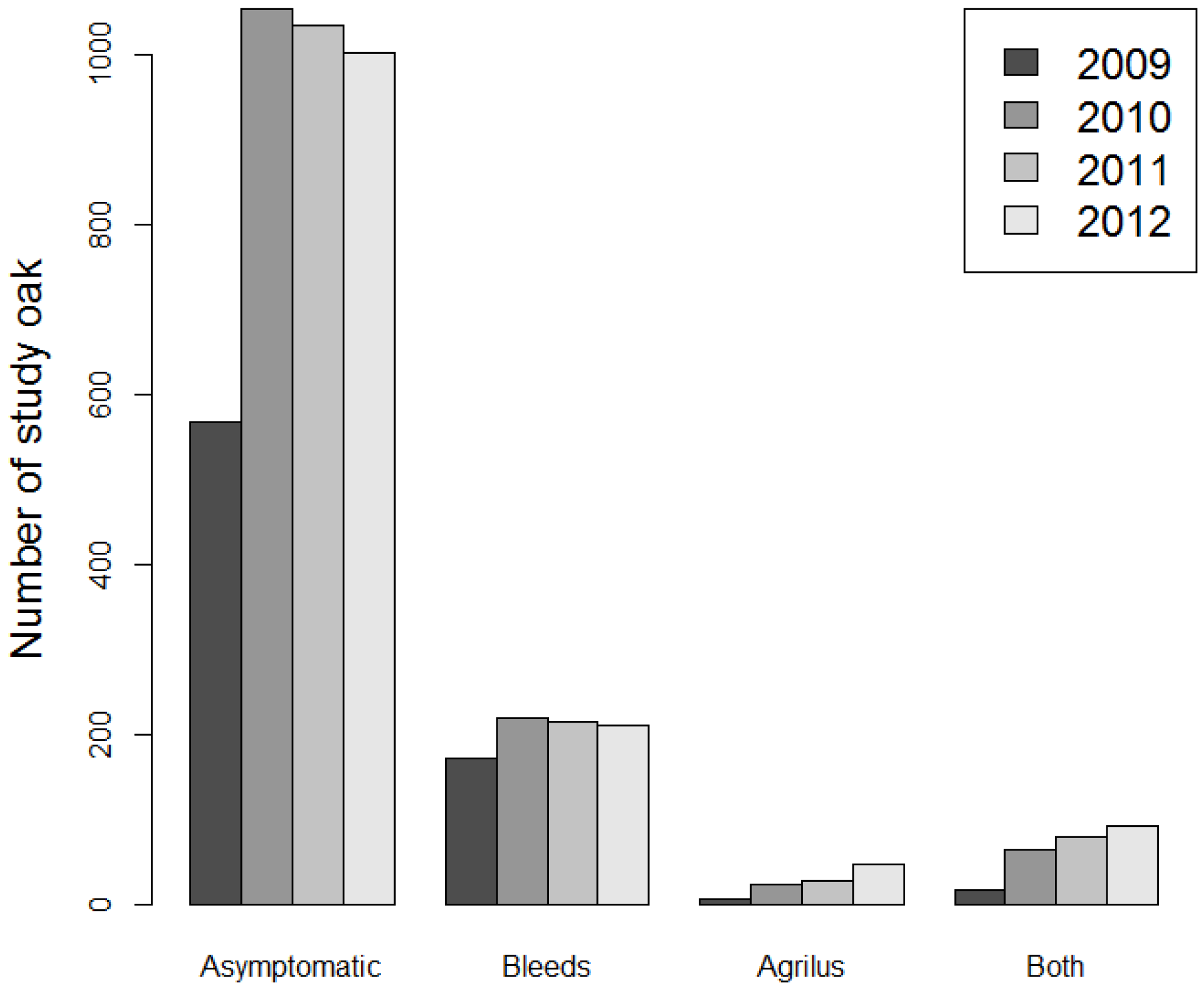
3.1. Co-Occurrence of Stem Symptoms and Emergence Holes
3.2. Sequential Development
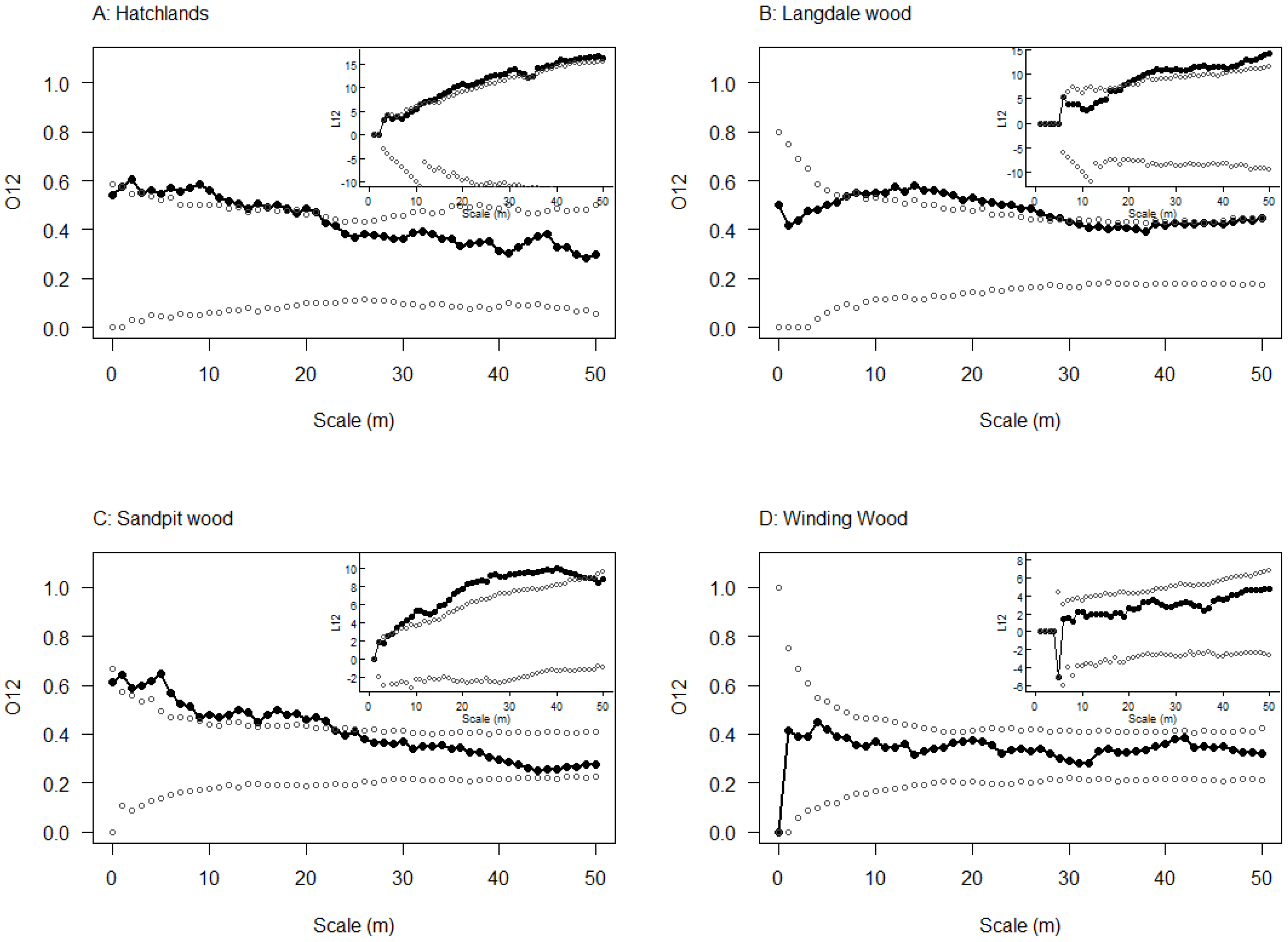
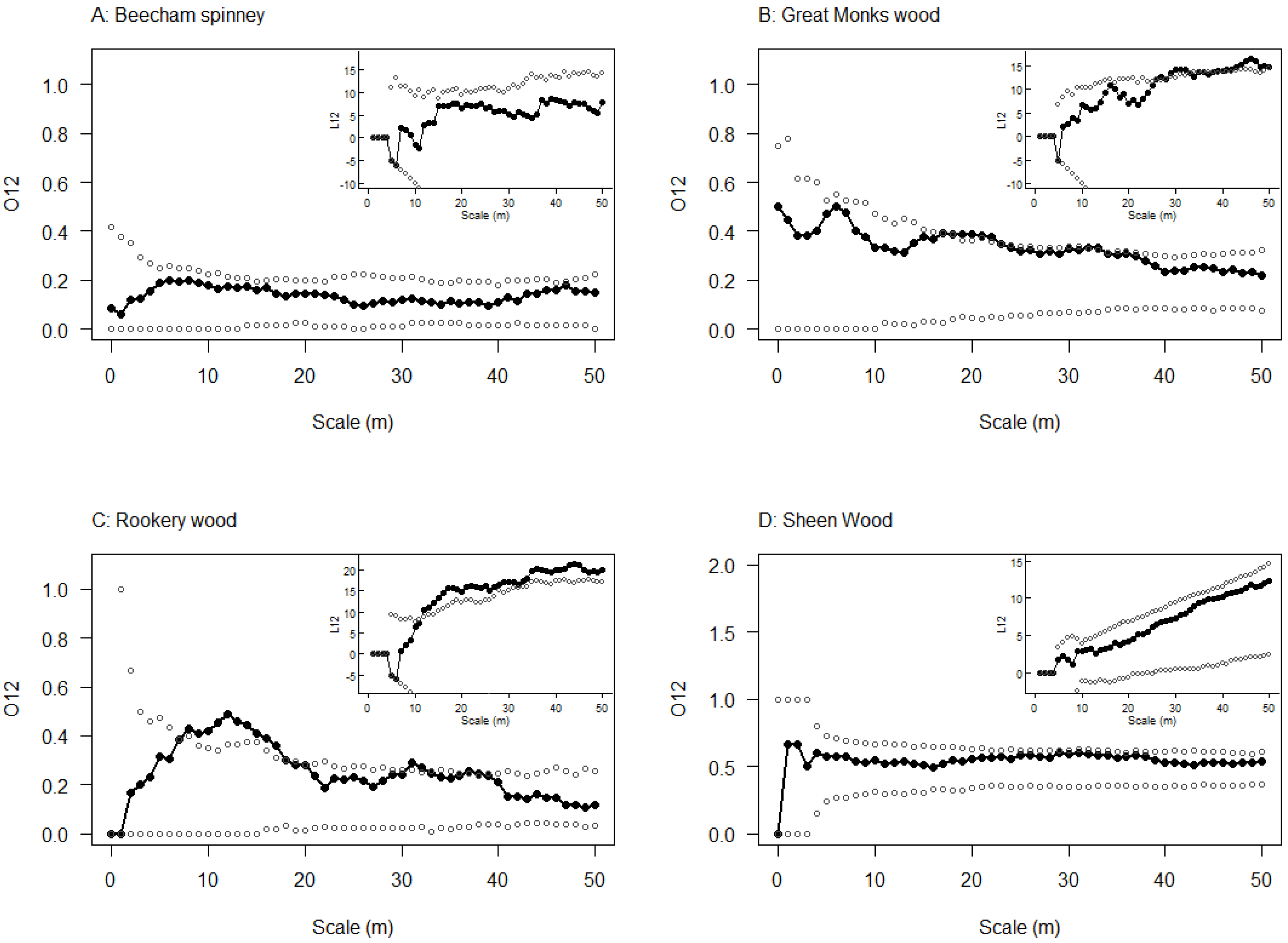
3.3. Spatial Relationship between Emergence Holes and Stem Bleeds
3.4. Within-Year Symptom Development
3.5. Trapping Experiments
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Denman, S.; Brown, N.; Kirk, S.; Jeger, M.; Webber, J. A description of the symptoms of Acute Oak Decline in Britain and a comparative review on causes of similar disorders on oak in Europe. Forestry 2014, 87, 535–551. [Google Scholar] [CrossRef]
- Brown, N.; Inward, D.J.G.; Jeger, M.; Denman, S. A review of Agrilus biguttatus in UK forests and its relationship with acute oak decline. Forestry 2015, 88, 53–63. [Google Scholar] [CrossRef]
- Denman, S.; Plummer, S.; Kirk, S.; Peace, A.; McDonald, J.E. Isolation studies reveal a shift in the cultivable microbiome of oak affected with Acute Oak Decline. Syst. Appl. Microbiol. 2016, 39, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Brewer, A.; Ditchburn, B. NFI Statistical Analysis Report: 50-Year Forecast of Hardwood Timber Availability; Forestry Commission: Edinburgh, UK, 2014. [Google Scholar]
- Manion, P. Tree Disease Concepts; Prentice-Hall: Englewood Cliffs, NJ, USA, 1991. [Google Scholar]
- Anderegg, W.R.L.; Hicke, J.A.; Fisher, R.A.; Allen, C.D.; Aukema, J.; Bentz, B.; Hood, S.; Lichstein, J.W.; Macalady, A.K.; McDowell, N.; et al. Tree mortality from drought, insects, and their interactions in a changing climate. New Phytol. 2015, 208, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Millar, C.I.; Stephenson, N.L. Temperate forest health in an era of emerging megadisturbance. Science 2015, 349, 823–826. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Jactel, H.; Petit, J.; Desprez-Loustau, M.-L.; Delzon, S.; Piou, D.; Battisti, A.; Koricheva, J. Drought effects on damage by forest insects and pathogens: A meta-analysis. Glob. Chang. Biol. 2012, 18, 267–276. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Desprez-Loustau, M.-L.; Marçais, B.; Nageleisen, L.-M.; Piou, D.; Vannini, A. Interactive effects of drought and pathogens in forest trees. Ann. For. Sci. 2006, 63, 597–612. [Google Scholar] [CrossRef]
- Oliva, J.; Stenlid, J.; Martínez-Vilalta, J. The effect of fungal pathogens on the water and carbon economy of trees: Implications for drought-induced mortality. New Phytol. 2014, 203, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Flower, C.E.; Gonzalez-Meler, M.A. Responses of Temperate Forest Productivity to Insect and Pathogen Disturbances. Ann. Rev. Plant Biol. 2015, 66, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.M. Recent advances in cause-effect research on oak decline in Europe. CAB Rev. 2008, 3, 1–22. [Google Scholar] [CrossRef]
- Thomas, F.M.; Blank, R.; Hartmann, G. Abiotic and biotic factors and their interactions as causes of oak decline in Central Europe. For. Pathol. 2002, 32, 277–307. [Google Scholar] [CrossRef]
- Denman, S.; Barrett, G.; Kirk, S.A.; McDonald, J.E.; Coetzee, M.P.A. Identification of Armillaria species on declined oak in Britain: Implications for oak health. Forestry 2017, 90, 148–161. [Google Scholar] [CrossRef]
- Denman, S.; Brady, C.; Kirk, S.; Cleenwerck, I.; Venter, S.; Coutinho, T.; De Vos, P. Brenneria goodwinii sp. nov., associated with acute oak decline in the UK. Int. J. Syst. Evol. Microbiol. 2012, 62, 2451–2456. [Google Scholar] [CrossRef] [PubMed]
- Brady, C.; Denman, S.; Kirk, S.; Venter, S.; Rodriguez-Palenzuela, P.; Coutinho, T. Description of Gibbsiella quercinecans gen. nov., sp. nov., associated with Acute Oak Decline. Syst. Appl. Microbiol. 2010, 33, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Brady, C.L.; Cleenwerck, I.; Denman, S.; Venter, S.N.; Rodríguez-Palenzuela, P.; Coutinho, T.A.; De Vos, P. Proposal to reclassify Brenneria quercina (Hildebrand and Schroth 1967) Hauben et al. 1999 into a new genus, Lonsdalea gen. nov., as Lonsdalea quercina comb. nov., descriptions of Lonsdalea quercina subsp. quercina comb. nov., Lonsdalea quercina subsp. ib. Int. J. Syst. Evol. Microbiol. 2012, 62, 1592–1602. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.; Jeger, M.; Kirk, S.; Xu, X.; Denman, S. Spatial and temporal patterns in symptom expression within eight woodlands affected by Acute Oak Decline. For. Ecol. Manag. 2016, 360, 97–109. [Google Scholar] [CrossRef]
- Vansteenkiste, D.; Tirry, L.; Van Acker, J.; Stevens, M. Predispositions and symptoms of Agrilus borer attack in declining oak trees. Ann. For. Sci. 2004, 61, 815–823. [Google Scholar] [CrossRef]
- Salle, A.; Nageleisen, L.M.; Lieutier, F. Bark and wood boring insects involved in oak declines in Europe: Current knowledge and future prospects in a context of climate change. For. Ecol. Manag. 2014, 328, 79–93. [Google Scholar] [CrossRef]
- Falck, R. Oak Decline in Lödderitz Forest District and in Westphalia. Z. Forst Jagdwes 1918, 50, 123–132. [Google Scholar]
- Jacquiot, C. Relationships between attacks by Agrilus biguttatus Fab. and some cases of decline in oak. Rev. Pathol. Veg. d’Entomol. Agric. Fr. 1949, 39, 172–182. [Google Scholar]
- Hartmann, G.; Blank, R. Winter frost, insect defoliation and Agrilus biguttatus Fabr. as causal factors of oak decline in northern Germany. Forst Holtz 1992, 47, 443–452. [Google Scholar]
- Kowalski, T. Oak decline: I. Fungi associated with various disease symptoms on overground portions of middle-aged and old oak (Quercus robur L.). Eur. J. For. Pathol. 1991, 21, 136–151. [Google Scholar] [CrossRef]
- Kehr, R.D.; Wulf, A. Fungi associated with above-ground portions of declining oaks (Quercus robur) in Germany. Eur. J. For. Pathol. 1993, 23, 18–27. [Google Scholar] [CrossRef]
- Kaus, A.; Schmitt, V.; Simon, A.; Wild, A. Microscopical and mycological investigations on wood of pendunculate oak (Quercus robur L.) relative to the occurrence of oak decline. J. Plant Physiol. 1996, 148, 302–308. [Google Scholar] [CrossRef]
- Moraal, L.; Hilszczanski, J. The oak buprestid beetle (Agrilus biguttatus) (F.) (Col., buprestidae), a recent factor in oak decline in Europe. J. Pest Sci. 2000, 73, 134–138. [Google Scholar]
- Dunn, J.P.; Potter, D.A.; Kimmerer, T.W. Carbohydrate reserves, radial growth, and mechanisms of resistance of oak trees to phloem-boring insects. Oecologia 1990, 83, 458–468. [Google Scholar] [CrossRef]
- Crook, D.J.; Khrimian, A.; Francese, J.A.; Fraser, I.; Poland, T.M.; Sawyer, A.J.; Mastro, V.C. Development of a host-based semiochemical lure for trapping emerald ash borer Agrilus planipennis (Coleoptera: Buprestidae). Environ. Entomol. 2008, 37, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Crook, D.J.; Mastro, V.C. Chemical ecology of the emerald ash borer Agrilus planipennis. J. Chem. Ecol. 2010, 36, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Vuts, J.; Woodcock, C.M.; Sumner, M.E.; Caulfield, J.C.; Reed, K.; Inward, D.J.G.; Leather, S.R.; Pickett, J.A.; Birkett, M.A.; Denman, S. Responses of the two-spotted oak buprestid, Agrilus biguttatus (Coleoptera: Buprestidae), to host tree volatiles. Pest Manag. Sci. 2016, 72, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Haack, R.A.; Jendak, E.; Houping, L.; Marchant, K.R.; Petrice, T.R.; Poland, T.M.; Ye, H. The emerald ash borer: A new exotic pest in North America. Newsl. Mich. Entomol. Soc. 2002, 47, 1–5. [Google Scholar]
- Coleman, T.W.; Seybold, S.J. Previously unrecorded damage to oak, Quercus spp., in southern California by the goldspotted oak borer, Agrilus coxalis Waterhouse (Coleoptera: Buprestidae). Pan-Pac. Entomol. 2008, 84, 288–300. [Google Scholar] [CrossRef]
- Flower, C.E.; Knight, K.S.; Rebbeck, J.; Gonzalez-Meler, M.A. The relationship between the emerald ash borer (Agrilus planipennis) and ash (Fraxinus spp.) tree decline: Using visual canopy condition assessments and leaf isotope measurements to assess pest damage. For. Ecol. Manag. 2013, 303, 143–147. [Google Scholar] [CrossRef]
- Knight, K.S.; Brown, J.P.; Long, R.P. Factors affecting the survival of ash (Fraxinus spp.) trees infested by emerald ash borer (Agrilus planipennis). Biol. Invasions 2013, 15, 371–383. [Google Scholar] [CrossRef]
- Leach, J.G. Insect Transmission of Plant Diseases; McGraw-Hill: New York, NY, USA, 1940. [Google Scholar]
- Al Adawi, A.O.; Al Jabri, R.M.; Deadman, M.L.; Barnes, I.; Wingfield, B.; Wingfield, M.J. The mango sudden decline pathogen, Ceratocystis manginecans, is vectored by Hypocryphalus mangiferae (Coleoptera: Scolytinae) in Oman. Eur. J. Plant Pathol. 2013, 135, 243–251. [Google Scholar] [CrossRef]
- Jewell, N.P. Statistics for Epidemiology; Chapman & Hall: Boca Raton, FL, USA, 2004. [Google Scholar]
- Wiegand, T.; Moloney, K. Rings, circles, and null models for point pattern analysis in ecology. Oikos 2004, 104, 209–229. [Google Scholar] [CrossRef]
- Xu, X.; Hu, X. Distance-based statistics for analysing spatial point pattern data. In Exercises in Plant Disease Epidemiology; Stevenson, K.L., Jeger, M., Eds.; APS Press: St. Paul, MN, USA, 2015; pp. 59–64. [Google Scholar]
- Ripley, B. Spatial Statistics; John Wiley & Sons: New York, NY, USA, 1981. [Google Scholar]
- Xu, X.; Harwood, T.D.; Pautasso, M.; Jeger, M.J. Spatio-temporal analysis of an invasive plant pathogen (Phytophthora ramorum) in England and Wales. Ecography 2009, 32, 504–516. [Google Scholar] [CrossRef]
- Wiegand, T. User Manual for the Programita Software; Helmholtz Centre for Environmental Research—UFZ: Leipzig, Germany, 2014. [Google Scholar]
- Baddeley, A.B.; Diggle, P.J.; Hardegen, A.; Lawrence, T.; Milne, R.K. On tests of spatial pattern based on simulation envelopes. Ecol. Monogr. 2014, 84, 477–489. [Google Scholar] [CrossRef]
- Wiegand, T.; Grabarnik, P.; Stoyan, D. Envelope tests for spatial point patterns with and without simulation. Ecosphere 2016, 7, 1–18. [Google Scholar] [CrossRef]
- Francese, J.; Oliver, J.B.; Fraser, I.; Lance, D.R.; Youssef, N.; Sawyer, A.J.; Mastro, V.C. Influence of trap placement and design on capture of the emerald ash borer (Coleoptera: Buprestidae). J. Econ. Entomol. 2008, 101, 1831–1837. [Google Scholar] [CrossRef] [PubMed]
- Siegert, N.W.; Mercader, R.J.; McCullough, D.G. Spread and dispersal of emerald ash borer (Coleoptera: Buprestidae): Estimating the spatial dynamics of a difficult-to-detect invasive forest pest. Can. Entomol. 2015, 147, 338–348. [Google Scholar] [CrossRef]
- Kovacs, K.F.; Haight, R.G.; McCullough, D.G.; Mercader, R.J.; Siegert, N.W.; Liebhold, A.M. Cost of potential emerald ash borer damage in U.S. communities, 2009–2019. Ecol. Econ. 2010, 69, 569–578. [Google Scholar] [CrossRef]
- Francese, J.A.; Crook, D.J.; Lance, D.R.; Sawyer, A.J.; Mastro, V.C. Optimization of trap color for emerald ash borer (Coleoptera: Buprestidae). J. Econ. Entomol. 2010, 103, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Bily, S. The Buprestidae (Coleoptera) of Fennoscandia and Denmark; Scandinavian Science Press: Klampenborg, Denmark, 1982. [Google Scholar]
- Habermann, M.; Preller, J. Studies on the biology and control of two-spotted lichen buprestid (Agrilus biguttatus Fabr.). Forst Holtz 2003, 58, 215–220. [Google Scholar]
- Jennings, D.E.; Duan, J.J.; Shrewsbury, P.M. Biotic mortality factors affecting emerald ash borer (Agrilus planipennis) are highly dependent on life stage and host tree crown condition. Bull. Entomol. Res. 2015, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Haavik, L.J.; Billings, S.A.; Guldin, J.M.; Stephen, F.M. Emergent insects, pathogens and drought shape changing patterns in oak decline in North America and Europe. For. Ecol. Manag. 2015, 354, 190–205. [Google Scholar] [CrossRef]
- Villari, C.; Herms, D.A.; Whitehill, J.G.A.; Cipollini, D.; Bonello, P. Progress and gaps in understanding mechanisms of ash tree resistance to emerald ash borer, a model for wood-boring insects that kill angiosperms. New Phytol. 2016, 209, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Whitehill, J.G.A.; Hill, A.L.; Opiyo, S.O.; Cipollini, D.; Herms, D.A.; Bonello, P. Effects of water availability on emerald ash borer larval performance and phloem phenolics of Manchurian and black ash. Plant Cell Environ. 2014, 37, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Haavik, L.J.; Flint, M.L.; Coleman, T.W.; Venette, R.C.; Seybold, S.J. Goldspotted oak borer effects on tree health and colonization patterns at six newly-established sites. Agric. For. Entomol. 2015, 17, 147–157. [Google Scholar] [CrossRef]
- James, T. Agrilus sulcicollis Lacordaire (Buprestidae) a jewel beetle new to Britain. Coleopterist 1994, 3, 33–35. [Google Scholar]
- Coleman, T.W.; Chen, Y.; Graves, A.D.; Hishinuma, S.M.; Grulke, N.E.; Flint, M.L.; Seybold, S.J. Developing monitoring techniques for the invasive goldspotted oak borer (Coleoptera: Buprestidae) in California. Environ. Entomol. 2014, 43, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Crook, D.J.; Francese, J.; Zylstra, K.E.; Fraser, I.; Sawyer, A.J.; Bartels, D.W.; Lance, D.R.; Mastro, V.C. Laboratory and field response of the emerald ash borer (Coleoptera: Buprestidae), to selected regions of the electromagnetic spectrum. J. Econ. Entomol. 2009, 102, 2160–2169. [Google Scholar] [CrossRef] [PubMed]
- Francese, J.; Fraser, I.; Rietz, M.L.; Crook, D.J.; Lance, D.R.; Mastro, V.C. Relation of color, size, and canopy placement of prism traps in determining capture of emerald ash borer (Coleoptera: Buprestidae). Can. Entomol. 2010, 142, 596–600. [Google Scholar] [CrossRef]
- Domingue, M.J.; Imrei, Z.; Lelito, J.P.; Muskovits, J.; Janik, G.; Csoka, G.; Mastro, V.C.; Baker, T.C. Trapping of European buprestid beetles in oak forests using visual and olfactory cues. Entomol. Exp. Appl. 2013, 148, 116–129. [Google Scholar] [CrossRef]
- Wermelinger, B.; Flueckiger, P.F.; Obrist, M.K.; Duelli, P. Horizontal and vertical distribution of saproxylic beetles (Col., Buprestidae, Cerambycidae, Scolytinae) across sections of forest edges. J. Appl. Entomol. 2007, 131, 104–114. [Google Scholar] [CrossRef]
- Allen, A.A. Notes on Agrilus pannonicus Pill. & Mitt. (Col.: Buprestidae) in 1985. Entomol. Rec. J. Var. 1988, 100, 25–28. [Google Scholar]
- Domingue, M.J.; Csoka, G.; Toth, M.; Vetek, G.; Penzes, B.; Mastro, V.; Baker, T.C. Field observations of visual attraction of three European oak buprestid beetles toward conspecific and heterospecific models. Entomol. Exp. Appl. 2011, 140, 112–121. [Google Scholar] [CrossRef]
- Gibbs, J.N.; Greig, B.J.W. Biotic and abiotic factors affecting the dying back of pedunculate oak Quercus robur L. Forestry 1997, 70, 399–406. [Google Scholar] [CrossRef]
| Year Study Began | Site | Oak Species | Mean Oak Diameter at 1.3 m (±SD) (cm) | Number of Live Study Oak | Density of Oak (Stems/ha) | Density of Tree Cover (All Stems/ha) |
|---|---|---|---|---|---|---|
| 2009 | Hatchlands | Q. robur Q. petraea Q. cerris | 84.63 (±26.59) | 140 | 6.14 | 8.02 |
| Langdale Wood | Q. robur Q. cerris | 66.00 (±10.58) | 260 | 30.95 | 31.79 | |
| Sandpit Wood | Q. robur | 56.68 (±17.84) | 162 | 114.39 | 132.75 | |
| Winding wood | Q. robur | 56.21 (±13.54) | 201 | 87.12 | 141.74 | |
| 2010 | Beecham Spinney | Q. robur Q. cerris | 41.19 (±18.13) | 186 | 84.48 | 239.37 |
| Great Monks Wood | Q. robur Q. cerris | 60.91 (±16.08) | 145 | 43.85 | 58.98 | |
| Rookery Wood | Q. robur | 55.91 (±38.33) | 115 | 78.50 | 133.11 | |
| Sheen Wood | Q. robur | 76.96 (±19.26) | 152 | 38.32 | 40.84 |
| Year | Prior Condition | New EH | Susceptible | Conditional Probability | RR | CI− 95% | CI+ 95% |
|---|---|---|---|---|---|---|---|
| 2010 | With stem bleeds | 21 | 171 | 0.122 | 13.98 | 5.34 | 36.56 |
| No symptoms | 5 | 569 | 0.009 | ||||
| 2011 | With stem bleeds | 13 | 216 | 0.060 | 3.78 | 1.84 | 7.74 |
| No symptoms | 16 | 1004 | 0.016 | ||||
| 2012 | With stem bleeds | 18 | 213 | 0.085 | 4.76 | 2.49 | 9.09 |
| No symptoms | 17 | 958 | 0.018 |
| Site | A. biguttatus | A. sulcicollis | A. laticornis | A. angustulus |
|---|---|---|---|---|
| Essex (site 1), 2010 | 24 | 51 | 123 | 5 |
| Essex (site 2), 2011 | 4 | 7 | 1 | 0 |
| Shropshire (site 1), 2011 | 1 | 0 | 1 | 0 |
| Shropshire (site 2), 2011 | 46 | 0 | 1 | 1 |
| Suffolk (site 1), 2011 | 4 | 23 | 3 | 0 |
| Suffolk (site 2), 2011 | 36 | 6 | 0 | 0 |
| 2010 Colour Trial | 2011 Experiment | ||||
|---|---|---|---|---|---|
| Factor | Mean | SE | Factor | Mean | SE |
| Black | 0.25 | 0.24 | Edge | 3.90 | 0.55 |
| Red | 0.63 | 0.3 | Wood | 0.65 | 0.21 |
| Blue | 0.63 | 0.3 | |||
| Green | 0 | 0 | 3 m | 2.80 | 0.54 |
| Purple | 1.5 | 0.4 | 6 m | 1.75 | 0.38 |
| 3 m | 0.92 | 0.21 | Lure | 2.25 | 0.51 |
| 10 m | 0.88 | 0.21 | No Lure | 2.30 | 0.45 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, N.; Jeger, M.; Kirk, S.; Williams, D.; Xu, X.; Pautasso, M.; Denman, S. Acute Oak Decline and Agrilus biguttatus: The Co-Occurrence of Stem Bleeding and D-Shaped Emergence Holes in Great Britain. Forests 2017, 8, 87. https://doi.org/10.3390/f8030087
Brown N, Jeger M, Kirk S, Williams D, Xu X, Pautasso M, Denman S. Acute Oak Decline and Agrilus biguttatus: The Co-Occurrence of Stem Bleeding and D-Shaped Emergence Holes in Great Britain. Forests. 2017; 8(3):87. https://doi.org/10.3390/f8030087
Chicago/Turabian StyleBrown, Nathan, Mike Jeger, Susan Kirk, David Williams, Xiangming Xu, Marco Pautasso, and Sandra Denman. 2017. "Acute Oak Decline and Agrilus biguttatus: The Co-Occurrence of Stem Bleeding and D-Shaped Emergence Holes in Great Britain" Forests 8, no. 3: 87. https://doi.org/10.3390/f8030087
APA StyleBrown, N., Jeger, M., Kirk, S., Williams, D., Xu, X., Pautasso, M., & Denman, S. (2017). Acute Oak Decline and Agrilus biguttatus: The Co-Occurrence of Stem Bleeding and D-Shaped Emergence Holes in Great Britain. Forests, 8(3), 87. https://doi.org/10.3390/f8030087







