Phenotypical and Molecular Characterisation of Fusarium circinatum: Correlation with Virulence and Fungicide Sensitivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultural, Morphological, and Molecular Characterization
2.2. Temperature-Growth Response
2.3. Virulence Tests
2.4. Fungicide Sensitivity
2.5. Statistical Methods
3. Results
3.1. Cultural, Morphological and Growth Rate Results
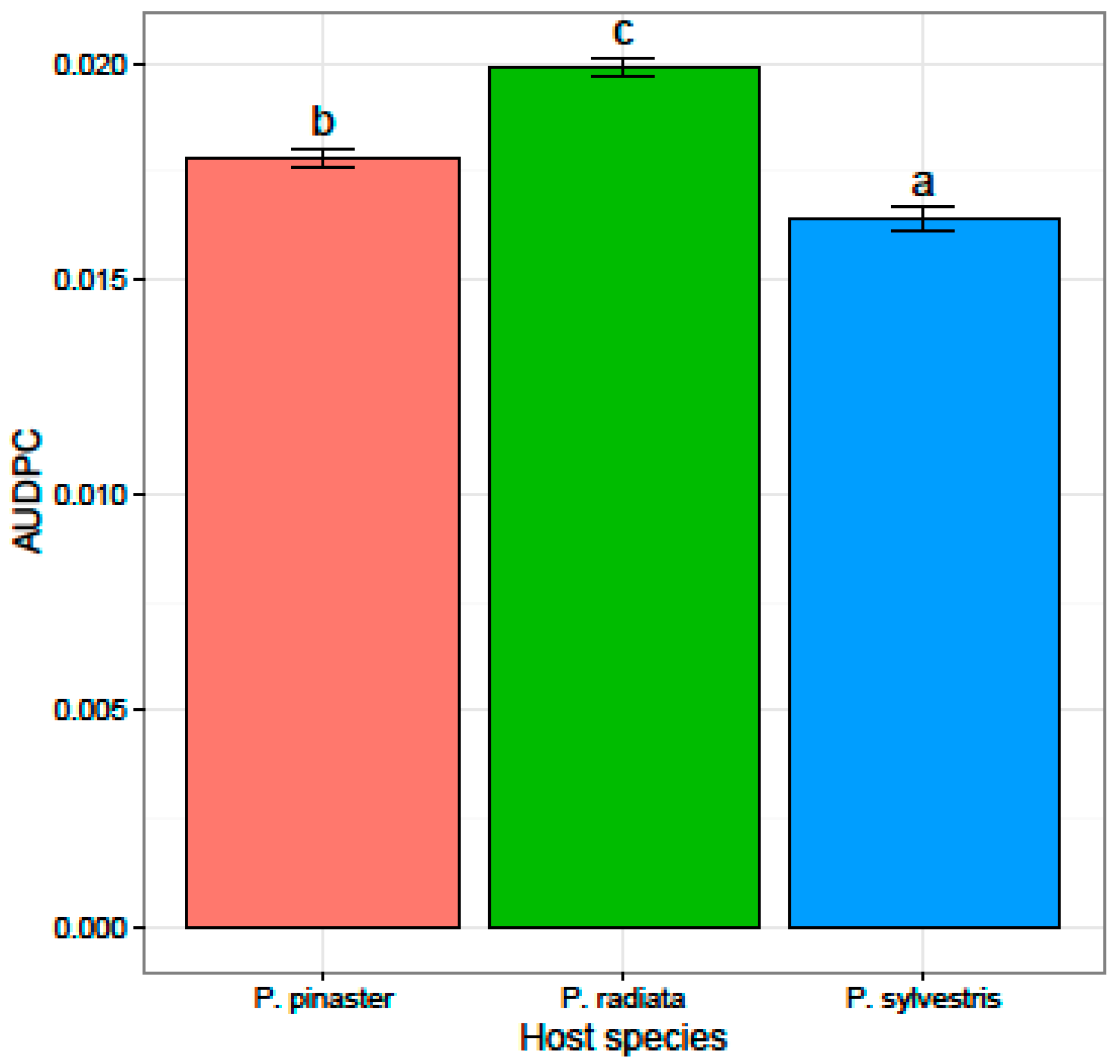
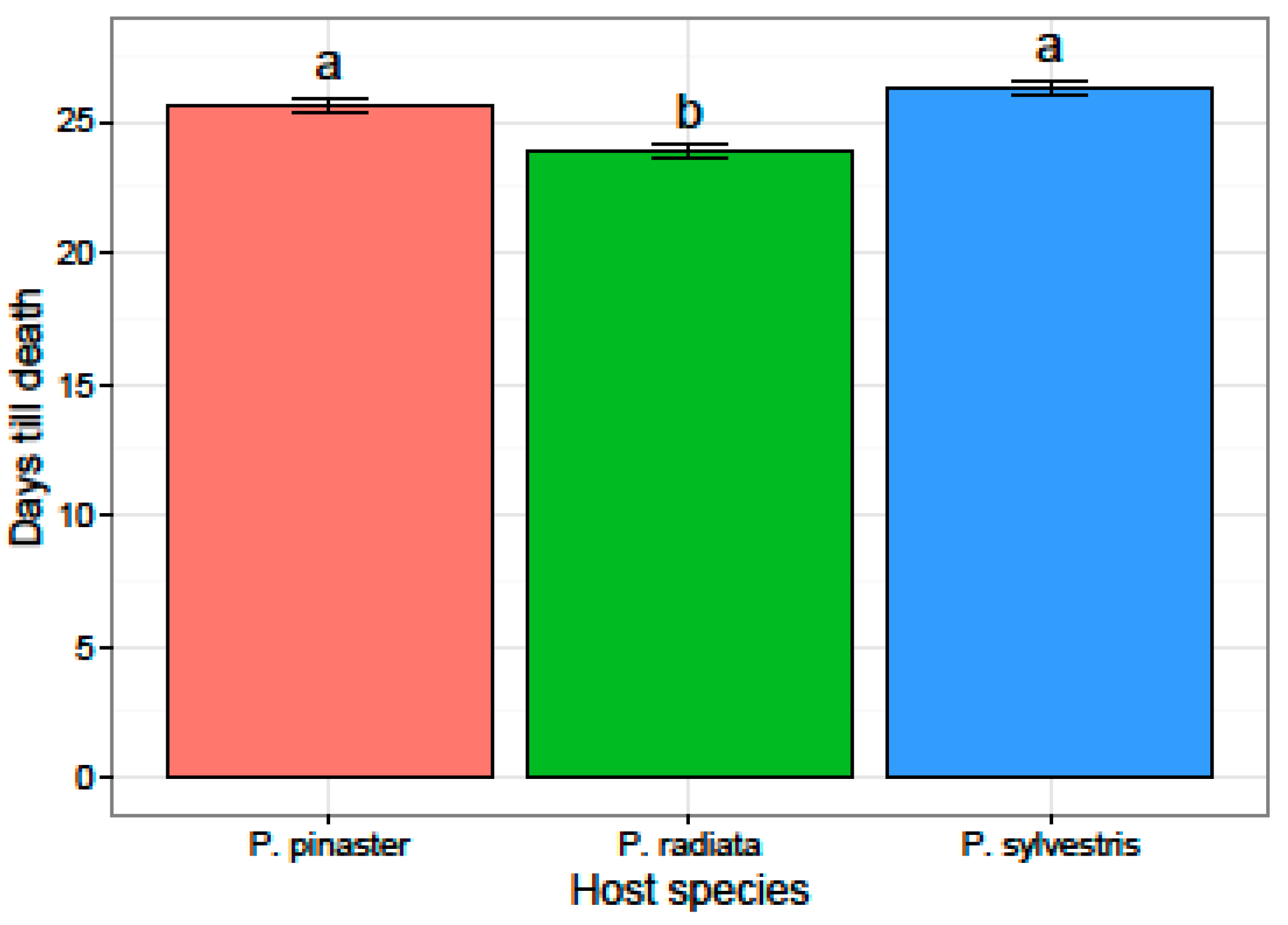
3.2. Virulence and Fungicide Sensitivity Tests
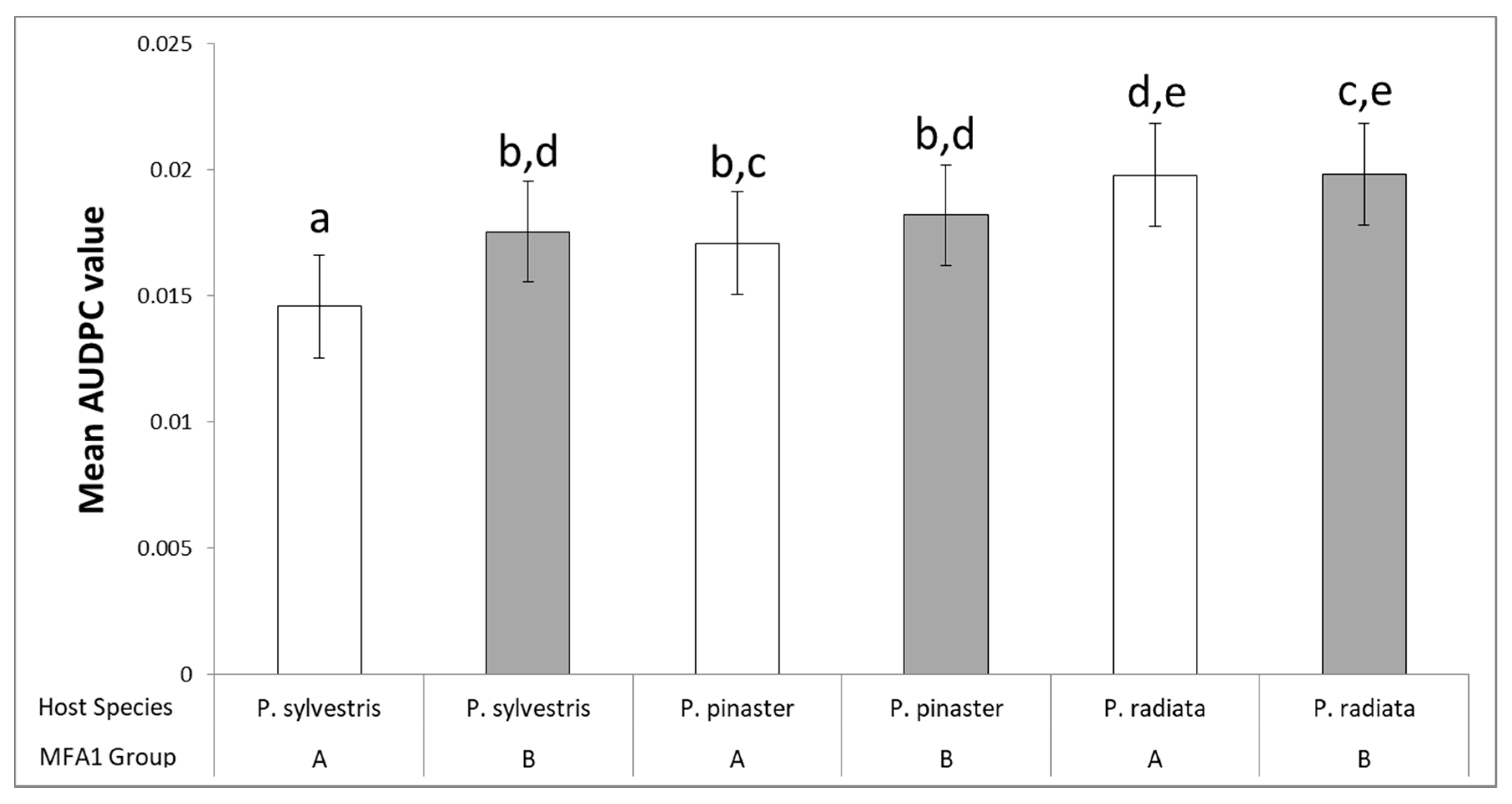
3.3. Clustering Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Isolate Code | Host Species | Country | Area | Colony Colour 2 | Colony Growth Rate 3 | Sterile Hyphae Morphology 4 | Mating Type | Detailed Growth Rates | MLG 5 | DAPC 6 Cluster | MFA1 7 Group | MFA2 7 Group | Fungicide Sensitivity Tests | Virulence Tests |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M-4058 | Pinus strobus | Canada | Ontario | W | F | A | 1 | - | 67 | nd | na | na | + | |
| 2010-1038213985 | Pinus radiata | Chile | Constitución | WP | F | A | 2 | + | 9 | 4 | A | E | + | |
| 2010-1249816808 | Pinus radiata | Chile | Constitución | P | F | A | 2 | + | 9 | 4 | A | E | ||
| 3549451339 | Pinus radiata | Chile | Curicó | WP | F | A | 2 | + | 9 | 4 | A | E | ||
| 2010-1454319498 | Pinus radiata | Chile | Linares | WP | F | A | 2 | + | 9 | 4 | A | E | ||
| 2010-1308417545 | Pinus radiata | Chile | Parral | P | F | A | 2 | + | 9 | 4 | A | E | ||
| 441463764 | Pinus radiata | Chile | Santa Cruz | P | F | A | 2 | + | 9 | 4 | A | E | ||
| 4246161911 | Pinus radiata | Chile | Valdivia | P | F | A | 2 | + | 13 | 4 | A | E | ||
| LNPV217 | Pinus radiata | France | Cote d’armor | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| LNPV211 | Pinus sp. | France | Perpignan | W | F | C | 1 | + | 24 | 2 | B | C | ||
| LNPV216 | Pinus radiata | France | Vendée | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| NRRL29945 MAFF237756 | Pinus luchuensis | Japan | Amami Ohshima | WPC | F | A | 1 | + | 39 | 5 | B | D | ||
| NRRL26431 MAFF 236397 | Pinus luchuensis | Japan | Kagoshima | W | F | C | 1 | + | 38 | nd | B | na | + | |
| MAFF239859 | Pinus luchuensis | Japan | Okinawa | WPC | F | A | 1 | + | 38 | nd | B | na | ||
| NRRL26432 MAFF236399 | Pinus luchuensis | Japan | Okinawa | WP | F | A | 1 | + | 39 | 5 | A | D | ||
| E2 | Pinus greggii | Mexico | Eastern Mexico | WPC | F | C | 2 | + | 9 | 4 | A | E | + | |
| A5 | Pinus patula | Mexico | Eastern Mexico | WY | F | C | 1 | + | 10 | 4 | B | B | ||
| JAL03 | Pinus douglasiana | Mexico | Jalisco | WP | F | C | 1 | + | 50 | 4 | B | E | ||
| L-J | Pinus leiophylla | Mexico | Michoacan | WY | F | C | 1 | + | 14 | 4 | B | B | ||
| Teo1 | Pinus teocote | Mexico | Michoacan | WPC | F | C | 1 | + | 7 | 4 | B | E | ||
| Teo3 | Pinus teocote | Mexico | Michoacan | WP | F | C | 2 | + | 8 | 4 | A | E | ||
| 264 | Pinus halepensis | Portugal | Portugal | WP | F | C | 1 | + | 62 | 1 | B | A | ||
| 275 | Pinus halepensis | Portugal | Portugal | W | F | C | 1 | + | 59 | 1 | B | A | ||
| 276 | Pinus nigra | Portugal | Portugal | W | F | C | 1 | + | 60 | 1 | B | A | ||
| 236 | Pinus pinaster | Portugal | Portugal | WP | F | C | 1 | + | 60 | 1 | B | A | ||
| 240 | Pinus radiata | Portugal | Portugal | W | F | C | 1 | + | 66 | 1 | B | A | + | |
| 252 | Pinus radiata | Portugal | Portugal | W | F | C | 1 | + | 62 | 1 | B | A | ||
| 273 | Pinus radiata | Portugal | Portugal | WP | F | C | 1 | + | 65 | 1 | B | A | ||
| 274 | Pinus radiata | Portugal | Portugal | W | F | C | 1 | + | 64 | 1 | B | A | ||
| FCC0497 K47 9 | Pinus sp. | South Africa | Mpumalanga Ngodwana | WPC | F | C | 2 | + | 12 | 4 | B | E | ||
| CBS119864 | Pinus patula | South Africa | South Africa | W | F | C | 1 | + | 3 | 4 | B | E | + | |
| CBS119865 | Pinus patula | South Africa | South Africa | WY | F | NC | 2 | + | 4 | 4 | A | E | + | + |
| CMWF10 FCC309 K203 5 | Pinus patula | South Africa | South Africa | WPC | F | C | 1 | + | 16 | 2 | B | C | ||
| NRRL25333 M-8575 | Pinus patula | South Africa | South Africa | WP | F | NC | 2 | + | 4 | 4 | A | E | + | |
| NRRL25621 CMWF7 FCC140 MAFF240075 | Pinus patula | South Africa | South Africa | WP | F | C | 2 | + | 26 | 2 | A | C | ||
| CMWF674 KS17 4 | Pinus radiata | South Africa | South Africa | WP | F | C | 1 | + | 2 | 2 | B | C | ||
| CMWF23 FCC514 K43 8 | Pinus sp. | South Africa | South Africa | WP | F | C | 2 | + | 28 | 2 | A | C | ||
| CMWF31 FCC133 10 | Pinus sp. | South Africa | South Africa | WPC | F | C | 2 | + | 1 | 2 | B | C | ||
| CMWF35 FCC124 K42 7 | Pinus sp. | South Africa | South Africa | WPC | F | C | 1 | + | 6 | 2 | B | C | ||
| CMWF498 FCC116 FGSC9023 2 | Pinus sp. | South Africa | South Africa | WP | F | C | 2 | + | 4 | 4 | A | E | ||
| 636/06-1 | Pinus nigra | Spain | Asturias | WY | F | C | 1 | + | 62 | 1 | B | B | ||
| 310/06-1 | Pinus palustris | Spain | Asturias | WPC | F | C | 1 | + | 43 | 5 | B | D | + | |
| 104 | Pinus pinaster | Spain | Asturias | W | S | C | 1 | + | 60 | 1 | B | A | + | |
| 125 | Pinus pinaster | Spain | Asturias | WP | F | C | 1 | + | 66 | 1 | B | A | ||
| 129 | Pinus pinaster | Spain | Asturias | WP | S | C | 1 | + | 52 | 1 | B | A | ||
| 165 | Pinus pinaster | Spain | Asturias | WP | S | C | 1 | + | 59 | 1 | B | A | ||
| 182 | Pinus pinaster | Spain | Asturias | nd | nd | nd | 2 | - | nd | nd | na | na | + | |
| 202 | Pinus pinaster | Spain | Asturias | nd | nd | nd | 2 | - | nd | nd | na | na | + | |
| 215 | Pinus pinaster | Spain | Asturias | WP | S | NC | 2 | + | 32 | 3 | A | F | ||
| 217 | Pinus pinaster | Spain | Asturias | WPC | F | C | 1 | + | 59 | 1 | B | A | + | |
| 07/0070-1 | Pinus pinaster | Spain | Asturias | WP | F | C | 1 | + | 59 | 1 | B | A | ||
| 07/0649-1a | Pinus pinaster | Spain | Asturias | WPC | S | C | 1 | + | 66 | 1 | B | A | ||
| 07/0649-1b | Pinus pinaster | Spain | Asturias | WP | F | C | 1 | - | 66 | 1 | na | na | + | |
| 488/06 | Pinus pinaster | Spain | Asturias | WP | S | C | 1 | + | 59 | 1 | B | A | ||
| 72 | Pinus radiata | Spain | Asturias | WPC | F | C | 1 | + | 59 | 1 | B | A | ||
| 96 | Pinus radiata | Spain | Asturias | WPC | F | C | 1 | + | 49 | 1 | B | A | ||
| 122 | Pinus radiata | Spain | Asturias | WP | F | NC | 2 | - | 62 | 1 | na | na | + | |
| 137 | Pinus radiata | Spain | Asturias | WP | S | C | 1 | + | 59 | 1 | B | A | ||
| 160 | Pinus radiata | Spain | Asturias | WP | F | C | 1 | + | 59 | 1 | B | A | + | |
| 161 | Pinus radiata | Spain | Asturias | W | F | C | 1 | + | 59 | 1 | B | A | ||
| 214 | Pinus radiata | Spain | Asturias | WPC | S | C | 1 | + | 59 | 1 | B | A | ||
| 229 | Pinus radiata | Spain | Asturias | WP | F | C | 1 | + | 63 | 1 | B | A | + | |
| 244 | Pinus radiata | Spain | Asturias | W | F | C | 1 | + | 59 | 1 | B | A | ||
| 07/0531-1 | Pinus radiata | Spain | Asturias | WP | S | NC | 2 | + | 32 | 3 | A | F | ||
| 07/0650-1 | Pinus radiata | Spain | Asturias | WPC | F | C | 1 | + | 66 | 1 | B | A | ||
| 07/0650-2 | Pinus radiata | Spain | Asturias | WY | F | C | 1 | + | 59 | 1 | B | B | ||
| 487/06 1 | Pinus radiata | Spain | Asturias | WP | F | C | 1 | + | 59 | 1 | B | A | ||
| 499/06-1 | Pinus radiata | Spain | Asturias | WY | F | C | 1 | + | 64 | 1 | B | B | ||
| 639/06-1 | Pinus radiata | Spain | Asturias | WPC | S | C | 1 | + | 62 | 1 | B | A | ||
| 639/06-2 | Pinus radiata | Spain | Asturias | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| 639/06-7 | Pinus radiata | Spain | Asturias | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| 700/05-2 | Pinus radiata | Spain | Asturias | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| 164 | Pinus sylvestris | Spain | Asturias | W | F | C | 1 | + | 59 | 1 | B | A | + | + |
| 524/06-2 | Pseudotsuga | Spain | Asturias | WP | F | C | 1 | + | 59 | 1 | B | A | ||
| 433 | Pinus nigra subsp. corsicana | Spain | Cantabria | WP | F | NC | 2 | + | 32 | 3 | A | F | + | |
| 76 | Pinus radiata | Spain | Cantabria | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| 194 | Pinus radiata | Spain | Cantabria | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| 221 | Pinus radiata | Spain | Cantabria | WP | F | NC | 2 | + | 32 | 3 | A | F | + | |
| 430 | Pinus radiata | Spain | Cantabria | WP | F | NC | 2 | + | 32 | 3 | A | F | + | |
| 431 | Pinus radiata | Spain | Cantabria | WP | F | NC | 2 | + | 32 | 3 | A | F | + | |
| 434 | Pinus radiata | Spain | Cantabria | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| 435 | Pinus radiata | Spain | Cantabria | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| 437 | Pinus radiata | Spain | Cantabria | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| 438 | Pinus radiata | Spain | Cantabria | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| 439 | Pinus radiata | Spain | Cantabria | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| 441 | Pinus radiata | Spain | Cantabria | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| 442 | Pinus radiata | Spain | Cantabria | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| 443 | Pinus radiata | Spain | Cantabria | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| 444 | Pinus radiata | Spain | Cantabria | WPC | F | A | 2 | + | 32 | 3 | A | E | ||
| 445 | Pinus radiata | Spain | Cantabria | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| 448 | Pinus radiata | Spain | Cantabria | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| 450 | Pinus radiata | Spain | Cantabria | WPC | S | NC | 2 | + | 32 | 3 | A | F | ||
| 452 | Pinus radiata | Spain | Cantabria | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| 723 | Pinus radiata | Spain | Cantabria | WPC | F | C | 1 | + | 58 | 1 | B | A | ||
| 1894-08 | Pinus radiata | Spain | Cantabria | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| 1896-08 | Pinus radiata | Spain | Cantabria | WPC | F | NC | 2 | + | 32 | 3 | A | F | ||
| 1900-08 | Pinus radiata | Spain | Cantabria | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| 389 | Pinus nigra | Spain | Castilla León | WP | S | C | 1 | + | 53 | 1 | B | A | + | |
| 453 | Pinus nigra | Spain | Castilla León | WPC | F | NC | 2 | + | 32 | 3 | A | F | + | |
| 649 | Pinus nigra | Spain | Castilla León | WPC | F | C | 1 | + | 61 | 1 | B | A | + | |
| 678 | Pinus nigra | Spain | Castilla León | WPC | F | C | 1 | + | 59 | 1 | B | A | + | |
| 821 | Pinus nigra | Spain | Castilla León | WY | S | C | 1 | + | 59 | 1 | B | B | ||
| 729 | Pinus pinea | Spain | Castilla León | WPC | F | C | 1 | + | 62 | 1 | B | A | ||
| 810 | Pinus pinea | Spain | Castilla León | WY | F | C | 1 | + | 59 | 1 | B | B | ||
| 623 | Pinus radiata | Spain | Castilla León | WP | F | NC | 2 | + | 32 | 3 | A | F | + | |
| 625 | Pinus radiata | Spain | Castilla León | WP | F | NC | 2 | - | 32 | 3 | na | na | + | |
| 982 | Pinus radiata | Spain | Castilla León | WP | F | C | 1 | + | 59 | 1 | B | A | ||
| 985 | Pinus radiata | Spain | Castilla León | WPC | F | C | 1 | + | 51 | 1 | B | A | ||
| 390 | Pinus sylvestris | Spain | Castilla León | WPC | F | NC | 2 | + | 32 | 3 | A | F | + | |
| 116 | Pinus nigra | Spain | Galicia | WP | F | NC | 2 | + | 32 | 3 | A | F | + | |
| 250 | Pinus nigra | Spain | Galicia | WPC | F | NC | 2 | - | 32 | 3 | na | na | + | |
| 253 | Pinus nigra | Spain | Galicia | WPC | L | C | 1 | + | 62 | 1 | B | A | + | + |
| 255 | Pinus nigra | Spain | Galicia | W | F | C | 1 | - | 62 | 1 | na | na | + | |
| 822 | Pinus pinaster | Spain | Galicia | WP | S | C | 1 | + | 62 | 1 | B | A | + | + |
| 823 | Pinus pinaster | Spain | Galicia | WP | F | C | 1 | + | 41 | 5 | B | D | ||
| 825 | Pinus pinaster | Spain | Galicia | W | F | C | 1 | + | 59 | 1 | B | A | + | + |
| 827 | Pinus pinaster | Spain | Galicia | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| 828 | Pinus pinaster | Spain | Galicia | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| 829 | Pinus pinaster | Spain | Galicia | WY | S | C | 1 | + | 60 | 1 | B | B | + | |
| 830 | Pinus pinaster | Spain | Galicia | WY | S | C | 1 | + | 59 | 1 | B | B | + | |
| 831 | Pinus pinaster | Spain | Galicia | WP | F | NC | 2 | + | 32 | 3 | A | F | + | |
| 100 | Pinus radiata | Spain | Galicia | nd | nd | nd | 1 | - | nd | nd | na | na | + | |
| G1 | Pinus radiata | Spain | Galicia | WY | F | C | 1 | + | 61 | 1 | B | B | ||
| M-8486 | Pinus radiata | Spain | País Vasco | WP | F | NC | 2 | + | 32 | 3 | A | F | + | |
| M-8487 | Pinus radiata | Spain | País Vasco | WP | F | NC | 2 | - | 32 | 3 | na | na | + | |
| pv1 | Pinus radiata | Spain | País Vasco | WP | F | NC | 2 | + | 32 | 3 | A | F | + | |
| pv14 | Pinus radiata | Spain | País Vasco | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| pv15 | Pinus radiata | Spain | País Vasco | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| pv2 | Pinus radiata | Spain | País Vasco | WP | F | NC | 2 | + | 32 | 3 | A | F | ||
| pv3 | Pinus radiata | Spain | País Vasco | WP | F | NC | 2 | + | 32 | 3 | A | F | + | |
| pv4 | Pinus radiata | Spain | País Vasco | WPC | F | NC | 2 | + | 32 | 3 | A | F | ||
| pv8 | Pinus radiata | Spain | País Vasco | WP | F | NC | 2 | + | 32 | 3 | A | F | + | |
| pv9 | Pinus radiata | Spain | País Vasco | P | F | NC | 2 | + | 32 | 3 | A | E | ||
| F1 2053 | Pinus taeda | Uruguay | Uruguay | WPC | F | C | 1 | + | 48 | nd | B | na | ||
| F1 2054 | Pinus taeda | Uruguay | Uruguay | WPC | F | C | 1 | + | 53 | 1 | B | A | ||
| F1 2186 | Pinus taeda | Uruguay | Uruguay | WPC | F | C | 1 | + | 47 | nd | B | na | ||
| F1 2187 | Pinus taeda | Uruguay | Uruguay | WPC | F | NC | 1 | + | 31 | 3 | B | F | ||
| D115 | Pinus virginiana | USA | Alabama | WPC | F | NC | 1 | + | 36 | 3 | B | F | ||
| M-3834 | Pinus radiata | USA | Berkeley, California | WPC | S | C | 1 | + | 24 | 2 | B | C | + | + |
| NRRL25331 M-8386 | Pinus radiata | USA | California | WP | S | C | 1 | - | 24 | 2 | na | na | + | + |
| CMWF350 FCC986 Fsp34 3 | Pinus sp. | USA | California | WP | F | C | 1 | + | 23 | 2 | B | C | ||
| FL102 | Pinus elliottii | USA | Florida | WP | F | C | 2 | + | 45 | 5 | A | D | ||
| FL3 | Pinus elliottii | USA | Florida | WPC | F | C | 1 | + | 46 | 5 | B | D | ||
| FL88 | Pinus elliottii | USA | Florida | WPC | F | C | 2 | + | 44 | 5 | B | D | ||
| M-1001 | Pinus elliottii | USA | Florida | WPC | S | C | 2 | + | 39 | 5 | B | D | + | |
| M-1025 | Pinus elliottii | USA | Florida | WP | F | C | 2 | + | 5 | 5 | A | D | ||
| M-1290 | Pinus elliottii | USA | Florida | WP | F | A | 1 | + | 39 | 5 | A | D | ||
| FK165 | Pinus elliottii | USA | Georgia | W | F | NC | 1 | + | 18 | 2 | B | C | ||
| M-0956 | Pinus elliottii | USA | Georgia | WPC | F | NC | 1 | + | 42 | 5 | B | D | ||
| M-0879 | Pinus palustris | USA | Georgia | WPC | F | C | 1 | + | 43 | 5 | B | D | ||
| FK867 | Pinus taeda | USA | Georgia | WPC | F | C | 2 | - | 17 | 3 | na | na | + | |
| M-0889 | Pinus taeda | USA | Georgia | WY | F | A | 2 | + | 34 | 3 | A | E | ||
| NRLL25332 MAFF240076 | Pinus taeda | USA | Georgia | WP | F | C | 2 | + | 17 | 3 | A | F | + | + |
| LA4 | Pinus radiata | USA | Los Angeles county, California | W | F | C | 1 | + | 29 | 2 | B | C | ||
| FSP606 | Pinus radiata | USA | Marin county, California | WP | F | C | 1 | + | 24 | 2 | B | C | ||
| M-0874 | Pinus taeda | USA | Mississippi | WPC | F | C | 1 | + | 54 | 1 | B | A | ||
| M-0887 | Pinus taeda | USA | Mississippi | WPC | F | C | 1 | + | 56 | 1 | B | A | ||
| FSP388 | Pinus radiata | USA | Monterey county, California | WP | F | NC | 1 | + | 43 | 5 | B | D | + | |
| FSP487 | Pinus radiata | USA | Monterey county, California | W | F | C | 1 | + | 25 | 2 | B | C | ||
| NRRL25707 MAFF240077 | Pinus caribaea | USA | North Carolina | WPC | F | NC | 1 | + | 19 | 2 | B | C | ||
| M-0873 | Pinus taeda | USA | North Carolina | WPC | F | NC | 1 | + | 22 | 4 | B | E | ||
| M-0912 | Pinus taeda | USA | North Carolina | WPC | S | NC | 1 | + | 31 | 3 | B | F | ||
| NRRL25708 MAFF 240078 | Pinus taeda | USA | North Carolina | WY | F | NC | 1 | + | 20 | 3 | B | B | ||
| FSP227 | Pinus radiata | USA | San Luis Obispo county, California | WP | F | C | 1 | + | 11 | 4 | B | E | ||
| FSP587 | Pinus radiata | USA | San Luis Obispo county, California | W | F | C | 2 | + | 30 | 4 | B | E | ||
| FSP607 | Pinus radiata | USA | Santa Cruz county, California | WP | F | C | 1 | + | 27 | 2 | B | C | ||
| FSP255 | Pinus radiata | USA | Sonoma county, California | W | F | C | 2 | + | 15 | 4 | B | E | ||
| FSP360 | Pinus radiata | USA | Sonoma county, California | WP | S | C | 1 | + | 39 | 5 | B | D | + | |
| M-1450 | Pinus virginiana | USA | South Carolina | WPC | F | NC | 2 | + | 21 | 4 | A | E | + | |
| FK313 | Pinus taeda | USA | Texas | WPC | F | C | 2 | + | 55 | 1 | B | A | ||
| M-1177 | Pinus taeda | USA | Texas | WPC | S | C | 1 | + | 35 | 3 | B | A | ||
| M-1061 | Pinus taeda | USA | USA | WY | F | C | 2 | + | 57 | 1 | B | B | ||
| M-1057 | Pinus virginiana | USA | USA | WPC | F | C | 1 | + | 37 | 3 | B | A | ||
| M-0875 | Pinus sp. | USA | Virginia | WP | F | NC | 2 | + | 33 | 3 | A | F |
References
- Martín-Rodrigues, N.; Espinel, S.; Sanchez-Zabala, J.; Ortíz, A.; González-Murua, C.; Duñabeitia, M.K. Spatial and temporal dynamics of the colonization of Pinus radiata by Fusarium circinatum, of conidiophora development in the pith and of traumatic resin duct formation. New Phytol. 2013, 198, 1215–1227. [Google Scholar] [CrossRef]
- Wingfield, M.J.; Hammerbacher, A.; Ganley, R.J.; Steenkamp, E.T.; Gordon, T.R.; Wingfield, B.D.; Coutinho, T.A. Pitch canker caused by Fusarium circinatum—A growing threat to pine plantations and forests worldwide. Australas. Plant Pathol. 2008, 37, 319–334. [Google Scholar] [CrossRef]
- Dwinell, L.D.; Barrows-Broaddus, J.; Kuhlman, E.G. Pitch Canker: A Disease Complex of Southern Pines. Plant Dis. 1985, 69, 270. [Google Scholar] [CrossRef]
- Viljoen, A.; Wingfield, M.J.; Marasas, W.F.O. First Report of Fusarium subglutinans f. sp. pini on Pine Seedlings in South Africa. Plant Dis. 1994, 78, 309–312. [Google Scholar] [CrossRef]
- Jacobs, A.; Coutinho, T.A.; Wingfield, M.J.; Ahumada, R.; Wingfield, B.D. Characterization of the pitch canker fungus, Fusarium circinatum, from Chile. S. Afr. J. Sci. 2007, 103, 253–257. [Google Scholar]
- European and Mediterranean Plant Protection Organization (EPPO) Global Database. Fusarium circinatum (GIBBCI). Available online: https://gd.eppo.int/taxon/GIBBCI/distribution (accessed on 7 October 2017).
- Ministerio de Agricultura, Pesca y Alimentación (MAPA). Informe de la Reunión Del Grupo de Trabajo de Laboratorio de Diagnóstico Y Prospecciones Fitosanitarias; Report; MAPA: Madrid, Spain, 1996. [Google Scholar]
- Dwinell, L.D.; Adams, D.; Guerra-Santos, J.J.; Aguirre, J.R.M. Pitch canker disease of Pinus radiata. In ICPP98—7th International Congress of Plant Pathology: Abstracts, Proceedings of the 7th International Congress of Plant Pathology, Edinburgh, UK, 9–16 August 1998; British Society for Plant Pathology: London, UK, 1998; pp. 9–16. Available online: http://www.bspp.org.uk/icpp98/3.7/30.html (accessed on 25 September 2017).
- Landeras, E.; García, P.; Fernández, Y.; Braña, M.; Fernández-Alonso, O.; Méndez-Lodos, S.; Pérez-Sierra, A.; León, M.; Abad-Campos, P.; Berbegal, M.; et al. Outbreak of Pitch Canker Caused by Fusarium circinatum on Pinus spp. in Northern Spain. Plant Dis. 2005, 89, 1015. [Google Scholar] [CrossRef]
- Pérez-Sierra, A.; Landeras, E.; León, M.; Berbegal, M.; García-Jiménez, J.; Armengol, J. Characterization of Fusarium circinatum from Pinus spp. in northern Spain. Mycol. Res. 2007, 111, 832–839. [Google Scholar] [CrossRef]
- Carlucci, A.; Colatruglio, L.; Frisullo, S. First Report of Pitch Canker Caused by Fusarium circinatum on Pinus halepensis and P. pinea in Apulia (Southern Italy). Plant Dis. 2007, 91, 1683. [Google Scholar] [CrossRef]
- Bragança, H.; Diogo, E.; Moniz, F.; Amaro, P. First Report of Pitch Canker on Pines Caused by Fusarium circinatum in Portugal. Plant Dis. 2009, 93, 1079. [Google Scholar] [CrossRef]
- EPPO PQR—EPPO Database on Quarantine Pests. Available online: http://www.eppo.int (accessed on 25 September 2017).
- Berbegal, M.; Pérez-Sierra, A.; Armengol, J.; Grünwald, N.J. Evidence for multiple introductions and clonality in Spanish populations of Fusarium circinatum. Phytopathology 2013, 103, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.R.; Okamoto, D.; Storer, A.J.; Wood, D.L. Susceptibility of five landscape pines to pitch canker disease, caused by Fusarium subglutinans f. sp. pini. HortScience 1998, 33, 868–871. [Google Scholar]
- Hodge, G.R.; Dvorak, W.S. Differential responses of Central American and Mexican pine species and Pinus radiata to infection by the pitch canker fungus. New For. 2000, 19, 241–258. [Google Scholar] [CrossRef]
- Roux, J.; Eisenberg, B.; Kanzler, A.; Nel, A.; Coetzee, V.; Kietzka, E.; Wingfield, M.J. Testing of selected South African Pinus hybrids and families for tolerance to the pitch canker pathogen, Fusarium circinatum. New For. 2007, 33, 109–123. [Google Scholar] [CrossRef]
- Iturritxa, E.; Mesanza, N.; Elvira-Recuenco, M.; Serrano, Y.; Quintana, E.; Raposo, R. Evaluation of genetic resistance in Pinus to pitch canker in Spain. Australas. Plant Pathol. 2012, 41, 601–607. [Google Scholar] [CrossRef]
- Martínez-Álvarez, P.; Pando, V.; Diez, J.J. Alternative species to replace Monterey pine plantations affected by pitch canker caused by Fusarium circinatum in northern Spain. Plant Pathol. 2014, 63, 1086–1094. [Google Scholar] [CrossRef]
- Schmale, D.G.; Gordon, T.R. Variation in susceptibility to pitch canker disease, caused by Fusarium circinatum, in native stands of Pinus muricata. Plant Pathol. 2003, 52, 720–725. [Google Scholar] [CrossRef]
- Kuhlman, E.G.; Cade, S. Pitch canker disease of loblolly and pond pines in North Carolina plantation. Plant Dis. 1985, 69, 175–176. [Google Scholar] [CrossRef]
- Elvira-Recuenco, M.; Iturritxa, E.; Majada, J.; Alia, R.; Raposo, R. Adaptive Potential of Maritime Pine (Pinus pinaster) Populations to the Emerging Pitch Canker Pathogen, Fusarium circinatum. PLoS ONE 2014, 9, e114971. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, A.; Wingfield, M.J.; Kemp, G.H.J.; Marasas, W.F.O. Susceptibility of pines in South Africa to the pitch canker fungus Fusarium subglutinans f.sp. pini. Plant Pathol. 1995, 44, 877–882. [Google Scholar] [CrossRef]
- Muñoz-Adalia, E.J.; Flores-Pacheco, J.A.; Martínez-Álvarez, P.; Martín-García, J.; Fernández, M.; Diez, J.J. Effect of mycoviruses on the virulence of Fusarium circinatum and laccase activity. Physiol. Mol. Plant Pathol. 2016, 94, 8–15. [Google Scholar] [CrossRef]
- Martínez-Álvarez, P.; Vainio, E.J.; Botella, L.; Hantula, J.; Diez, J.J. Three mitovirus strains infecting a single isolate of Fusarium circinatum are the first putative members of the family Narnaviridae detected in a fungus of the genus Fusarium. Arch. Virol. 2014, 159, 2153–2155. [Google Scholar] [CrossRef]
- Agusti-Brisach, C.; Perez-Sierra, A.; Armengol, J.; Garcia-Jimenez, J.; Berbegal, M. Efficacy of hot water treatment to reduce the incidence of Fusarium circinatum on Pinus radiata seeds. Forestry 2012, 85, 629–635. [Google Scholar] [CrossRef]
- Berbegal, M.; Landeras, E.; Sánchez, D.; Abad-Campos, P.; Pérez-Sierra, A.; Armengol, J. Evaluation of Pinus radiata seed treatments to control Fusarium circinatum: Effects on seed emergence and disease incidence. For. Pathol. 2015, 45, 525–533. [Google Scholar] [CrossRef]
- Brasier, C.M. Fitness, continuous variation and selection in fungal populations: An ecological perspective. In The Structure of Fungal Populations; Worrall, J., Ed.; Kluwer Academic Publishers: Boston, MA, USA; New York, NY, USA, 1998; pp. 289–318. [Google Scholar]
- Milgroom, M.G. Population Biology of Plant Pathogens: Genetics, Ecology, and Evolution; APS Press, The American Phytopathological Society: St. Paul, MN, USA, 2015; ISBN 978-0-89054-450-1. [Google Scholar]
- Van Poucke, K.; Franceschini, S.; Webber, J.F.; Vercauteren, A.; Turner, J.A.; McCracken, A.R.; Heungens, K.; Brasier, C.M. Discovery of a fourth evolutionary lineage of Phytophthora ramorum: EU2. Fungal Biol. 2012, 116, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Brasier, C.M.; Franceschini, S.; Vettraino, A.M.; Hansen, E.M.; Green, S.; Robin, C.; Webber, J.F.; Vannini, A. Four phenotypically and phylogenetically distinct lineages in Phytophthora lateralis. Fungal Biol. 2012, 116, 1232–1249. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, S.; Webber, J.F.; Sancisi-Frey, S.; Brasier, C.M. Gene × environment tests discriminate the new EU2 evolutionary lineage of Phytophthora ramorum and indicate that it is adaptively different. For. Pathol. 2014, 44, 219–232. [Google Scholar] [CrossRef]
- Robin, C.; Brasier, C.; Reeser, P.; Sutton, W.; Vannini, A.; Vettraino, A.M.; Hansen, E. Pathogenicity of Phytophthora lateralis Lineages on Different Selections of Chamaecyparis lawsoniana. Plant Dis. 2015, 99, 1133–1139. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Ames, IA, USA, 2006. [Google Scholar]
- Campbell, C.L.; Madden, L.V. Introduction to Plant Disease Epidemiology; John Wiley & Sons: New York, NY, USA, 1990. [Google Scholar]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 2015. Available online: http://tarwi.lamolina.edu.pe/~fmendiburu (accessed on 25 September 2017).
- Husson, F.; Josse, J.; Pages, J. Principal Component Methods—Hierarchical Clustering—Partitional Clustering: Why Would We Need to Choose for Visualizing Data? Technical Report; Agrocampus: Rennes, France, 2010. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Development Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; ISBN 3-900051-07-0.
- Kim, Y.-S.; Woo, K.-S.; Koo, Y.-B.; Yeo, J.-K. Variation in susceptibility of six pine species and hybrids to pitch canker caused by Fusarium circinatum. For. Pathol. 2008, 38, 419–428. [Google Scholar] [CrossRef]
- Runion, G.B.; Bruck, R.I. Effects of Thiabendazole-DMSO Treatment of Longleaf Pine Seed Contaminated with Fusarium subglutinans on Germination and Seedling Survival. Plant Dis. 1988, 72, 872–874. [Google Scholar] [CrossRef]
- Allen, T.W.; Enebak, S.A.; Carey, W.A. Evaluation of fungicides for control of species of Fusarium on longleaf pine seed. Crop Prot. 2004, 23, 979–982. [Google Scholar] [CrossRef]
- Chung, W.-H.; Ishii, H.; Nishimura, K.; Fukaya, M.; Yano, K.; Kajitani, Y. Fungicide Sensitivity and Phylogenetic Relationship of Anthracnose Fungi Isolated from Various Fruit Crops in Japan. Plant Dis. 2006, 90, 506–512. [Google Scholar] [CrossRef]
- Secor, G.A.; Rivera, V.V.; Khan, M.F.R.; Gudmestad, N.C. Monitoring Fungicide Sensitivity of Cercospora beticola of Sugar Beet for Disease Management Decisions. Plant Dis. 2010, 94, 1272–1282. [Google Scholar] [CrossRef]
- Nirenberg, O.H.; O’Donnell, K. New Fusarium species and combinations within the Gibberella fujikuroi species complex. Mycologia 1998, 90, 434–458. [Google Scholar] [CrossRef]
- Inman, A.R.; Kirkpatrick, S.C.; Gordon, T.R.; Shaw, D.V. Limiting effects of low temperature on growth and spore germination in Gibberella circinata, the cause of pitch canker in pine species. Plant Dis. 2008, 92, 542–545. [Google Scholar] [CrossRef]
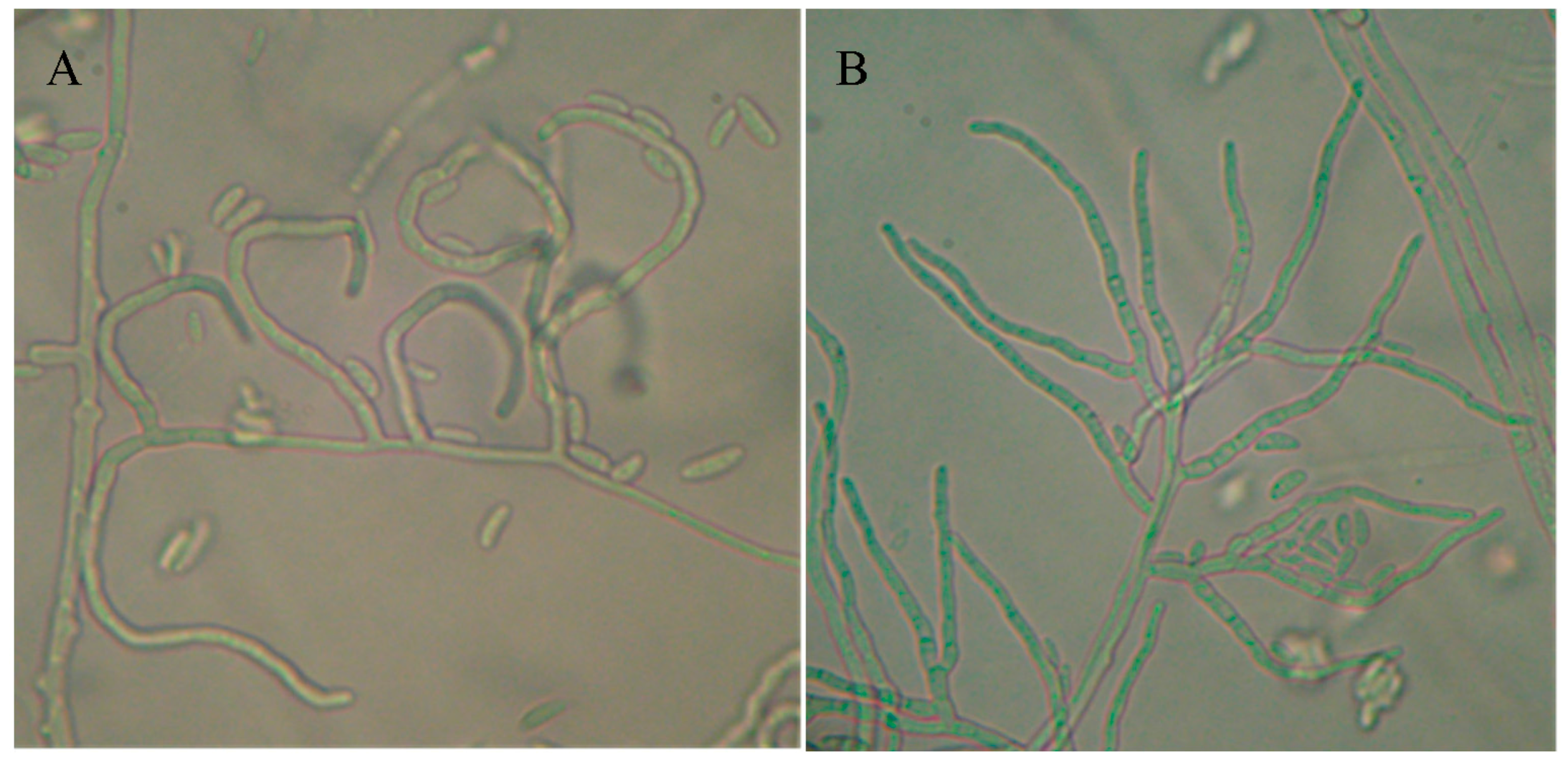
| Temperature | Minimum | Mean | Maximum |
|---|---|---|---|
| 5 °C | 0.060 | 0.262 | 0.500 |
| 10 °C | 0.560 | 1.042 | 1.556 |
| 15 °C | 1.798 | 3.137 | 4.181 |
| 20 °C | 2.492 | 5.057 | 6.772 |
| 25 °C | 3.175 | 5.856 | 8.679 |
| 30 °C | 2.167 | 4.346 | 7.573 |
| 35 °C | 0.127 | 0.699 | 2.202 |
| Isolate Code | Fludioxonil | Pyraclostrobin |
|---|---|---|
| M-4058 | 38.6 | 0.1 |
| NRRL26431/MAFF 236397 | 40 | 0.1 |
| CBS119864 | 53 | 1 |
| CBS119865 | 9 | 5 |
| 104 | 69 | <0.1 |
| 182 | 50 | <0.1 |
| 202 | >100 | 0.4 |
| 217 | 47 | 6.6 |
| 122 | >100 | 2 |
| 160 | 30 | <0.1 |
| 229 | >100 | 18 |
| 164 | >100 | >100 |
| 433 | 65 | <0.1 |
| 430 | >100 | <0.1 |
| 431 | >100 | <0.1 |
| 389 | 15 | <0.1 |
| 453 | >100 | <0.1 |
| 649 | 61 | <0.1 |
| 678 | 54 | 0.2 |
| 623 | 45 | 1 |
| 625 | 48 | 0.6 |
| 390 | 43 | <0.1 |
| 250 | >100 | 0.1 |
| 253 | >100 | >100 |
| 255 | >100 | >100 |
| 822 | >100 | 5 |
| 825 | 22 | <0.1 |
| 829 | 40 | 78 |
| 830 | 9 | 30 |
| 831 | >100 | 75 |
| 100 | >100 | >100 |
| M-8486 | 60 | <0.1 |
| M-8487 | >100 | <0.1 |
| pv1 | >100 | <0.1 |
| pv3 | >100 | 0.1 |
| pv8 | >100 | <0.1 |
| M-3834 | >100 | 2 |
| NRRL25331 M-8386 | >100 | <0.1 |
| NRLL25332/MAFF240076 | 60 | >100 |
| M-1450 | 20 | <0.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mullett, M.; Pérez-Sierra, A.; Armengol, J.; Berbegal, M. Phenotypical and Molecular Characterisation of Fusarium circinatum: Correlation with Virulence and Fungicide Sensitivity. Forests 2017, 8, 458. https://doi.org/10.3390/f8110458
Mullett M, Pérez-Sierra A, Armengol J, Berbegal M. Phenotypical and Molecular Characterisation of Fusarium circinatum: Correlation with Virulence and Fungicide Sensitivity. Forests. 2017; 8(11):458. https://doi.org/10.3390/f8110458
Chicago/Turabian StyleMullett, Martin, Ana Pérez-Sierra, Josep Armengol, and Mónica Berbegal. 2017. "Phenotypical and Molecular Characterisation of Fusarium circinatum: Correlation with Virulence and Fungicide Sensitivity" Forests 8, no. 11: 458. https://doi.org/10.3390/f8110458
APA StyleMullett, M., Pérez-Sierra, A., Armengol, J., & Berbegal, M. (2017). Phenotypical and Molecular Characterisation of Fusarium circinatum: Correlation with Virulence and Fungicide Sensitivity. Forests, 8(11), 458. https://doi.org/10.3390/f8110458







