Complex Challenges of Maintaining Whitebark Pine in Greater Yellowstone under Climate Change: A Call for Innovative Research, Management, and Policy Approaches
Abstract
:1. Introduction
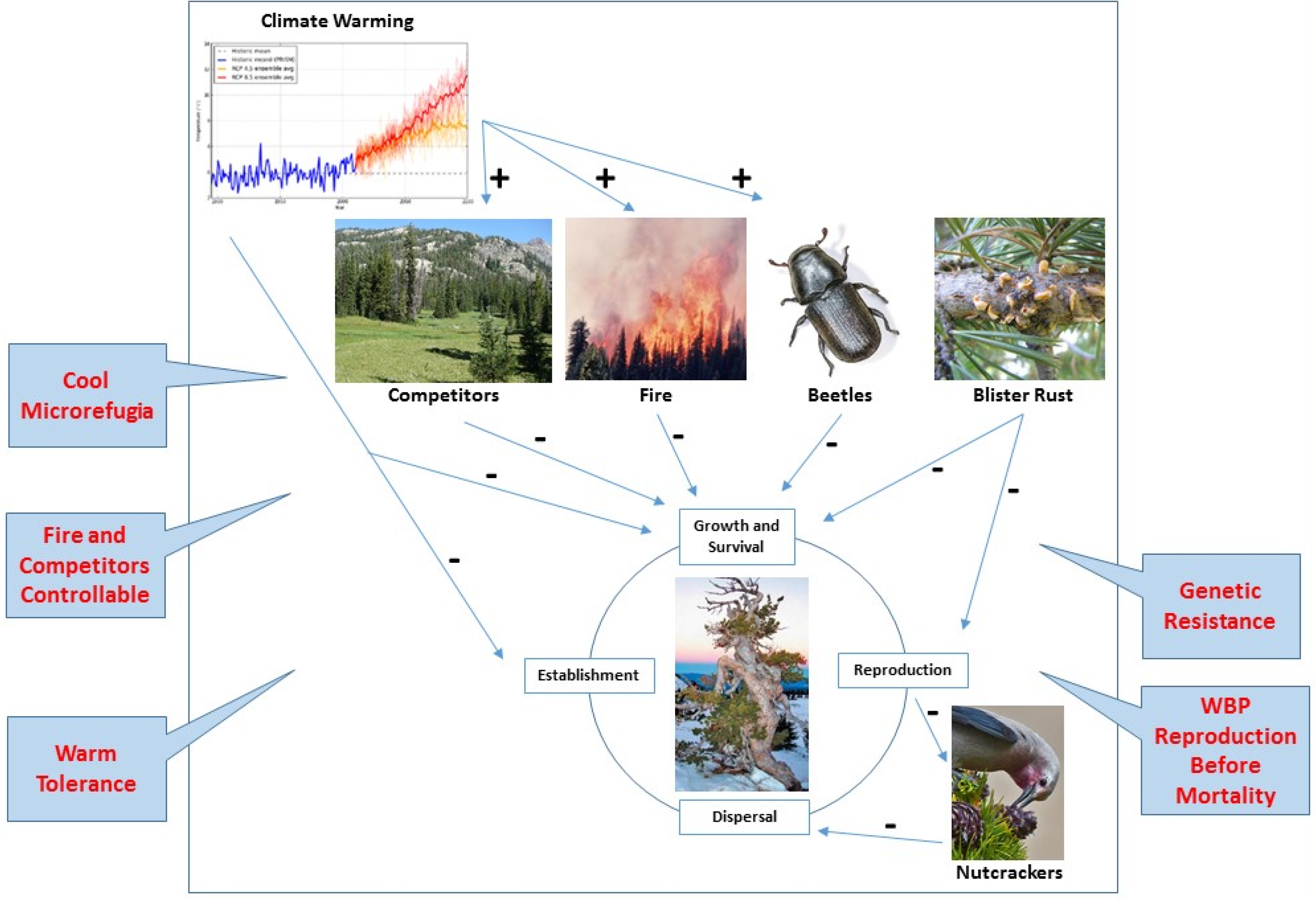
2. Complex Interactions that Limit WBP under Climate Change
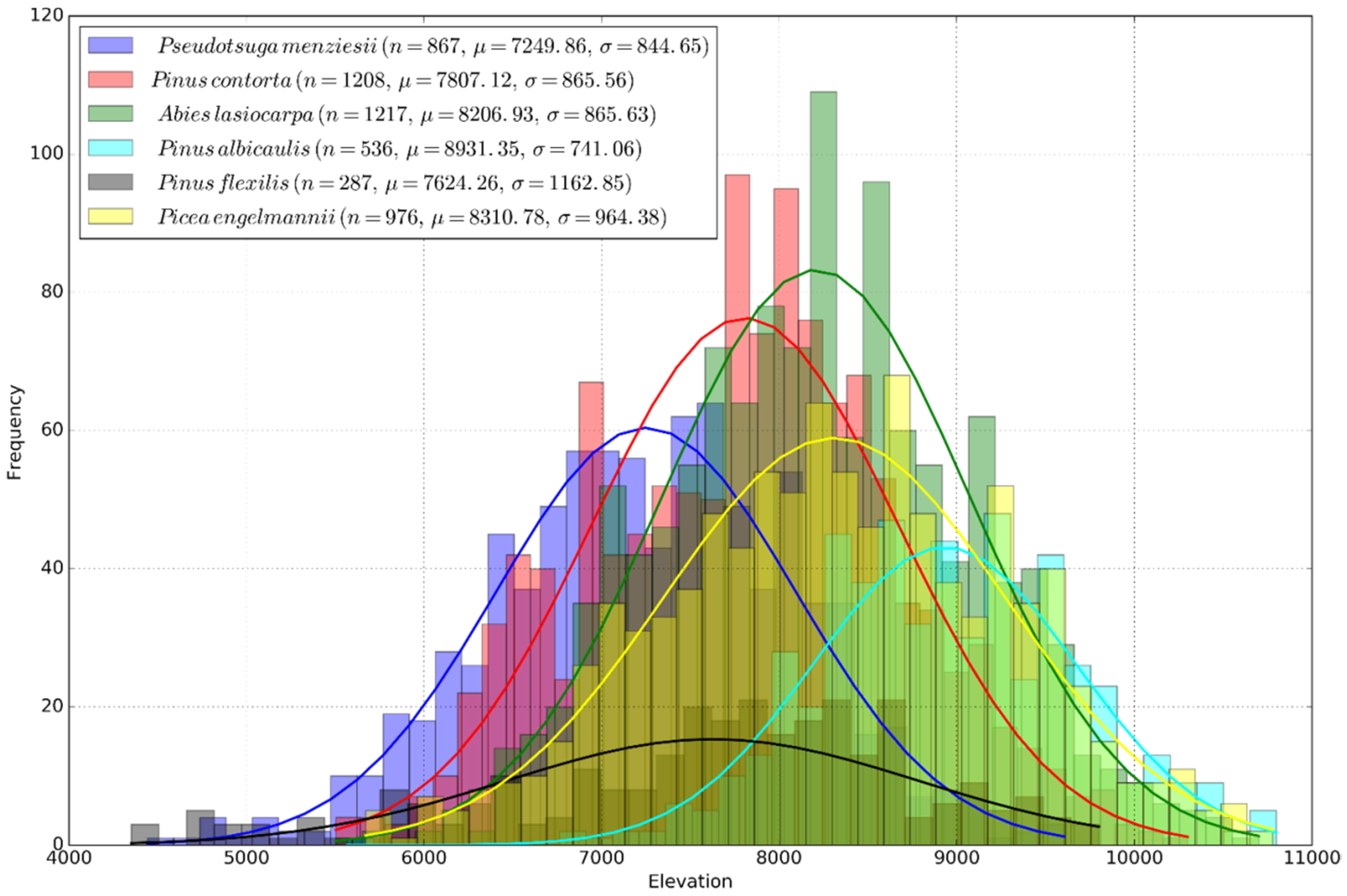
2.1. Climate Suitability
2.2. Competition
2.3. Fire
2.4. Mountain Pine Beetle
2.5. White Pine Blister Rust
2.6. Seed Predation and Dispersal
3. Current Management Approach and Status
- Monitoring. The goal is to quantify the status and trends in WBP condition and use results to guide management. This goal is achieved by monitoring WBP survival, reproduction, and mortality agents.
- Protection. The goal is to prevent or minimize damage to existing trees and stands from insects, disease, and fire. Strategies to realize this goal include protecting genetically disease resistant trees, cone-producing trees, and trees exhibiting blister rust resistance through use of anti-aggregation pheromones (verbenone), insecticides (carbaryl), and pruning. Additionally, blister rust resistant trees and stands are protected from wildland fire.
- Restoration. The goal is to restore WBP stands by replanting or by creating conditions that favor natural regeneration and dominance of WBP. Methods include planting blister-rust resistant seedlings, creating openings conducive to the natural regeneration of WBP, and removing competing vegetation.
- Tree Improvement. The goal is to identify and propagate genotypes that have resistance or tolerance to adverse factors such as drought and white pine blister rust. This is realized by collecting seeds from WBP trees having potential resistance to white pine blister rust, propagating the seeds in nurseries, testing for blister rust resistance, and using seeds from trees that show resistance to populate a seed orchard to produce resistant seedlings for planting.
4. New Perspectives on WBP Dynamics under Climate Change
4.1. Microrefugia
4.2. Temperature Niche
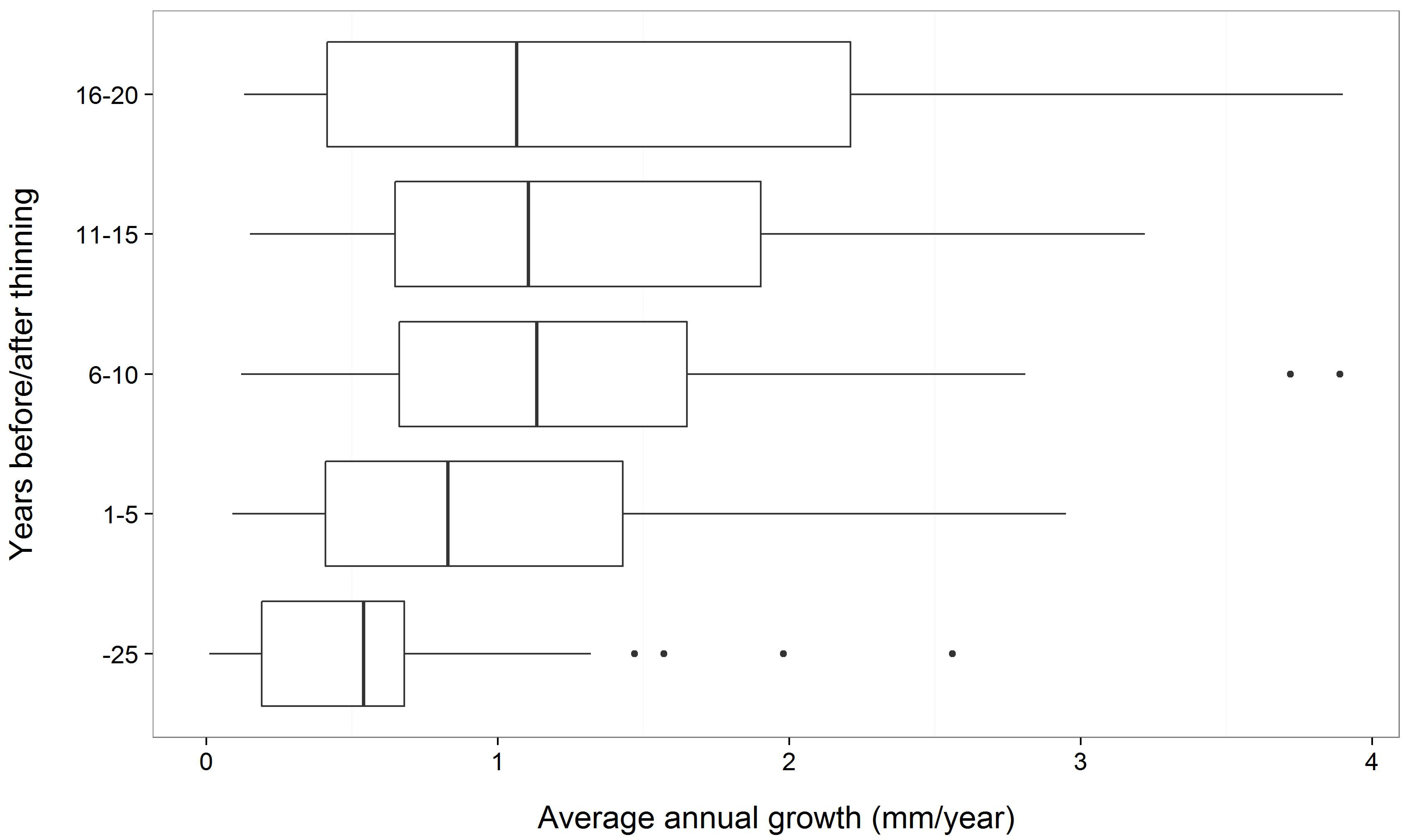
4.3. Release from Competition
4.4. Shifting Fire Regime
4.5. Mountain Pine Beetle Escape
4.6. White Pine Blister Rust Resistance
5. Research, Management, and Policy Needs
5.1. Research Needs
5.2. Management and Policy Recommendations
5.2.1. Spatial Distribution of Treatments
5.2.2. Adaptive Management
5.2.3. Policy Evaluation for Restricted Federal Land Allocations
6. Prognosis
- Research to better understand the direct and indirect effects of climate change and mechanisms that may allow the species to remain viable under changing climate, especially if aided by management;
- Development of management strategies that are spatially and temporally organized in the context of future climate suitability for the target species and the organisms that influence that species;
- Adaptive management where treatments are arrayed across key biophysical gradients and monitored to quantify effectiveness and tailor future management to biophysical conditions;
- Use of demographic and mechanistic models to project treatments forward in time.
- Re-evaluation of legal and policy constraints regarding active management in high elevation locations; and
- Interagency collaboration to allow unified management approaches across the larger spatial scales relevant to managing these species under climate change.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C. Climate change threats to plant diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef] [PubMed]
- Rehfeldt, G.E.; Crookston, N.L.; Saenz-Romero, C.; Campbel, E.M. North American vegetation model for land-use planning in a changing climate: A solution to large classification problems. Ecol. Appl. 2012, 22, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C.; Agudo, R. The future of species under climate change: Resilience or decline? Science 2013, 341, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Elsen, P.R.; Tingley, M.W. Global mountain topography and the fate of montane species under climate change. Nat. Clim. Chang. 2015, 5, 772–776. [Google Scholar] [CrossRef]
- Dobrowski, S.Z. A climatic basis for microrefugia: The influence of terrain on climate. Glob. Chang. Biol. 2010, 17, 1022–1035. [Google Scholar] [CrossRef]
- Dullinger, S.; Gattringer, A.; Thuiller, W.; Moser, D.; Zimmermann, N.E.; Guisan, A.; Willner, W.; Plutzar, C.; Leitner, M.; Mang, T.; et al. Extinction debt of high-mountain plants under twenty-first-century climate change. Nat. Clim. Chang. 2015, 2, 619–622. [Google Scholar] [CrossRef]
- Landres, P. Let it be: A Hands-off Approach to Preserving Wildness in Protected Areas. In Beyond Naturalness: Rethinking Park and Wilderness Stewardship in an Era of Rapid Change; Cole, D.N., Yung, L., Eds.; Island Press: Washington, DC, USA, 2010; pp. 88–105. [Google Scholar]
- Colwell, R.; Avery, S.; Berger, J.; Davis, G.E.; Hamilton, H.; Lovejoy, T.; Malcom, S.; McMullen, A.; Novacek, M.; Roberts, R.J.; et al. Revisiting Leopold: Resource Stewardship in the National Parks; National Park System Advisory Board Science Committee: Washington, DC, USA, 2012. [Google Scholar]
- National Park Service (NPS). National Park Service Policy 2006. Doi, nps. Isbn 0–16–076874–8; National Park Service (NPS): Washington, DC, WA, USA, 2006; p. 169.
- U.S. Forest Service (USFS). FSM 2000, National Forest Resource Management—Chapter 2070 Vegetation Ecology. Amendment no. 2000-2008-1; p. 12. Available online: http://www.fs.fed.us/cgi-bin/Directives/get_dirs/fsm?2000 (accessed on 20 June 2015).
- Chapin, F.S., III; Matson, P.A.; Vitousek, P.M. Principles of Terrestrial Ecosystem Ecology, 2nd ed.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Keane, R.E.; Holsinger, L.M.; Mahalovich, M.F.; Tomback, D.F. Restoring Whitebark Pine Ecosystems in the Face of Climate Chang; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, General Technical Report RMRS-GTR-XXX: Fort Collins, CO, USA, In Press.
- Keane, R.E.; Tomback, D.F.; Aubry, C.A.; Bower, A.D.; Campbel, E.M.; Cripps, C.L.; Jenkins, M.B.; Mahalovich, M.F.; Manning, M.; McKinney, S.T.; et al. A Range-Wide Restoration Strategy for Whitebark Pine (Pinus albicaulis); U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, General Technical Report RMRS-GTR-279: Fort Collins, CO, USA, 2012.
- Tomback, D.F.; Achuff, P. Blister rust and western forest biodiversity: Ecology, values and outlook for white pines. For. Pathol. 2010, 40, 186–225. [Google Scholar] [CrossRef]
- Tomback, D.F.; Arno, S.F.; Keane, R.E. Whitebark Pine Communities: Ecology and Restoration; Island Press: Washington, DC, USA, 2001; p. 328. [Google Scholar]
- Hamann, A.; Wang, T. Potential effects of climate change on ecosystem and tree species distribution in British Columbia. Ecology 2006, 87, 2773–2786. [Google Scholar] [CrossRef]
- Crookston, N.L.; Rehfeldt, G.E.; Dixon, G.E.; Weiskittel, A.R. Addressing climate change in the forest vegetation simulator to assess impacts on landcape forest dynamics. For. Ecol. Manag. 2010, 260, 1198–1211. [Google Scholar] [CrossRef]
- Coops, N.C.; Waring, R.H. Estimating the vulnerability of fifteen tree species under changing climate in northwest North America. Ecol. Model. 2011, 222, 2119–2129. [Google Scholar] [CrossRef]
- Whitlock, C. Postglacial vegetation and climate of Grand Teton and southern Yellowstone National parks. Ecol. Monogr. 1993, 63, 173–198. [Google Scholar] [CrossRef]
- O’Connell, B.M.; LaPoint, E.B.; Turner, J.A.; Ridley, T.; Pugh, S.A.; Wilson, A.M.; Waddell, K.L.; Conkling, B.L. The Forest Inventory and Analysis Database: Database Description and User Guide Version 6.0.2 for Phase 2; U.S. Department of Agriculture, Forest Service, 2015. Available online: http://www.fia.fs.fed.us/library/database-documentation (accessed on 14 January 2014).
- Bechtold, W.A.; Patterson, P.L. The Enhanced Forest Inventory and Analysis Program—National Sampling Design and Estimation Procedures; U.S. Department of Agriculture, Forest Service, Southern Research Station, General Technical Report SRS-80: Asheville, NC, USA, 2005; p. 85.
- Gibson, K.; Shov, K.; Kegley, S.; Jorgensen, C.; Smith, S.; Witcosky, J. Mountain Pine Beetle Impacts in High-Elevation Five-Needle Pines: Current Trends and Challenges; U.S. Department of Agriculture, Forest Service, Northern Region. R1–08–020: Missoula, MT, USA, 2008.
- Macfarlane, W.W.; Logan, J.A.; Kern, W.R. Using the Landscape Assessment System (LAS) to Assess Mountain Pine Beetle-Caused Mortality of White Bark Pine Beetle-Caused Mortality of Whitebark Pine, Greater Yellowstone Ecosystem, 2009; Project Report Prepared for the Greater Yellowstone Coordinating Committee; Whitebark Pine Subcommittee: Jackson, WY, USA, 2010. [Google Scholar]
- Larson, E.R.; Kipfmueller, K.F. Ecological disaster or the limits of observation? Reconciling modern declines with the long-term dynamics of whitebark pine communities. Geogr. Compass 2012, 6/4, 189–214. [Google Scholar] [CrossRef]
- Schrag, A.M.; Bunn, A.G.; Graumlich, L.J. Influence of bioclimatic variables on tree-line conifer distribution in the Greater Yellowstone Ecosystem: Implications for species of conservation concern. J. Biogeogr. 2008, 35, 698–710. [Google Scholar] [CrossRef]
- Chang, T.; Hansen, A.J.; Piekielek, N. Patterns and variability of projected bioclimatic habitat for Pinus albicaulis in the Greater Yellowstone Area. PLoS ONE 2014, 9, e111669. [Google Scholar] [CrossRef] [PubMed]
- Bartlein, P.J.; Whitlock, C.; Shafer, S. Future climate in the Yellowstone National Park region and its potential impact on vegetation. Conser. Biol. 1997, 11, 782–792. [Google Scholar]
- Romme, W.H.; Turner, M.G. Implications of global climate change for biogeographic patterns in the Greater Yellowstone Ecosystem. Conserv. Biol. 1991, 5, 373–386. [Google Scholar] [CrossRef]
- U.S. Fish and Wildlife Service (USFWS). 2011. Available online: http://www.Fws.Gov/mountain-prairie/species/plants/whitebarkpine (accessed on 20 June 2015).
- Greater Yellowstone Coordinating Committee Whitebark Pine Subcommittee. Whitebark Pine Strategy for the Greater Yellowstone Area; Greater Yellowstone Coordinating Committee Whitebark Pine Subcommittee, 2011; p. 41. [Google Scholar]
- McLane, S.C.; Aitken, S.N. Whitebark pine (Pinus albicaulis) assisted migration potential: Testing establishment north of the species range. Ecol. Appl. 2012, 22, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.; Larson, B.M.H. Should we move the whitebark pine? Assisted migration, ethics, and global environmental change. Environ. Values 2014, 23, 641–662. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Franklin, J. Mapping Species Distributions: Spatial Inference and Prediction; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Warwell, M.V.; Rehfeldt, G.E.; Crookston, N.L. Modeling contemporary climate profiles of whitebark pine (Pinus albicaulis) and predicting responses to global warming. In Proceedings of the Conference on Whitebark Pine: A Pacific Coast Perspective, U.S. Department of Agriculture, Forest Service R6-NR-FHP-2007–01, Ashland, OR, USA, 26–27 August 2006; Goheen, E.M., Sniezko, R.A., Eds.; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2007. [Google Scholar]
- Hansen, A.J.; Phillips, L. Which tree species and biome types are most vulnerable to climate change in the US Northern Rocky Mountains? For. Ecol. Manag. 2015, 338, 68–83. [Google Scholar] [CrossRef]
- Pfister, R.D.; Kovalchik, B.L.; Arno, S.F.; Presby, R.C. Forest habitat types of Montana; USDA Forest Service General Technical Report INT; US Forest Service: Washington, DC, USA, 1977.
- Weaver, T.; Dale, D. Pinus albicaulis in central Montana: Environment, vegetation and production. Am. Midl. Nat. 1974, 222–230. [Google Scholar] [CrossRef]
- Piekielek, N.; Hansen, A.J.; Chang, T. Using custom scientific workflow software and GIS to inform protected area climate adaptation planning across the Greater Yellowstone. Ecol. Inf. 2015, 30, 40–48. [Google Scholar] [CrossRef]
- Campbell, E.M.; Antos, J.A. Postfire succession in Pinus albicaulis—Abies lasiocarpa forests of southern British Columbia. Can. J. Bot. 2003, 81, 383–397. [Google Scholar] [CrossRef]
- Morgan, P.; Bunting, S.; Keane, R.E.; Arno, S. Fire ecology of whitebark pine (Pinus albicaulis) forests in the Rocky Mountains, USA. In WC Schmidt and F.-K. Holtmeier (comps), Proceedings—International workshop on Subalpine Stone Pines and Their Environment: The Status of our Knowledge. USDA Forest Service General Technical Report INT-309; USDA Forest Service General Technical Report INT-309: St. Moritz, Switzerland, 1994; pp. 136–142. [Google Scholar]
- Murray, M.P.; Bunting, S.C.; Morgan, P. Landscape trends (1753–1993) of whitebark pine (Pinus albicaulis) forests in the west Big Hole range of Idaho/Montana, USA. Arct. Antarct. Alp. Res. 2000, 412–418. [Google Scholar] [CrossRef]
- Tomback, D.F.; Sund, S.K.; Hoffman, L.A. Post-fire regeneration of Pinus albicaulis: Height-age relationships, age structure, and microsite characteristics. Can. J. For. Res. 1993, 23, 113–119. [Google Scholar] [CrossRef]
- Romme, W.H.; Despain, D. Historical perspective on the Yellowstone fires of 1988. Bioscience 1989, 39, 695–699. [Google Scholar] [CrossRef]
- Keane, R.E.; Loehman, R.A. Understanding the Role of Wildland Fire, Insects, and Disease in Predicting Climate Change Effects on Whitebark Pine: Simulating Vegetation, Disturbance, and Climate Dynamics in a Northern Rocky Mountain Landscape. Abstract #nh33b-06; In AGU Fall Meeting Abstracts; American Geophysical Union: San Francisco, CA, USA, 2010; Volume 1, p. 6. [Google Scholar]
- Loehman, R.A.; Corrow, A.; Keane, R.E. Modeling Climate Changes and Wildfire Interactions: Effects on Whitebark pine (Pinus albicaulis) and Implications for Restoration, Glacier National Park, Montana, USA. In The Future of High-Elevation, Five-Needle White Pines in Western North America: Proceedings of the High Five Symposium; Keane, R.E., Tomback, D.F., Murray, M.P., Smith, C.M., Eds.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Missoula, MT, USA, 2011; Volume Proceedings RMRS-P-63, pp. 176–189. [Google Scholar]
- Westerling, A.L.; Turner, M.G.; Smithwick, E.A.H.; Romme, W.H.; Ryan, M.G. Continued warming could transform Greater Yellowstone fire regimes by mid-21st century. Proc. Natl. Acad. Sci. 2011, 108, 13165–13170. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.S.; Keane, R.E.; Loehman, R.A. Climate changes and wilfire alter forest composition of Yellowstone National Park, but forest cover persists. Clim. Chang. 2015. In press. [Google Scholar]
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Turner, M.G.; Romme, W.H. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: The dynamics of bark beetle eruptions. Bioscience 2008, 58, 501–517. [Google Scholar] [CrossRef]
- Logan, J.A.; Macfarlane, W.W.; Willcox, L. Whitebark pine vulnerability to climate-driven mountain pine beetle disturbance in the Greater Yellowstone Ecosystem. Ecol. Appl. 2010, 20, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Logan, J.A.; Macfarlane, W.W.; Willcox, L. Effective monitoring as a basis for adaptive management: A case history of mountain pine beetle in Greater Yellowstone Ecosystem whitebark pine. Iforest-Biogeosci. For. 2009, 2, 19–22. [Google Scholar] [CrossRef]
- Perkins, D.L.; Swetnam, T.W. A dendroecological assessment of whitebark pine in the Sawtooth-Salmon River region, Idaho. Can. J. For. Res. Revue Can. Rech. For. 1996, 26, 2123–2133. [Google Scholar] [CrossRef]
- Hicke, J.A.; Logan, J.A.; Powell, J.; Ojima, D.S. Changing temperatures influence suitability for modeled mountain pine beetle (Dendroctonus ponderosae) outbreaks in the western United States. J. Geophys. Res. Biogeosci. 2006, 111. [Google Scholar] [CrossRef]
- Logan, J.A.; Powell, J.A. Ghost forest, global warming, and the mountain pine beetle (Coleoptera: Scolytidae). Am. Entomol. 2001, 47, 160–173. [Google Scholar] [CrossRef]
- Bentz, B.J.; Regniere, J.; Fettig, C.J.; Hansen, E.M.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Negron, J.F.; Seybold, S.J. Climate change and bark beetles of the western United States and Canada: Direct and indirect effects. Bioscience 2010, 60, 602–613. [Google Scholar] [CrossRef]
- Geils, B.W.; Vogler, D. A Natural History of Cronartium ribicola. In The Future of High-Elevation, Five-Needle White Pines in Western North America: Proceedings of the High Five Symposium; Keane, R.E., Tomback, D.F., Murray, M.P., Smith, C.M., Eds.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fort Collins, Co., Proceedings RMRS-P-63: Missoula, MT, USA, 2011. [Google Scholar]
- Schwandt, J.W.; Kearns, H.S.J.; Byler, J.W. Impacts of White Pine Blister Rust and Competition on Natural Whitebark Pine Regeneration in Northern IDAHO 1995–2012; Forest Health Protection, Report Number 13–09, 2013. [Google Scholar]
- Shanahan, E.; Irvine, K.M.; Roberts, D.W.; Litt, A.; Legg, K.; Daley, R. Status of Whitebark Pine in the Greater Yellowstone Ecosystem: A step-Trend Analysis Comparing 2004–2007 to 2008–2011; Natural Resource Technical Report NPS/GRYN/NRTR—2014/917; U.S. Department of the Interior, National Park Service: Fort Collins, CO, USA, 2014.
- Hatala, J.A.; Dietze, M.C.; Crabtree, R.L.; Kendall, K.; Six, D.; Moorcroft, P.R. An ecosystem-scale model for the spread of a host-specific forest pathogen in the Greater Yellowstone Ecosystem. Ecol. Appl. 2010, 21, 1138–1153. [Google Scholar] [CrossRef]
- Lanner, R.M. Adaptations of whitebark pine for seed dispersal by Clark's nutcracker. Can. J. For. Res. 1982, 12, 391–402. [Google Scholar] [CrossRef]
- Lorenz, T.J.; Sullivan, K.A.; Bakian, A.V.; Aubry, C.A. Cache-site selection in Clark's nutcracker. Auk 2011, 128, 237–247. [Google Scholar] [CrossRef]
- Tomback, D.F. Dispersal of whitebark pine seeds by Clark’s nutcracker: A mutualism hypothesis. J. Anim. Ecol. 1982, 51, 451–467. [Google Scholar] [CrossRef]
- McKinney, D.W.; Fieldler, C. Tree squirrel habitat selection and predispersal seed predation in a declining subalpine conifer. Oecologia 2010, 162, 697–707. [Google Scholar] [CrossRef] [PubMed]
- McKinney, D.W.; Tomback, D.F. Altered Community Dynamics in Rocky Mountain Whitebark Pine Forests and the Potential for Accelerating Declines. In Mountain Ecosystems: Dynamics, Management and Conservation; Richards, K.E., Ed.; Nova Science Publishers: Hauppage, NY, USA, 2011; pp. 45–78. [Google Scholar]
- Buermeyer, K.; Reinhart, D.P.; Legg, K.; Kelly, V. Case study: Whitebark Pine in GYE. In Climate Change in Wildlands: Pioneering Approaches to Science and Management in the Rocky Mountains and Appalachians; Hansen, A.J., Theobald, D.M., Oliff, T., Monihan, W., Eds.; Island Press: Washington, DC, USA, In Press.
- Keane, R.E. The Importance of Wilderness to Whitebark Pine Research and Management. In Proceedings of the Symposium: Wilderness Science: In a Time for Change. Volume 3: Wilderness As a Place for Scientific Inquiry; USDA Forest Service General Technical Report RMRS-P-15-VOL-3: Missoula, MT, USA, 2000; pp. 84–93. [Google Scholar]
- Greater Yellowstone Whitebark Pine Monitoring Working Group (GYWPMWG). Interagency Whitebark pine Monitoring Protocol for the Greater Yellowstone Ecosystem. Version 1.1; Greater Yellowstone Coordinating Committee: Bozeman, MT, USA, 2011. [Google Scholar]
- Daly, C.; Conklin, D.R.; Unsworth, M.H. Local atmospheric decoupling in complex topography alters climate change impacts. Int. J. Climatol. 2010, 30, 1857–1864. [Google Scholar] [CrossRef]
- Bansal, S.; Reinhardt, K.; Germino, M.J. Linking carbon balance to establishment patterns: Comparison of whitebark pine and Engelmann spruce seedlings along an herb cover exposure gradient at treeline. Plant Ecol. 2011, 212, 219–228. [Google Scholar] [CrossRef]
- Franklin, J.; Davis, F.W.; Ikegami, M.; Syphard, A.D.; Flint, L.E.; Flint, A.L.; Hannah, L. Modeling plant species distributions under future climates: How fine scale do climate projections need to be? Glob. Chang. Biol. 2013, 19, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Millar, C.I.; Westfall, R.D.; Delany, D.L.; Bokach, M.J.; Flint, A.L.; Flint, L.E. Forest mortality in high-elevation whitebark pine (Pinus albicaulis) forests of eastern California, USA; influence of environmental context, bark beetles, climatic water deficit, and warming. Can. J. For. Res. Rev. Can. Rech. For. 2012, 42, 749–765. [Google Scholar] [CrossRef]
- Thoma, D.; Irvine, K.; Shovic, H.; Shanahan, E.; Legg, K. Climatic controls on mountain pine beetle mediated mortality in whitebark pine in the GYE. In The 12th Biennial Scientific Conference on the Greater Yellowstone Ecosystem, Mammoth, WY, USA, 6–8 October 2014.
- Buotte, P.C.; Hicke, J.A.; Preisler, H.K.; Abatzoglou, J.T.; Raffa, K.F.; Logan, J.A. Historical and future climate influences on mountain pine beetle outbreaks in whitebark pine forests of the Greater Yellowstone Ecosystem. Proc. Natl. Acad. Sci. USA 2015. In press. [Google Scholar]
- Dolanc, C.R.; Thorne, J.H.; Safford, H.D. Widespread shifts in the demographic structure of subalpine forests in the Sierra Nevada, California, 1934 to 2007. Glob. Ecol. Biogeogr. 2013, 22, 264–276. [Google Scholar] [CrossRef]
- Iglesias, V.; Krause, T.R.; Whitlock, C. Complex response of pine to past environmental variability increases understanding of its future vulnerability. PLos ONE 2015, 10, e0124439. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.; Weaver, T. Effects of Temperature and Temperature Preconditioning on Seedling Performance of Whitebark Pine. In Symposium on Whitebark Pine Ecosystems: Ecology and Management of a High-Mountain Resource; Schmidt, W.C., McDonald, K.J., Eds.; U.S. Department of Agriculture, Forest Service, Intermountain Research Station, General Technical Report INT-GTR-270: Ogden, UT, USA, 1990; pp. 134–139. [Google Scholar]
- Keane, R.E. Restoration of whitebark pine forests in the Northern Rocky Mountains, USA. In The future of High-Elevation, Five-Needle White Pines in Western North America: Proceedings of the High Five Symposium; Keane, R.E., Tomback, D.F., Murray, M.P., Smith, C.M., Eds.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Missoula, MT, USA, 2011; Volume Proceedings RMRS-P-63, pp. 338–347. [Google Scholar]
- Callaway, R.M. Competition and facilitation on elevation gradients in subalpine forests of the northern Rocky Mountains, USA. Oikos 1998, 82, 561–573. [Google Scholar] [CrossRef]
- Keane, R.E.; Gray, K.L.; Dickinson, L.J. Whitebark Pine Diameter Growth Response to Removal of Competition; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2007; Vol. Research Note RMRS-RN-32, p. 9.
- Barmore, W.J.; Taylor, D.L.; Hayden, P. Ecological Effects and Biotic Succession Following the 1974 Waterfalls Canyon fire in Grand Teton National Park; Unpublished Report; GTNP, 1976. [Google Scholar]
- Keane, R.E.; Parsons, R. Restoring whitebark pine forests of the northern Rocky Mountains, USA. Ecol. Restor. 2010, 28, 56–70. [Google Scholar] [CrossRef]
- Bockino, N.K.; Tinker, D.B. Interactions of white pine blister rust and mountain pine beetle in whitebark pine ecosystems in the southern Greater Yellowstone Area. Nat. Areas J. 2012, 32, 31–40. [Google Scholar] [CrossRef]
- Fiedler, C.E.; McKinney, S.T. Forest structure, health, and mortality in two Rocky Mountain whitebark pine ecosystems: Implications for restoration. Nat. Areas J. 2014, 34, 290–299. [Google Scholar] [CrossRef]
- Larson, E.R. Influences of the biophysical environment on blister rust and mountain pine beetle, and their interactions, in whitebark pine forests. J. Biogeogr. 2011, 38, 453–470. [Google Scholar] [CrossRef]
- Perkins, D.L.; Roberts, D.W. Predictive models of whitebark pine mortality from mountain pine beetle. For. Ecol. Manag. 2003, 174, 495–510. [Google Scholar] [CrossRef]
- Gillette, N.F.; Wood, D.L.; Hines, S.J.; Runyon, J.B.; Negrón, J.F. The once and future forest: Consequences of mountain pine beetle treatment decisions. For. Sci. 2014, 60, 527–538. [Google Scholar]
- Larson, E.R.; Kipfmueller, K.F. Patterns in whitebark pine regeneration and their relationships to biophysical site characteristics in southwest Montana, central Idaho, and Oregon, USA. Can. J. For. Res. 2010, 40, 476–487. [Google Scholar] [CrossRef]
- Sniezko, R.A.; Mahalovich, M.F.; Schoettle, A.W.; Vogler, D.R. Past and Current Investigations of the Genetic Resistance to Cronartium ribicola in High-Elevation Five-Needle Pines. In The future of high-elevation, five-needle white pines in Western North America: Proceedings of the High Five Symposium; Keane, R.E., Tomback, D.F., Murray, M.P., Smith, C.M., Eds.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fort Collins, Co., Proceedings RMRS-P-63: Missoula, MT, USA, 2011. [Google Scholar]
- Hoff, R.J.; Ferguson, D.E.; McDonald, G.I.; Keane, R.E. Chapter 17: Strategies for Managing Whitebark Pine in the Presence of White Pine Blister Rust. In Whitebark Pine Communitites: Ecology and Restoration; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, DC, USA, 2001; p. 441. [Google Scholar]
- Schoettle, A.W.; Sniezko, R.A. Proactive intervention to sustain high-elevation pine ecosystems threatened by white pine blister rust. J. For. Restor. 2007, 12, 327–336. [Google Scholar] [CrossRef]
- Rull, V. Microrefugia. J. Biogeogr. 2009, 36, 481–484. [Google Scholar] [CrossRef]
- Gollan, J.R.; Ramp, D.; Ashcroft, M.B. Assessing the distribution and protection status of two types of cool environment to facilitate their conservation under climate change. Conserv. Biol. 2014, 28, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Randin, C.F.; Engler, R.; Normand, S.; Zappa, M.; Zimmermann, N.E.; Pearman, P.B.; Vittoz, P.; Thuiller, W.; Guisan, A. Climate change and plant distribution: Local models predict high-elevation persistence. Glob. Chang. Biol. 2009, 15, 1557–1569. [Google Scholar] [CrossRef]
- Maher, E.L.; Germino, M.J. Microsite differentiation among conifer species during seedling establishment at alpine treeline. Ecoscience 2006, 13, 334–341. [Google Scholar] [CrossRef]
- Weaver, T. Whitebark Pine and its Environment. In Whitebark Pine Communities: Ecology and Restoration; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, DC, USA, 2001; pp. 45–73. [Google Scholar]
- Smith-McKenna, E.K.; Tomback, D.F.; Zhang, H.; Malanson, G.P. Topographic influences on the distribution of white pine blister rust in Pinus albicaulis treeline communities. Ecoscience 2013, 20, 215–229. [Google Scholar] [CrossRef]
- Arno, S.F.; Hoff, R.J. Pinus Albicaulis Engelm. Whitebark Pine. In Silvics of North America. Volume 1. Conifers; Burns, R.M., Honkala, B.H., Eds.; Agricultural Handbook 654; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1990; Volume 1. Conifers, pp. 268–279. [Google Scholar]
- Keane, R.E.; Arno, S.F. Rapid decline of whitebark pine in western Montana: Evidence from 20-year remeasurements. West. J. Appl. For. 1993, 8, 44–47. [Google Scholar]
- Berryman, A.A. Population Dynamics of Forest Insects. In Forest Insects: Principles and Practice of Population Management; Springer: New York, NY, USA, 1986; pp. 51–77. [Google Scholar]
- Larsson, S.; Oren, R.; Waring, R.H.; Barret, J.W. Attacks of mountain pine beetle as related to tree vigor of ponderosa pine. For. Sci. 1983, 29, 395–402. [Google Scholar]
- Raffa, K.F.; Berryman, A.A. The role of host plant resistance in the colonization behavior and ecology of bark beetles (Coleoptera: Scolytidae). Ecol. Monogr. 1983, 53, 27–49. [Google Scholar] [CrossRef]
- Waring, R.H.; Pitman, G.B. A Simple Model of Host Resistance to Bark Beetles; Oregon State University, School of Forestry: Corvallis, OR, USA, 1980. [Google Scholar]
- Christiansen, E.; Waring, R.H.; Berryman, A.A. Resistance of conifers to bark beetle attack—Searching for general relationships. For. Ecol. Manag. 1987, 22, 89–106. [Google Scholar] [CrossRef]
- Lohr, S. Sampling Design and Analysis, 2nd ed.; Brooks/Cole Cengage Learning: Boston, MA, USA, 2010. [Google Scholar]
- McKinney, D.W.; Fiedler, C.E.; Tomback, D.F. Invasive pathogen threatens bird-pine mutualism: Implications for sustaining a high-elevation ecosystem. Ecol. Appl. 2009, 19, 597–607. [Google Scholar] [CrossRef] [PubMed]
- National Park Service (NPS). Summary of Whitebark Pine Status and Cone Presence Used in Analysis for Management Paper; Interagency Whitebark Pine Long-Term Monitoring Database; 2014.
- McCaughey, W.; Scott, G.L.; Izlar, K.L. Whitebark pine planting guidelines. West. J. Appl. For. 2009, 24, 163–166. [Google Scholar]
- Stein, B.A.; Glick, P.; Edelson, N.; Staudt, A. Climate-Smart Conservation: Putting Adaptation Principles into Practice; National Wildlife Federation: Washington, DC, WA, USA, 2014. [Google Scholar]
- Walters, C. Adaptive Management of Renewable Resources; Macmillan: New York, NY, USA, 1986. [Google Scholar]
- Hickler, T.; Vohland, K.; Feehan, J.; Miller, P.A.; Smith, B.; Costa, L.; Giesecke, T.; Fronzek, S.; Carter, T.R.; Cramer, W.; et al. Projecting the future distribution of European potential natural vegetation zones with a generalized, tree species-based dynamic vegetation model. Glob. Ecol. Biogeogr. 2012, 21, 50–63. [Google Scholar] [CrossRef]
- Menges, E.S. Population viability analysis in plants: Challenges and opportunities. Trends Ecol. Evolut. 2000, 15, 51–56. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. IPCC, 2013: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Chong, G.; Battllori, E.; Coop, J.; Haire, S.; Krawchuck, M.A.; Miller, C.; Parisien, M.A.; Whitman, E. Great Northern LCC Fire Refugia Project. Available online: https://griffingroups.com/groups/profile/23823/great-northern-lcc-fire-refugia-project (accessed on 6 January 2016).
- Long, E.; Biber, E. The wilderness act and climate change adaptation. Environ. Law 2014, 44, 632–691. [Google Scholar]
- Stephenson, N.L.; Millar, C.I. Climate change: Wilderness’ greatest challenge. Park Sci. 2012, 28, 34–38. [Google Scholar] [CrossRef]
- Schullary, P. Searching for Yellowstone: Ecology and wonder in the Last Wilderness; Montana Historical Society Press: Helena, MT, USA, 1997. [Google Scholar]
- Stephenson, N.L. Making the transition to the third era of natural resources management. GWS J. Parks Prot. Areas Cult. Sites 2014, 31, 227–235. [Google Scholar]
- Watson, A.; Martin, S.; Christensen, N.; Fauth, G.; Williams, D. The relationship between perceptions of wilderness character and attitudes toward management intervention to adapt biophysical resources to a changing climate and nature restoration at Sequoia and Kings Canyon National parks. J. Environ. Manag. 2015, 56, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, E.A.; (Department of Political Science, Montana State University, Bozeman, MT, USA.). Personal communication, 2015.
- Monahan, W.B.; Cook, T.; Melton, F.; Connor, J.; Bobowski, B. Forecasting distributional responses of limber pine to climate change at management-relevant scales in Rocky Mountain National Park. PLos ONE 2013, 8, e83163. [Google Scholar] [CrossRef] [PubMed]
- Zolkos, S.G.; Jantz, P.; Cormier, T.; Iverson, L.R.; McKenney, D.W.; Goetz, S.J. Projected tree species redistribution under climate change: Implications for ecosystem vulnerability across protected areas in the eastern United States. Ecosystems 2015, 18, 202–220. [Google Scholar] [CrossRef]
- Hennon, P.E.; D’Amore, D.V.; Schaberg, P.G.; Witter, P.G.; Shanley, C.S. Shifting climate, altered niche, and a dynamic conservation strategy for yellow-cedar in the North Pacific coastal rainforest. Bioscience 2012, 62, 147–158. [Google Scholar]
- Colwell, R.; Brehm, G.; Cardelús, C.L.; Gilman, A.C.; Longino, J.T. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 2008, 322, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.M.; Bradford, J.B.; Lauenroth, W.K. Early indicators of change: Divergent climate envelopes between tree life stages imply range shifts in the western United States. Glob. Ecol. Biogeogr. 2014, 23, 168–180. [Google Scholar] [CrossRef]
- Iverson, L.R.; Prasad, A.M.; Matthews, S.N.; Peters, M. Estimating potential habitat for 134 eastern US tree species under six climate scenarios. For. Ecol. Manag. 2008, 254, 390–406. [Google Scholar] [CrossRef]
- Aubry, C.A.; Devine, W.; Shoal, R.; Bower, A.D.; Miller, J.; Maggiulli, N. Climate Change and Forest Biodiversity: A Vulnerability Assessment and Action Plan for National Forests in Western Washington; U.S. Department of Agriculture, Forest Service, PNW Region: Portland, OR, USA, 2011.
- Gray, L.K.; Hamman, A. Tracking suitable habitat for tree populations under climate change in western North America. Clim. Chang. 2013, 117, 289–303. [Google Scholar] [CrossRef]
- McKinney, D.W.; Pedlar, J.H.; Rood, R.B.; Price, D. Revisiting projected shifts in the climate envelopes of North American trees using updated general circulation models. Glob. Chang. Biol. 2011, 17, 2720–2730. [Google Scholar] [CrossRef]
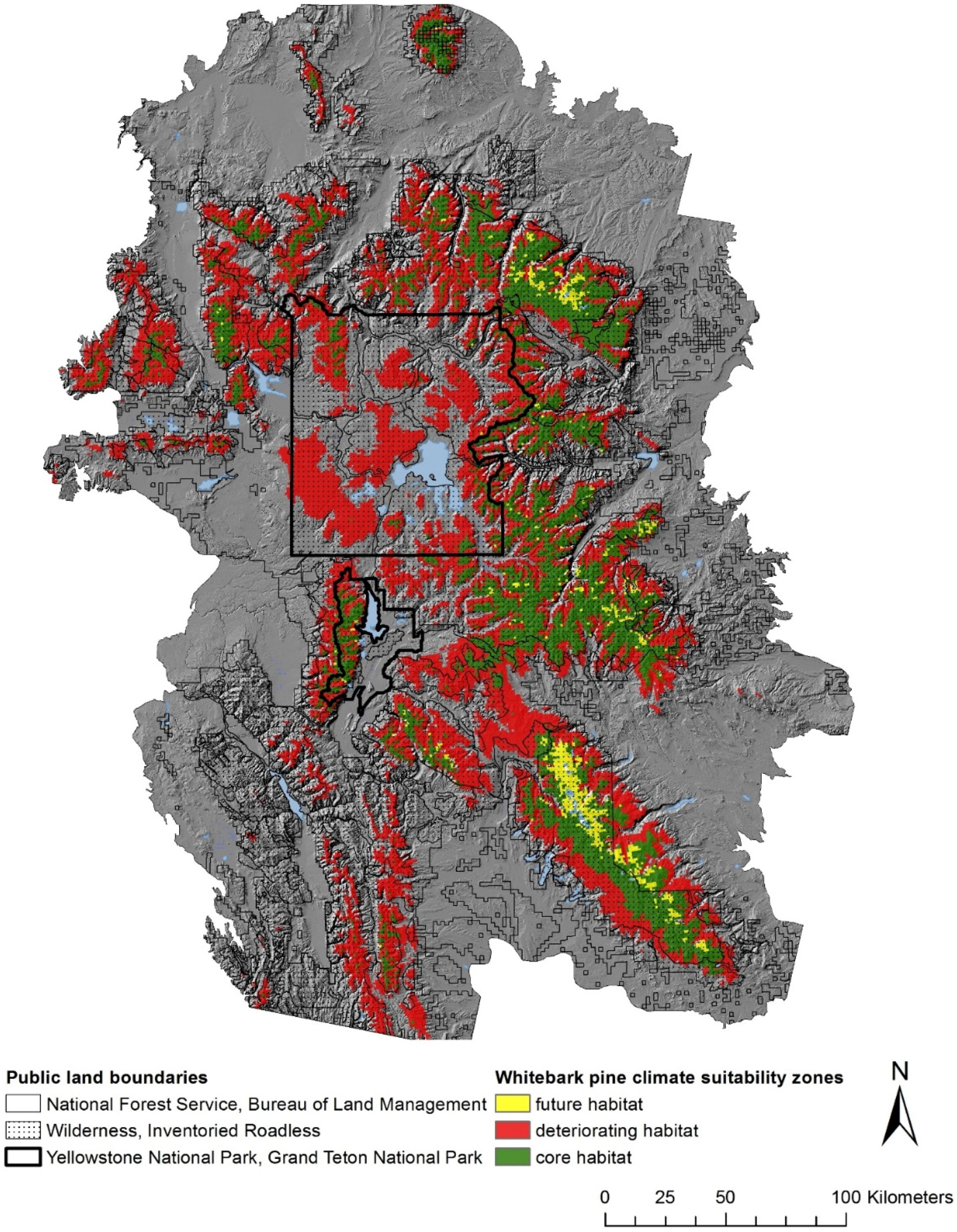
| Land Allocation Type | Agency | Legal Direction/Management Philosophy | Proportion of GYE WBP Aerial Extent |
|---|---|---|---|
| Multiple Use | National Forest Service; Bureau of Land Management | Multiple use while maintaining ecological integrity | 8.09% |
| Non-wilderness | National Park Service | Preserve unimpaired natural resources for the enjoyment, education, and inspiration of this and future generations. | 0.14% |
| Wilderness (Designated, Proposed, Recommended, Study Area) | National Forest Service; Bureau of Land Management; National Park Service; U.S. Fish and Wildlife Service | Maintain natural and untrammeled conditions | 68.18% |
| Inventoried Roadless Area | National Forest Service | Roads and timber harvest prohibited. Forest health treatments allowed. | 23.38% |
| Management Activity | Land Allocation Type | ||
|---|---|---|---|
| Multiple Use | Wilderness 1 | Inventoried Roadless Areas | |
| Research/monitoring | 101 plots | 105 plots | 83 plots |
| Reforestation monitoring | 700 ha total | 0 ha | 351 ha |
| Protection from mountain pine beetle | 688 trees and 890 ha annually | 398 trees and 34 ha annually | 76 trees and 157 ha annually |
| Planting seeds/seedlings | 302 ha total | 4 ha total | 351 ha total |
| Mechanical pruning/thinning | 428 ha total | 0 ha total | 8 ha total |
| Targeted fire suppression | 0 ha total | 0 ha total | 0 ha total |
| Wildland/prescribed fire use | 53 ha total | 0 ha total | 0 ha total |
| Seed collection | 166 bushels total | 20 bushels total | 101 bushels total |
| Limiting Factors | Mechanism | Weight of Evidence (Citations) | Research Needs | Management Implications (Citations) |
|---|---|---|---|---|
| Climate change | WBP persistence in microrefugia | Strong [68,69,70,71,72] | Finer resolution modeling to identify microrefugia and monitoring of WBP performance | Remove competing species in cold/wet settings; protect from mountain pine beetles in cold/dry settings; protect microrefugia from severe fire [71,73] |
| Climate tolerances | Fundamental temperature niche broader than currently perceived | Moderate [71,74,75,76] | Physiological studies of temperature, precipitation limits | Remove competing species to reduce competitive exclusion of WBP in warmer settings [12,13,24,30,65,77] |
| Competing tree species | Release from competition improves WBP viability | Strong [40,78,79] | Species/age specific competitive effects on WBP | |
| Fire | Enhanced moderate severity fire favors WBP | Weak [77,80] | Projections of fire severity in WBP habitat; WBP sensitivity to fire | Protect rust-resistant trees, especially those whose seeds are being harvested; allow more wildland fire use fires during moderate years; [12,13,48,65,81] |
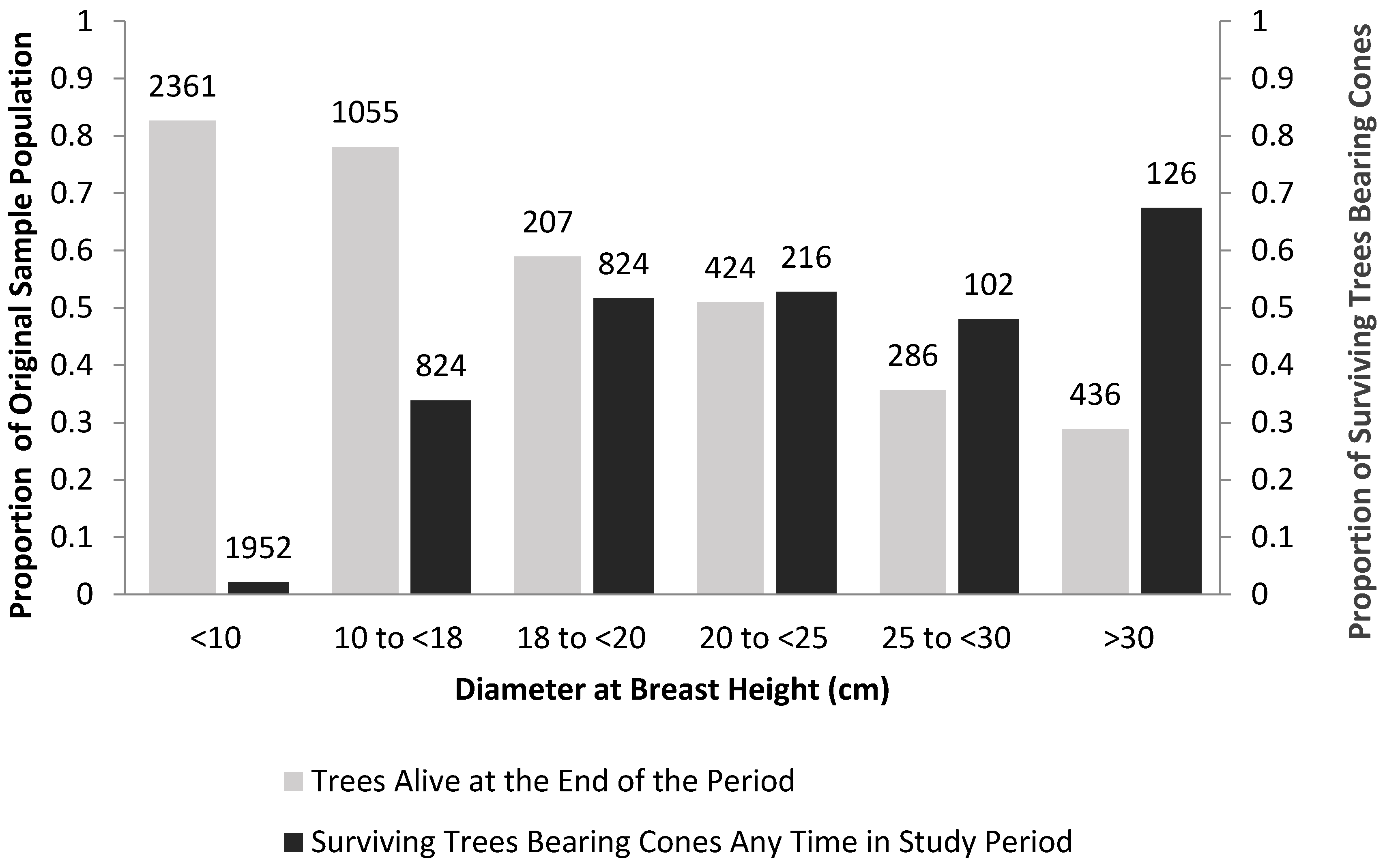
| Mountain Pine Beetle | Medium sized WBP 1 resist beetles and reproduce | Strong [82,83,84,85], Figure 4 | Rates of reproduction and survival of medium sized WBP1 relative to minimum population size | Remove competing vegetation and perhaps thin existing WBP to increase vigor of reproducing trees [12,13,65,86] |
| White Pine Blister Rust | Natural and artificial selection allows persistence | Well known [87,88] | Long term monitoring of rust resistance | Plant rust-resistant seedlings; protect known rust-resistant individuals from MPB and fire [89,90] |
| Habitat Type | Goals | Objectives | Strategies |
|---|---|---|---|
| Core | Maintain currently viable populations | Reduce probability of stand replacing disturbances | Exclude stand replacing fires |
| Maximize tree vigor to enhance survival and reproduction | Use prescribed fire and thinning to reduce competition | ||
| Minimize beetle populations | Apply insecticides to reduce mortality due to beetles | ||
| Maximize seed dispersal to future habitats | Maximize tree vigor to enhance survival and reproduction | Use prescribed fire and thinning to promote WBP cone production to swamp predation and maintain dispersers | |
| Produce seedlings for planting in future habitats | Grow rust-resistant and warm-tolerant stock | Collect seed from rust-resistant and/or warm tolerant WBP trees and rear in nurseries | |
| Deteriorating | Retain WBP population functions as long as possible | Same objectives as in core habitat as resources allow | Same strategies as in core habitat as resources allow |
| Future | Encourage establishment of WBP | Create suitable sites for seedling establishment | Use prescribed fire and mechanical treatment to create suitable openings |
| Establish new stands from nursery stock | Plant rust-resistant and/or warm tolerant WBP trees and rear in nurseries | ||
| Encourage development of viable populations | Same objectives as in core habitat as resources allow | Same strategies as in core habitat as resources allow | |
| All | Improve knowledge and management effectiveness | Refine management effectiveness | Research, monitoring, adaptive management |
| Climate Zone | Future Fire Risk | Treatment and Priority: 1-High, 2-Moderate, 3-Low, 4-not Appropriate | ||||
|---|---|---|---|---|---|---|
| Planting | Thinning | MPB Protection | Prescribed Fire | Wildland Fire Use | ||
| Core | Low | 1-plant to regenerate WBP stands following mortality from MPB or blister rust | 2-reduce competition to increase vigor | 1-protect rust-resistant individuals from MPB mortality | 3-reduce competition to increase vigor | 1-reduce fuels, increase vigor, and create landscape heterogeneity |
| High | 1-plant favorable burned areas with rust-resistant seedlings | 2-reduce canopy and surface fuels to lower potential fire-caused mortality | 1-protect rust-resistant individuals from MPB mortality | 3-use prescribed fire to reduce competition and fuel loads | 1-reduce fuels, increase vigor, and create landscape heterogeneity | |
| Deteriorating | Low | 3-plant rust-resistant seedlings to keep the species in historical lands to monitor for success | 4-benefits will be minimal | 3-protect rust-resistant individuals | 3-reduce competition for rust-resistant individuals | 2-promote landscape heterogeneity |
| High | 4-seedlings have a high risk of being burned | 3-may promote growth of rust-resistant individuals | 4-existing trees have a high risk of being killed by fire | 3-reduce canopy and surface fuels to protect rust-resistant individuals | 3-reduce fuels and promote landscape heterogeneity | |
| Future | Low | 3-plant burns with rust-resistant seedlings | 4-most high elevation stands competition free | 2-protect rust-resistant individuals | 3-lower fuels to reduce fire risk to rust-resistant individuals | 2-Promote landscape hetereogeneity and reduce fuels |
| High | 3-plant to ensure future competitive advantage and mimic nutcracker dispersal | 4-most high elevation stands competition free | 3-Protect rust-resistant individuals | 4-probably won't need additional fire | 2-promote landscape heterogeneity, reduce fuels | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, A.; Ireland, K.; Legg, K.; Keane, R.; Barge, E.; Jenkins, M.; Pillet, M. Complex Challenges of Maintaining Whitebark Pine in Greater Yellowstone under Climate Change: A Call for Innovative Research, Management, and Policy Approaches. Forests 2016, 7, 54. https://doi.org/10.3390/f7030054
Hansen A, Ireland K, Legg K, Keane R, Barge E, Jenkins M, Pillet M. Complex Challenges of Maintaining Whitebark Pine in Greater Yellowstone under Climate Change: A Call for Innovative Research, Management, and Policy Approaches. Forests. 2016; 7(3):54. https://doi.org/10.3390/f7030054
Chicago/Turabian StyleHansen, Andrew, Kathryn Ireland, Kristin Legg, Robert Keane, Edward Barge, Martha Jenkins, and Michiel Pillet. 2016. "Complex Challenges of Maintaining Whitebark Pine in Greater Yellowstone under Climate Change: A Call for Innovative Research, Management, and Policy Approaches" Forests 7, no. 3: 54. https://doi.org/10.3390/f7030054
APA StyleHansen, A., Ireland, K., Legg, K., Keane, R., Barge, E., Jenkins, M., & Pillet, M. (2016). Complex Challenges of Maintaining Whitebark Pine in Greater Yellowstone under Climate Change: A Call for Innovative Research, Management, and Policy Approaches. Forests, 7(3), 54. https://doi.org/10.3390/f7030054





