Development of Ash Dieback in South-Eastern Germany and the Increasing Occurrence of Secondary Pathogens
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Sites
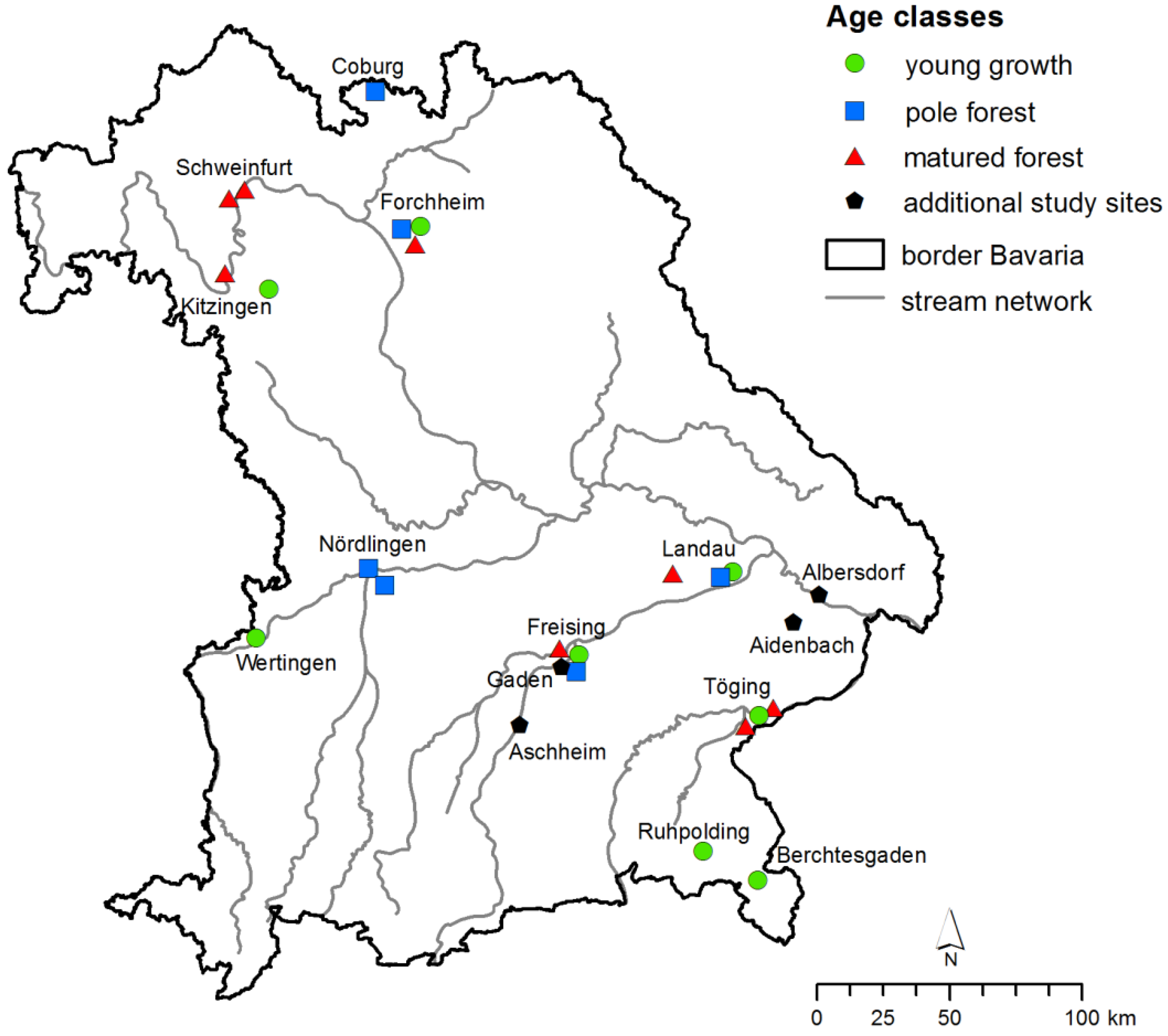
2.2. Measurement of Vitality and Diameters at Breast Height
| Young Growth Stands | Height (m) | Pole Forests | DBH (cm) | Matured Forests | DBH (cm) |
|---|---|---|---|---|---|
| Berchtesgaden (1) | 4.3 | Coburg (2) | 11.9 | Forchheim (3) | 26.6 |
| Forchheim (5) | 2.5 | Forchheim (4) | 8.7 | Freising (6) | 37.9 |
| Freising (8) | 2.1 | Freising (7) | 12.7 | Kitzingen (10) | 38.8 |
| Kitzingen (11) | 2.4 | Landau (12) | 5.7 | Landau (14) | 35.8 |
| Landau (13) | 2.4 | Nördlingen I (16) | 13.0 | Schweinfurt I (19) | 36.2 |
| Ruhpolding (18) | 1.9 | Nördlingen II (17) | 14.7 | Schweinfurt II (20) | 33.0 |
| Töging (23) | 4.2 | Töging I (21) | 45.7 | ||
| Wertingen (24) | 3.8 | Töging II (22) | 52.0 | ||
| Average | 3.0 | 11.1 | 38.3 |
| Pole Forests | Mean Vitality | Mean DBH (cm) | Minimum DBH (cm) | Maximum DBH (cm) |
|---|---|---|---|---|
| Gaden | 3.11 | 14.1 | 7.0 | 33.5 |
| Aschheim | 1.82 | 15.6 | 7.8 | 25.7 |
| Aidenbach | 1,46 | 16.6 | 8.9 | 22.7 |
| Albersdorf | 2.19 | 11.7 | 6.2 | 19.7 |
| Average | 2.15 | 14.5 | 7.5 | 25.4 |
2.3. Isolation and Cultivation of H. Fraxineus from Infected Branches
| Study Site | Type of Tissue | No. of Samples | Cultivation/Detection | PCR |
|---|---|---|---|---|
| Young growth stands | ||||
| Berchtesgaden (1) | Infected branches | 10 | 8 | - |
| Forchheim (5) | Infected branches | 4 | 2 | +* |
| Freising (8) | Infected branches | 10 | 3 | +* |
| Kitzingen (11) | Infected branches | 10 | 3 | +* |
| Landau (13) | Infected branches | 10 | 6 | +* |
| Ruhpolding (18) | Infected branches | 10 | 8 | - |
| Töging (23) | Infected branches | 10 | 4 | +* |
| Wertingen (24) | Infected branches | 10 | 6 | +* |
| Pole forests | ||||
| Coburg (2) | Infected branches | 3 | 1 | + |
| Forchheim (4) | Infected branches | 3 | 1 | + |
| Freising (7) | Fruiting bodies | 6 | 6 | + |
| Landau (12) | Infected branches | 3 | 1 | + |
| Nördlingen I (16) | Infected branches | 3 | 2 | + |
| Nördlingen II (17) | Infected branches | 3 | 1 | + |
| Matured forests | ||||
| Forchheim (3) | Infected branches | 3 | 1 | + |
| Freising (6) | Dead petioles | 4 | 3 | + |
| Kitzingen (10) | Infected branches | 3 | 0 | - |
| Landau (14) | Infected branches | 3 | 3 | + |
| Schweinfurt I (19) | Infected branches | 3 | 2 | + |
| Schweinfurt II (20) | Infected branches | 3 | 3 | + |
| Töging I (21) | Infected branches | 3 | 1 | + |
| Töging II (22) | Infected branches | 3 | 1 | + |
2.4. Measurements of Stem Necroses and Their Collar Circumferences
2.5. Isolation of Armillaria spp. from Collar Lesions of Ash Trees
2.6. PCR Method
2.7. Assessment of Bark Beetle Infestations
2.8. Calculations and Statistical Analysis
3. Results
3.1. Development of Vitality in Ash Stands from 2010 to 2014
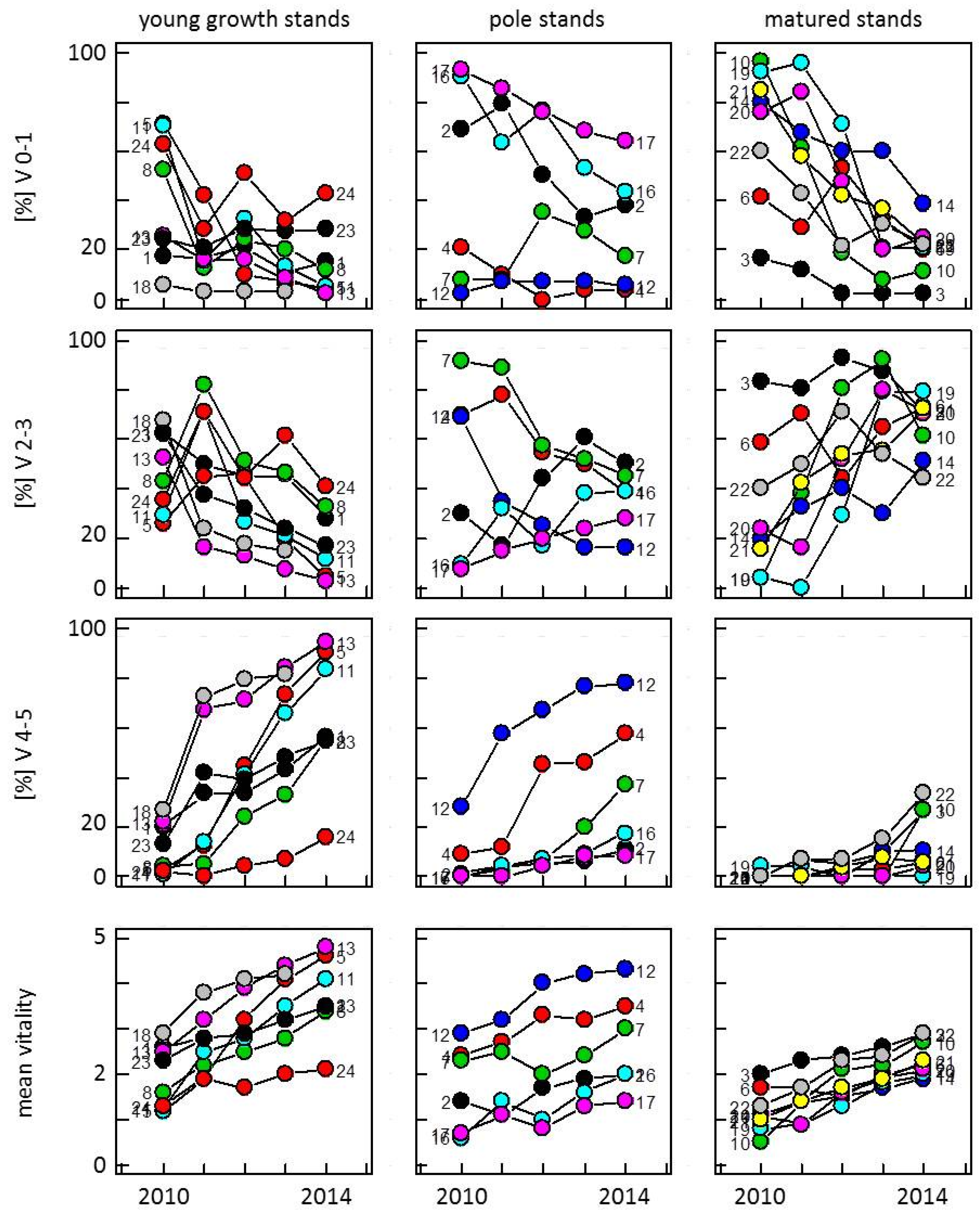
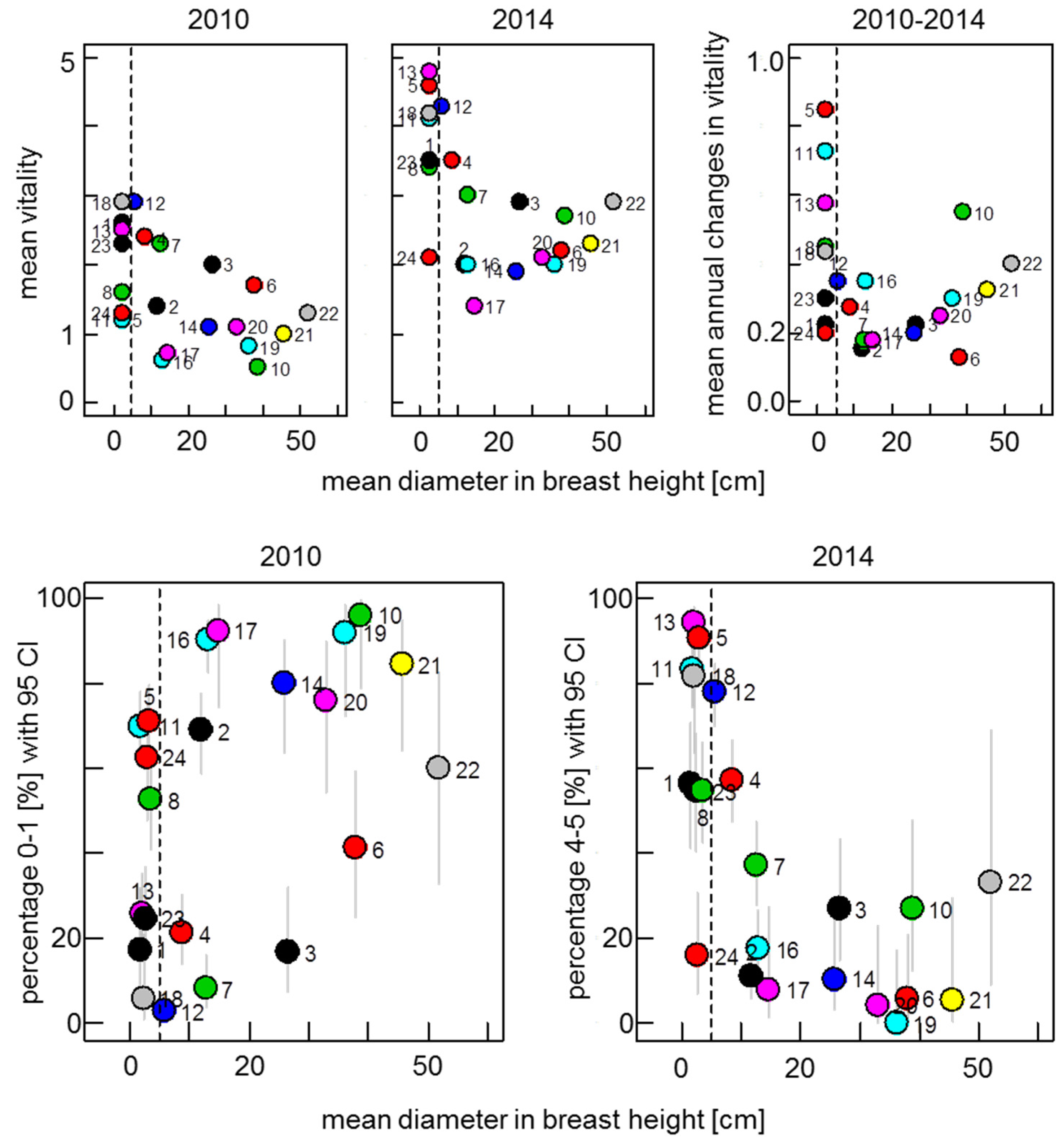
3.2. Screening for Potentially Resistant Trees
| Young Growth Stands | Tolerant Trees | n | % |
|---|---|---|---|
| Berchtesgaden (1) | 2 | 42 | 5 |
| Forchheim (5) | 2 | 77 | 3 |
| Freising (8) | 1 | 70 | 1 |
| Kitzingen (11) | 2 | 97 | 2 |
| Landau (13) | 1 | 78 | 1 |
| Ruhpolding (18) | 0 | 31 | 0 |
| Töging (23) | 1 | 57 | 2 |
| Wertingen (24) | 8 | 48 | 17 |
| Sum | 17 | 500 | 4 |
| Pole stands | Tolerant trees | n | % |
| Coburg (2) | 28 | 99 | 28 |
| Forchheim (4) | 1 | 108 | 1 |
| Freising (7) | 1 | 96 | 1 |
| Landau (12) | 2 | 124 | 2 |
| Nördlingen (16) | 35 | 98 | 36 |
| Nördlingen (17) | 16 | 25 | 64 |
| Sum | 83 | 550 | 22 |
| Matured stands | Tolerant trees | n | % |
| Forchheim (3) | 0 | 41 | 0 |
| Freising (6) | 4 | 34 | 12 |
| Kitzingen (10) | 3 | 26 | 12 |
| Landau (14) | 14 | 39 | 36 |
| Schweinfurt (19) | 5 | 24 | 21 |
| Schweinfurt (20) | 5 | 24 | 21 |
| Töging (21) | 4 | 17 | 24 |
| Töging (22) | 1 | 9 | 11 |
| Sum | 36 | 214 | 17 |
3.3. Extent of Armillaria Infection in Reference to the Lengths and Circumferences of Collar Lesions
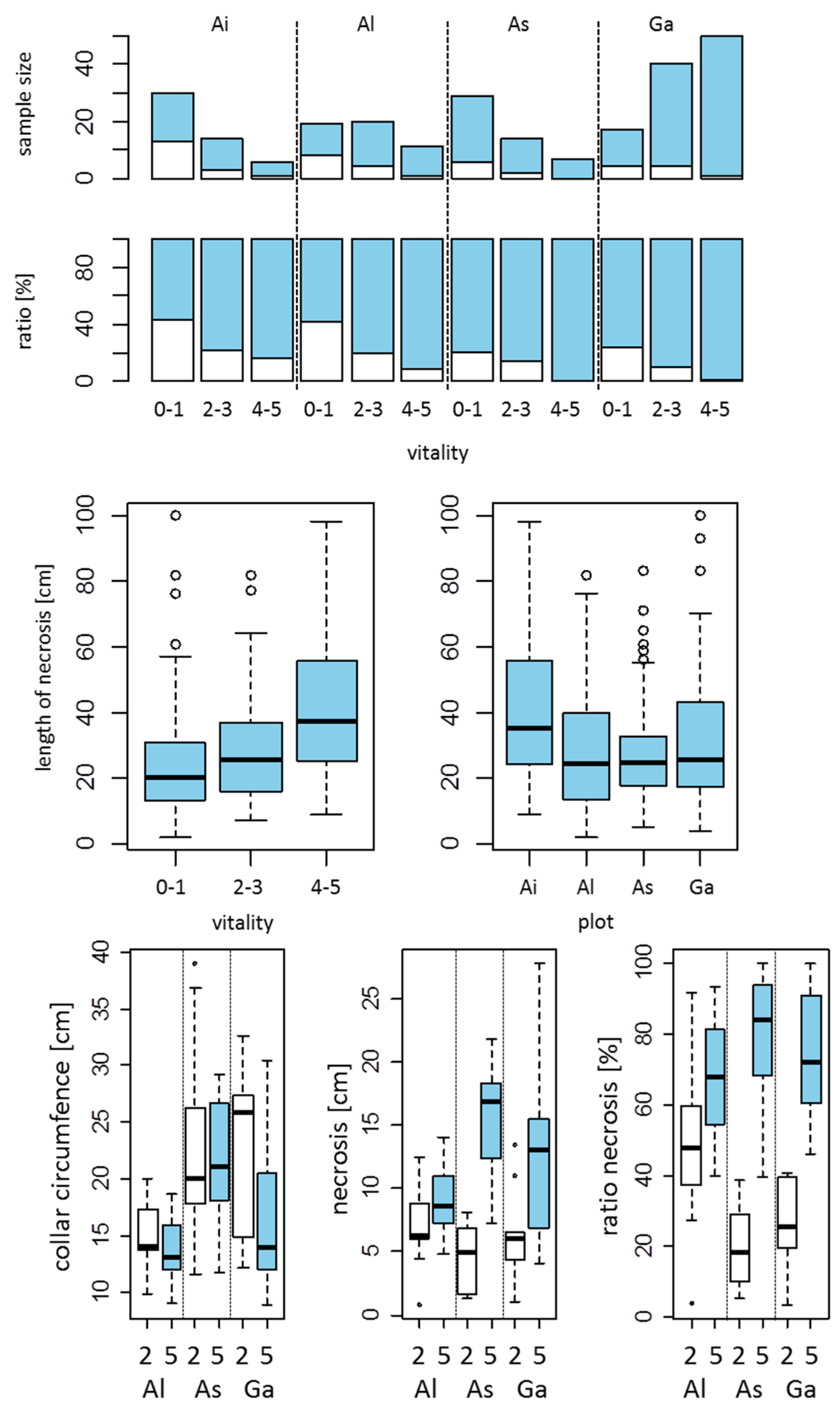
3.4. Armillaria Conditioned Mortality
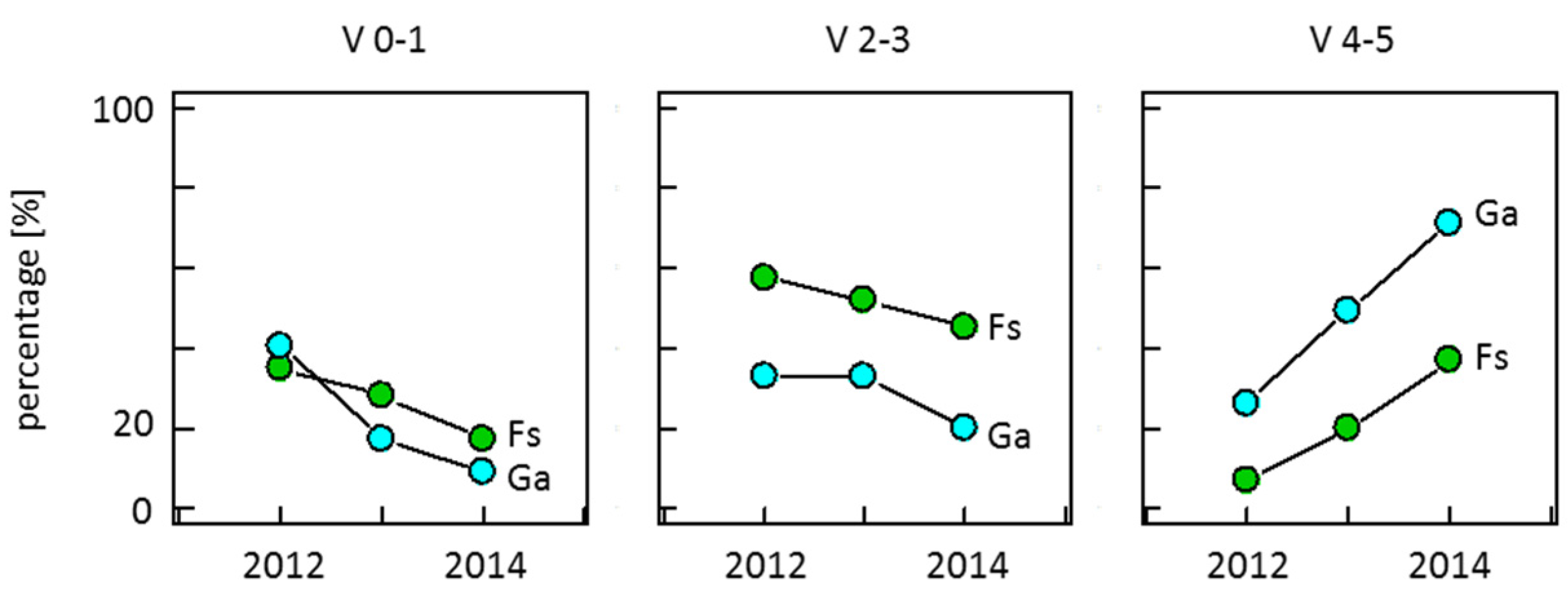
| Vitality Dev. | 2012–2013 | 2013–2014 | ||
|---|---|---|---|---|
| Gaden | Freising | Gaden | Freising | |
| 0–5 | 0 | 0 | 0 | 0 |
| 1–5 | 4 | 0 | 2 | 0 |
| 2–5 | 0 | 1 | 4 | 0 |
| 3–5 | 4 | 3 | 0 | 1 |
| 4–5 | 8 | 3 | 13 | 3 |
| 5–5 | 13 | 3 | 29 | 10 |
| Sum | 29 | 10 | 48 | 14 |
3.5. Breeding Galleries of Ash Bark Beetles Only in Recently Dead Trees
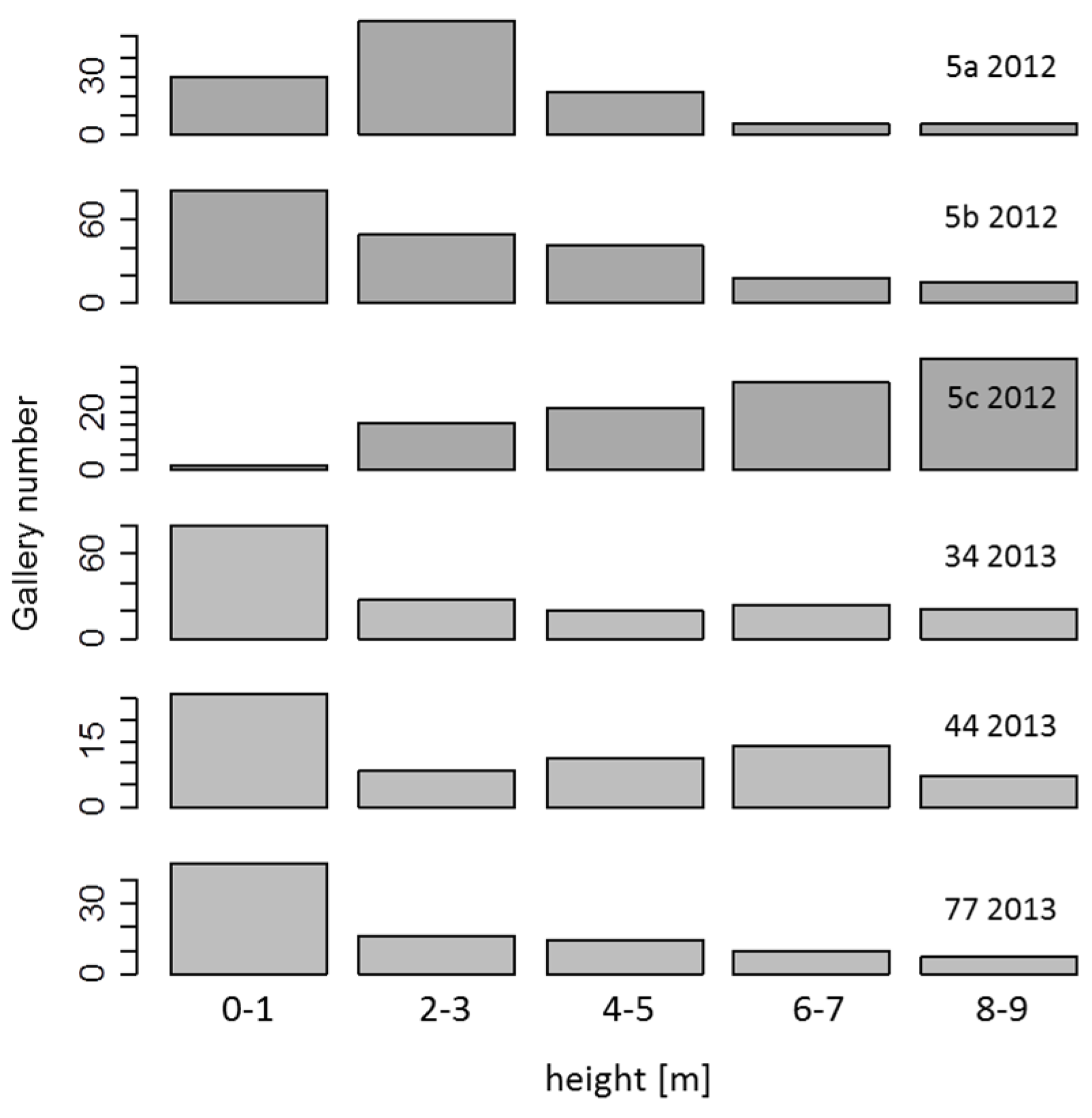
| Feature/Sample Area | Ga | As | Ai | Al |
|---|---|---|---|---|
| Total amount of vitality 5-trees | 29 | 13 | 4 | 15 |
| Greenish under the bark | 7 | 10 | 2 | 14 |
| Brownish under the bark | 22 | 3 | 2 | 1 |
| Recently generated breeding galleries | 7 | 10 | 2 | 13 |
| Vitality 5-trees with greenish bark and breeding galleries [%] | 100 | 100 | 100 | 93 |
4. Discussion
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baral, H.O.; Queloz, V.; Hosoya, T. Hymenoscyphus fraxineus, the correct scientific name for the fungus causing ash dieback in Europe. IMA Fungus 2014, 5, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.J.; Hosoya, T.; Baral, H.O.; Hosaka, K.; Kakishima, M. Hymenoscyphus pseudoalbidus, the correct name for Lambertella albida reported from Japan. Mycotaxon 2013, 122, 25–41. [Google Scholar] [CrossRef]
- Zheng, H.D.; Zhuang, W.Y. Hymenoscyphus albidoides sp. nov. and H. pseudoalbidus from China. Mycol. Prog. 2014, 13, 625–638. [Google Scholar] [CrossRef]
- Han, J.G.; Shrestha, B.; Hosoya, T.; Lee, K.H.; Sung, G.H.; Shin, H.D. First report of the ash dieback pathogen Hymenoscyphus fraxineus in Korea. Mycobiology 2014, 42, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Queloz, V.; Grünig, C.R.; Berndt, R.; Kowalski, T.; Sieber, T.N.; Holdenrieder, O. Cryptic speciation in Hymenoscyphus albidus. For. Pathol. 2011, 41, 133–142. [Google Scholar] [CrossRef]
- Timmermann, V.; Borja, I.; Hietala, A.M.; Kirisits, T.; Solheim, H. Ash dieback: Pathogen spread and diurnal patterns of ascospore dispersal, with special emphasis on Norway. EPPO Bull. 2011, 41, 14–20. [Google Scholar] [CrossRef]
- Skovsgaard, J.P.; Thomsen, I.M.; Skovgaard, I.M.; Martinussen, T. Associations among symptoms of dieback in even-aged stands of ash (Fraxinus excelsior L.). For. Pathol. 2010, 40, 7–18. [Google Scholar] [CrossRef]
- Kjær, E.D.; McKinney, L.V.; Nielsen, L.R.; Hansen, L.N.; Hansen, J.K. Adaptive potential of ash (Fraxinus excelsior) populations against the novel emerging pathogen Hymenoscyphus pseudoalbidus. Evol. Appl. 2012, 5, 219–228. [Google Scholar] [CrossRef] [PubMed]
- McKinney, L.V.; Thomsen, I.M.; Kjaer, E.D.; Nielsen, L.R. Genetic resistance to Hymenoscyphus pseudoalbidus limits fungal growth and symptom occurrence in Fraxinus excelsior. For. Pathol. 2012, 42, 69–74. [Google Scholar] [CrossRef]
- Metzler, B.; Baumann, M.; Baier, U.; Heydeck, P.; Bressem, U.; Lenz, H. Bundesweite Zusammenstellung: Handlungsempfehlungen beim Eschentriebsterben. AFZ-Der Wald 2013, 68, 17–20. [Google Scholar]
- Kunca, A.; Leontovyc, R.; Zúbrik, M.; Gubka, A. Bark beetle outbreak on weakened ash trees and applied control measures. EPPO Bull. 2011, 41, 11–13. [Google Scholar] [CrossRef]
- Schedl, K.E. Scolytidae. In Die Käfer Mitteleuropas: Band 10; Freude, H., Harde, K.W., Lohse, G.A., Eds.; Goecke & Evers Verlag: Krefeld, Germany, 1981; pp. 34–99. [Google Scholar]
- Sauvard, D. General biology of bark beetles. In Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis; Lieutier, F., Day, K.R., Battisti, A., Grégoire, J.C., Evans, H.F., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2004; pp. 63–88. [Google Scholar]
- Pfister, A. Aktuelle Schäden durch Eschenbastkäfer in der Steiermark. Forstsch. Aktuell 2012, 54, 22–25. [Google Scholar]
- Keča, N.; Klopfenstein, N.B.; Kim, M.S.; Solheim, H.; Woodward, S. Initial characterization of an unidentified Armillaria isolate from Serbia using LSU-IGS1 and TEF-1-α genes. For. Pathol. 2015, 45, 120–126. [Google Scholar] [CrossRef]
- Longa, C.M.O.; La Porta, N. Rapid identification of Armillaria species by PCR-DGGE. J. Microbiol. Methods 2014, 107, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Prospero, S.; Holdenrieder, O.; Rigling, D. Comparison of the virulence of Armillaria cepistipes and Armillaria ostoyae on four Norway spruce provenances. For. Pathol. 2004, 34, 1–14. [Google Scholar] [CrossRef]
- Lygis, V.; Vasiliauskas, R.; Larsson, K.H.; Stenlid, J. Wood-inhabiting fungi in stems of Fraxinus excelsior in declining ash stands of northern Lithuania, with particular reference to Armillaria cepistipes. Scand. J. For. Res. 2005, 20, 337–346. [Google Scholar] [CrossRef]
- Enderle, R.; Peters, F.; Nakou, A.; Metzler, B. Temporal development of ash dieback symptoms and spatial distribution of collar rots in a provenance trial of Fraxinus excelsior. Eur. J. For. Res. 2013, 132, 865–876. [Google Scholar] [CrossRef]
- Kelley, M.B.; Fierke, M.K.; Stephen, F.M. Identification and distribution of Armillaria species associated with an oak decline event in the Arkansas Ozarks. For. Pathol. 2009, 39, 397–404. [Google Scholar] [CrossRef]
- Solla, A.; Tomlinson, F.; Woodward, S. Penetration of Picea sitchensis root bark by Armillaria mellea, Armillaria ostoyae and Heterobasidion annosum. For. Pathol. 2002, 32, 55–70. [Google Scholar] [CrossRef]
- Roll-Hansen, F. The Armillaria species in Europe: A literature review. Eur. J. For. Pathol. 1985, 15, 22–31. [Google Scholar] [CrossRef]
- Lenz, H.D.; Straßer, L.; Baumann, M.; Baier, U. Boniturschlüssel zur Einstufung der Vitalität von Alteschen. AFZ-DerWald 2012, 3, 18–19. [Google Scholar]
- Kowalski, T. Chalara fraxinea sp. nov. associated with dieback of ash (Fraxinus excelsior) in Poland. For. Pathol. 2006, 36, 264–270. [Google Scholar] [CrossRef]
- Gherghel, F.; Fussi, B.; Donges, K.; Haustein, M.; Jakob, K.M.; Müller, K.; Piškur, B.; Hauptman, T.; Lenz, H.D.; Konnert, M.; et al. The development of a species-specific test to detect Hymenoschyphus pseudoalbidus in ash tissues. For. Pathol. 2014, 44, 137–144. [Google Scholar] [CrossRef]
- Maphosa, L.; Wingfield, B.D.; Coetzee, M.P.A.; Mwenje, E.; Wingfield, M.J. Phylogenetic relationships among Armillaria species inferred from partial elongation factor 1-alpha DNA sequence data. Australas. Plant Pathol. 2006, 35, 513–520. [Google Scholar] [CrossRef]
- Johansson, S.B.K.; Vasaitis, R.; Ihrmark, K.; Barklund, P.; Stenlid, J. Detection of Chalara fraxinea from tissue of Fraxinus excelsior using species-specific ITS primers. For. Pathol. 2010, 40, 111–115. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Schumacher, J.; Wulf, A.; Leonhard, S. Erster Nachweis von Chalara fraxinea T. Kowalski in Deutschland- ein Verursacher neuartiger Schäden an Eschen. Deutsch. Pflanzenschutzd. 2007, 59, 121–123. [Google Scholar]
- Leonhard, S.; Straßer, L.; Nannig, A.; Blaschke, M.; Schumacher, J.; Immler, T. Neues Krankheitsphänomen an der Esche: Das von Chalara fraxinea verursachte Eschentriebsterben ist auch in Bayern nachgewiesen. LWF Aktuell 2009, 71, 60–63. [Google Scholar]
- Pliura, A.; Lygis, V.; Suchockas, V.; Bartkevicius, E. Performance of twenty four European Fraxinus excelsior populations in three Lithuanian progeny trials with a special emphasis on resistance to Chalara fraxinea. Balt. For. 2011, 17, 17–34. [Google Scholar]
- McKinney, L.V.; Nielsen, L.R.; Hansen, J.K.; Kjaer, E.D. Presence of natural genetic resistance in Fraxinus excelsior (Oleraceae) to Chalara fraxinea (Ascomycota): An emerging infectious disease. Heredity 2011, 106, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Stener, L.G. Clonal differences in susceptibility to the dieback of Fraxinus excelsior in southern Sweden. Scand. J. For. Res. 2013, 28, 205–216. [Google Scholar] [CrossRef]
- Rytkönen, A.; Lilja, A.; Drenkhan, R.; Gaitnieks, T.; Hantula, J. First record of Chalara fraxinea in Finland and genetic variation among isolates sampled from Aland, mainland Finland, Estonia and Latvia. For. Pathol. 2011, 41, 169–174. [Google Scholar] [CrossRef]
- Entry, J.A.; Martin, N.E.; Cromack, K.; Stafford, S.G. Light and nutrient limitation in Pinus monticola: Seedling susceptibility to Armillaria infection. For. Ecol. Manag. 1986, 17, 189–198. [Google Scholar] [CrossRef]
- Bakys, R.; Vasiliauskas, A.; Ihrmark, K.; Stenlid, J.; Menkis, A.; Vasaitis, R. Root rot, associated fungi and their impact on health condition of declining Fraxinus excelsior stands in Lithuania. Scand. J. For. Res. 2011, 26, 128–135. [Google Scholar] [CrossRef]
- Husson, C.; Caël, O.; Grandjean, J.P.; Nageleisen, L.M.; Marçais, B. Occurrence of Hymenoscyphus pseudoalbidus on infected ash logs. Plant Pathol. 2012, 61, 889–895. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenz, H.D.; Bartha, B.; Straßer, L.; Lemme, H. Development of Ash Dieback in South-Eastern Germany and the Increasing Occurrence of Secondary Pathogens. Forests 2016, 7, 41. https://doi.org/10.3390/f7020041
Lenz HD, Bartha B, Straßer L, Lemme H. Development of Ash Dieback in South-Eastern Germany and the Increasing Occurrence of Secondary Pathogens. Forests. 2016; 7(2):41. https://doi.org/10.3390/f7020041
Chicago/Turabian StyleLenz, Heike D., Bernadett Bartha, Ludwig Straßer, and Hannes Lemme. 2016. "Development of Ash Dieback in South-Eastern Germany and the Increasing Occurrence of Secondary Pathogens" Forests 7, no. 2: 41. https://doi.org/10.3390/f7020041
APA StyleLenz, H. D., Bartha, B., Straßer, L., & Lemme, H. (2016). Development of Ash Dieback in South-Eastern Germany and the Increasing Occurrence of Secondary Pathogens. Forests, 7(2), 41. https://doi.org/10.3390/f7020041





