4.1. How Resistant to Drought Are Seedlings of the Minor Tree Species?
A synoptic evaluation of the species’ drought tolerance is not straightforward since the measured variables did not always provide a clear picture for individual species and were not necessarily consistent for the duration of the experiment. In addition, there is no agreed formula for the combination of these variables into an index. However, we attempted such a synthesis of the main outcomes of the experiment (
Table 6). The selection and ranking of the variables was based on their validity to express drought impacts on plants. Shoot dieback was selected as the most important variable to rank drought tolerance because it integrates the influence of many different processes and, if it translates into mortality, has a high relevance for the higher hierarchical level, the population [
24,
59,
85]. Shedding of leaves or twigs may be regarded as the ultimate measure of drought resistance, although without further information one might not be able to identify the cause of dieback such as hydraulic failure or carbon starvation [
21]. Biomass increment under drought conditions was selected as the second most important variable as it integrates various manifestations of assimilation, physiology, and metabolism [
86,
87]. Therefore, biomass increment under conditions of water shortage serves as a proxy to evaluate drought tolerance, but single physiological parameters like
Amax,
E,
gs, and WUE are nonetheless important [
59,
63,
64], especially the relationship between
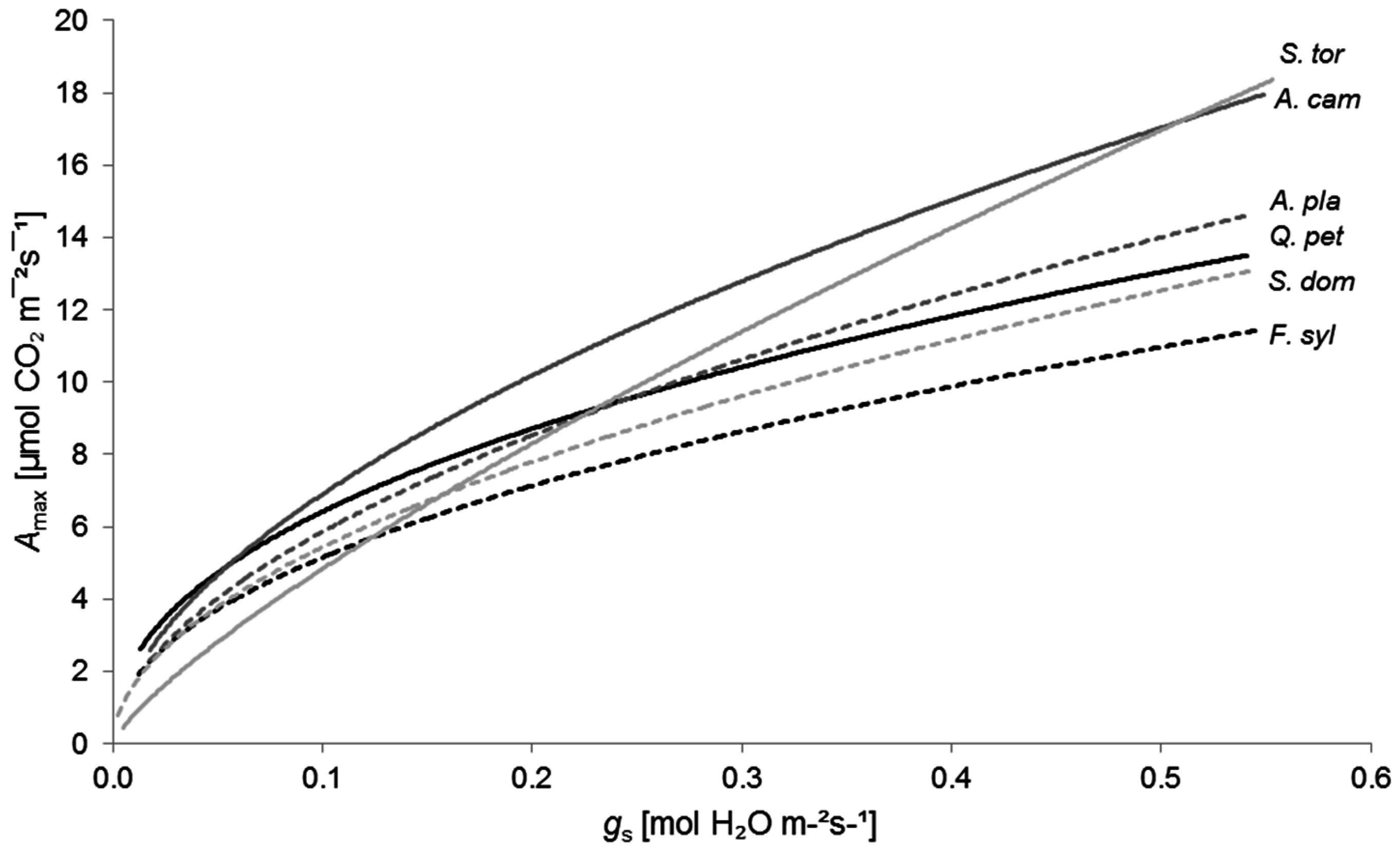
Amax and
gs [
49,
57,
84]. The relation of both variables is a straightforward measure of physiological drought stress, as
Amax decreases with declining
gs values owing to water shortage [
84]. Further variables like the root-to-shoot (r/s) ratio could also be selected to convey drought tolerance [
88,
89]. Although initial biomass of the seedlings had no significant influence on the root-to-shoot ratio, it remains unclear if 69 days of artificial drought were sufficient to alter within-plant carbon allocation of the seedlings expressed in the calculated r/s ratios. Therefore, we did not add it to the relative ranking of the species’ drought tolerance. The drought tolerance ranking of tree species in
Table 6 would not change if the r/s ratio was also taken into account. However, the two
Acer species were the only ones where the r/s ratio did not decline significantly from control to dry treatment.
In summary, the
Acer species and
Quercus petraea obtained a higher drought tolerance ranking than
Fagus sylvatica and the
Sorbus species. This ranking is somewhat unexpected when considering the ecosystems in which the species occur naturally, which might suggest a higher ranking of the
Sorbus species. Both
Sorbus species typically occur in xerothermic Mediterranean ecosystems ([
90,
91];
Table 1), whereas
Acer platanoides is often regarded as a temperate to sub-boreal species [
92,
93] with a broad range of suitable site conditions [
32]. However, the southern distribution limit of
A. platanoides comes close to those of the
Sorbus species (
Table 1). Although the seedlings of both
Acer species were very resistant to drought,
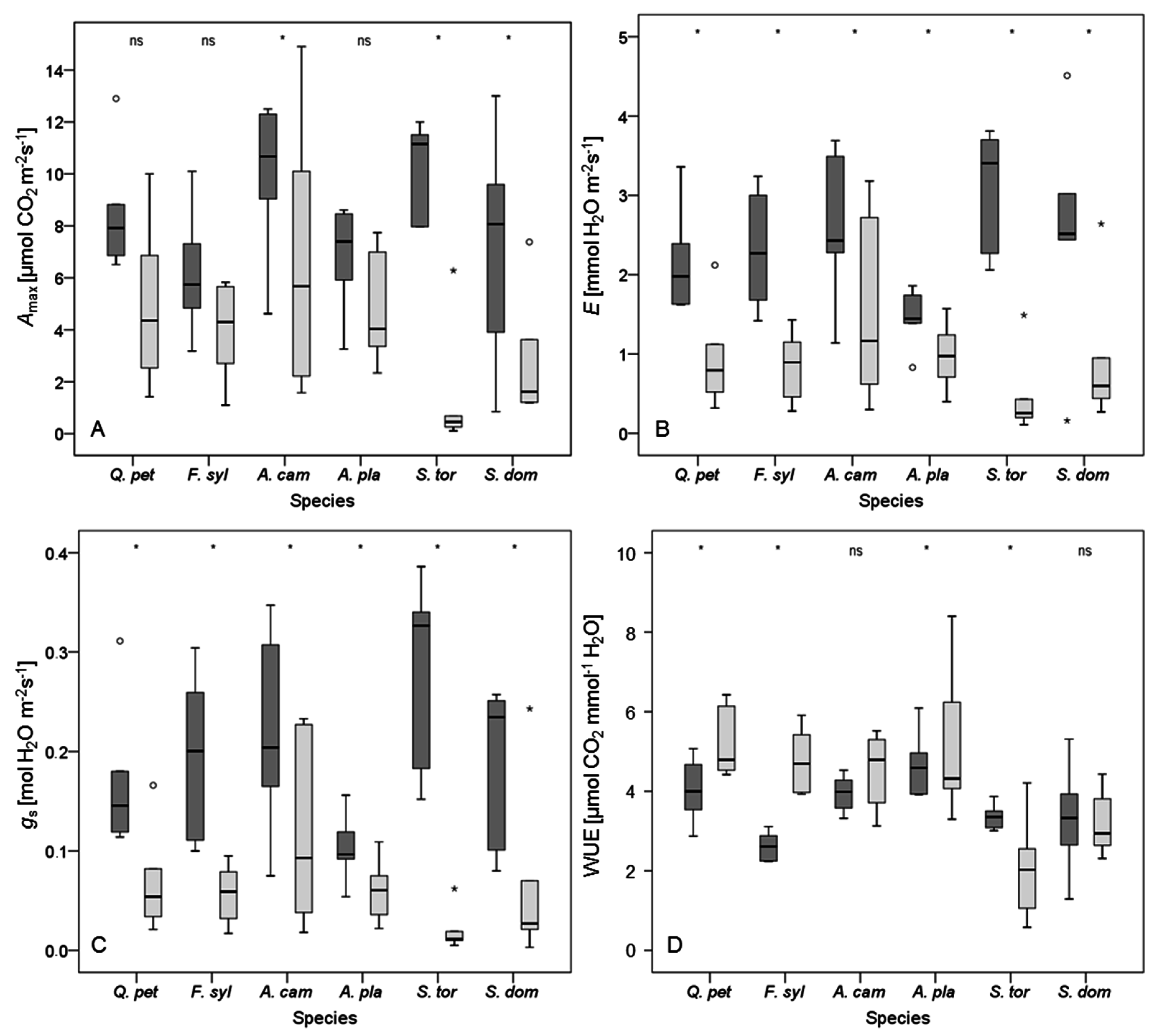
Amax,
E, and
gs of
Acer platanoides were slightly less affected by water stress than respective physiological properties in
A. campestre (
Table 4). However, the latter species gained more biomass, aboveground and belowground, attained the highest root-to-shoot ratios, and its
Rtc values showed no statistically significant deviations from unity for all tested variables under severe drought conditions (
Table 3 and
Table 4). In addition, and in contrast to all the other species studied, no
Acer seedlings died in this experiment, even if they were not watered at all.
Only a few published studies are available for seedlings of the minor tree species for direct comparisons with our results. Transpiration of
A. campestre was similar to values measured by [
94], whereas
Amax and the root-to-shoot ratio were much higher in plants from our experiment. High root-to-shoot ratios of
A. platanoides were in accordance with the values provided by [
88], but some differences in gas exchange were noticeable. Maximum photosynthesis rates in this species were notably higher when compared to rates quantified by [
57,
77,
88], while
E and
gs were slightly higher in other studies [
88,
95] than in our experiment. In contrast to [
57],
E values were higher in our pot experiment, while values of
gs and WUE were slightly higher in their study. Based on measurements of xylem hydraulic conductivity, vessel density, and vessel grouping,
A. platanoides was regarded as better adapted to lower water availability than
A. campestre [
96]. However, other studies found similar hydraulic conductivity in the xylem of the two
Acer species [
97]. This may indicate that the variation in these properties that may be caused by tree age, site conditions or genetics has not been captured by the available studies so far. The fact that no shoot dieback occurred in both
Acer species indicates a high resistance to hydraulic failure [
98]. Although
A. campestre was ranked the most drought tolerant species, the results of our experiment do not suggest a clear differentiation between the two
Acer species according to their drought tolerance, since they differed only in their physiological performance under drought stress (
Table 6).
In contrast to the
Acer species, drought resistance of the
Sorbus seedlings was surprisingly low. The resistance of
Sorbus domestica was comparable to that of
Fagus sylvatica, but
S. torminalis was the least drought-resistant tree species within this study as indicated by a rapid decline of physiological performance as soon as the water supply became limited (
Table 4). The measured values for
gs are in accordance with those of another study, where
S. torminalis restricted
gs rapidly with increasing drought stress and was highly vulnerable to xylem embolism [
90]. However, we could not detect indications of lasting xylem embolism for
S. torminalis, since the species recovered quickly after drought stress (
Table 5), which might demonstrate that the hydraulic conductivity was either not completely lost [
24] or that the seedlings may have the capacity to actively ‘refill’ their possibly embolised vessels with water rapidly [
63]. When sufficient water was available, the seedlings of
S. torminalis showed even higher maximum photosynthetic rates than adult trees [
78]. Likewise,
S. domestica seedlings of the control group showed higher values of
Amax,
E, and
gs compared to similar studies [
76]. However, seedlings of the
Sorbus species were the largest at the beginning of the experiment and this may also explain why they experienced drought stress more strongly than the other species. However, differences in plant height of more than 100 cm between species were accepted in comparable pot experiments [
57], whereas mean seedling height differed by less than 23 cm between
S. torminalis and
Q. petraea here (
Table S1).
Considering all measured variables, the seedlings of
Quercus petraea were less resistant to drought than the
Acer species, but obviously more resistant than
Fagus sylvatica and the
Sorbus species (
Figure 3,
Table 6). This classification of oak in relation to beech is consistent with other studies that have documented the higher physiological performance of oak under conditions of water shortage [
52,
99]. The root-to-shoot ratios measured in this study were in accordance with values determined for
F. sylvatica [
100], but slightly higher for
Q. petraea than in other studies [
101].
In the long term, high root-to-shoot ratios can be regarded as an adaptation to dry sites [
89]. In the short-term, however, the root-to-shoot ratio may decline with increasing drought stress in seedlings as was found also in other studies [
53,
102]. This might indicate that carbon (C) assimilation is so strongly reduced by drought that there is not sufficient C to facilitate its allocation to below-ground structures. Only the
Acer species were able to maintain their C allocation patterns between aboveground and belowground structures, which might be one explanation for their distinct drought resistance observed in our study. Hence, only these species may have been able to invest into further aboveground biomass.
4.2. How Well do Seedlings of the Minor Tree Species Recover after Drought?
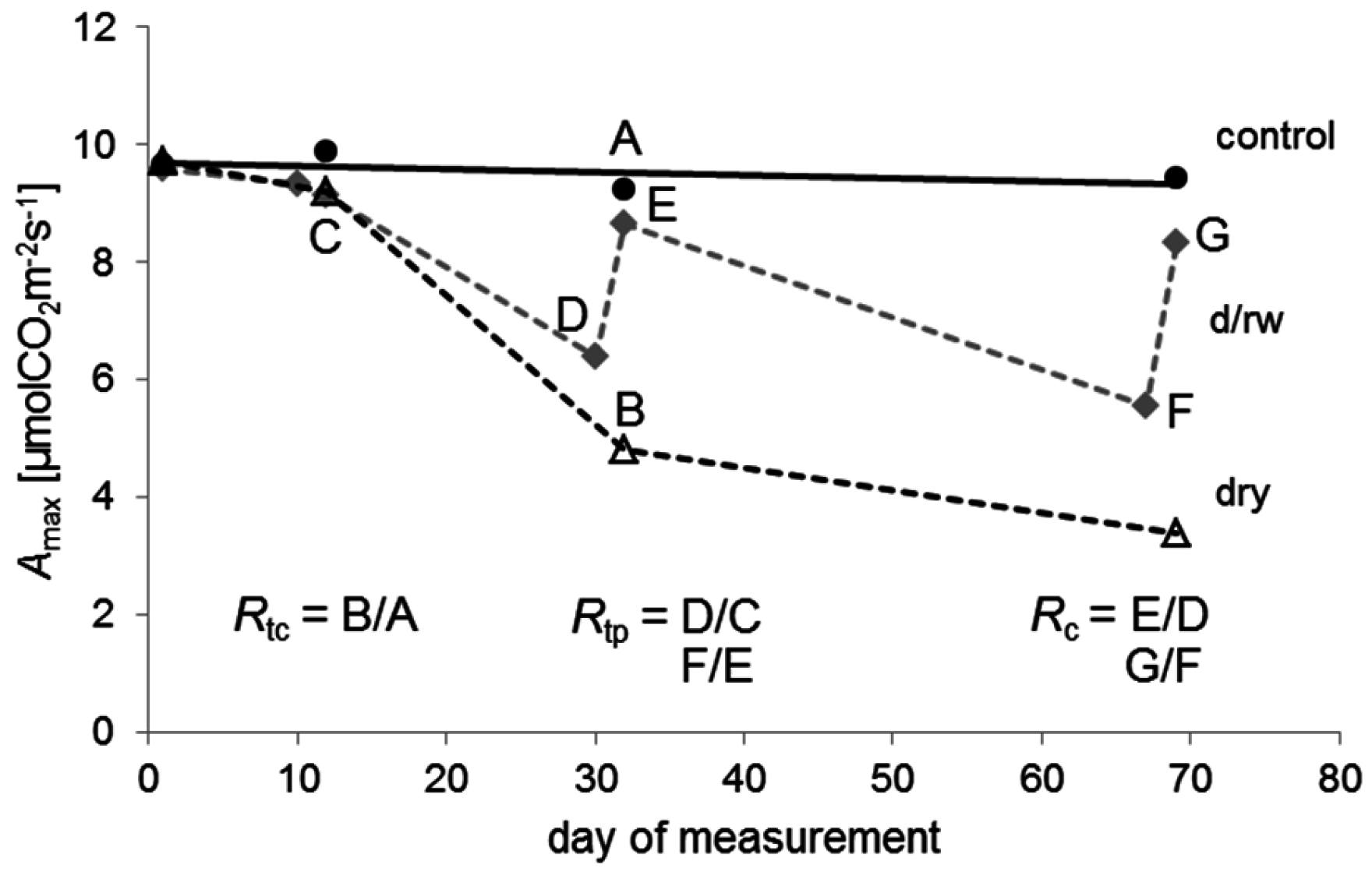
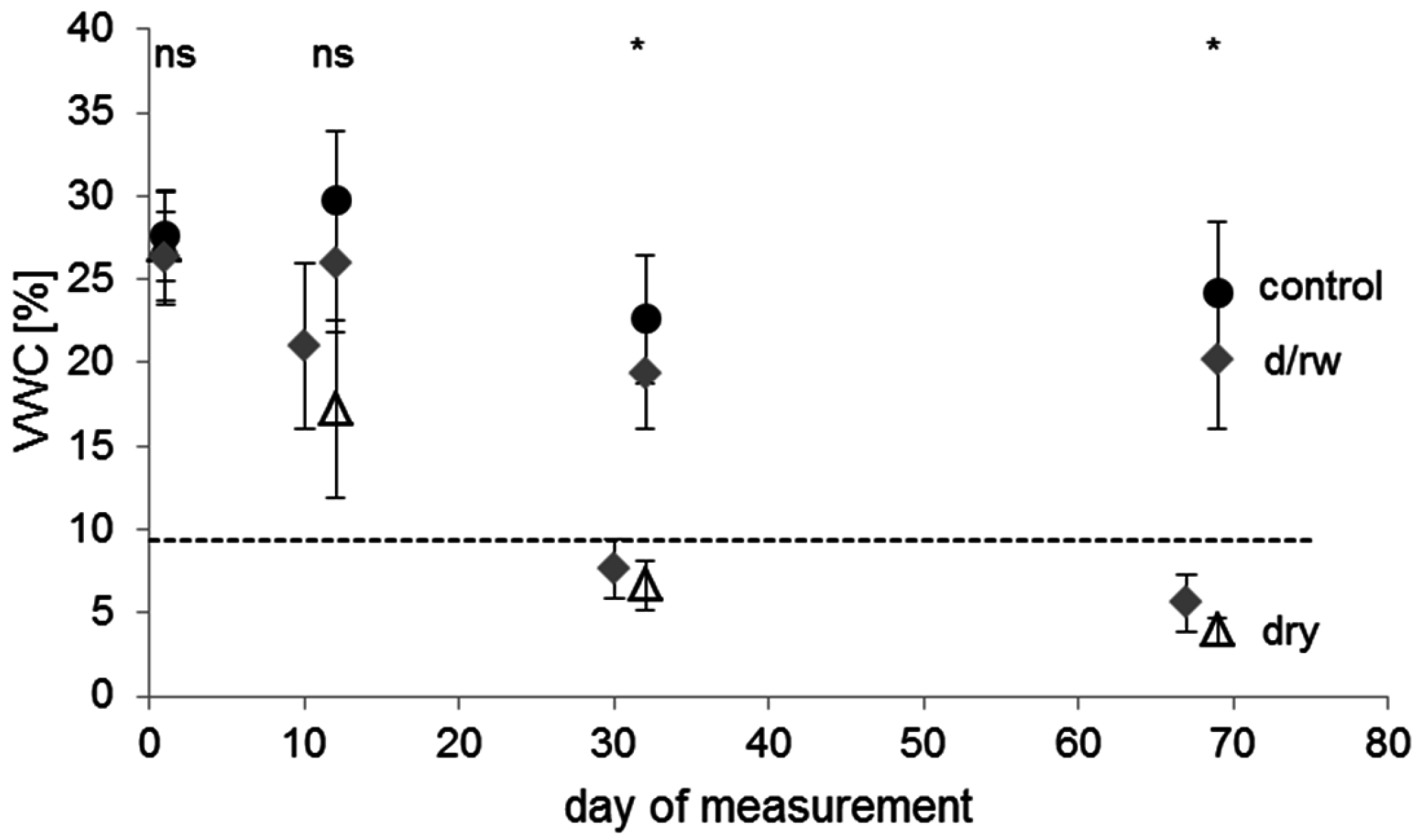
Surprisingly, some of the least drought-resistant species in our study such as
Sorbus domestica recovered most quickly or fully from drought (
Table 5). Also,
Fagus sylvatica and
S. torminalis showed a high ability to recover, especially from less severe droughts. The fact that seedlings were able to recover, although the soil matric potential had dropped below the permanent wilting point (−1.5 MPa), indicates that this value does not represent a true physiological wilting point for the entire woody seedlings. Here, the PWP may instead be regarded as an indicator of severe drought stress. The swift recovery also indicates that these species did obviously not suffer from a high degree of fine-root mortality or xylem embolism during drought [
67,
97,
103]. Unfortunately, these processes, which may also explain the slower recovery of the
Acer species and
Quercus petraea, were not investigated in our study. The ability to recover quickly after periods of water shortage may be a particular advantage for tree species growing in environments with erratic or highly fluctuating precipitation patterns during the growing season. This may be true on shallow soils, where the available water depends largely on recent rainfall [
99,
104]. Such warm and dry forest sites, mostly with calcareous and rendzic soils, are very often occupied by
Acer and
Sorbus species [
34].
Our findings are in accordance with experiments that have documented the ability of
Fagus sylvatica to regain high values of
Amax and
gs with rewetting following drought [
54,
105]. In our study,
F. sylvatica recovered quickly within three days following drought. Contradictory results have been observed previously in a comparative study with
Quercus petraea and
F. sylvatica [
99]. However, in that study, the physiological variables were measured only one day after rewetting, which might be too short for
F. sylvatica to recover fully. Unfortunately, physiological and growth performance of seedlings during repeated droughts has been studied in few experiments only and thus it is difficult to compare our results for the investigated species with those of other studies.
4.3. Are There Different Mechanisms that Enable Seedlings of Minor Tree Species to Withstand Droughts?
On the basis of this experiment, different strategies of the investigated tree species to cope with drought stress are discernible. The maintenance of high values for
E and
gs even under intensifying drought stress, followed by a breakdown of
Amax,
E, and
gs under severe drought conditions in
Sorbus torminalis (
Table 4), suggests anisohydric behavior [
21,
106]. The low values of
Amax with increasing drought conditions (
Figure 3), and the early onset of leaf wilting and abscission also fit into this strategy.
Although species that maintain stomatal opening under drought conditions bear a high risk of damage or death by cavitation, they are expected to be more tolerant to prolonged drought of intermediate intensity, because the risk of carbon starvation is reduced [
21,
107]. The maintenance of stomatal conductance, which is characteristic of an anisohydric behavior, is often found in deep-rooted species like tuart gum (
Eucalyptus gomphocephala DC.) or northern red oak (
Quercus rubra L.), which may maintain water access for longer periods following the onset of drought conditions [
108,
109,
110]. As deep roots are thought to be a key factor for plants to survive droughts [
85,
111], this adaptation could not be expressed in the potted seedlings of this experiment, and thus the attribution of strategies to cope with drought and the comparisons between species has to be viewed with caution. In nature, the vast majority of regeneration of the two
Sorbus species consists of root sprouts, which would be supported by the mother trees that have a deep root system [
112]. Whether this would aid the maintenance of transpiration or just prevent carbon starvation by lateral transport of assimilates has, to our knowledge, not been researched.
In contrast to the anisohydric behaviour of
Sorbus torminalis, growth and physiology of
Acer platanoides is representative of an isohydric behavior. This is indicated by high water-use efficiency (
Figure 3) and a constant reduction in
gs as drought conditions become more severe. On the one hand, this response to drought reduces the risk of hydraulic failure through cavitation [
21]. On the other hand, mortality of isohydric species through carbon starvation is more likely during prolonged droughts of intermediate intensity [
21]. Obviously, the drought periods in our experiment were not long enough to induce carbon starvation in the
Acer species.
Species with isohydric or anisohydric behavior might have different preferences or realized niches with regard to soil water availability. However, co-occurrences of
Acer campestre,
A. platanoides,
Sorbus torminalis, and
S. domestica within one forest stand are not uncommon [
34]. Whether or not these different strategies of water use under drought conditions might be a mechanism of complementary resource use that leads to an overall higher resistance and resilience of the whole tree community in relation to drought stress (e.g., [
113]) is not known.
Not all plants can be clearly classified into those with anisohydric or isohydric behavior, however. Instead, the physiological behavior of plants should be seen as a continuum between these discrete categories [
47]. There are exceptions to these extreme expressions of anisohydric or isohydric behavior, and some species take an intermediate position [
21]. Classifications according to physiological properties alone are difficult, especially when pronounced changes in
Amax [
17,
114] or
gs [
115] between seedlings and adult trees are not uncommon. Even between species of the same genus, obvious differences in drought avoidance strategies exist. In our study, the response of transpiration and stomatal conductance to drought in
Sorbus domestica might indicate an isohydric strategy (
Figure 3), whereas the rapid breakdown of leaf gas exchange under conditions of severe water shortage of
S. torminalis could be indicative of an anisohydric strategy. To classify species according to these different water-spending or water-saving behaviors during drought, it is necessary to monitor their physiological processes over extended drought periods and in situations where their morphological adaptations such as the development of a deep root system are not restricted. Hence, our greenhouse experiment with potted seedlings can provide only a first indication. For the short drought cycles that seedlings experienced in the drying-rewetting cycles of this trial, the indices of drought resistance and recovery appear to be more appropriate. In addition, drought cycles of different lengths are important to assess the relative success of the different mechanisms to cope with drought under different conditions.
4.4. How Suitable Are Pot Experiments to Extrapolate and Transfer Results to the Field?
Although pot experiments cannot replicate the processes in a natural setting, they are frequently used for comparative plant physiological studies under controlled conditions [
69,
116]. Such studies might be particularly helpful to improve our understanding of highly understudied species like
Sorbus torminalis or
S. domestica.
Based on a widespread evaluation of pot experiments, a number of recommendations to improve transferability of the experimental results to field conditions have been provided [
58]. An adequate adjustment period before starting the experiment, the use of natural soils in contrast to standard potting mix, and a sufficient pot size were identified as crucial factors [
58]. The ratio of the total plant biomass to rooting volume (BVR) has been suggested as an index to assess the adequacy of pot sizes [
117], where BVR ratios less than 2 indicate that pots are of sufficient size [
118]. In this experiment, all species except for
S. torminalis had mean BVR values of less than 2 at the end of the experiment (
Table S4). The higher values of
S. torminalis seedlings can be explained by higher biomass at the onset of the experiment. The high initial biomass of this species might also explain its low resistance to drought. However, we tested for potential effects of initial plant biomass on physiological performance and growth under drought stress. Since there were no effects of initial plant biomass within and between species, differences in physiology and biomass increment are explained by species and/or treatments. Apart from this, all further recommendations by [
58] had been followed in our experiment.
The validity and relevance of results might have been enhanced through an extension of the experiment beyond one vegetation period. Then, long-term effects like delayed changes in biomass increment and partitioning, carbon allocation or resprouting could have been observed. Also, only one provenance has been studied here, whereby effects like plasticity, adaptation or niche segmentation are mostly neglected. However, our results provide first indications of contrasting mechanisms in the seedlings of different species to withstand drought.