Abstract
Characterization of decomposition dynamics of fine roots is essential for understanding vegetation–soil feedbacks and predicting ecosystem responses to future climate scenarios, given their more rapid turnover rates. Using a branch-order classification, we separated the fine root systems of Larix gmelinii into two classes: first- and second-order roots combined into one (lower-order); third- and fourth-order roots combined into another (higher-order). In a field experiment, we conducted a litterbag study to investigate fine root decomposition and its relationship with root order class and soil depth over 17 months. Despite their lower C:N ratio and smaller diameter, lower-order roots decomposed more slowly compared with higher-order roots over this period. This pattern also seems to hold true at each different depths (10, 20 and 30 cm) in the soil profile. Our data suggest that the slow decomposition rate of lower-order roots may result from their poor carbon quality. Moreover, we found that the decomposition rates of both lower-order and higher-order roots decreased linearly from 10 cm to 30 cm, which implied that a substantially larger fraction of fine root mass would be stabilized as soil organic carbon in the deeper rather than the upper soil layers.
1. Introduction
Globally, up to more than 50% of terrestrial net primary production is returned to the soil via the decomposition of plant tissues [1]. Therefore, identifying general mechanisms underlying litter decomposition has major implications for accurate forecasts of the future interplay between global change and terrestrial C cycles [2,3,4]. The decomposition of leaf litter has been well studied at local and global scales. However, despite the fact that the dominant input of plant material into soil may be driven from the turnover of fine roots [5,6,7,8], understanding of the factors that control fine root decomposition remains limited. Specifically, published reports of the predictive principles to describe the controls on fine root decomposition yielded large discrepancies. For example, in global data sets of a large range of species, Silver and Miya [9] found fine root chemistry (particularly C:N ratio and Ca concentration) to be the factor most closely linked to root decomposition rates. Other studies suggested that the initial C:N ratio, N concentration, or Ca concentration explained no variation in fine root decomposition rates in many ecosystems [10,11,12,13,14]. Our inability to determine generalized drivers of root decomposition across species limits both our understanding of vegetation–soil feedbacks and our ability to predict ecosystem responses to global change.
For nearly half a century, fine roots have been defined as a homogenous pool according to an ambiguous diameter size class, most commonly ≤2 mm. Increasing evidence suggests, however, that fine roots defined in this way probably include a large number of individual root segments that differ markedly in their morphology, chemistry, and physiology [8,15,16,17,18,19,20]. For example, different root branch orders strongly influence tissue chemical properties (e.g., C:N ratio, and concentrations of N, lignin, cellulose, and phenolic compounds), and the relative contribution of different branch orders to a fine-root sample ≤2 mm may vary considerably across species [11,17,19]. However, studies of fine root-mortality and decomposition have not really accounted for the functional heterogeneity among different diameter classes or branching orders within the fine root system, and even fewer have concentrated on the most short-lived absorptive roots that dominate root mortality.
So far, to our knowledge, only three published papers have quantified the relationship between root branch order and decomposition rates and all found that across the studied tree species [11,14,21], lower-order roots (first and second) decayed at a lower rate than higher-order roots (third and fourth order) despite lower-order roots generally having a lower C:N ratio and smaller diameter (higher surface:mass ratio). Clearly, more experimental research is needed for validating the commonality of the phenomenon that lower-order roots decay at a lower rate compared with higher-order roots.
Besides root order or root diameter, another variable that may influence root decomposition is soil depth. While the controls and patterns of plant litter and soil organic carbon turnover are well established for upper soil depth, our understanding of the controls of turnover in subsoil horizons remains limited [22], despite the fact that more than 50% of the total soil C stock is stored in deep soil horizons [23,24]. While the controls over and patterns of plant litter and soil organic carbon turnover are well established for upper soil depths, our understanding of the controls over of turnover in subsoil horizons remain still limited. Gill and Burke [25] found that fine root decomposition rates declined linearly with soil depth in a shortgrass steppe soil profile. In contrast, some studies have shown that litter decomposed more rapidly in the lower soil profile compared to the upper soil depth [26,27]. Additionally, the depth distribution of fine roots in different branching orders varies, which probably has important implications for the amount of root-derived C being processed. For example, the majority of short-lived lower-order roots was generally distributed in shallower horizons compared with long-lived higher-order roots [19].
The main objectives of this study were to: (1) examine the effects of root order on root tissue chemistry and decomposition rates in a temperate forest; and (2) assess to what extent the root decomposition rates are influenced by depth classes. We hypothesized that lower-order roots (first and second) would decompose more slowly than higher-order roots, and fine root decomposition would decline with soil depth because of variation in the physical environment and soil community through the soil profile. We tested these hypotheses by conducting a field litterbag study to examine the relationships of fine root decomposition rates with root order and soil depth using root material from Larix gmelinii Rupr., the most important plantation species in Northeastern China.
2. Materials and Methods
2.1. Site Description
The study site was located at Laoshan Forest Research Station of Northeast Forestry University in Heilongjiang Province, northeastern China (127°30′–127°34′ E, 45°20′–45°25′ N). The site has a continental monsoon climate with a strong monsoon windy spring, a warm and humid summer, and a dry and cold winter. The mean annual precipitation is 730 mm, most of which falls in summer. The average annual air temperature is 2.8 °C, with a mean monthly maximum of 20.9 °C and a mean monthly minimum of −19.6 °C (unpublished data from Laoshan Forest Research Station). Soils at the site are Hap-Boric Luvisols (dark brown forest soil in the Chinese Soil Taxonomic System) with high organic matter content and nitrogen content. The soil organic matter was 13.71% ± 0.82%, 8.96% ± 0.73% and 4.64% ± 0.61% for the 0–10, 10–20 and 20–30 cm soil depth intervals, respectively. Soil pH was 6.15 ± 0.04, 5.87 ± 0.04 and 5.58 ± 0.05 for the 0–10, 10–20 and 20–30 cm soil depth intervals, respectively. Soil texture was loam, sandy loam and clay loam for the 0–10, 10–20 and 20–30 cm soil depth intervals, respectively. More details of soil characteristics and site information can be found in Sun et al. [28,29].
Our field decomposition experiments were conducted in a 40-year-old Larix gmelinii Rupr. plantation. This forest type was selected because it represents the dominant natural forest and a key species of most plantations in Northeastern China [30]. Three plots, each 8 m × 8 m, were chosen for this study. The mean diameter at breast height was 18.7 ± 0.93 cm at the time of root sampling. The respective average root diameter (mm) (mean values ± standard error) from first-order to fourth-order roots of Larix gmelinii was 0.26 ± 0.02, 0.28 ± 0.01, 0.46 ± 0.05, and 0.85 ± 0.09.
2.2. Decomposition Experiment
We quantified lower-order (first and second) and higher-order root (third and fourth order) decomposition rates for Larix gmelinii buried at three soil depths. In late April 2014, we excavated root branching samples from the upper 30-cm of the soil profile and then carefully separated lateral root branches from the soil, ensuring the finest root branching orders were still intact and remained attached to higher-order roots. While there may be some differences in root morphology, chemistry, and physiology between shallow and subsoil roots, the primary objective of this study is to focus on the edaphic conditions and environmental variables that may alter root decay rates rather than the differences caused by plant allocation patterns. Following the method proposed by Pregitzer et al. [17], the first four root orders were identified, and the adhering soil particles were carefully removed using magnifying glasses and tweezers. In the present study, first- and second-order roots were combined into one class, and third- and fourth-order roots were combined into a second class. Furthermore, we selected the fresh roots as root samples in our decomposition experiment because there is currently no method for the collection of a sufficient number of dead fine roots that have just died and have not already begun to decay, although lower-order roots may gradually lose their functions as they age [31] and are associated with saprotrophic fungi when still living [32,33].
Oven-dried (65 °C) root material of approximately 1.0 g of each root class was placed into 10 × 10-cm nylon decomposition bags (120-μm mesh). Such a small mesh size of the litterbags would undoubtedly exclude all meso- and macrofauna, both important processors of detritus in various forests [34,35,36,37], so this study focused on microbially mediated decomposition. For each root class, 12 litterbags containing root material were horizontally deployed in late May 2014 at each soil depth in each of the three replicated forest plots, and three different litterbags of each root class at each soil depth under each plot were harvested in August and October 2014 and in May and October 2015. A total of 54 litterbags for each root class were collected at each harvest. There were 432 root litterbags in total. Upon harvest, decomposed root samples were carefully removed from the litterbags, dried (65 °C) and weighed.
2.3. Chemical Analyses
A subsample of roots used from each root class for chemistry analyses at the beginning of litterbag experiment was ground to obtain a uniform particle size. Total C was determined using a multi N/C 3100 analyzer and HT1300 Solids Module (Analytik Jena AG, Jena, Germany). Concentrations of initial litter nutrients such as potassium (K), calcium (Ca) and magnesium (Mg) were analyzed by a novAA 350 atomic absorption spectrometer analyzer (Analytik Jena AG, Jena, Germany) following digestion in molar HCl. Total N in the digested solution was analyzed by the Semimicro-Kjeldahl method and total phosphorus (P) was determined by the vanadomolybdate yellow color method, after root samples were digested in a solution of H2SO4 (98%)–HClO4 (72%) (10:1; V:V). Analysis of initial root C fractions for each root class followed the technique of Ryan et al. [38]. Root C-fractions encompassing “extractives” (i.e., accessible labile C compounds consisting of nonpolar constituents, such as fats, oils, waxes, and polar constituents, such as nonstructural carbohydrates and polyphenols were removed using a two-stage extraction in dichloromethane and boiling water, respectively), “acid-hydrolyzable” structural components (i.e., moderately degradable C compounds consisting primarily of cellulose and hemicellulose were removed using a two-stage digestion in 72% and 2.5% sulfuric acid, respectively), and “acid-unhydrolyzable” structural components (i.e., highly inhibiting C compounds consisting of lignin and other highly reduced compounds, such as suberin, cutin, and tannin protein complexes, which are residues of the two-stage sulfuric acid digestion minus ash mass) were determined by the forest products serial digestion technique [38]. Concentrations of total non-structural carbohydrate (TNC) of root samples were determined following the technique of Seifter et al. [39].
2.4. Statistical Analysis
Analysis of covariance (ANCOVA) was used to explore the effects of root class on initial tissue chemistry concentrations and ratios. We calculated the decomposition constant (k) by fitting the percentage of remaining ash-free dry mass for each root class against time using a linear decay model:
where X is the fraction of initial mass remaining at time (t), t is time in years, and k is the decomposition rate constant in year−1. Here, we employed the linear decay model because it provided a better fit than the single-exponential, double-exponential, and asymptotic decay models over the short duration of the 17-month observation (only initial stages of decomposition). It is important to note that this study focused exclusively on the early stage of decomposition and thus the decay rate constant k (year−1) in our paper actually represents the initial mass loss constant. Two-way analysis of variance (ANOVA) was used to assess the influence of root class and soil depth on root decomposition rates. All statistical analyses were performed using SPSS software (2001, ver. 13.0, SPSS Inc., Chicago, IL, USA).
X = 1 − kt
3. Results
3.1. Initial Substrate Chemistry
Clear and significant differences in initial concentrations of most determined compounds were found among different root classes at the start of the litterbag experiment (Table 1). Initial nutrient concentrations were generally higher in first- and second-order roots compared with third- and fourth-order roots. For example, initial N concentrations in first- and second-order roots were almost twice those of third- and fourth-order roots (p < 0.0001). The contrasting concentrations of N among different root classes resulted in opposing gradients in C:N ratios (p < 0.0001). In addition, first- and second-order roots had higher concentrations of acid-unhydrolyzable fractions than third- and fourth-order roots (p < 0.0001). In contrast, initial concentrations of acid-hydrolyzable fraction and total non-structural carbohydrate (TNC) were much lower in first- and second-order roots compared with third- and fourth-order roots (p < 0.0001).

Table 1.
Initial root chemistry parameters (means and SE) at the start of the litterbag experiment of Larix gmelinii.
3.2. Effects of Root Class on Decomposition
The linear decomposition model fits the actual rates of mass loss well for both lower-order and higher-order roots over the 17 months of decomposition (Figure 1). The calculated mass remaining using a single lineal decay model fits the actual mass remaining for lower-order roots with R2 = 0.90 and higher-order roots with an R2 = 0.88. Despite their lower C:N ratio and smaller diameter (higher surface :mass ratio), first- and second-order roots decayed at a lower rate than third- and fourth-order roots, as reflected by both root classes (p < 0.001 in both cases). Furthermore, this pattern was observed for roots buried at 10, 20, and 30 cm in the soil profile. For example, after 17 months of decomposition, mass loss was 22% in third- and fourth-order roots while it was 16% in first- and second-order roots at 10 cm depth (Figure 1). At the same time, the decomposition constant (k) was 0.11/year for the first- and second-order roots and 0.17/year for third- and fourth-order roots at 10 cm depth (Table 2).
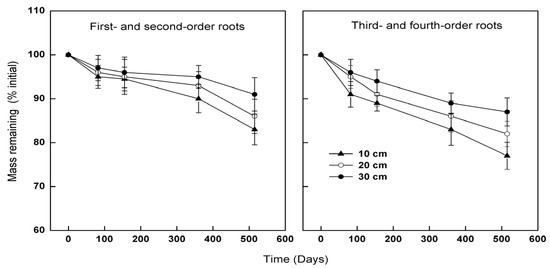
Figure 1.
Effects of root order and soil depth on mass remaining (% initial) of Larix gmelinii during 17 months of exposure in field. Error bars represent ± SE (n = 3).

Table 2.
Decomposition rate coefficients obtained by fitting a linear decomposition model to Larix gmelinii root data at three soil depths after 17 months of field exposure.
3.3. Effects of Soil Depth on Decomposition
After 17 months of decomposition, the decomposition rate of both lower-order and higher-order root material decreased significantly from 10 to 30 cm in the soil profile (p = 0.006 and 0.009 in lower- and higher-order roots, respectively). This pattern is reflected in both the decay rate constant (k) and the root mass remaining at the end of the decomposition experiment.
Over the time period of 17 months, for lower-order roots, 84% of the root samples incubated at the 10 cm depth remained, while 91% of the root samples incubated at the 30 cm depth persisted (Figure 1). Low-order roots buried at the 20 cm depth had an intermediate mass-loss rate, with 86% of the original roots persisting (Figure 1). The decay rate constant (k) of lower-order roots was also significantly higher for roots incubated at the 10 cm depth than for roots incubated at the 30 cm depth (Table 2). At the 10 cm depth, for lower-order roots, k was 0.11 year−1, while at the 30 cm depth, k was 0.06 year−1 (Table 2). For higher-order roots, 78% of the roots incubated at the 10 cm depth remained, while 87% of the roots incubated at the 30 cm depth persisted (Figure 1). Further, the decay rate constant (k) of higher-order roots was 0.17, 0.14 and 0.10 year−1 at the 10, 20 and 30 cm soil depth, respectively.
4. Discussion
In complex root systems, different root orders serve different functions with the first to third orders used primarily for water and nutrient absorption, and higher orders used mainly for transport, anchorage, and storage [17,19,20,40]. These functional divergences between lower-order and higher-order roots would strongly influence tissue substrate chemistry and thus further affect root decomposition rates [20]. Our analysis of root decay along more functionally based criteria (i.e., root order) among the fine root systems of Larix gmelinii, revealed that lower-order roots decomposed at a lower rate than higher-order roots over 17 months of decomposition. These results are in agreement with three other recently published reports [11,14,21], in which all studied tree species demonstrated lower decomposition rates in lower-order compared with higher-order roots. Another separate study, which examined decomposition of root tips from 35 co-occurring woody species in a Chinese temperate forest, showed that on average, only 35% of the initial root tip mass was lost after six years of field decomposition (T. Sun et al., unpublished data). Other experimental studies based on diameter classes also found that the finest roots decayed more slowly compared with somewhat coarser roots [23,24,41,42]. As a consequence, the slow decomposition rate of the finest root branching orders probably seems to be a common pattern in the woody species examined so far.
One of the mechanisms responsible for the lower decomposition of lower-order roots may be their higher concentrations of the acid-unhydrolyzable fraction, which indicates a lower energy substrate supply for maintenance of the active and abundant decomposer community. Other studies also observed that lower-order roots had higher concentrations of acid-unhydrolyzable matter than higher-order roots. For example, Xiong et al. [14] reported that acid-unhydrolyzable concentrations were significantly higher in lower-order roots than in higher-order roots across eight tree species. Sun et al. [12] found that very fine roots <0.5 mm had higher concentrations of the acid-unhydrolyzable fraction as compared to coarser roots (0.5–2.0 mm). Given that lower-order roots generally lack secondary (wood) development such as the formation of secondary xylem, the formation of lignin is expected to be very low [19,20,40]. Besides true molecular lignin, the acid-unhydrolyzable fraction must capture also other highly reduced compounds, such as aliphatics and suberin [38,43,44]. An alternative explanation for faster decomposition of higher-order roots maybe their typical role in storage of non-structural carbohydrates, which corresponds with their storage and transport functions [19,20,28]. These easily degradable and labile C substrates may provide necessary energy for the decomposer community, promoting the degradation of many complex C compounds in the higher-order roots [34,45].
Another factor besides root order that may influence the decomposition process is soil depth. Our data revealed that decomposition rates of both lower-order and higher-order roots were highest in the shallow soil profile and tended to decrease with soil depth through the soil profile. Other studies in semiarid grasslands and forests have also reported a decrease in fine root decomposition rates with soil depth, with the highest decomposition rates occurring in the upper soil depth [25]. On the contrary, few studies have found that plant litter decomposed faster in deep soil horizons than in shallow soil [26,27]. Soil bulk density, temperature, moisture availability, pH, clay content, nutrient limitations and microbial community composition generally change with soil depth in the soil profile [25,26,27,46], and fine root decomposition rates are probably affected by these parameters.
5. Conclusions
The decomposition results presented here add to growing evidence that short-lived lower-order roots decay at a lower rate compared with long-lived higher-order roots after 17 months of decomposition. This pattern holds true across three different soil depths in a soil profile. We suggest that the reason for such slow decomposition in lower-order roots may be their poor carbon quality, more specifically, because of their higher concentration of recalcitrant C (e.g., acid-unhydrolyzable) and less labile C (e.g., non-structural carbohydrates). Given that lower-order roots turnover fastest and may dominate root production, their exceptionally slow decomposition probably implies that these roots are a major driver of soil C storage and dynamics. Furthermore, our results showed decomposition rates of both lower-order and higher-order roots decreased with depth through the soil profile, which probably suggests that a higher proportion of root litter may be stabilized in deeper soil horizons.
Acknowledgments
This work was supported by the State Key Program of China (2016YFD0300904 and 2016YFA0600800), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB15010400), and Natural Science Foundation of China (31500361).
Author Contributions
Tao Sun conceived and designed the experiments; Tao Sun, Hongguang Zhang and Lili Dong performed the experiments; Tao Sun, Qingkui Wang and Yuanyuan Li analyzed the data; Tao Sun, Lili Zhang, Zhiejie Wu and Zhengwen Wang contributed reagents/materials/analysis tools; Tao Sun wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; van der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: A triangular relationship. Oikos 1997, 79, 439–449. [Google Scholar] [CrossRef]
- Aerts, R. The freezer defrosting: Global warming and litter decomposition rates in cold biomes. J. Ecol. 2006, 94, 713–724. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.C.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Pérez-Harguindeguy, N. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.A.; Jackson, R.B. Global patterns of root turnover for terrestrial ecosystems. New Phytol. 2000, 147, 13–31. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Mommer, L.; de Vries, F.T. Going underground: Root traits as drivers of ecosystem processes. Trends Ecol. Evol. 2014, 29, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Freschet, G.T.; Cornwell, W.K.; Wardle, D.A.; Elumeeva, T.G.; Liu, W.; Jackson, B.G.; Onipchenko, V.G.; Soudzilovskaia, N.A.; Tao, J.; Cornelissen, J.H. Linking litter decomposition of above-and below-ground organs to plant-soil feedbacks worldwide. J. Ecol. 2013, 101, 943–952. [Google Scholar] [CrossRef]
- Xia, M.; Talhelm, A.F.; Pregitzer, K.S. Fine roots are the dominant source of recalcitrant plant litter in sugar maple-dominated northern hardwood forests. New Phytol. 2015, 208, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Silver, W.L.; Miya, R.K. Global patterns in root decomposition: Comparisons of climate and litter quality effects. Oecologia 2001, 129, 407–419. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Oleksyn, J.; Eissenstat, D.M.; Reich, P.B. Fine root decomposition rates do not mirror those of leaf litter among temperate tree species. Oecologia 2010, 162, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Goebel, M.; Hobbie, S.E.; Bulaj, B.; Zadworny, M.; Archibald, D.D.; Oleksyn, J.; Reich, P.B.; Eissenstat, D.M. Decomposition of the finest root branching orders: Linking belowground dynamics to fine-root function and structure. Ecol. Monogr. 2011, 81, 89–102. [Google Scholar] [CrossRef]
- Sun, T.; Mao, Z.; Dong, L.; Hou, Y.; Wang, X. Further evidence for slow decomposition of very fine roots using two methods: Litterbags and intact cores. Plant Soil 2013, 366, 633–646. [Google Scholar] [CrossRef]
- Sun, T.; Mao, Z.; Han, Y. Slow decomposition of very fine roots and some factors controlling the process: A 4-year experiment in four temperate tree species. Plant Soil 2013, 372, 445–458. [Google Scholar] [CrossRef]
- Xiong, Y.; Fan, P.; Fu, S.; Zeng, H.; Guo, D. Slow decomposition and limited nitrogen release by lower order roots in eight Chinese temperate and subtropical trees. Plant Soil 2013, 363, 19–31. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Wells, C.E.; Yanai, R.D.; Whitbeck, J. Building roots in a changing environment: Implications for root longevity. New Phytol. 2000, 147, 33–42. [Google Scholar] [CrossRef]
- Wells, C.E.; Eissenstat, D.M. Marked differences in survivorship among apple roots of different diameters. Ecology 2001, 82, 882–892. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; DeForest, J.L.; Burton, A.J.; Allen, M.F.; Ruess, R.W.; Hendrick, R.L. Fine root architecture of nine North American trees. Ecol. Monogr. 2002, 72, 293–309. [Google Scholar] [CrossRef]
- Guo, D.; Mitchell, R.J.; Withington, J.M.; Fan, P.P.; Hendricks, J.J. Endogenous and exogenous controls of root life span, mortality and nitrogen flux in a longleaf pine forest: Root branch order predominates. J. Ecol. 2008, 96, 737–745. [Google Scholar] [CrossRef]
- Guo, D.; Xia, M.; Wei, X.; Chang, W.; Liu, Y.; Wang, Z. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol. 2008, 180, 673–683. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Guo, D. Slow decomposition of lower order roots: A key mechanism of root carbon and nutrient retention in the soil. Oecologia 2010, 163, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.H.; Burke, I.C.; Lauenroth, W.K. Soil organic matter and nutrient availability responses to reduced plant inputs in shortgrass steppe. Ecology 1996, 77, 2516–2527. [Google Scholar] [CrossRef]
- Paul, E.A.; Follett, R.F.; Leavitt, S.W.; Halvorson, A.; Peterson, G.A. Radiocarbon dating for determination of soil organic matter pool sizes and dynamics. Soil Sci. Soc. Am. J. 1997, 61, 1058–1067. [Google Scholar] [CrossRef]
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- Gill, R.A.; Burke, I.C. Influence of soil depth on the decomposition of Bouteloua gracilis roots in the shortgrass steppe. Plant Soil 2002, 241, 233–242. [Google Scholar] [CrossRef]
- Rovira, P.; Vallejo, V.R. Organic carbon and nitrogen mineralization under Mediterranean climatic conditions: The effects of incubation depth. Soil Biol. Biochem. 1997, 29, 1509–1520. [Google Scholar] [CrossRef]
- Rovira, P.; Vallejo, V.R. Mineralization of carbon and nitrogen from plant debris, as affected by debris size and depth of burial. Soil Biol. Biochem. 2002, 34, 327–339. [Google Scholar] [CrossRef]
- Sun, T.; Dong, L.; Wang, Z.; Lü, X.; Mao, Z. Effects of long-term nitrogen deposition on fine root decomposition and its extracellular enzyme activities in temperate forests. Soil Biol. Biochem. 2016, 93, 50–59. [Google Scholar] [CrossRef]
- Sun, T.; Dong, L.; Mao, Z.J. Simulated Atmospheric Nitrogen Deposition Alters Decomposition of Ephemeral Roots. Ecosystems 2015, 18, 1240–1252. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, D.; Wang, X.; Gu, J.; Mei, L. Fine root architecture, morphology, and biomass of different branch orders of two Chinese temperate tree species. Plant Soil 2006, 288, 155–171. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Volder, A. The efficiency of nutrient acquisition over the life of a root. In Nutrient Acquisition by Plants: An Ecological Perspective; BarririRad, H., Ed.; Ecological Studies 191; Springer: New York, NY, USA, 2004; pp. 185–220. [Google Scholar]
- Li, A.; Fahey, T.J.; Pawlowska, T.E.; Fisk, M.C.; Burtis, J. Fine root decomposition, nutrient mobilization and fungal communities in a pine forest ecosystem. Soil Biol. Biochem. 2015, 83, 76–83. [Google Scholar] [CrossRef]
- Koide, R.T.; Fernandez, C.W.; Peoples, M.S. Can ectomycorrhizal colonization of Pinus resinosa roots affect their decomposition? New Phytol. 2011, 191, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Hättenschwiler, S.; Coq, S.; Barantal, S.; Handa, I.T. Leaf traits and decomposition in tropical rainforests: Revisiting some commonly held views and towards a new hypothesis. New Phytol. 2011, 189, 950–965. [Google Scholar] [CrossRef] [PubMed]
- Hättenschwiler, S.; Gasser, P. Soil animals alter plant litter diversity effects on decomposition. Proc. Natl. Acad. Sci. USA 2005, 102, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Handa, I.T.; Aerts, R.; Berendse, F.; Berg, M.P.; Bruder, A.; Butenschoen, O.; Chauvet, E.; Gessner, M.O.; Jabiol, J.; Makkonen, M. Consequences of biodiversity loss for litter decomposition across biomes. Nature 2014, 509, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Hobbie, S.E.; Reich, P.B.; Oleksyn, J.; Ogdahl, M.; Zytkowiak, R.; Hale, C.; Karolewski, P. Tree species effects on decomposition and forest floor dynamics in a common garden. Ecology 2006, 87, 2288–2297. [Google Scholar] [CrossRef]
- Ryan, M.G.; Melillo, J.M.; Ricca, A. A comparison of methods for determining proximate carbon fractions of forest litter. Can. J. For. Res. 1990, 20, 166–171. [Google Scholar] [CrossRef]
- Seifter, S.; Dayton, S.; Novic, B.; Muntwyler, E. The estimation of glycogen with the anthrone reagent. Arch. Biochem. 1950, 25, 191–200. [Google Scholar] [PubMed]
- Valenzuela-Estrada, L.R.; Vera-Caraballo, V.; Ruth, L.E.; Eissenstat, D.M. Root anatomy, morphology, and longevity among root orders in Vaccinium corymbosum (Ericaceae). Am. J. Bot. 2008, 95, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- McClaugherty, C.A.; Aber, J.D.; Mellilo, J.M. Decomposition dynamics of fine roots in forested ecosystems. Oikos 1984, 42, 378–386. [Google Scholar] [CrossRef]
- Lõhmus, K.; Ivask, M. Decomposition and nitrogen dynamics of fine roots of Norway spruce (Picea abies (L.) Karst.) at different sites. Plant Soil 1995, 168, 89–94. [Google Scholar] [CrossRef]
- Berg, B. Decomposition of root litter and some factors regulating the process: Long-term root litter decomposition in a Scots pine forest. Soil Biol. Biochem. 1984, 16, 609–617. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant Litter. Decomposition, Humus Formation, Carbon Sequestration, 3rd ed.; Springer: Heidelberg/Berlin, Germany, 2014; pp. 171–187. [Google Scholar]
- Tamura, M.; Tharayil, N. Plant litter chemistry and microbial priming regulate the accrual, composition and stability of soil carbon in invaded ecosystems. New Phytol. 2014, 203, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Pregitzer, K.S.; Laskowski, M.J.; Burton, A.J.; Lessard, V.C.; Zak, D.R. Variation in sugar maple root respiration with root diameter and soil depth. Tree Physiol. 1998, 18, 665–670. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).