4.2. Temperature Effects
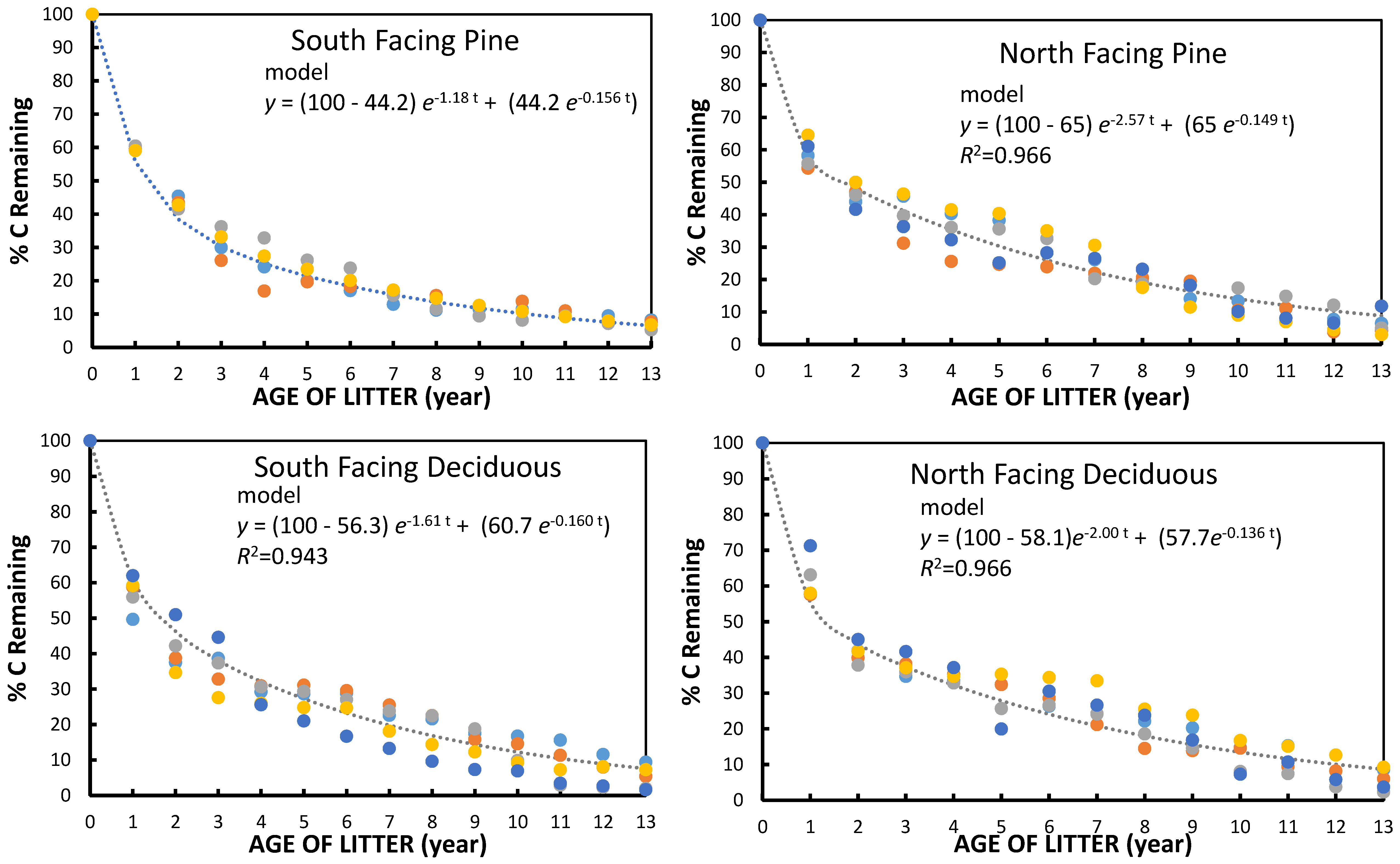
The small but significant increase in turnover rate on both South facing watersheds, as compared to North facing watersheds, was consistent with what might be expected with a 2 °C difference in litter temperature compared to the North facing slope. Although it was impossible to continuously measure litter and soil temperature in the plots during the study, the continuous monitoring on both the S. and North facing deciduous watersheds has consistently shown a difference in temperature in all seasons. The differences corresponded to a Q
10 of 2.1 which is well within the range of Q
10 values from a number of litter decomposition studies that were cited by Bothwell et al. [
6], including Scowcroft et al. [
27], Giardina et al. [
2] for soil C efflux, and Hyvonen et al. [
28] for wheat straw. Bothwell et al. [
6] used the elevation gradient approach but was able to choose sites that gave a narrow range of precipitation, and they noted many studies in which moisture was a confounding variable (e.g., [
5]). One of the advantages of the paired watershed approach was that they were similar in elevation and precipitation and both North facing watersheds abutted one another, as did both South facing watersheds (
Table 1). Precipitation did average 10.4% less on the two South facing watersheds but this difference, if significant, would tend to work in the opposite direction from the observed differences in turnover rate. It is also possible that the more direct radiation on the litter layer on the South facing watershed might make it dryer on the surface, but the relatively high precipitation rates may ameliorate this factor. One of the advantages of this study is that it not only measured differences in short term decomposition rates but also showed higher C pools in nearly the entire forest floor.
One other aspect of the study suggests that there was no long-term pool of very old humus (older than 13 years) that serves as a very long-term storage of C, unlike what is often assumed for soils. This suggests that a future increase in temperature of about 3 °C would simply lead to a lower mass of the forest floor of similar magnitude to the differences observed between the current N. and South facing watersheds. If we substitute the “slow” decomposition k values and litterfall rates into Olson’s equation for the time to reach a 95% steady state forest floor mass [
22], then the times are on the order of 15–20 years, which is less than the time since the last disturbances, so it was likely the forest floor C was near steady state on all watersheds. However, theses “steady state” masses are likely to decline as long-term temperatures rise. Succession in the pine forest is also a factor affecting litterfall.
4.3. Kinetics of Litter vs. Humus Mineralization
One of the most important findings of the study was that the exponential rates of decomposition did not continue to decrease after the first couple of years. The substrate was presumably getting lower in quality. A previous study of chemical functional group content using
13NMR and humic substance content in subsamples taken from the North facing pine WS plots showed C in fresh litter contained 15.6% humic substances (mostly polyphenols and fulvic acid) [
20]. However, that percentage grew to 39.5% of the C by 13 years, indicating a decline in substrate quality [
20]. However, in units of g/m
2, the humic substance fraction declined in mass from year 1 to 13, suggesting that after one year there were enzyme systems at work that could degrade humic substances and probably the lignin that initially comprised 31% [
19] of the mass of the senescent pine litter (
Table 5). Thus, even in the intermediate stages of decomposition there were already microorganisms degrading “low quality” substrate and these were sufficient to prevent a large decline in decomposition rate in the “humus” phase. There was no indication that decomposition rate declined almost to a stop at any point.
The oldest net provided a rare opportunity to delineate the humus that had accumulated on the forest floor that was older than 13 years. It is possible that a portion of the material was fine particles that had migrated downward, but the presence of the 13 year old layer in most plots suggested there would be a small 14th and 15th year layer as well. This layer had the appearance of Oa horizon material (humus). This older humus was only 0.7% to 1.9% of the forest floor C mass in the two deciduous watersheds and 2.4% to 2.7% in the two pine watersheds. It was remarkable that at least some of this material was a layer of recently shed pine needles or deciduous litter when the first net had been laid down 13 years before.
However, there are other sites where long-term accumulation of stable humus occurs, as documented by Berg and McClaugherty [
4]. They give an extensive discussion of the question “Does humus accumulate and where?”. In one example of very deep accumulations of O horizon litter and humus on boreal forested islands, roots and ericoid mycorrhizae have been shown to account for a large proportion of the accumulated organic matter, in part because the rooting zone was above the very deep mineral soil boundary [
29]. In the case of the Coweeta site, it has a humid temperate/subtropical climate, the soil never freezes, and the soils are not extremely nutrient poor [
13], factors that Berg and McClaugherty discuss in relation to properties that may lead to the development of extremely refractory humus layers. Mn concentrations in the litter of the two deciduous watersheds averaged 1.1 mg/g for litter and 1.7 mg/g for humus [
24]. Mn concentrations may be critical for Mn dependent lignin and humic acid peroxidation [
30,
31]. The range in the deciduous litter and humus was intermediate compared to the ranges of most concentrations of the litter in the late stages of decomposition in the study of Berg et al. (1–2 mg/g) which showed a relationship with mass loss in the later stages of decomposition [
30].
There have been other long-term studies that have comparable results for long-term kinetics. In the 10 year litterbag LIDET study [
32] mass remaining over a period of time was shown for litterfall from a group of four to five species in multiple different deciduous and coniferous forest sites across a climatic gradient. The % mass remaining for the mean of the deciduous forest sites was quite similar to the data in
Figure 1 up until six to seven years. Thereafter, the decomposition curve in the LIDET study tended to “level” out to a greater extent in the last three to four years whereas the curve from
Figure 1 of this study continued its trend. We might attribute some of this difference to the use of weight remaining in the LIDET study while this study used % C remaining. The percentage of ash became more concentrated in the latter years and would have obscured the observed continued decomposition of C. Also, the Coweeta site had a climatic index [
32] higher than the two other deciduous forest sites in the LIDET study. The mean decomposition rates for the litter incubated in temperate coniferous forests were lower than those for pine litter in this study, but again, the Coweeta site tended to be warmer that the sites in the LIDET study.
Binkley [
33] used the same technique (developed by Jorgensen et al. [
20]) with a
Pinus taeda forest floor over 10 years and found that 30% of the organic matter remained after 10 years, giving a k rate of −0.166. This rate was lower than that for the South facing pine decomposition rate at Coweeta where at 10 years only about 11% of the C remained. Binkley’s rate coefficient (k), however, was similar to our “slow” fraction rate. However, the study showed a continuation of the decomposition rate during the latter phases, as was the case for the Coweeta study.
4.4. Species Differences
Many studies have identified important differences in decomposition rates characteristic of different dominant species and the effect on O horizon C stock [
34]. For example, in a study of a common garden, experiment plantations of 14 different tree species in Poland demonstrated some fairly large differences on both O and mineral horizon carbon stocks under different species [
13]. Augusto et al. [
35] used a meta-analysis of the number of studies including common garden studies comparing the influence of evergreen gymnosperm and deciduous angiosperm species. They found that while litterfall was comparable, forest floor mass was higher in most evergreen gymnosperm forests with some exceptions. However, they speculated that while deciduous angiosperm litter may exhibit faster decomposition in the early stages, it could enable greater stabilization in the late stages (see also similar conclusions by Prescott [
36]).
In the Coweeta study, the species composition on the paired watersheds was intentionally manipulated by planting two pine plantations adjacent to two non-manipulated deciduous tree dominated watersheds. The basis for the hypothesis that the turnover rate for the pine forest floor would be lower was based on the findings of other studies that
Pinus strobus had a relatively low first year decomposition rate, a relatively high lignin content, and a relatively high ratio of lignin to N compared to most deciduous species in the same studies (see
Table 7) [
19,
37]. In the common garden experiment in Poland, two of the highest accumulations of O horizon carbon were under the two pine species [
13]. In the case of the Coweeta study the effect of pine vs. deciduous forest was insignificant. In the Coweeta study, however, two of the most common species were
Quercus velutina and
Quercus prinus, both with relatively low first year decomposition rates in other studies [
18] and
Table 7. In the Polish common garden,
Quercus rubra (in the same subgenus as
Quercus velutina) had large O horizon C stocks that were similar to the two
Pinus species.
Quercus prinus also had a relatively high ratio of lignin to N in the study of Cromack and Monk [
19]. Not all
Quercus species have a uniformly low rate of decomposition, as shown by the decomposition rates from Cromack and Monk [
19] and the accumulation of O horizon of
Quercus species in the common garden experiment in Poland (
Quercus robar vs.
Quercus rubra) [
13].
There was no a priori reason that the species mixture on the North and South facing deciduous watersheds would confound the difference in turnover rate due to temperature differences. The applicability of the prediction of the weighted average species decomposition calculation (
Table 2) was supported by the fact that Cromack and Monk’s [
19] mixed species litterbag exhibited almost the same decomposition rate (see
Table 7). One co-dominant species in the Coweeta deciduous forest was
Acer rubrum, which had one of the highest first year decomposition rates in the study of Cromack and Monk [
19], and also had the highest content of water soluble C (40.7% of initial C as opposed to an average of 27.2% for weighted species mix) in the study of Qualls et al. [
38].
Acer rubrum was almost equally important in the two deciduous watersheds, and that factor, along with the similar distribution of oaks, helped make the predicted decomposition rate shown in
Table 7 on the two deciduous watersheds similar. The importance of
Acer rubrum in forest floor decomposition was shown by Alexander [
12] who showed changes on forest floor biomass with succession to
Acer rubrum dominance in the central hardwoods forest of the U.S. In addition, Ma et al. [
39] predict a major shift from oak-hickory dominance to maple dominance in the central hardwoods forest of the U.S. due to climate change and fire suppression. Because so much of the initial decomposition rate of
Acer rubrum is due to its high soluble organic C content, it is possible that C is simply translocated to the mineral soil where it can be adsorbed and perhaps become more resistant to decomposition. Nevertheless, the impact of climatic warming on O horizon C storage can be influenced not only by warming but by shifts in species range.
4.5. The Fate of Litterfall on a Deciduous Forest Watershed
The South facing deciduous watershed was chosen to compile a summary of the stocks and fluxes of the forest floor based on measurements summarized in the Tables of this paper. In addition, there were also detailed measurements of dissolved organic C (DOC) flux from the forest floor on the South facing deciduous WS and measurements of the stock of C in the A horizon available from other studies.
Figure 2 places the forest floor inputs, outputs, and stocks in a quantitative perspective.
The diagram indicates the following major points:
- (1)
The mean residence time of the whole forest floor was 3.1 years.
- (2)
Only 1.9% of the whole forest floor (O horizon) is more than 13 years old, and there was no large accumulation of older humus.
- (3)
Fine particle fluxes onto the surface or into the A horizon were significant but small compared to litterfall (2.2% compared to litterfall), the mass of the forest floor, and the leaching of DOC.
- (4)
The net flux of DOC from the forest floor is by far the largest input into the A horizon.
- (5)
The net deposition of DOC in the A horizon (influx minus efflux) is a major fate for litterfall and was 18% as large as mineralization (measured by difference).
- (6)
C storage in the A horizon was 84% of that in the O and A horizons combined.
A few qualifications should be noted about the budgetary diagram (
Figure 2). First, the mineralization represents only the C flux from the forest floor and not the mineral soil. It is expected that the CO
2 flux from the mineral soil and root respiration may be much larger. Also, the forest floor mass represents mass in summer before the next autumnal litterfall, and is the annual minimum. That factor should be noted when comparing the mass of forest floor to other literature. This paper concentrates on foliar litter masses and does not include woody litter, but the small woody litterfall is indicated in the diagram to put it in perspective. Some small proportion of the DOC flux and fine particle flux, however, could originate from woody litter. The arrows for DOC leaching and fine particle flux are shown bypassing the thin layer of older humus simply for clarity in the diagram, but likely traverse the layer. Bioturbation was not explicitly measured in this study, and so is indicated by arrows with a question mark instead of a quantity.
The small transfer of particulate C into the A horizon and the small size of the older humus layer suggest that transport of DOC and deposition of root litter may be more important as origins for the C in the A horizon. Where earthworms are common, bioturbation has a dramatic effect in the redistribution of O horizon organic matter into the mineral soil [
13]. However, earthworms were not common in these acidic soils [
10] and macroscopic bioturbation was not obvious with the mor O horizon and the clear smooth boundary between the O and A horizon. However, other types of litter and soil fauna such as enchytraeids, collembollans, millipedes, mites, and nematodes have been found to be abundant [
10,
19]. The translocation and adsorption of refractory DOC from the forest floor to the mineral soil may be a means for enhancing the residence time [
42].