Wood Density-Climate Relationships Are Mediated by Dominance Class in Black Spruce (Picea mariana (Mill.) B.S.P.)
Abstract
:1. Introduction
2. Materials and Methods
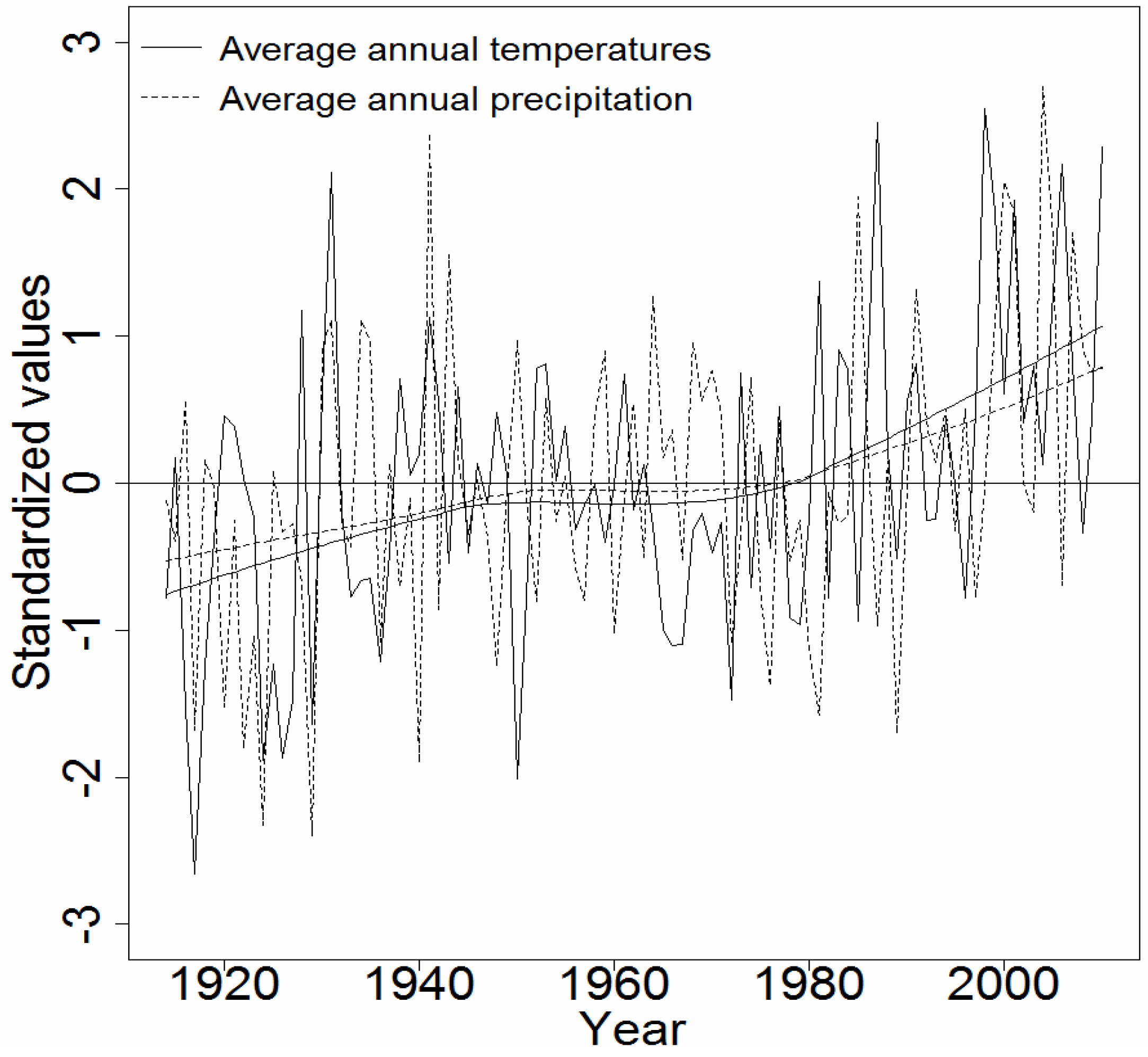
2.1. Sampling and Climate Data
2.2. Annual Ring Width and Wood Density Measurements
| Abbreviations | Description (unit) |
|---|---|
| MnD | Minimum ring density (kg m−3) |
| MxD | Maximum ring density (kg m−3) |
| RD | Ring density (kg m−3) |
| RW | Ring width (mm) |
| CA | Cambial age (years) |
| Tmean.i | Mean daily temperature of month i in the current year (°C) |
| Tmin.i | Average minimum daily temperature of month i in the current year (°C) |
| Tmax.i | Average maximum daily temperature of month i in the current year (°C) |
| ExTmax.i | Extreme maximum daily temperature of month i in the current year (°C) |
| Prec.i | Precipitation of month i in the current year (mm) |
| Prec.i.p | Precipitation of month i in the previous year (mm) |
| Dominance Class | Tree Age (years) | DBH (cm) | Height (m) | Number of Rings | RW (mm) | Mean Ring Density (kg/m3) | Minimum Ring Density (kg/m3) | Maximum Ring Density (kg/m3) |
|---|---|---|---|---|---|---|---|---|
| Dominant | 84 | 19.7 | 17.6 | 1015 | 1.16 (0.48) | 481.76 (58.59) | 348.25 (45.51) | 776.96 (101.44) |
| Co-dominant | 81 | 13.3 | 14.7 | 968 | 0.78 (0.35) | 529.68 (66.53) | 384.35 (61.72) | 784.16 (86.44) |
| Intermediate | 79 | 11.5 | 14.5 | 472 | 0.64 (0.24) | 535.07 (65.53) | 396.38 (64.81) | 750.28 (81.51) |
2.3. Data Analysis

3. Results
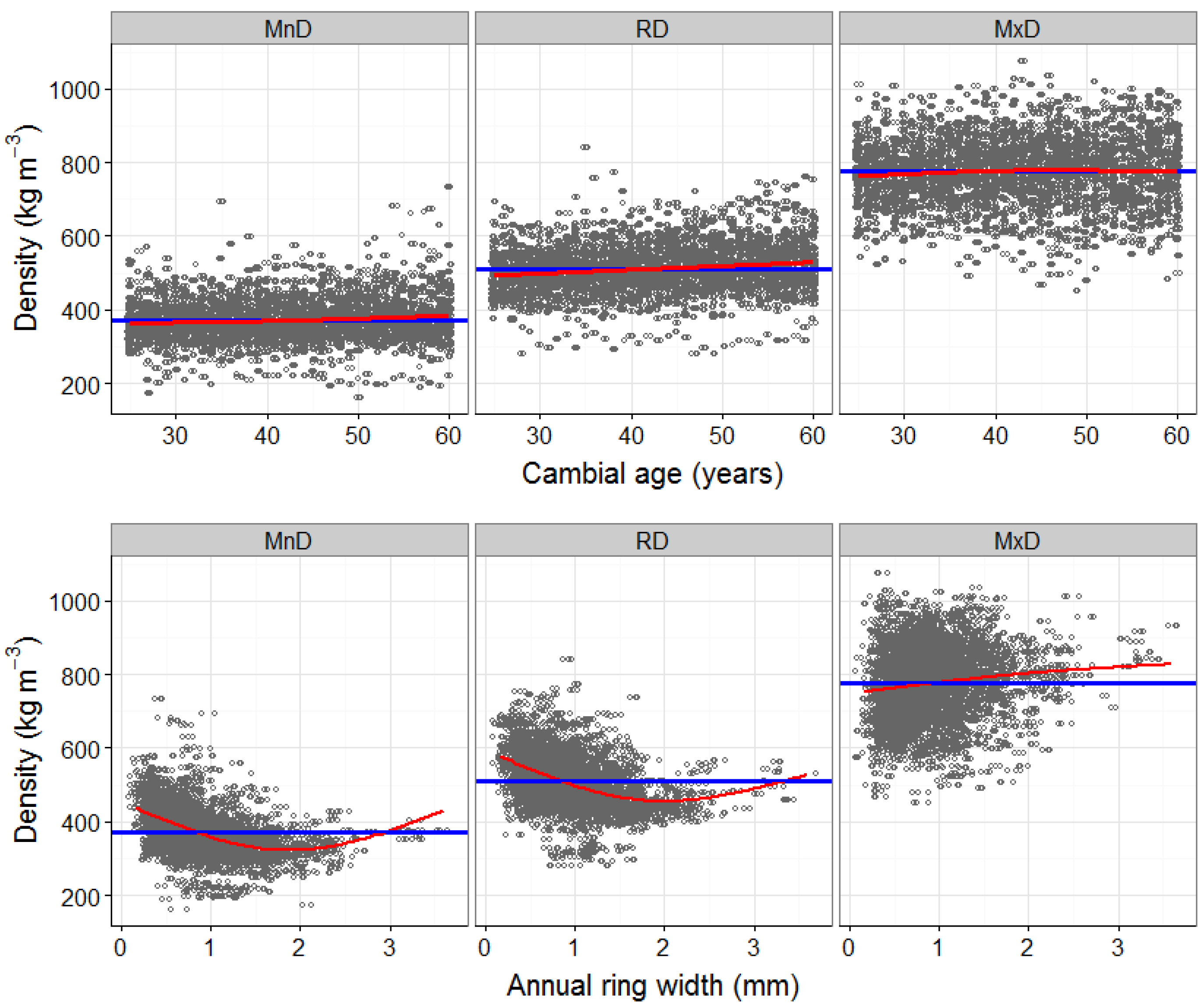
3.1. Detrending the Effects of Cambial Age and Ring Width on Wood Density Components (Model 1)
3.2. Inclusion of Climate Variables in the Models (Model 2)
| Minimum Density | Pooled | Dominant | Co-dominant | Intermediate | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 2 | Model 2 | Model 2 | ||||||
| Est. | S.E. | Est. | S.E. | Est. | S.E. | Est. | S.E. | Est. | S.E. | |
| (Intercept) | 277.2 *** | 7.8 | 291.7 *** | 10.2 | 302.8 *** | 14.1 | 273.8 *** | 14.0 | 291.8 *** | 21.9 |
 | 81.8 *** | 4.2 | 83.3 *** | 4.1 | 59.5 *** | 6.6 | 84.9 *** | 6.2 | 100.5 *** | 9.1 |
| Tmin.1 | −0.7 *** | 0.1 | −0.7 *** | 0.2 | −0.8 ** | 0.3 | ||||
| Tmin.5 | 2.4 *** | 0.2 | 2.2 *** | 0.3 | 2.3 *** | 0.5 | 1.8 *** | 0.6 | ||
| Tmin.7 | −1.7 *** | 0.4 | −1.9 *** | 0.5 | −2.1 * | 1.1 | ||||
| Prec.5 | −0.1 *** | 0.0 | −0.1 *** | 0.0 | −0.1 *** | 0.0 | ||||
| Prec.6 | −0.1 *** | 0.0 | −0.1 *** | 0.0 | −0.1 *** | 0.0 | −0.1 * | 0.0 | ||
| Prec.3p | −0.2 *** | 0.0 | −0.2 *** | 0.0 | −0.2 ** | 0.1 | ||||
| Prec.10p | 0.0* | 0.0 | 0.1 * | 0.0 | ||||||
| Coefficients of Determination | ||||||||||
| Fixed | 0.22 | 0.24 | 0.05 | 0.25 | 0.14 | |||||
| Site | 0.31 | 0.33 | 0.51 | 0.36 | 0.25 | |||||
| Tree | 0.72 | 0.73 | 0.72 | 0.73 | 0.62 | |||||
| Error Statistics Calculated from the Fixed Effects | ||||||||||
| ME% | 0.2 | 0.2 | ||||||||
| |ME|% | 10.3 | 10.1 | ||||||||
| Maximum Density | Pooled | Dominant | Co-dominant | Intermediate | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 2 | Model 2 | Model 2 | ||||||
| Est. | S.E. | Est. | S.E. | Est. | S.E. | Est. | S.E. | Est. | S.E. | |
| (Intercept) | 945.6 *** | 20.1 | 928.8 *** | 29.4 | 753.5 *** | 25.2 | 961.3 *** | 32.6 | 1077.8 *** | 53.8 |
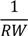 | −45.9 *** | 3.1 | −45.4 *** | 3.1 | −53.9 *** | 8.0 | −47.2 *** | 4.3 | −39.1 *** | 4.5 |
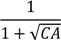 | −802.4 *** | 123.9 | −638.8 *** | 122.3 | −1033 *** | 180.9 | −1161.4 *** | 228.4 | ||
| Tmean.4 | 2.0 *** | 0.6 | 3.2** | 1.2 | ||||||
| Tmean.5 | 3.8 *** | 0.5 | 4.5 *** | 0.8 | 4.8 *** | 0.8 | 3.8 *** | 1.1 | ||
| Tmean.6 | 1.8 * | 0.7 | 2.8 ** | 1.1 | ||||||
| ExTmax.8 | −1.8 *** | 0.6 | −3.4 *** | 1.1 | ||||||
| Prec.7 | −0.1 *** | 0.0 | −0.1 * | 0.1 | −0.2 *** | 0.1 | ||||
| Prec.8 | −0.2 *** | 0.0 | −0.2 *** | 0.0 | −0.1 *** | 0.0 | −0.2 *** | 0.1 | ||
| Prec.5.p | 0.1 *** | 0.0 | 0.2 *** | 0.1 | ||||||
| Coefficients of determination | ||||||||||
| Fixed | 0.01 | 0.03 | 0.03 | 0.04 | 0.07 | |||||
| Site | 0.01 | 0.03 | 0.21 | 0.04 | 0.41 | |||||
| Tree | 0.53 | 0.56 | 0.61 | 0.49 | 0.54 | |||||
| Error statistics calculated from the fixed effects | ||||||||||
| ME% | 0.3 | 0.3 | ||||||||
| |ME|% | 9.4 | 9.2 | ||||||||
| Ring Density | Pooled | Dominant | Co-dominant | Intermediate | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 2 | Model 2 | Model 2 | ||||||
| Est. | S.E. | Est. | S.E. | Est. | S.E. | Est. | S.E. | Est. | S.E. | |
| (Intercept) | 556.6 *** | 21.5 | 562.8 *** | 24.1 | 500.6 *** | 34.6 | 564.0 *** | 39.1 | 621.2 *** | 32.8 |
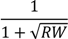 | 68.4 ** | 26.3 | 86.2 *** | 25.4 | 77.3 * | 39.1 | 104.6 * | 41.8 | ||
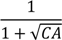 | −617.0 *** | 77.8 | −621.8 *** | 75.4 | −335.7 *** | 112.4 | −661.7 *** | 121.6 | −1045.0 *** | 185.6 |
| Tmin.1 | −0.7 *** | 0.2 | −0.6 * | 0.2 | −0.7 * | 0.3 | −1.05 * | 0.4 | ||
| Tmean.4 | 0.9 *** | 0.3 | 1.2 *** | 0.4 | ||||||
| Tmean.5 | 2.8 *** | 0.3 | 2.5 *** | 0.4 | 3.0 *** | 0.5 | 3.1 *** | 0.7 | ||
| Tmin.7 | −1.7 *** | 0.5 | −1.4 * | 0.7 | ||||||
| Tmean.9 | −1.7 *** | 0.5 | −1.9 *** | 0.6 | −2.7 *** | 0.8 | ||||
| Prec.4 | 0.1 * | 0.0 | 0.2 *** | 0.1 | ||||||
| Prec.6 | −0.1 *** | 0.0 | −0.1 *** | 0.0 | −0.1 *** | 0.0 | ||||
| Prec.8 | −0.1 *** | 0.0 | −0.1 *** | 0.0 | −0.1 *** | 0.0 | ||||
| Prec.3.p | −0.1 * | 0.0 | −0.1 * | 0.1 | ||||||
| Prec.5.p | 0.1 *** | 0.0 | 0.1 * | 0.0 | 0.2 *** | 0.0 | ||||
| Prec.8.p | −0.1 *** | 0.0 | −0.1 *** | 0.0 | ||||||
| Prec.12.p | −0.1 ** | 0.0 | −0.2 * | 0.1 | ||||||
| Coefficients of determination | ||||||||||
| Fixed | 0.07 | 0.11 | 0.08 | 0.12 | 0.09 | |||||
| Site | 0.19 | 0.23 | 0.48 | 0.25 | 0.40 | |||||
| Tree | 0.70 | 0.73 | 0.72 | 0.69 | 0.64 | |||||
| Error statistics calculated from the fixed effects | ||||||||||
| ME% | 0.4 | 0.3 | ||||||||
| |ME|% | 9.9 | 9.6 | ||||||||
| Density Component | Dominance Classes | Correlation | G-Score | ||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | ||
| MnD | Dominant | 0.23 | 0.30 | 0.58 | 0.62 |
| Co-dominant | 0.54 | 0.55 | 0.65 | 0.66 | |
| Intermediate | 0.35 | 0.38 | 0.52 | 0.54 | |
| Dominant | 0.09 | 0.22 | 0.54 | 0.56 | |
| MxD | Co-dominant | 0.24 | 0.28 | 0.57 | 0.58 |
| Intermediate | 0.22 | 0.33 | 0.64 | 0.66 | |
| Dominant | 0.20 | 0.36 | 0.54 | 0.59 | |
| RD | Co-dominant | 0.35 | 0.36 | 0.56 | 0.62 |
| Intermediate | 0.25 | 0.30 | 0.42 | 0.54 | |
4. Discussion
4.1. The Effects of Cambial Age and Ring Width on Wood Density Components
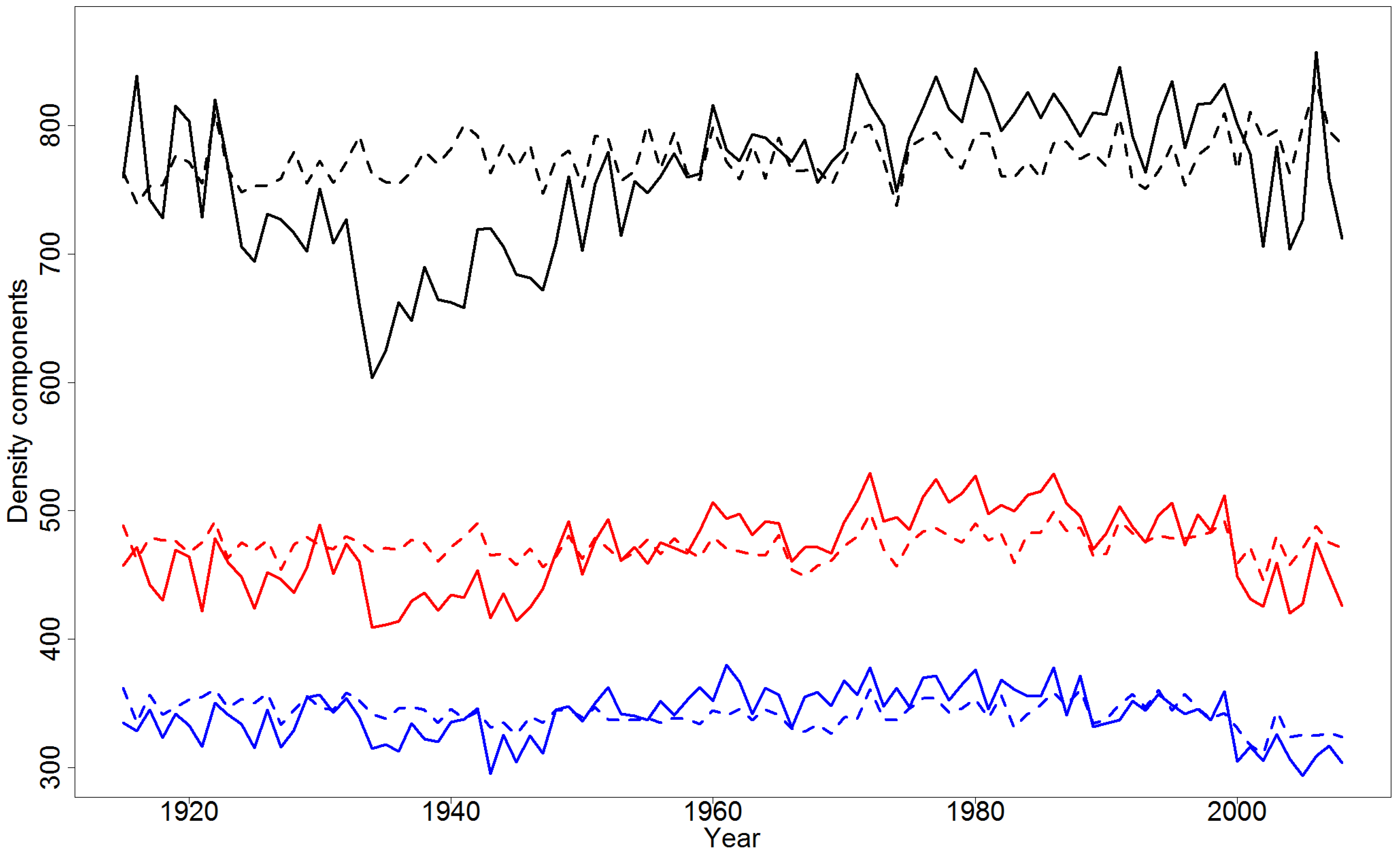
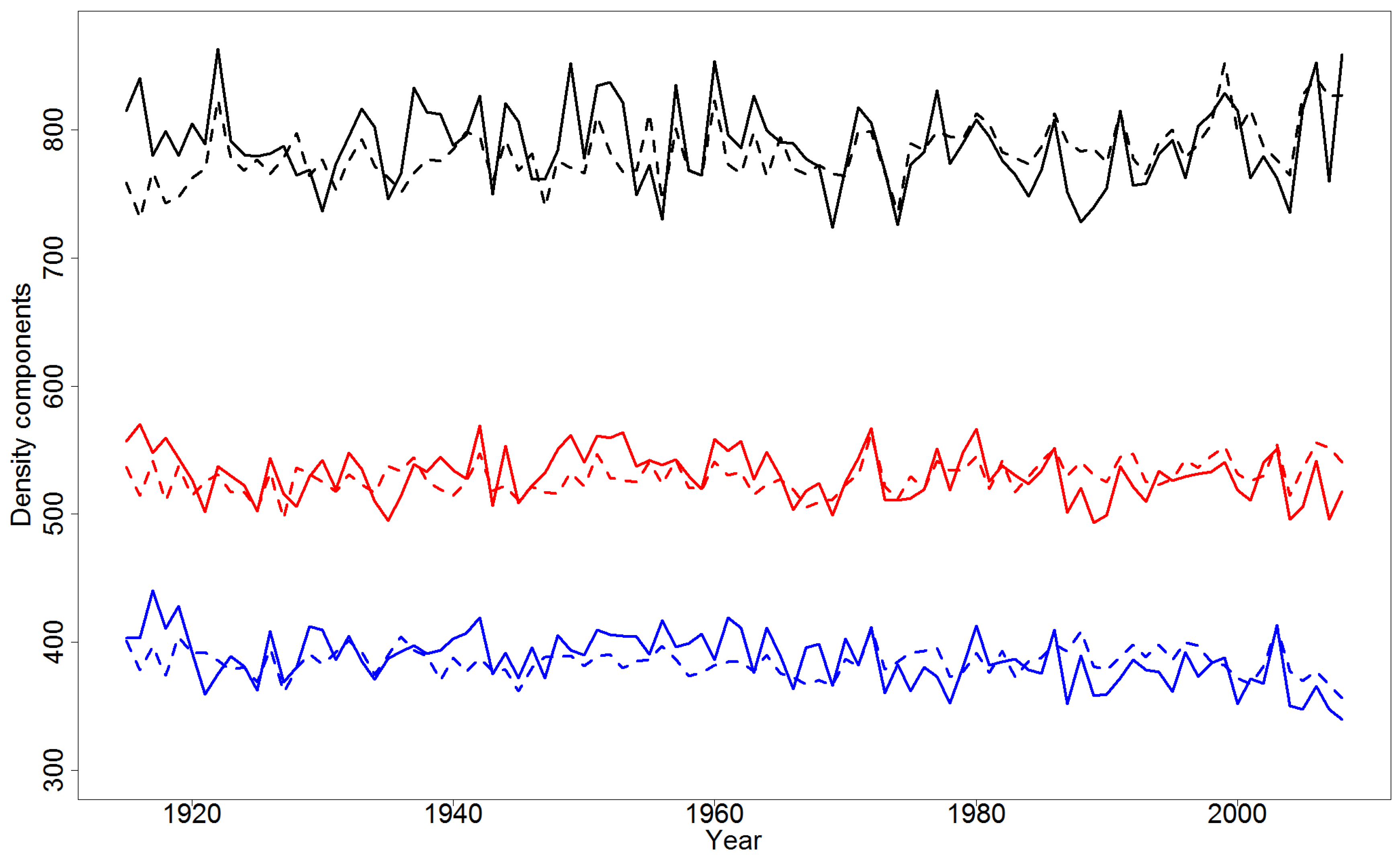
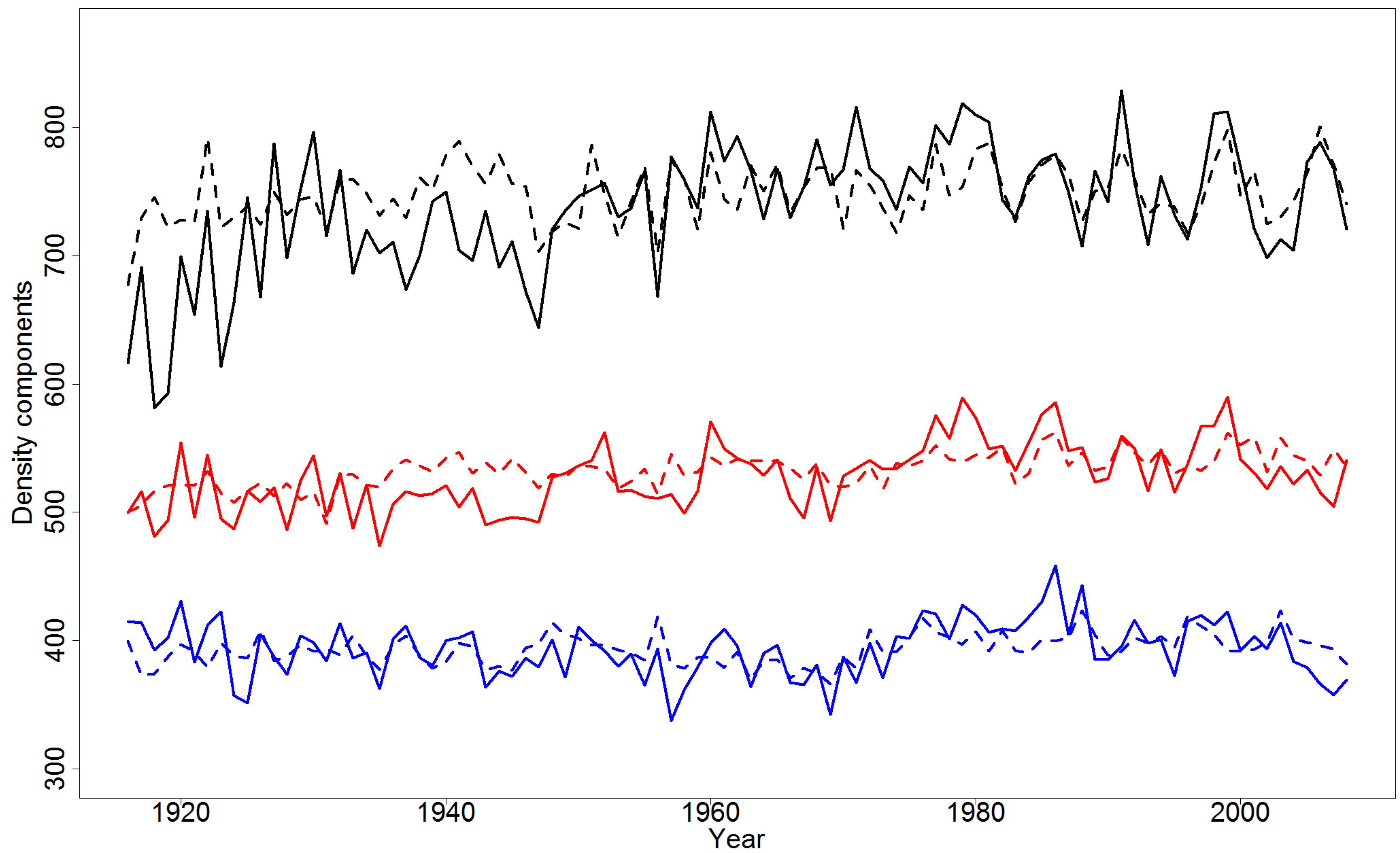
4.2. Effect of Climatic Variables on Wood Density Components in the Pooled Data
4.3. The Effects Dominance Class on Density-Climate Relationships
4.4. Simulations of Yearly Variation in Wood Density
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Boisvenue, C.L.; Running, S.W. Impacts of climate change on natural forest productivity—Evidence since the middle of the 20th century. Glob. Chang. Biol. 2006, 12, 862–882. [Google Scholar] [CrossRef]
- Bouriaud, O.; Bréda, N.; Dupouey, J.L.; Granier, A. Is ring width a reliable proxy for stem-biomass increment? A case study in European beech. Can. J. For. Res. 2005, 35, 2920–2933. [Google Scholar] [CrossRef]
- Hogg, E.H.; Brandt, J.P.; Michaelian, M. Impacts of a regional drought on the productivity, dieback, and biomass of Western Canadian aspen forests. Can. J. For. Res. 2008, 38, 1373–1384. [Google Scholar] [CrossRef]
- D’Arrigo, R.D.; Jacoby, G.C. Secular trends in high northern latitude temperature reconstructions based on tree rings. Clim. Chang. 1993, 25, 163–177. [Google Scholar] [CrossRef]
- Mäkinen, H.; Nöjd, P.; Kahle, H.P.; Neumann, U.; Tveite, B.; Mielikäinen, K.; Röhle, H.; Spiecker, H. Large-scale climatic variability and radial increment variation of Picea abies (L.) Karst. in central and Northern Europe. Trees 2003, 17, 173–184. [Google Scholar]
- Savva, Y.; Koubaa, A.; Tremblay, F.; Bergeron, Y. Effects of radial growth, tree age, climate, and seed origin on wood density of diverse jack pine populations. Trees 2010, 24, 53–65. [Google Scholar] [CrossRef]
- Jyske, T.; Hölttä, T.; Mäkinen, H.; Nöjd, P.; Lumme, I.; Spiecker, H. The Effect of artificially induced drought on radial increment and wood properties of Norway spruce. Tree Physiol. 2010, 30, 103–115. [Google Scholar] [CrossRef]
- Kilpeläinen, A.; Peltola, H.; Ryyppö, A.; Sauvala, K.; Laitinen, K.; Kellomäki, S. Wood properties of scots pines (Pinus sylvestris) grown at elevated temperature and carbon dioxide concentration. Tree Physiol. 2003, 23, 889–897. [Google Scholar] [CrossRef]
- Kilpeläinen, A.; Peltola, H.; Ryyppö, A.; Kellomäki, S. Scots pine responses to elevated temperature and carbon dioxide concentration: Growth and wood properties. Tree Physiol. 2005, 25, 75–83. [Google Scholar] [CrossRef]
- Ketterings, Q.M.; Coe, R.; van Noordwijk, M.; Ambagau, Y.; Palm, C.A. Reducing uncertainty in the use of allometric biomass equations for predicting above-ground tree biomass in mixed secondary forests. For. Ecol. Manag. 2001, 146, 199–209. [Google Scholar] [CrossRef]
- Pussinen, A.; Nabuurs, G.J.; Wieggers, H.J.J.; Reinds, G.J.; Wamelink, G.W.W.; Kros, J.; Mol-Dijkstra, J.P.; de Vries, W. Modelling long-term impacts of environmental change on mid-and high-latitude european forests and options for adaptive forest management. For. Ecol. Manag. 2009, 258, 1806–1813. [Google Scholar] [CrossRef]
- Zobel, B.J.; van Buijtenen, J.P. Wood Variation:Its Causes and Control; Springer-Verlag: Berlin, Germany; New York, NY, USA, 1989. [Google Scholar]
- Baker, T.R.; Phillips, O.L.; Malhi, Y.; Almeida, S.; Arroyo, L.; di Fiore, A.; Erwin, T.; Killeen, T.J.; Laurance, S.G.; Laurance, W.F. Variation in wood density determines spatial patterns in Amazonian forest biomass. Glob. Chang. Biol. 2004, 10, 545–562. [Google Scholar] [CrossRef]
- Decoux, V.; Varcin, É.; Leban, J.M. Relationships between the intra-ring wood density assessed by X-ray densitometry and optical anatomical measurements in conifers. Consequences for the cell wall apparent density determination. Ann. For. Sci. 2004, 61, 251–262. [Google Scholar] [CrossRef]
- Fritts, H.C.; Vaganov, E.E.; Scviderskaya, I.V.; Shashkin, A.V. Climate variation and tree-ring structure in conifers. Empirical and mechanistic models of tree-ring width, number of cells, cell size, cell-wall thickness and wood density. Clim. Res. 1991, 1, 97–116. [Google Scholar] [CrossRef]
- Larson, P.R.; Kretschmann, D.E.; Clark, A., III; Isebrands, J.G. Formation and Properties of Juvenile Wood in Southern Pines: A Synopsis; Series: General Technical Reports; U.S. Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2001. [Google Scholar]
- Begum, S.; Nakaba, S.; Bayramzadeh, V.; Oribe, Y.; Kubo, T.; Funada, R. Temperature responses of cambial reactivation and xylem differentiation in hybrid poplar (Populus sieboldii × P. grandidentata) under natural conditions. Tree Physiol. 2008, 28, 1813–1819. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Gričar, J.; Seo, J.W.; Rathgeber, C.B.; Anfodillo, T.; Morin, H.; Levanic, T.; Oven, P.; Jalkanen, R. Critical temperatures for xylogenesis in conifers of cold climates. Glob. Ecol. Biogeogr. 2008, 17, 696–707. [Google Scholar] [CrossRef]
- Begum, S.; Nakaba, S.; Yamagishi, Y.; Oribe, Y.; Funada, R. Regulation of cambial activity in relation to environmental conditions: Understanding the role of temperature in wood formation of trees. Physiol. Plant 2013, 147, 46–54. [Google Scholar] [CrossRef]
- Bouriaud, O.; Leban, J.M.; Bert, D.; Deleuze, C. Intra-annual variations in climate influence growth and wood density of Norway spruce. Tree Physiol. 2005, 25, 651–660. [Google Scholar] [CrossRef]
- Wimmer, R.; Grabner, M. A comparison of tree-ring features in picea abies as correlated with climate. IAWA J. 2000, 21, 403–416. [Google Scholar] [CrossRef]
- Wang, L.; Payette, S.; Begin, Y. Relationships between anatomical and densitometric characteristics of black spruce and summer temperature at tree line in Northern Quebec. Can. J. For. Res. 2002, 32, 477–486. [Google Scholar] [CrossRef]
- Briffa, K.R.; Schweingruber, F.H.; Jones, P.D.; Osborn, T.J.; Shiyatov, S.G.; Vaganov, E.A. Reduced sensitivity of recent tree-growth to temperature at high northern latitudes. Nature 1998, 391, 678–682. [Google Scholar] [CrossRef]
- Schweingruber, F.H.; Briffa, K.R.; Nogler, P. A tree-ring densitometric transect from Alaska to Labrador. Int. J. Meteorol. 1993, 37, 151–169. [Google Scholar]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Martinez-Vilalta, J.; Vanderklein, D.; Mencuccini, M. Tree height and age-related decline in growth in Scots pine (Pinus sylvestris L.). Oecologia 2007, 150, 529–544. [Google Scholar] [CrossRef]
- Peñuelas, J. Plant physiology: A big issue for trees. Nature 2005, 437, 965–966. [Google Scholar] [CrossRef]
- Mencuccini, M.; Martínez–Vilalta, J.; Vanderklein, D.; Hamid, H.A.; Korakaki, E.; Lee, S.; Michiels, B. Size–mediated ageing reduces vigour in trees. Ecol. Lett. 2005, 8, 1183–1190. [Google Scholar] [CrossRef]
- Mérian, P.; Lebourgeois, F. Size-mediated climate-growth relationships in temperate forests: A multi-species analysis. For. Ecol. Manag. 2011, 261, 1382–1391. [Google Scholar] [CrossRef]
- Castagneri, D.; Nola, P.; Cherubini, P.; Motta, R. Temporal variability of size–growth relationships in a Norway spruce forest: The influences of stand structure, logging, and climate. Can. J. For. Res. 2012, 42, 550–560. [Google Scholar] [CrossRef]
- De Luis, M.; Novak, K.; Čufar, K.; Raventós, J. Size mediated climate–growth relationships in Pinus Halepensis and Pinus Pinea. Trees 2009, 23, 1065–1073. [Google Scholar] [CrossRef]
- Chhin, S.; Hogg, E.H.; Lieffers, V.J.; Huang, S. Potential effects of climate change on the growth of lodgepole pine across diameter size classes and ecological regions. For. Ecol. Manag. 2008, 256, 1692–1703. [Google Scholar] [CrossRef]
- Koubaa, A.; Zhang, S.Y.; Isabel, N.; Beaulieu, J.; Bousquet, J. Phenotypic correlations between juvenile-mature wood density and growth in black spruce. Wood Fiber Sci. 2000, 32, 61–71. [Google Scholar]
- Zhang, S.Y.; Chauret, G.; Ren, H.Q.; Desjardins, R. Impact of initial spacing on plantation black spruce lumber grade yield, bending properties, and MSR yield. Wood Fiber Sci. 2002, 34, 460–475. [Google Scholar]
- Alteyrac, J.; Zhang, S.Y.; Cloutier, A.; Ruel, J.C. Influence of stand density on ring width and wood density at different sampling heights in black spruce (Picea Mariana (Mill.) BSP). Wood Fiber Sci. 2005, 37, 83–94. [Google Scholar]
- St-Germain, J.L.; Krause, C. Latitudinal variation in tree-ring and wood cell characteristics of Picea mariana across the continuous boreal forest in Quebec. Can. J. For. Res. 2008, 38, 1397–1405. [Google Scholar] [CrossRef]
- Ecoregions Working Group. Ecoclimatic Regions of Canada, First Approximation; Environment Canada: Ottawa, ON, Canada, 1989; p. 118. [Google Scholar]
- Xiang, W.; Leitch, M.; Auty, D.; Duchateau, E.; Achim, A. Radial trends in black spruce wood density can show an age-and growth-related decline. Ann. For. Sci. 2014. [Google Scholar] [CrossRef]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Bontemps, J.D.; Esper, J. Statistical modelling and RCS detrending methods provide similar estimates of long-term trend in radial growth of common beech in north-eastern France. Dendrochronologia 2011, 29, 99–107. [Google Scholar] [CrossRef]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS; Springer: New York, NY, USA, 2000; p. 528. [Google Scholar]
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK; New York, NY, USA; San Francisco, CA, USA, 1976. [Google Scholar]
- Briffa, K.R.; Osborn, T.J.; Schweingruber, F.H.; Jones, P.D.; Shiyatov, S.G.; Vaganov, E.A. Tree-ring width and density data around the northern hemisphere: Part 1, local and regional climate signals. Holocene 2002, 12, 737–757. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. The R Core Team (2009) Nlme: Linear and Nonlinear Mixed Effects Models; R Package Version 3.1–96; R Foundation for Statistical Computing: Vienna, Austria, 2010. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. Available online: http://cran.r-project.org (accessed on 11 May 2013).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Parresol, B.R. Assessing tree and stand biomass: A review with examples and critical comparisons. For. Sci. 1999, 45, 573–593. [Google Scholar]
- Eckstein, D.; Bauch, J. Beitrag zur rationalisierung eines dendrochronologischen verfahrens und zur analyse seiner aussagesicherheit. Forstwiss. Cent. 1969, 88, 230–250. [Google Scholar] [CrossRef]
- Bunn, A.G. Statistical and visual crossdating in R using the DplR library. Dendrochronologia 2010, 28, 251–258. [Google Scholar] [CrossRef]
- Jyske, T.; Mäkinen, H.; Saranpää, P. Wood density within Norway spruce stems. Silva Fenn. 2008, 42, 439–455. [Google Scholar]
- Franceschini, T.; Longuetaud, F.; Bontemps, J.D.; Bouriaud, O.; Caritey, B.D.; Leban, J.M. Effect of ring width, cambial age, and climatic variables on the within-ring wood density profile of Norway spruce Picea abies (L.) Karst. Trees 2013, 27, 913–925. [Google Scholar] [CrossRef]
- Mäkinen, H.; Jaakkola, T.; Piispanen, R.; Saranpää, P. Predicting wood and tracheid properties of Norway spruce. For. Ecol. Manag. 2007, 241, 175–188. [Google Scholar] [CrossRef]
- Deslauriers, A.; Morin, H.; Begin, Y. Cellular phenology of annual ring formation of Abies balsamea in the Quebec boreal forest (Canada). Can. J. For. Res. 2003, 33, 190–200. [Google Scholar] [CrossRef]
- Rathgeber, C.B.; Rossi, S.; Bontemps, J.D. Cambial activity related to tree size in a mature silver-fir plantation. Ann. Bot. 2011, 108, 429–438. [Google Scholar] [CrossRef]
- Gričar, J.; Zupančič, M.; Čufar, K.; Koch, G.; Schmitt, U.; Oven, P. Effect of local heating and cooling on cambial activity and cell differentiation in the stem of Norway spruce (Picea abies). Ann. Bot. 2006, 97, 943–951. [Google Scholar] [CrossRef]
- Rossi, S.; Morin, H.; Deslauriers, A.; Plourde, P.Y. Predicting xylem phenology in black spruce under climate warming. Glob. Chang. Biol. 2011, 17, 614–625. [Google Scholar] [CrossRef]
- Deslauriers, A.; Morin, H. Intra-annual tracheid production in balsam fir stems and the effect of meteorological variables. Trees 2005, 19, 402–408. [Google Scholar] [CrossRef]
- Mäkinen, H.; Saranpää, P.; Linder, S. Wood-density variation of Norway spruce in relation to nutrient optimization and fibre dimensions. Can. J. For. Res. 2002, 32, 185–194. [Google Scholar] [CrossRef]
- Grace, J.; Norton, D.A. Climate and growth of Pinus sylvestris at its upper altitudinal limit in Scotland: Evidence from tree growth-rings. J. Ecol. 1990, 78, 601–610. [Google Scholar] [CrossRef]
- Pederson, N.; Cook, E.R.; Jacoby, G.C.; Peteet, D.M.; Griffin, K.L. The influence of winter temperatures on the annual radial growth of six northern range margin tree species. Dendrochronologia 2004, 22, 7–29. [Google Scholar] [CrossRef]
- Ryan, D.A.J.; Allen, O.B.; McLaughlin, D.L.; Gordon, A.M. Interpretation of sugar maple (Acer saccharum) ring chronologies from central and southern Ontario using a mixed linear model. Can. J. For. Res. 1994, 24, 568–575. [Google Scholar] [CrossRef]
- Wilson, R.J.S.; Luckman, B.H. Dendroclimatic reconstruction of maximum summer temperatures from upper treeline sites in interior British Columbia, Canada. Holocene 2003, 13, 851–861. [Google Scholar] [CrossRef]
- Vaganov, E.A.; Schulze, E.D.; Skomarkova, M.V.; Knohl, A.; Brand, W.A.; Roscher, C. Intra-annual variability of anatomical structure and δ13C values within tree rings of spruce and pine in alpine, temperate and boreal Europe. Oecologia 2009, 161, 729–745. [Google Scholar] [CrossRef]
- Chen, F.; Yuan, Y.J.; Wei, W.S.; Yu, S.L.; Fan, Z.A.; Zhang, R.B.; Zhang, T.W.; Li, Q.; Shang, H.M. Temperature reconstruction from tree-ring maximum latewood density of Qinghai spruce in Middle Hexi Corridor, China. Theor. Appl. Climatol. 2012, 107, 633–643. [Google Scholar] [CrossRef]
- Lebourgeois, F.O.; Lévy, G.; Aussenac, G.; Clerc, B.; Willm, F. Influence of soil drying on leaf water potential, photosynthesis, stomatal conductance and growth in two black pine varieties. Ann. For. Sci. 1998, 55, 287–299. [Google Scholar] [CrossRef]
- Durre, I.; Wallace, J.M.; Lettenmaier, D.P. Dependence of extreme daily maximum temperatures on antecedent soil moisture in the contiguous united states during summer. J. Clim. 2000, 13, 2641–2651. [Google Scholar] [CrossRef]
- Yasue, K.; Funada, R.; Kobayashi, O.; Ohtani, J. The effects of tracheid dimensions on variations in maximum density of Picea glehnii and relationships to climatic factors. Trees 2000, 14, 223–229. [Google Scholar] [CrossRef]
- Splechtna, B.E.; Dobry, J.; Klinka, K. Tree-ring characteristics of subalpine fir (Abies lasiocarpa (Hook.) Nutt.) in relation to elevation and climatic fluctuations. Ann. For. Sci. 2000, 57, 89–100. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Martín-Benito, D.; Cherubini, P.; del Río, M.; Cañellas, I. Growth response to climate and drought in Pinus nigra Arn. trees of different crown classes. Trees 2008, 22, 363–373. [Google Scholar] [CrossRef]
- Kimmins, J.P. Forest Ecology: A Foundation for Sustainable Management; Prentice-Hall Inc.: New York, NY, USA, 1997. [Google Scholar]
- Pichler, P.; Oberhuber, W. Radial growth response of coniferous forest trees in an inner alpine environment to heat-wave in 2003. For. Ecol. Manag. 2007, 242, 688–699. [Google Scholar] [CrossRef]
- Olivar, J.; Bogino, S.; Spiecker, H.; Bravo, F. Climate impact on growth dynamic and intra-annual density fluctuations in aleppo pine (Pinus halepensis) trees of different crown classes. Dendrochronologia 2012, 30, 35–47. [Google Scholar] [CrossRef]
- Van Lear, D.H.; Kapeluck, P.R. Above-and below-stump biomass and nutrient content of a mature loblolly pine plantation. Can. J. For. Res. 1995, 25, 361–367. [Google Scholar] [CrossRef]
- Naidu, S.L.; DeLucia, E.H.; Thomas, R.B. Contrasting patterns of biomass allocation in dominant and suppressed loblolly pine. Can. J. For. Res. 1998, 28, 1116–1124. [Google Scholar] [CrossRef]
- Goldblum, D.; Rigg, L.S. Tree growth response to climate change at the deciduous boreal forest ecotone, Ontario, Canada. Can. J. For. Res. 2005, 35, 2709–2718. [Google Scholar] [CrossRef]
- Bonan, G.B.; Shugart, H.H. Environmental factors and ecological processes in boreal forests. Annu. Rev. Ecol. Syst. 1989, 20, 1–28. [Google Scholar]
- D’Arrigo, R.D.; Jacoby, G.C.; Free, R.M. Tree-ring width and maximum latewood density at the North American tree line: Parameters of climatic change. Can. J. For. Res. 1992, 22, 1290–1296. [Google Scholar] [CrossRef]
Appendix
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xiang, W.; Auty, D.; Franceschini, T.; Leitch, M.; Achim, A. Wood Density-Climate Relationships Are Mediated by Dominance Class in Black Spruce (Picea mariana (Mill.) B.S.P.). Forests 2014, 5, 1163-1184. https://doi.org/10.3390/f5061163
Xiang W, Auty D, Franceschini T, Leitch M, Achim A. Wood Density-Climate Relationships Are Mediated by Dominance Class in Black Spruce (Picea mariana (Mill.) B.S.P.). Forests. 2014; 5(6):1163-1184. https://doi.org/10.3390/f5061163
Chicago/Turabian StyleXiang, Wei, David Auty, Tony Franceschini, Mathew Leitch, and Alexis Achim. 2014. "Wood Density-Climate Relationships Are Mediated by Dominance Class in Black Spruce (Picea mariana (Mill.) B.S.P.)" Forests 5, no. 6: 1163-1184. https://doi.org/10.3390/f5061163
APA StyleXiang, W., Auty, D., Franceschini, T., Leitch, M., & Achim, A. (2014). Wood Density-Climate Relationships Are Mediated by Dominance Class in Black Spruce (Picea mariana (Mill.) B.S.P.). Forests, 5(6), 1163-1184. https://doi.org/10.3390/f5061163