Composition and Elevation of Spruce Forests Affect Susceptibility to Bark Beetle Attacks: Implications for Forest Management
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Species
2.2. Study Area and Naturality Index
2.3. Spruce Forest Types

| Spruce forest types | Code | Area (ha) | Area (%) | Elevation (m a.s.l.) | DBH 1 (cm) | Density 2 (trees per ha) | Naturality Index 3 |
|---|---|---|---|---|---|---|---|
| Pure spruce plantations | PLA | 4514 | 6.8 | 718 ± 147 | 31.4 ± 0.5 | 358 ± 17 | 1.0 |
| Pure spruce reforestations | REF | 4844 | 7.3 | 940 ± 299 | 35.5 ± 0.8 | 325 ± 8 | 1.3 |
| Pure spruce mountain forests | MOU | 3729 | 5.6 | 1022 ± 298 | 33.1 ± 0.3 | 265 ± 6 | 2.5 |
| Pure spruce alpine forests | ALP | 10,429 | 15.7 | 1502 ± 174 | 35.1 ± 0.4 | 250 ± 11 | 4.0 |
| Spruce-conifer mixed forests | Mix C | 7512 | 11.3 | 989 ± 282 | 34.1 ± 0.4 | 298 ± 7 | 3.0 |
| Spruce-broadleaf mixed forests | Mix B | 24,203 | 36.6 | 1175 ± 216 | 31.7 ± 0.4 | 278 ± 4 | 3.8 |
| Spruce-conifer-broadleaf mixed forests | Mix C-B | 10,846 | 16.4 | 1184 ± 174 | 31.5 ± 0.5 | 288 ± 9 | 4.0 |
| Total | ‒ | 66,080 | 100 | ‒ | ‒ | ‒ | ‒ |
2.4. Data Collection
2.5. Statistical Analysis
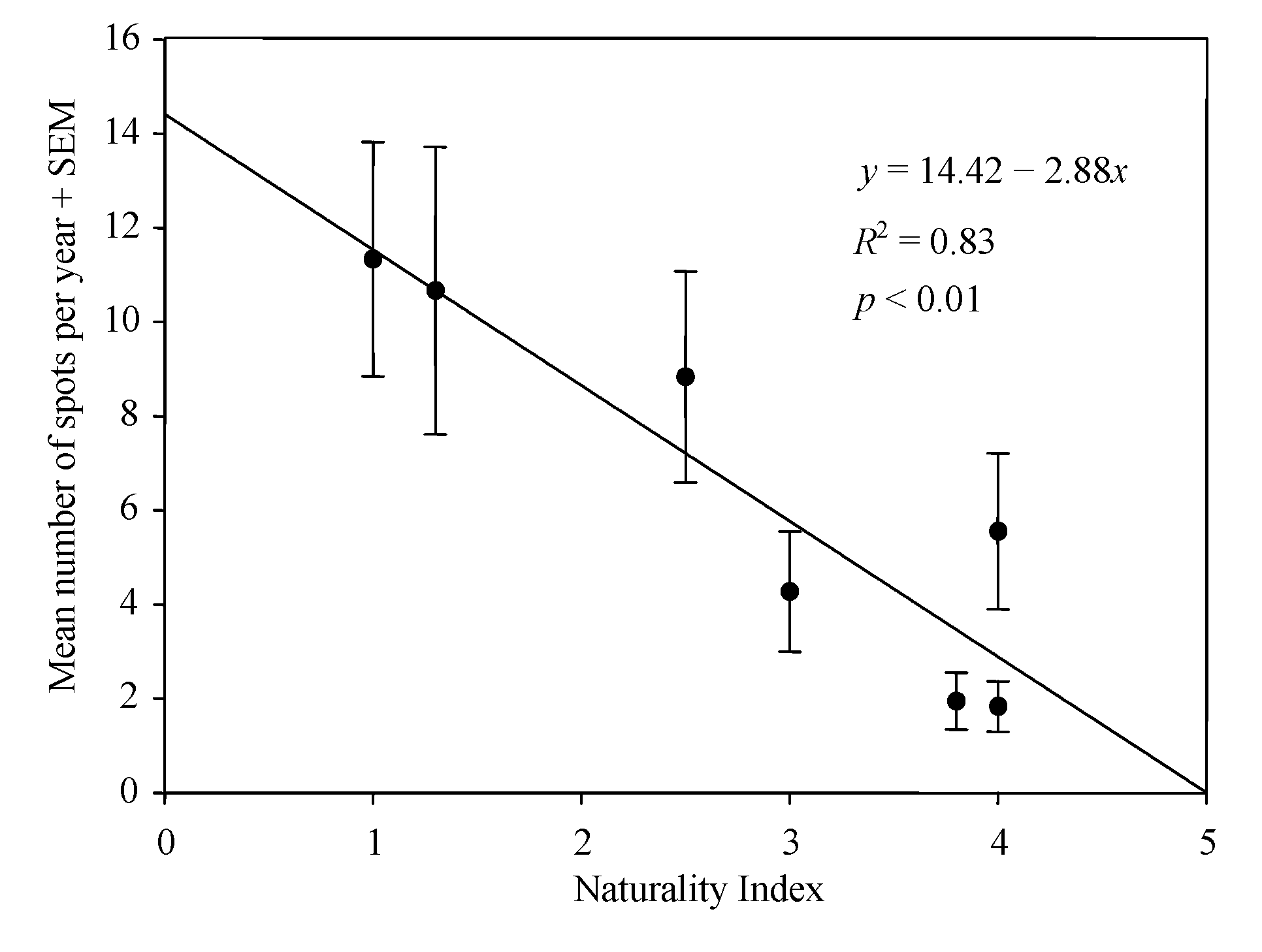
3. Results


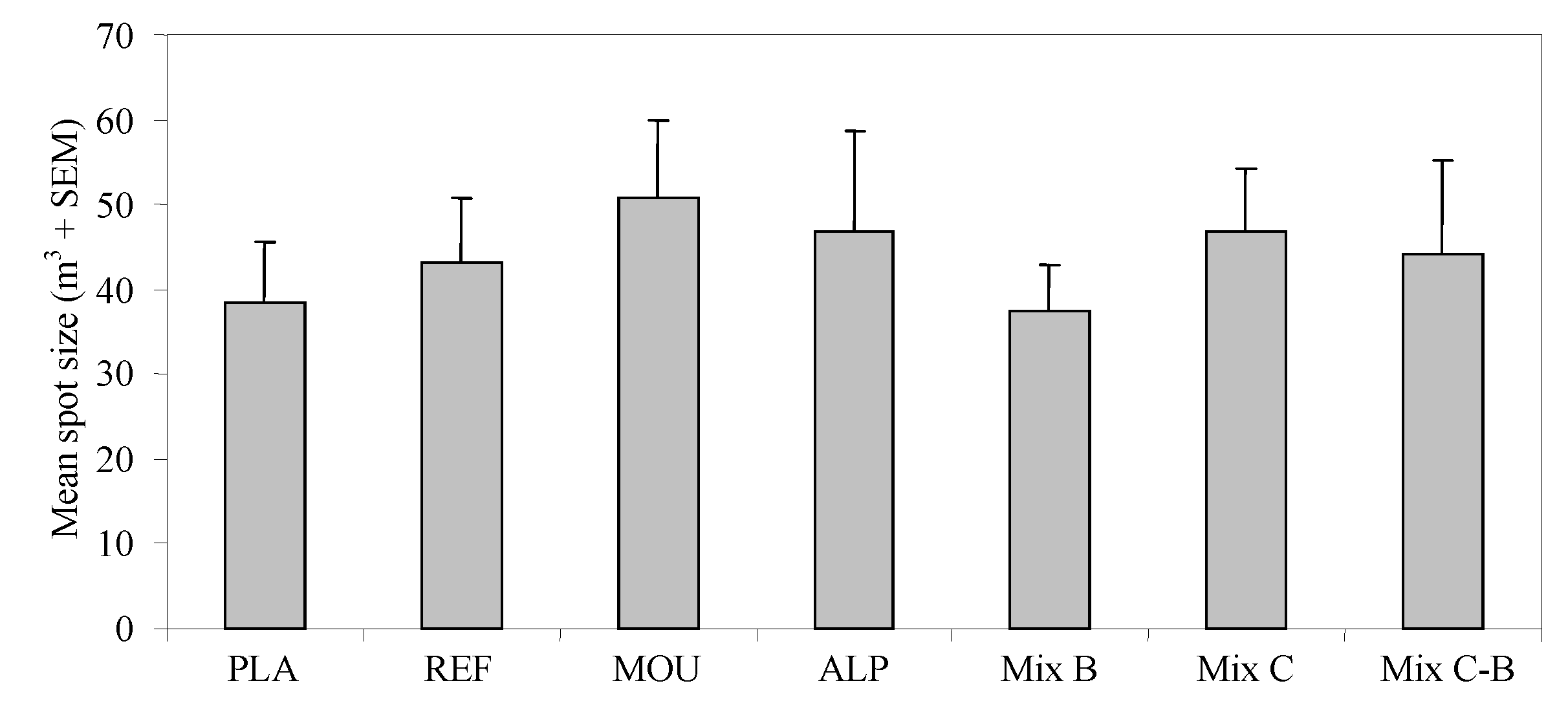
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Speight, M.R.; Wainhouse, D. Ecology and Management of Forest Insects; Clarendon Press: Oxford, UK, 1989; p. 374. [Google Scholar]
- Manion, P.D. Tree Disease Concepts, 2nd ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 1991; p. 402. [Google Scholar]
- Hlásny, T.; Turčáni, M. Persisting bark beetle outbreak indicates the unsustainability of secondary Norway spruce forests: Case study from Central Europe. Ann. For. Sci. 2013, 70, 481–491. [Google Scholar] [CrossRef]
- Netherer, S.; Nopp-Mayr, U. Predisposition Assessment Systems (PAS) as supportive tools in forest management: Rating of site and stand-related hazards of bark beetle infestation in the High Tatra Mountains as an example for system application and verification. For. Ecol. Manag. 2005, 207, 99–107. [Google Scholar] [CrossRef]
- Jönsson, A.M.; Schroeder, L.M.; Lagergren, F.; Anderbrant, O.; Smith, B. Guess the impact of Ips typographus—An ecosystem modelling approach for simulating spruce bark beetle outbreaks. Agric. For. Meteorol. 2012, 166–167, 188–200. [Google Scholar] [CrossRef]
- Overbeck, M.; Schmidt, M. Modelling infestation risk of Norway spruce by Ips typographus (L.) in the Lower Saxon Harz Mountains (Germany). For. Ecol. Manag. 2012, 266, 115–125. [Google Scholar] [CrossRef]
- Lieutier, F.; Day, K.R.; Battisti, A.; Grégoire, J.-C.; Evans, H.F. Bark and Wood Boring Insects in Living Trees in Europe, A Synthesis; Kluwer Academic Publishers: Dordrecht, UK, 2004; p. 569. [Google Scholar]
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Turner, M.G.; Romme, W.H. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: Dynamics of biome-wide bark beetle eruptions. BioScience 2008, 58, 501–517. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Jactel, H.; Petit, J.; Desprez-Loustau, M.-L.; Delzon, S.; Piou, D.; Battisti, A.; Koricheva, J. Drought effects on damage by forest insects and pathogens: A meta-analysis. Glob. Chang. Biol. 2012, 18, 267–276. [Google Scholar] [CrossRef]
- Wermelinger, B. Ecology and management of the spruce bark beetle, Ips typographus—A review of recent research. For. Ecol. Manag. 2004, 202, 67–82. [Google Scholar] [CrossRef]
- Jönsson, A.M.; Harding, S.; Krokene, P.; Lange, H.; Lindelöw, Å.; Økland, B.; Ravn, H.P.; Schroeder, L.M. Modelling the potential impact of global warming on Ips typographus voltinism and reproductive diapauses. Clim. Chang. 2011, 109, 695–718. [Google Scholar] [CrossRef]
- Marini, L.; Lindelöw, A.; Jönsson, A.M.; Wulff, S.; Schroeder, L.M. Population dynamics of the spruce bark beetle: A long-term study. Oikos 2013, 122, 1768–1776. [Google Scholar] [CrossRef]
- Faccoli, M.; Stergulc, F. Ips typographus (L.) pheromone trapping in south Alps: Spring catches determine damage thresholds. J. Appl. Entomol. 2004, 128, 307–311. [Google Scholar] [CrossRef]
- Faccoli, M.; Stergulc, F. A practical method for predicting the short-time trend of bivoltine populations of Ips typographus (L.) (Col., Scolytidae). J. Appl. Entomol. 2006, 130, 61–66. [Google Scholar] [CrossRef]
- Faccoli, M.; Stergulc, F. Damage reduction and performance of mass trapping devices for forest protection against the spruce bark beetle, Ips typographus (Coleoptera Curculionidae Scolytinae). Ann. For. Sci. 2008, 65, 309. [Google Scholar] [CrossRef]
- Faccoli, M. Effect of weather on Ips typographus (Coleoptera Curculionidae) phenology, voltinism and associated spruce mortality in the south-eastern Alps. Environ. Entomol. 2009, 38, 307–316. [Google Scholar] [CrossRef]
- Faccoli, M.; Bernardinelli, I. Breeding performance of the second generation in some bivoltine populations of Ips typographus (Coleoptera Curculionidae) in the south-eastern Alps. J. Pest Sci. 2011, 84, 15–23. [Google Scholar] [CrossRef]
- Marini, L.; Ayres, M.P.; Battisti, A.; Faccoli, M. Climate affects severity and altitudinal distribution of outbreaks in an eruptive bark beetle. Clim. Chang. 2012, 115, 327–341. [Google Scholar] [CrossRef]
- Bernardinelli, I.; Stergulc, F.; Frigimelica, G.; Zandigiacomo, P.; Faccoli, M. Spatial analysis of Ips typographus infestations in South-Eastern Alps. In Proceedings of the Workshop on Methodology of Forest Insect and Disease Survey in Central Europe (IUFRO Working Party 7.03.10), Forest Training Centre, Gmunden, Austria, 11–14 September 2006; Forster, B., Knízek, M., Grodzki, W., Eds.; Federal Research and Training Centre for Forests, Natural Hazards and Landscape (BFW): Gmunden, Austria, 2008; pp. 45–52. [Google Scholar]
- Christiansen, E.; Bakke, A. The Spruce Bark Beetle of Eurasia. In Dynamics of Forest Insect Populations; Berryman, A.A., Ed.; Plenum Publishing Corporation: New York, NY, USA, 1988; pp. 479–503. [Google Scholar]
- Grégoire, J.-C.; Evans, H.F. Damage and Control of BAWBILT Organisms, an Overview. In Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis; Lieutier, F., Day, K.R., Battisti, A., Grégoire, J.-C., Evans, H.F., Eds.; Kluwer Academic Publishers: London, UK, 2004; pp. 19–37. [Google Scholar]
- Annila, E. Influence of the temperature upon the development and voltinism of Ips typographus L. (Coleoptera, Scolytidae). Ann. Zool. Fenn. 1969, 6, 161–207. [Google Scholar]
- Faccoli, M. Winter mortality in sub-corticolous populations of Ips typographus (Coleoptera Scolytidae) and its parasitoids in the South-eastern Alps. J. Pest Sci. 2002, 75, 62–68. [Google Scholar]
- Franklin, A.; de Canniere, C.; Grégoire, J.-C. Can sales of infested timber be used to quantify attacks by Ips typographus (Coleoptera, Scolytidae)? A pilot study from Belgium. Ann. For. Sci. 2004, 61, 477–480. [Google Scholar] [CrossRef]
- Schmidt-Vogt, H. Die Fichte Band I. Taxonomie-Verbreitung-Morphologie-Ökologie-Waldgesellscahften; Verlag Paul Parey: Hamburg and Berlin, Germany, 1977; p. 647. [Google Scholar]
- Del Favero, R.; Poldini, L.; Bortoli, P.L.; Dreossi, G.; Lasen, C.; Vanone, G. La Vegetazione Forestale e la Selvicoltura Nella Regione Friuli Venezia Giulia; Regione Autonoma del Friuli Venezia Giulia, Direzione Regionale Foreste, Servizio Selvicoltura: Trieste, Italy, 1998; Volume 1, p. 490. [Google Scholar]
- Del Favero, R.; Poldini, L.; Bortoli, P.L.; Dreossi, G.; Lasen, C.; Vanone, G. La Vegetazione Forestale e la Selvicoltura Nella Regione Friuli Venezia Giulia; Regione Autonoma del Friuli Venezia Giulia, Direzione Regionale Foreste, Servizio Selvicoltura: Trieste, Italy, 1998; Volume 2, p. 303. [Google Scholar]
- Poldini, L. La Vegetazione del Carso Isontino e Triestino; LINT Edizioni: Trieste, Italy, 1989; p. 315. [Google Scholar]
- Poldini, L.; Pertot, M. Criteri di indicizzazione del valore naturalistico sull’esempio del Carso triestino-goriziano. Soc. Bot. Ital. 1989, 21, 133–151. [Google Scholar]
- von Hornstein, F. Wald und Mensch; O. Maier Verlag: Ravensburg, Germany, 1951. [Google Scholar]
- Remmert, H. Ökologie; Springer: Berlin, Heidelberg, Germany, New York, NY, USA, 1976. [Google Scholar]
- European Nature Information System, EUNIS. Available online: http://eunis.eea.europa.eu/habitats.jsp (accessed on 09 January 2014).
- BAUSINVE, Regione Autonoma Friuli Venezia Giulia. Available online: http://www.regione.fvg.it/rafvg/cms/RAFVG/economia-imprese/agricoltura-foreste/foreste/FOGLIA4/ (accessed on 09 January 2014).
- Stergulc, F.; Frigimelica, G.; Carpanelli, A. L’inventario Fitopatologico Forestale del Friuli-Venezia Giulia: Metodologie e Risultati di un Programma di Monitoraggio Permanente dello Stato Fitosanitario delle Foreste. In Proceedings of the Monitoraggio delle Stato Fitosanitario delle Foreste: Esperienze a Confronto, Florence, Italy, 12 Aprile 2002; Arsia, Toscana, Ed.; pp. 83–92.
- Zar, J.H. Biostatistical Analysis; Prentice Hall Press: Upper Saddle River, NJ, USA, 1999; p. 898. [Google Scholar]
- Paine, T.D.; Raffa, K.F.; Harrington, T.C. Interactions among scolytid bark beetles, their associated fungi, and live host conifers. Annu. Rev. Entomol. 1997, 42, 179–206. [Google Scholar] [CrossRef]
- Baier, P.; Führer, E.; Kirisits, T.; Rosner, S. Defence reactions of Norway spruce against bark beetles and the associated fungus Ceratocystis polonica in secondary pure and mixed species stands. For. Ecol. Manag. 2002, 159, 73–86. [Google Scholar] [CrossRef]
- Faccoli, M.; Blaženec, M.; Schlyter, F. Feeding response to host and non-host compounds by males and females of the spruce bark beetle Ips typographus in a tunnelling microassay. J. Chem. Ecol. 2005, 31, 745–759. [Google Scholar] [CrossRef]
- Byers, J.A.; Zhang, Q.H.; Schlyter, F.; Birgersson, G. Volatiles from nonhost birch trees inhibit pheromone response in spruce bark beetles. Naturwiss 1998, 85, 557–561. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Schlyter, F.; Anderson, P. Green leaf volatiles interrupt pheromone response of spruce bark beetle Ips typographus. J. Chem. Ecol. 1999, 25, 2847–2861. [Google Scholar] [CrossRef]
- Jactel, H.; Brockerhoff, E. Tree diversity reduces herbivory by forest insects. Ecol. Lett. 2007, 10, 835–848. [Google Scholar] [CrossRef]
- Chinellato, F.; Faccoli, M.; Marini, L.; Battisti, A. Distribution of Norway spruce bark and wood boring beetles along Alpine elevational gradients. Agric. For. Entomol. 2013. [Google Scholar] [CrossRef]
- kland, B.; Berryman, A. Resource dynamics plays a key role in regional fluctuations of the spruce bark beetle Ips typographus. Agric. For. Entomol. 2004, 6, 141–146. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment. In Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, New York, NY, USA, 2007; p. 996. [Google Scholar]
- Schelhaas, M.-J.; Nabuurs, G.-J.; Schuck, A. Natural disturbances in the European forests in the 19th and 20th centuries. Glob. Chang. Biol. 2003, 9, 1620–1633. [Google Scholar] [CrossRef]
- Lange, H.; Økland, B.; Krokene, P. Thresholds in the life cycle of the spruce bark beetle under climate change. Inter. J. Complex. Syst. 2006, 1648, 1–10. [Google Scholar]
- Baier, P.; Pennerstorfer, J.; Schopf, A. PHENIPS—A comprehensive phenology model of Ips typographus (L.) (Col., Scolytinae) as a tool for hazard rating of bark beetle infestation. For. Ecol. Manag. 2007, 249, 171–186. [Google Scholar] [CrossRef]
- Jönsson, A.M.; Harding, S.; Bärring, L.; Ravn, H.P. Impact of climate change on the population dynamics of Ips typographus in southern Sweden. Agr. For. Meteorol. 2007, 146, 70–81. [Google Scholar] [CrossRef]
- Jönsson, A.M.; Appelberg, G.; Harding, S.; Bärring, L. Spatiotemporal impact of climate change on the activity and voltinism of the spruce bark beetle, Ips typographus. Glob. Chang. Biol. 2009, 15, 486–499. [Google Scholar] [CrossRef]
- Wittwer, D.; Matthews, K.; Zogas, K.; Trummer, L.; Holsten, E.; Schulz, B.; Hennon, P.; Schultz, M.; Riggs, J.; Burnside, R. Forest Insect and Disease Conditions in Alaska, 1998; General Technical Report US Forest Service R10-TP-74; USDA Forest Service, Forest Health Protection-Alaska Region: Anchorage, AK, USA, November; 1988. [Google Scholar]
- Hansen, E.M.; Bentz, B.J. Comparison of reproductive capacity among univoltine, semivoltine, and re-emerged parent spruce beetles (Coleoptera: Scolytidae). Can. Entomol. 2003, 135, 697–712. [Google Scholar] [CrossRef]
- Berg, E.E.; Henry, J.D.; Fastie, C.L.; DeVolder, A.D.; Matsuoka, S.M. Spruce beetle outbreaks on the Kenai Peninsula, Alaska, and Kluane National Park and Reserve, Yukon Territory: Relationship to summer temperatures and regional differences in disturbance regimes. For. Ecol. Manag. 2006, 227, 219–232. [Google Scholar] [CrossRef]
- Colombari, F.; Schroeder, M.L.; Battisti, A.; Faccoli, M. Spatio-temporal dynamics of an Ips acuminatus outbreak and implications for management. Agric. For. Entomol. 2013, 15, 34–42. [Google Scholar] [CrossRef]
- Uniyal, S.K.; Uniyal, A. Climate change and large-scale degradation of spruce: Common pattern across the globe. Clim. Res. 2009, 38, 261–263. [Google Scholar] [CrossRef]
- Hlásny, T.; Sitková, Z. Spruce Forests Decline in the Beskids; National Forest Centre, Forest Research Institute Zvolen, Czech University of Life Sciences Prague, Forestry and Game Management Research Institute Jíloviště–Strnady: Zvolen, Czech Republic, 2010. [Google Scholar]
- Griess, V.C.; Acevedo, R.; Härtl, F.; Staupendahl, K.; Knoke, T. Does mixing tree species enhance stand resistance against natural hazards? A case study for spruce. For. Ecol. Manag. 2012, 267, 284–296. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Faccoli, M.; Bernardinelli, I. Composition and Elevation of Spruce Forests Affect Susceptibility to Bark Beetle Attacks: Implications for Forest Management. Forests 2014, 5, 88-102. https://doi.org/10.3390/f5010088
Faccoli M, Bernardinelli I. Composition and Elevation of Spruce Forests Affect Susceptibility to Bark Beetle Attacks: Implications for Forest Management. Forests. 2014; 5(1):88-102. https://doi.org/10.3390/f5010088
Chicago/Turabian StyleFaccoli, Massimo, and Iris Bernardinelli. 2014. "Composition and Elevation of Spruce Forests Affect Susceptibility to Bark Beetle Attacks: Implications for Forest Management" Forests 5, no. 1: 88-102. https://doi.org/10.3390/f5010088
APA StyleFaccoli, M., & Bernardinelli, I. (2014). Composition and Elevation of Spruce Forests Affect Susceptibility to Bark Beetle Attacks: Implications for Forest Management. Forests, 5(1), 88-102. https://doi.org/10.3390/f5010088




