Retrospective Analysis of a Large-Scale Gypsy Moth Outbreak in Hungary Combining Multi-Source Satellite and In Situ Data
Abstract
1. Introduction
- How effectively can MODIS-based NDVI and Z NDVI detect and quantify the level of defoliation caused by the gypsy moth, and what is the agreement between observed defoliation and NDVI and Z NDVI anomalies?
- How have the NDVI and LAI of different tree species changed in areas affected by gypsy moth gradation?
- How do field damage reports correlate with satellite data?
- How does the light trap catch data correlate with satellite data, and could it predict the gypsy moth gradation?
2. Materials and Methods
2.1. Gypsy Moth
2.2. Study Area
2.3. Field Datasets
2.3.1. Field Damage Reports
2.3.2. Light Traps
2.4. Land Cover Dataset
2.5. Remote Sensing Data
2.5.1. NDVI Datasets
2.5.2. Detecting Defoliated Pixels
2.5.3. Leaf Area Index (LAI) Datasets
2.5.4. Z NDVI Classification
- Z NDVI < −2: severe forest damage;
- Z NDVI < −1: moderate forest damage;
- Z NDVI < 0: neutral state;
- Z NDVI < 1: regeneration;
- Z NDVI > 2: strong regeneration.
2.5.5. Validation with Field Data
3. Results
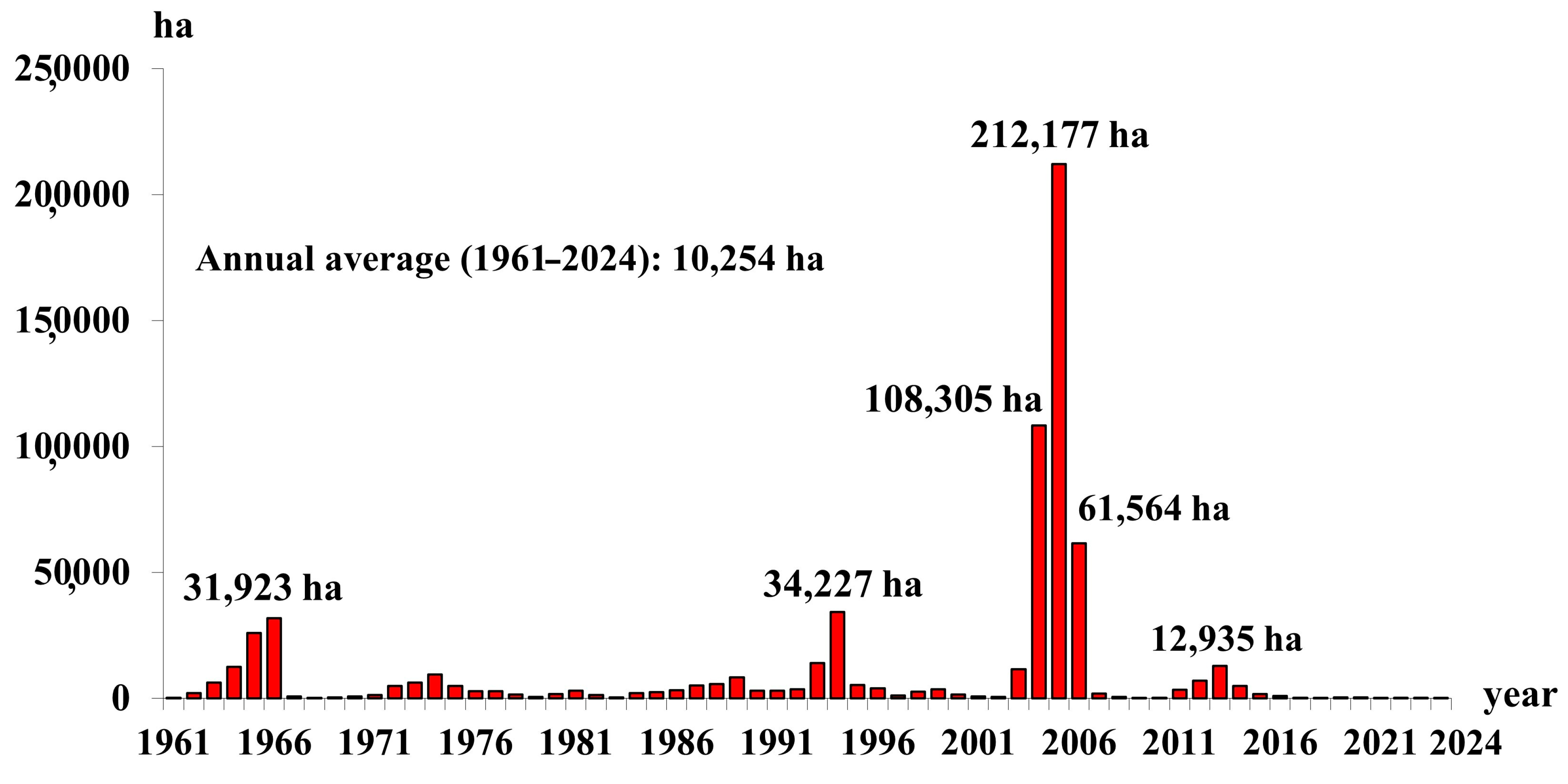
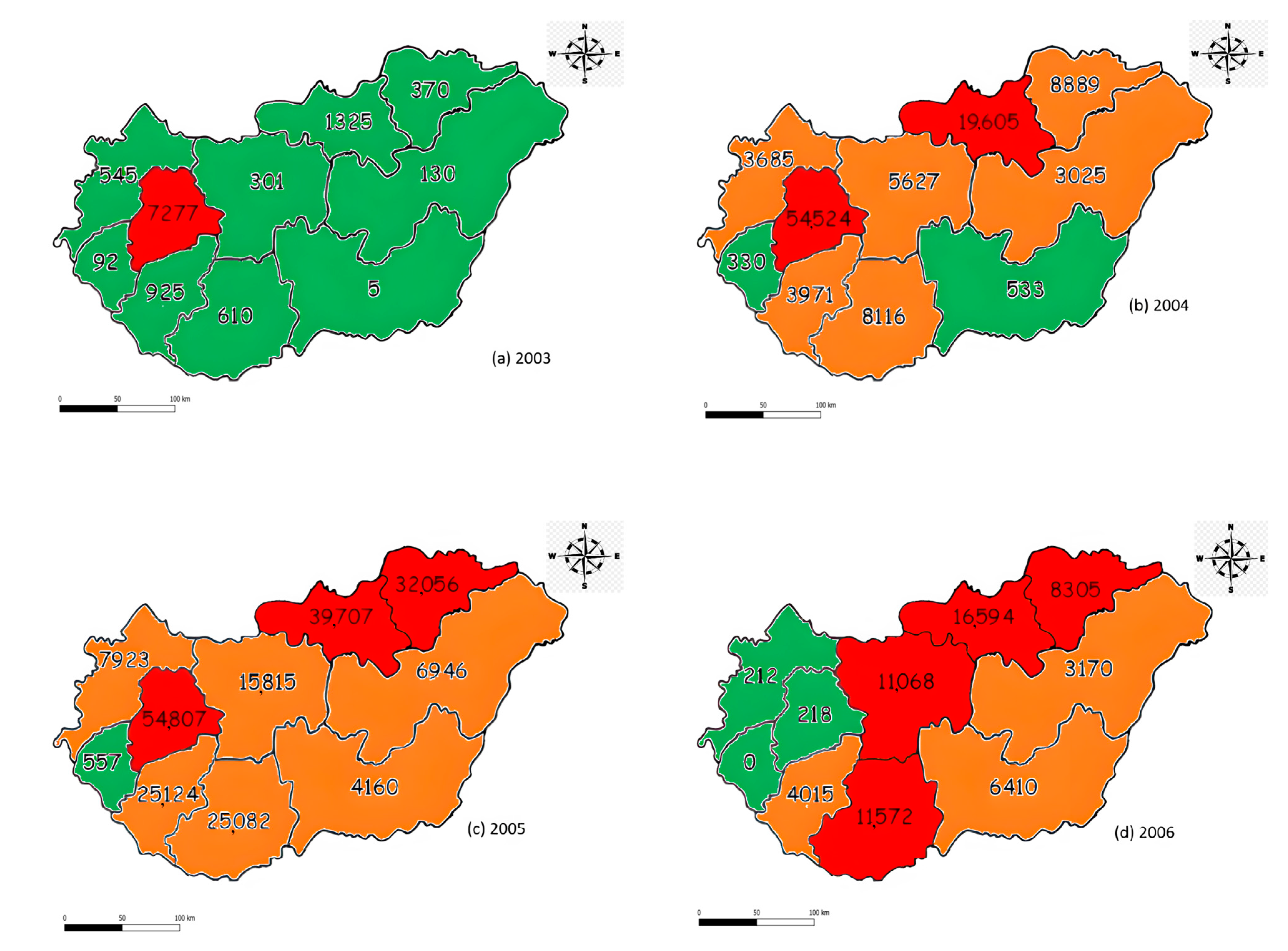
3.1. Field Damage
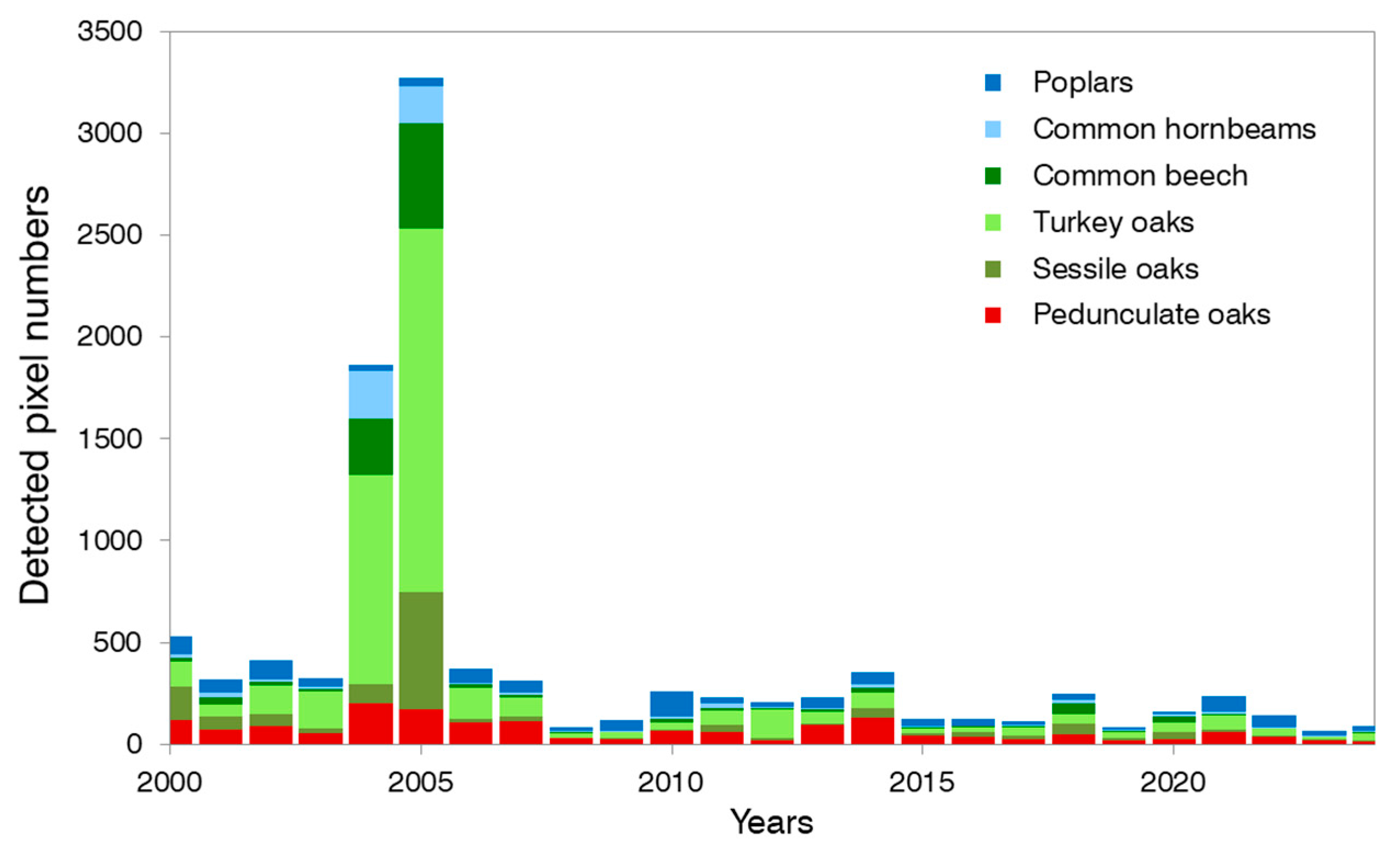
3.2. Detected Defoliated Pixels
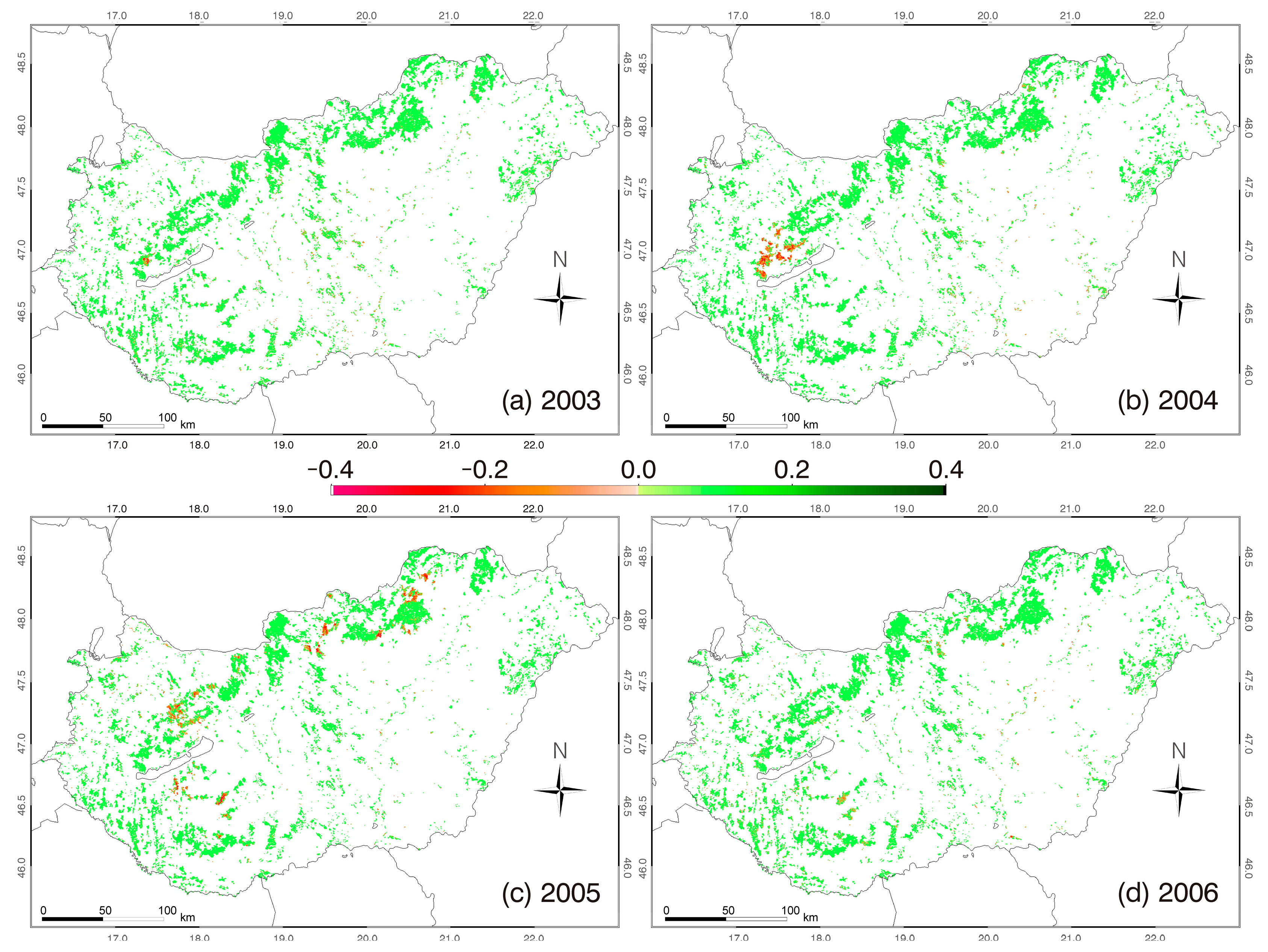
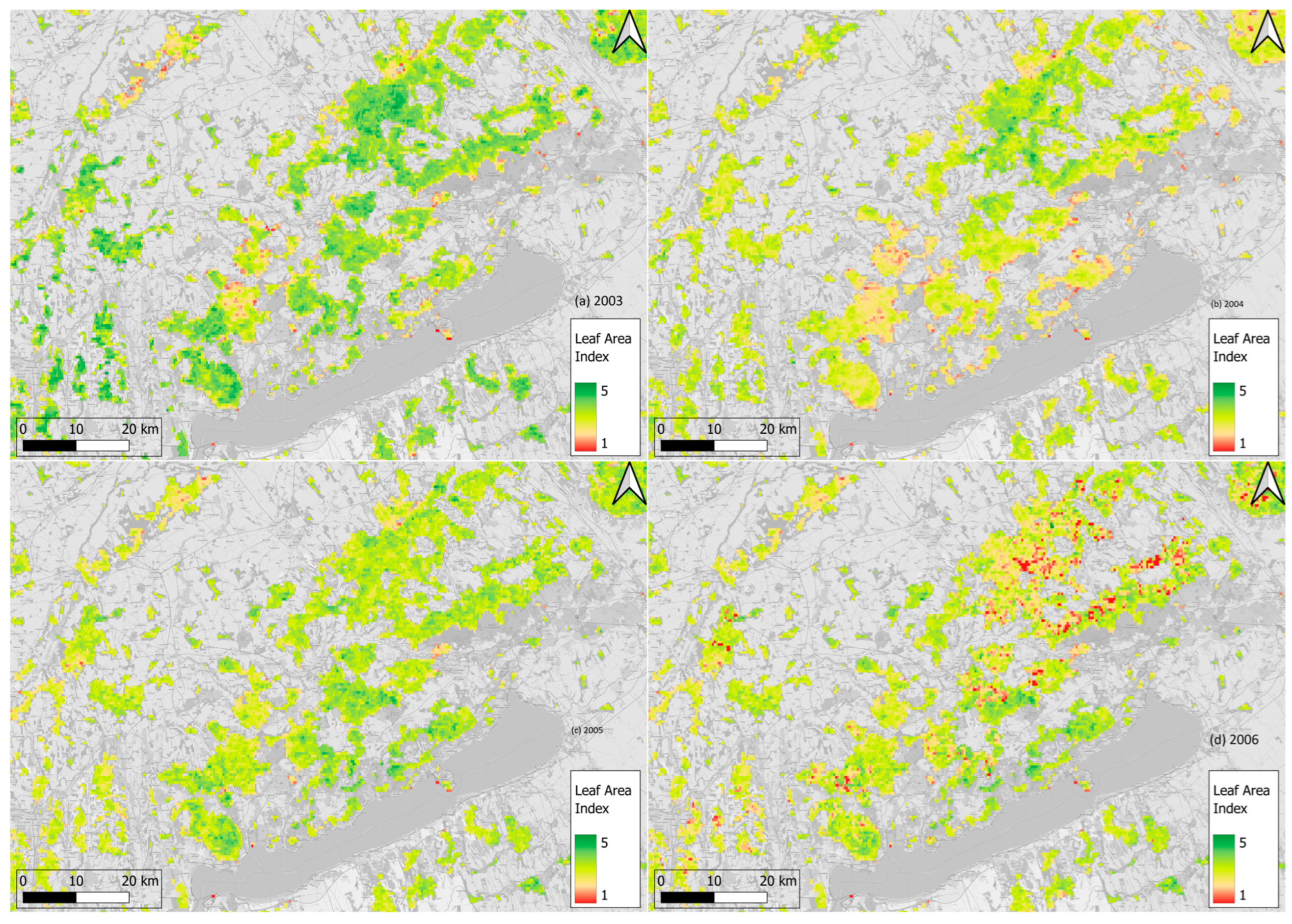
3.3. Z NDVI Patterns
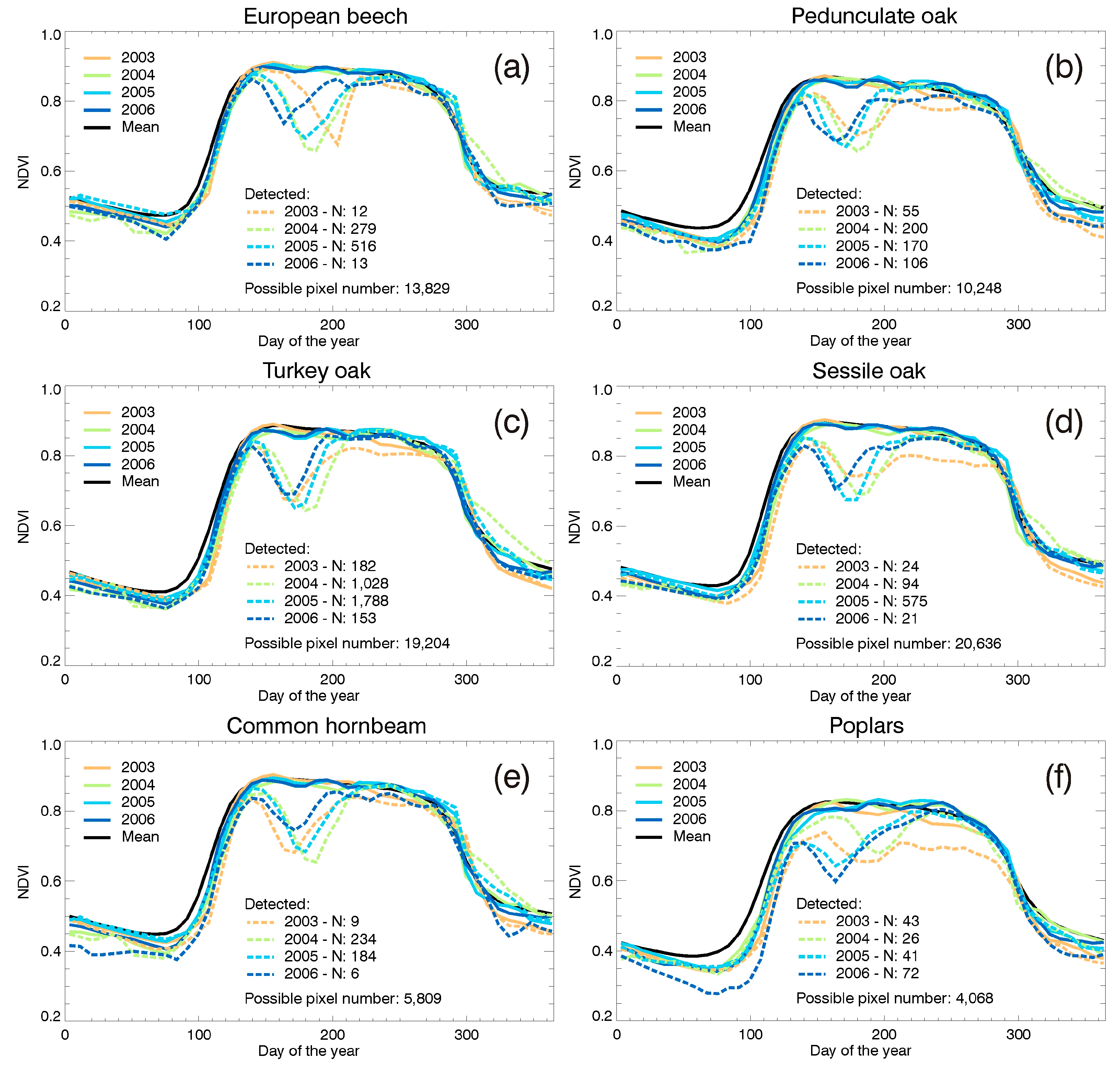
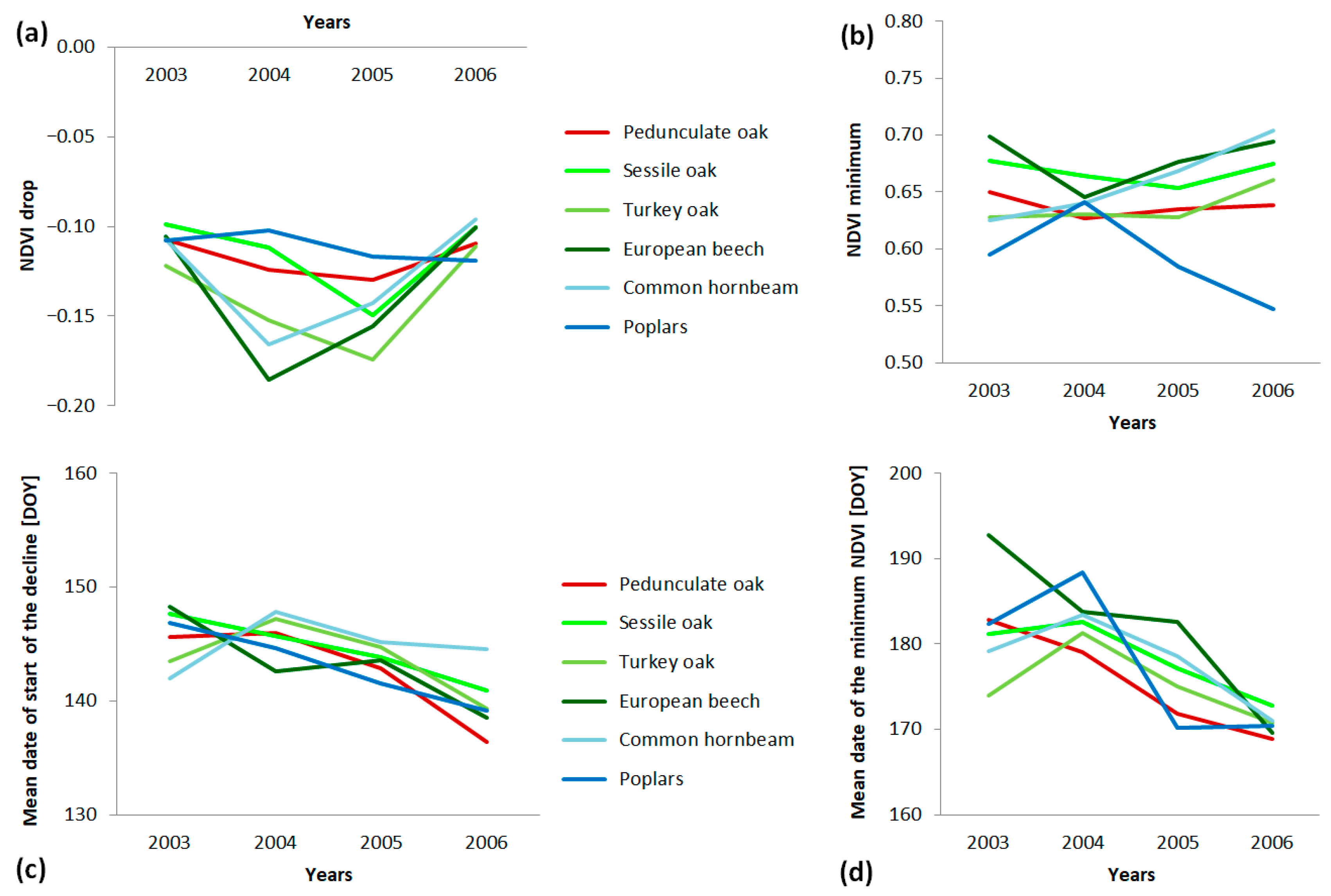
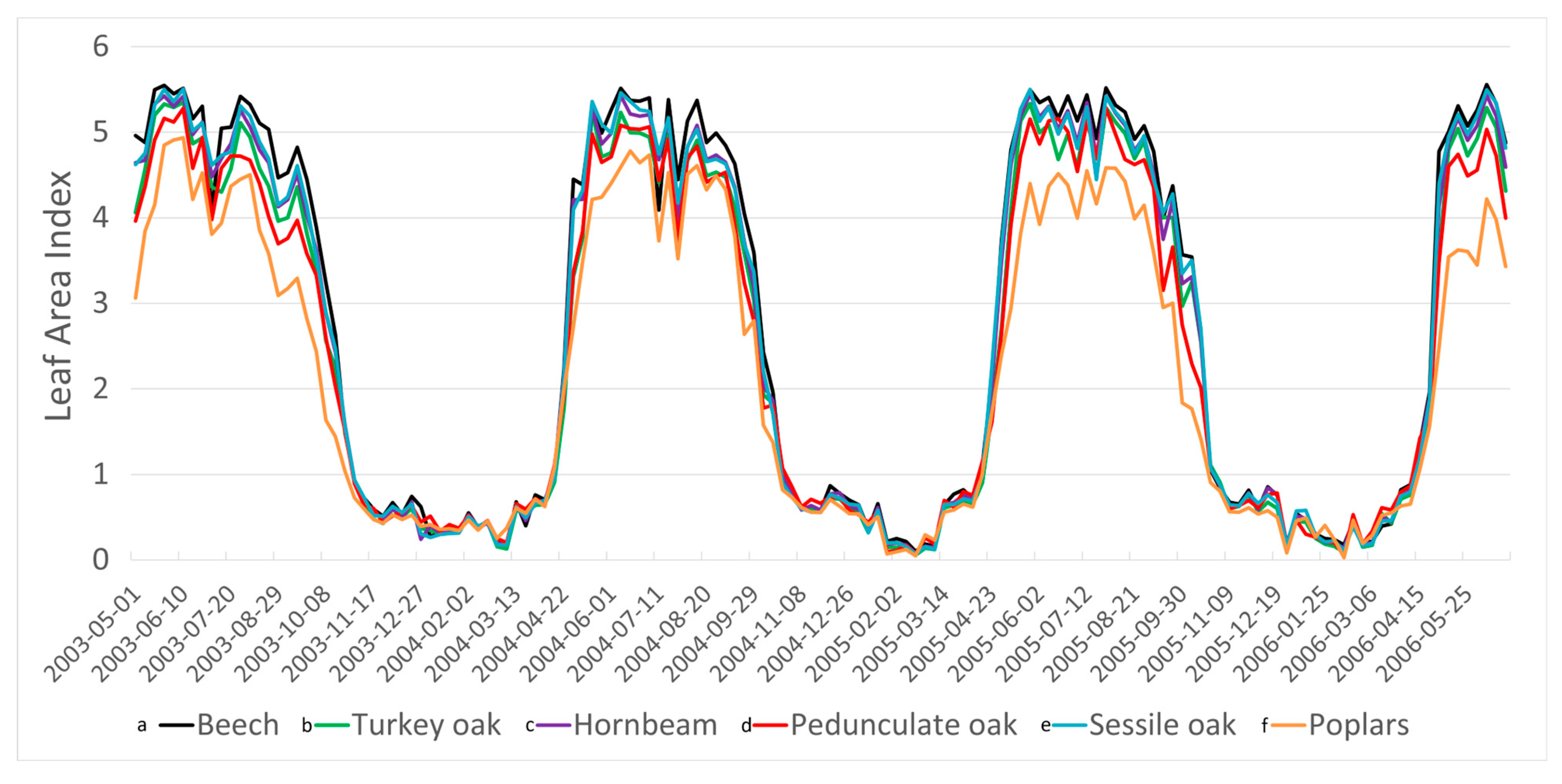
3.4. Leaf Area Index Changes
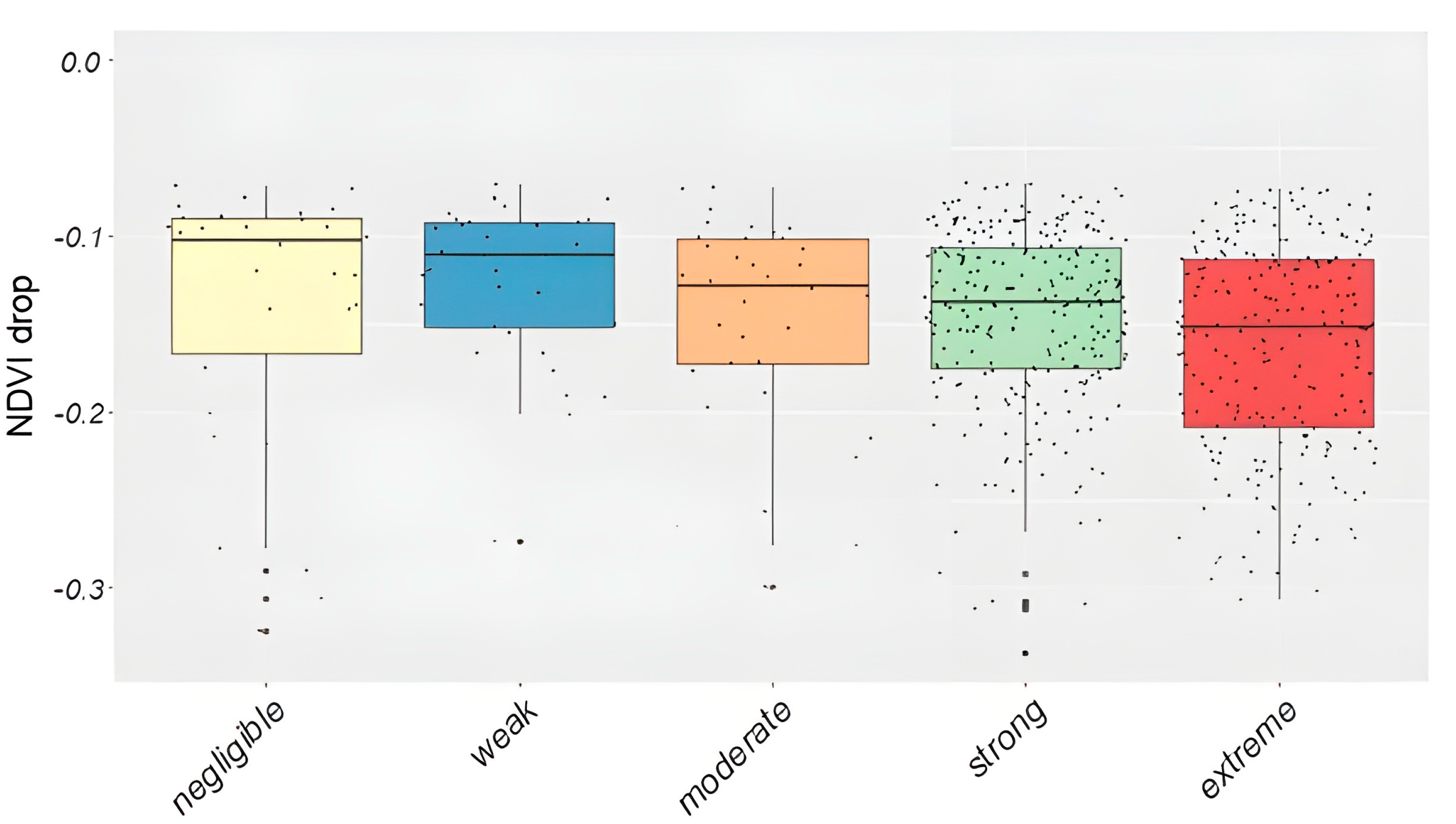
3.5. Validation Using Field Observations
4. Discussion
4.1. Effectiveness of Combined Remote Sensing and Field Observations
4.2. Limitations of the Moderate Spatial Resolution
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DOY | Day of Year |
| ESA | European Space Agency |
| EVI | Enhanced Vegetation Index |
| LAI | Leaf Area Index |
| MODIS | Moderate Resolution Imaging Spectroradiometer |
| NASA | National Aeronautics and Space Administration |
| NDII | Normalised Difference Infrared Index |
| NDVI | Normalised Difference Vegetation Index |
| Z NDVI | Standardised Difference Vegetation Index |
References
- Johnson, D.M.; Liebhold, A.M.; Bjorsnstad, O.N. Geographical variation in periodicity of gypsy moth outbreaks. Ecography 2006, 29, 367–374. [Google Scholar] [CrossRef]
- Castedo-Dorado, F.; Lago-Parra, G.; Lombardero, M.J.; Otero-Traba, M.A.; Pérez-Otero, J.F. European gypsy moth (Lymantria dispar dispar L.) completes development and defoliates exotic radiata pine plantations in Spain. New Zealand J. For. Sci. 2016, 46, 18. [Google Scholar] [CrossRef]
- Markóné Nagy, K. A Gyapjaslepke (Lymantria dispar L.) Tömegszaporodásának Elemzése, Valamint Táplálkozásbiológiai Vizsgálatok Gyapjaslepkével és Apácalepkével (Lymantria monacha L.). Ph.D. Thesis, Roth Gyula Doctoral School of Forestry and Wildlife Management, University of West Hungary, Sopron, Hungary, 2013. Available online: http://doktori.uni-sopron.hu/id/eprint/411/19/disszertacio(12).TextMark.pdf (accessed on 14 August 2025).
- Csóka, G.; Hirka, A. A gyapjaslepke (Lymantria dispar L.) legutóbbi tömegszaporodása Magyarországon. Növényvédelem 2009, 45, 196–201. [Google Scholar]
- Hajek, A.E.; Elkinton, J.S.; Humber, R.A.; May, B.; Walsh, S.R.A.; Silver, J.C. Entomophaga maimaiga sp. nov., a fungal pathogen of gypsy moth (Lymantria dispar) in North America and Japan. J. Invertebr. Pathol. 1990, 55, 357–370. [Google Scholar]
- Reardon, R.C.; Hajek, A.E.; Podgwaite, J.D.; Maddox, J.V. Control of gypsy moth, Lymantria dispar (L.), with the nuclear polyhedrosis virus product Gypchek. J. Pest Sci. 1996, 46, 103–110. [Google Scholar]
- Rhainds, M. Mass Trapping Lepidopteran Pests with Light Traps, with Focus on Tortricid Forest Pests: What If? Insects 2024, 15, 267. [Google Scholar] [CrossRef] [PubMed]
- Bjerge, K.; Nielsen, J.B.; Sepstrup, M.V.; Helsing-Nielsen, F.; Høye, T.T. An Automated Light Trap to Monitor Moths (Lepidoptera) Using Computer Vision-Based Tracking and Deep Learning. Sensors 2021, 21, 343. [Google Scholar] [CrossRef]
- Komatsu, M.; Kurihara, K.; Saito, S.; Domae, M.; Masuya, N.; Shimura, Y.; Kajiyama, S.; Kanda, Y.; Sugizaki, K.; Ebina, K.; et al. Management of flying insects on expressways through an academic–industrial collaboration: Evaluation of the effect of light wavelengths and meteorological factors on insect attraction. Zool. Lett. 2020, 6, 15. [Google Scholar] [CrossRef]
- Zúbrik, M.; Kunca, A.; Kulfan, J.; Rell, S.; Nikolov, C.; Galko, J.; Vakula, J.; Gubka, A.; Leontovyč, R.; Konôpka, B.; et al. Occurrence of gypsy moth (Lymantria dispar L.) in the Slovak Republic and its outbreaks during 1945–2020. Cent. Eur. For. J. 2021, 67, 3–13. [Google Scholar] [CrossRef]
- Leskó, K.; Szentkirályi, F.; Kádár, F. Gyapjaslepke (Lymantria dispar L.) populációinak fluctuációs mintázatai 1963–1993 közötti időszakban Magyarországon. Erd. Kut. 1994, 84, 163–176. [Google Scholar]
- McManus, M.; Csóka, G. History and Impact of Gypsy Moth in North America and Comparison to the Recent Outbreaks in Europe. Acta Silv. Lignaria Hung. 2007, 3, 47–64. [Google Scholar] [CrossRef]
- Leskó, K.; Szentkirályi, F.; Kádár, F. Araszoló lepkefajok fluktuáció-mintázatának elemzése hosszú távú (1961–1997) magyarországi fénycsapdázási és kártételi idősorokon. Erd. Kut. 1998, 88, 319–333. [Google Scholar]
- Leskó, K.; Szentkirályi, F.; Kádár, F. Aranyfarú szövőlepke (Euproctis chrysorrhoea L.) magyarországi populációinak hosszútávú fluctuációs mintázatai. Erd. Kut. 1995, 85, 169–184. [Google Scholar]
- Leskó, K.; Szentkirályi, F.; Kádár, F. A gyűrűsszövő (Malacosoma neustria L.) hosszú távú (1962–1996) populációingadozásai Magyarországon. Erd. Kut. 1997, 86–87, 207–220. [Google Scholar]
- Valtonen, A.; Hirka, A.; Szőcs, L.; Ayres, M.P.; Roininen, H.; Csóka, G. Long-term species loss and homogenization of moth communities in Central-Europe. J. Anim. Ecol. 2017, 86, 730–738. [Google Scholar] [CrossRef]
- Zeng, L.; Wardlow, B.D.; Xiang, D.; Hu, S.; Li, D. A review of vegetation phenological metrics extraction using time-series, multispectral satellite data. Remote Sens. Environ. 2020, 237, 11511. [Google Scholar] [CrossRef]
- Xue, J.; Su, B. Significant Remote Sensing Vegetation Indices: A Review of Developments and Applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef]
- Berra, E.F.; Gaulton, R. Remote sensing of temperate and boreal forest phenology: A review of progress, challenges and opportunities in the intercomparison of in-situ and satellite phenological metrics. For. Ecol. Manag. 2021, 480, 118663. [Google Scholar] [CrossRef]
- De Beurs, K.M.; Townsend, P.A. Estimating the effect of gypsy moth defoliation using MODIS. Remote Sens. Environ. 2008, 112, 3983–3990. [Google Scholar] [CrossRef]
- Spruce, J.P.; Sader, S.; Ryan, R.E.; Smoot, J.; Kuper, P.; Ross, K.; Prados, D.; Russell, J.; Gasser, G.; McKellip, R.; et al. Assessment of MODIS NDVI time series data products for detecting forest defoliation by gypsy moth outbreaks. Remote Sens. Environ. 2011, 115, 427–437. [Google Scholar] [CrossRef]
- Latifovic, L.; Arain, M.A. The impact of spongy moth (Lymantria dispar dispar) defoliation on the carbon balance of a temperate deciduous forest in North America. Agric. For. Meteorol. 2024, 354, 110076. [Google Scholar] [CrossRef]
- Olsson, P.-O. Monitoring Insect Defoliation in Forests with Time-Series of Satellite-Based Remote Sensing Data—Near Real-Time Methods and Impact on the Carbon Balance. Ph.D. Thesis, Department of Physical Geography and Ecosystem Science, Lund University, Lund, Sweden, 2016. [Google Scholar]
- Csóka, G.; Nádor, G. A gyapjaslepke kártétel monitorozása távérzékeléssel Veszprém, Somogy, és Nógrád megye területén 2005-ben. Agrofórum 2006, 17, 4–10. [Google Scholar]
- Somogyi, Z.; Koltay, A.; Molnár, T.; Móricz, N. Forest health monitoring system in Hungary based on MODIS products. In Az Elmélet és a Gyakorlat Találkozása a Térinformatikában IX: Theory Meets Practice in GIS; Molnár, V.E., Ed.; Debreceni Egyetem: Debrecen, Hungary, 2018; ISBN 978-963-318-723-4. [Google Scholar]
- Klapwijk, M.J.; Csóka, G.; Hirka, A.; Björkman, C. Forest insects and climate change: Long-term trends in herbivore damage. Ecol. Evol. 2013, 3, 4183–4196. [Google Scholar] [CrossRef]
- Tanács, E.; Belényesi, M.; Lehoczki, R.; Pataki, R.; Petrik, O.; Standovár, T.; Pásztor, L.; Laborczi, A.; Szatmári, G.; Molnár, Z.; et al. A national, high-resolution ecosystem basemap: Methodology, validation, and possible uses. Term. Közl. 2019, 25, 34–58. [Google Scholar] [CrossRef]
- NÖSZTÉP Ecosystem Map of Hungary. Available online: https://alapterkep.termeszetem.hu/ (accessed on 14 May 2025).
- Kern, A.; Marjanović, H.; Csóka, G.; Móricz, N.; Pernek, M.; Hirka, A.; Matošević, D.; Paulin, M.; Kovač, G. Detecting the oak lace bug infestation in oak forests using MODIS and meteorological data. Agric. For. Meteorol. 2021, 306, 108436. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Scheel, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. In Proceedings of the 3rd Earth Resource Technology Satellite (ERTS) Symposium, Washington, DC, USA, 10–14 December 1973; Volume 1, pp. 48–62. [Google Scholar]
- Justice, C.O.; Vermote, E.; Townshend, J.R.G.; Defries, R.; Roy, D.P.; Hall, D.K.; Salomonson, V.V.; Privette, J.L.; Riggs, G.; Strahler, A.; et al. The Moderate Resolution Imaging Spectroradiometer (MODIS): Land remote sensing for global change research. IEEE Trans. Geosci. Remote Sens. 1998, 36, 1228–1249. [Google Scholar] [CrossRef]
- Vermote, E. MODIS/Terra Surface Reflectance 8-Day L3 Global 250m SIN Grid V061. NASA EOSDIS Land Processes Distributed Active Archive Center. 2021. Available online: https://www.earthdata.nasa.gov/data/catalog/lpcloud-mod09q1-061 (accessed on 1 February 2025).
- LP DAAC (Land Processes Distributed Active Archive Center). MOD09A1, Collection 6.1. NASA EOSDIS Land Processes DAAC, USGS Earth Resources Observation and Science (EROS) Center, Sioux Falls, South Dakota. Available online: https://lpdaac.usgs.gov (accessed on 1 February 2025).
- Myneni, R.; Knyazikhin, Y.; Park, T. MODIS/Terra+Aqua Leaf Area Index/FPAR 8-Day L4 Global 500 m SIN Grid V061. NASA EOSDIS Land Processes Distributed Active Archive Center. 2021. Available online: https://www.earthdata.nasa.gov/data/catalog/lpcloud-mcd15a2h-061 (accessed on 11 May 2025).
- Chen, J.; Jönsson, P.; Tamura, M.; Gu, Z.; Matsushita, B.; Eklundh, L. A simple method for reconstructing a high-quality NDVI time-series data set based on the Savitzky–Golay filter. Remote Sens. Environ. 2004, 91, 332–344. [Google Scholar] [CrossRef]
- Peters, A.J.; Walter-Shea, E.A.; Viña, A.; Hayes, M.; Svoboda, M.D. Drought Monitoring with NDVI-Based Standardized Vegetation Index. Photogramm. Eng. Remote Sens. 2002, 68, 72–75. [Google Scholar]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-Scale Geospatial Analysis for Everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Hussain, N.; Gonsamo, A.; Wang, S.; Peng, Y.; Chhetri, S.G.; Chen, J.M.; Arain, M.A. Assessment of spongy moth infestation impacts on forest productivity and carbon loss using the Sentinel-2 satellite remote sensing and eddy covariance flux data. Ecol. Process. 2024, 13, 37. [Google Scholar] [CrossRef]
- Pasquarella, V.J.; Bradley, B.A.; Woodcock, C.E.; Pontius, J.A. Three decades of gypsy moth defoliation mapped using Landsat time series data. Remote Sens. Ecol. Conserv. 2021, 7, 293–305. [Google Scholar] [CrossRef]
- Wittich, D.; Rottensteiner, F.; Voelsen, M.; Heipke, C.; Müller, S. Deep Learning for the Detection of Early Signs for Forest Damage Based on Satellite Imagery. ISPRS Ann. Photogramm. Remote Sens. Spatial Inf. Sci. 2022, V-2-2022, 307–315. [Google Scholar] [CrossRef]
- Kirsch, M.; Wernicke, J.; Datta, P.; Preisach, C. Early Detection of Forest Calamities in Homogeneous Stands—Deep Learning Applied to Bark-Beetle Outbreaks. arXiv 2025, arXiv:2503.12883. [Google Scholar]
- Singh, H.; Ang, L.M.; Srivastava, S. Active Wildfire Detection via Satellite Imagery and Machine Learning: An Empirical Investigation of Australian Wildfires. Nat. Hazards 2025, 121, 9777–9800. [Google Scholar] [CrossRef]
- Pilarska, D.; McManus, M.; Pilarski, P.; Georgiev, G.; Mirchev, P.; Linde, A. Monitoring the establishment and prevalence of the fungal entomopathogen Entomophaga maimaiga in two Lymantria dispar populations in Bulgaria. J. Pest Sci. 2006, 79, 63–67. [Google Scholar] [CrossRef]
- Georgiev, G.; Mirchev, P.; Georgieva, M.; Kechev, M.; Belilov, S.; Matova, M.; Petrova, V.; Mateva, P.; Kirilova, M.; Mutafchiiski, I. Biological control of gypsy moth (Lymantria dispar) by the entomopathogenic fungus Entomophaga maimaiga in Bulgaria in 2021. Silva Balc. 2021, 22, 17–27. [Google Scholar] [CrossRef]
- Eklundh, L.; Johansson, T.; Solberg, S. Mapping insect defoliation in Scots pine with MODIS time-series data. Remote Sens. Environ. 2009, 113, 1566–1573. [Google Scholar] [CrossRef]
- Spruce, J.P.; Hicke, J.A.; Hargrove, W.W.; Grulke, N.E.; Meddens, A.J.H. Use of MODIS NDVI products to map tree mortality levels in forests affected by mountain pine beetle outbreaks. Forests 2019, 10, 811. [Google Scholar] [CrossRef]
- Olsson, P.-O.; Kantola, T.; Lyytikäinen-Saarenmaa, P.; Jönsson, A.M.; Eklundh, L. Development of a method for monitoring of insect induced forest defoliation—Limitation of MODIS data in Fennoscandian forest landscapes. Silva Fenn. 2016, 50, 1495. [Google Scholar] [CrossRef]
- European Space Agency (ESA). Sentinel-2 Mission Guide. Available online: https://www.esa.int/Applications/Observing_the_Earth/Copernicus/Sentinel-2 (accessed on 27 March 2025).
- Mori, N.; Kawatsu, K.; Noriyuki, S.; Kosilov, A.; Martemyanov, V.; Yamashita, M.; Inoue, M.N. Monitoring and prediction of the spongy moth (Lymantria dispar) outbreaks in mountain landscapes using a combination of Sentinel-2 images and nonlinear time series model. For. Ecol. Manag. 2024, 563, 121975. [Google Scholar] [CrossRef]
- Molnár, T.; Király, G. Forest Disturbance Monitoring Using Cloud-Based Sentinel-2 Satellite Imagery and Machine Learning. J. Imaging 2024, 10, 14. [Google Scholar] [CrossRef]
- Dalponte, M.; Solano-Correa, Y.T.; Frizzera, L.; Gianelle, D. Mapping a European Spruce Bark Beetle Outbreak Using Sentinel-2 Remote Sensing Data. Remote Sens. 2022, 14, 3135. [Google Scholar] [CrossRef]
- Senf, C.; Pflugmacher, D.; Wulder, M.A.; Hostert, P. Harmonizing Landsat and Sentinel-2 Time Series for Large-Area Disturbance Mapping. Remote Sens. Environ. 2021, 264, 112571. [Google Scholar] [CrossRef]
- Immitzer, M.; Atzberger, C.; Koukal, T. Early Detection of Bark Beetle Infestation in Norway Spruce (Picea abies) Forests Using Sentinel-2 Time Series. Remote Sens. 2021, 13, 3652. [Google Scholar] [CrossRef]
- Näsi, R.; Honkavaara, E.; Lyytikäinen-Saarenmaa, P.; Blomqvist, M.; Litkey, P.; Hakala, T.; Viljanen, N.; Kantola, T.; Tanhuanpää, T.; Holopainen, M. Remote Sensing of Forest Health Using Airborne Hyperspectral and Sentinel-2 Data. Remote Sens. 2018, 10, 470. [Google Scholar] [CrossRef]
- Schiller, C.; Költzow, J.; Schwarz, S.; Schiefer, F.; Fassnacht, F.E. Forest Disturbance Detection in Central Europe Using Transformers and Sentinel-2 Time Series. Remote Sens. Environ. 2024, 315, 114475. [Google Scholar]
- Assmann, J.J.; Pedersen, P.B.M.; Moeslund, J.E.; Senf, C.; Treier, U.A.; Corcoran, D.; Koma, Z.; Nord-Larsen, T.; Normand, S. Temperate forests of high conservation value are successfully identified by satellite and LiDAR data fusion. Conserv. Sci. Pract. 2025, 7, e13302. [Google Scholar] [CrossRef]
- Choi, W.-I.; Kim, E.-S.; Yun, S.-J.; Lim, J.-H.; Kim, Y.-E. Quantification of One-Year Gypsy Moth Defoliation Extent in Wonju, Korea, Using Landsat Satellite Images. Forests 2021, 12, 545. [Google Scholar] [CrossRef]
- Bernal, I.; Sánchez-Martínez, L.J.; Zambrano-Martínez, S.; Viejo, J.L. Application of Remote Sensing to the Quantification of Defoliation Caused by Lymantria dispar in “Los Alcornocales” Natural Park (Cádiz, Spain). SHILAP Rev. Lepidopterol. 2023, 51, 419–425. [Google Scholar]
- Viana-Soto, A.; Senf, C. The European Forest Disturbance Atlas: A forest disturbance monitoring system using the Landsat archive. Earth Syst. Sci. Data 2025, 17, 2373–2404. [Google Scholar] [CrossRef]
- Ciceu, A.; Bălăcenoiu, F.; de Groot, M.; Chakraborty, D.; Avtzis, D.N.; Barta, M.; Blaser, S.; Bracalini, M.; Castagneyrol, B.; Chernova, U.A.; et al. The ongoing range expansion of the invasive oak lace bug across Europe: Current occurrence and potential distribution under climate change. Sci. Total Environ. 2024, 949, 174950. [Google Scholar] [CrossRef] [PubMed]
| Plot | Latitude | Longitude | 2003 | 2004 | 2005 | 2006 |
|---|---|---|---|---|---|---|
| Acsád | 47.32 | 16.72 | 44 | 96 | 143 | 26 |
| Bakonybél | 47.25 | 17.76 | 162 | 3929 | 2083 | 5 |
| Bugac | 46.66 | 19.68 | 5 | 79 | 25 | 7 |
| Diósjenő | 47.95 | 19.02 | 24 | 269 | 460 | 204 |
| Egyházaskesző | 47.42 | 17.32 | 278 | 14,623 | 471 | 20 |
| Erdősmecske | 46.21 | 18.53 | 11 | 99 | 392 | 58 |
| Felsőtárkány | 47.98 | 20.43 | 49 | 361 | 2208 | 21 |
| Gyula | 46.70 | 21.32 | 47 | 175 | 176 | 80 |
| Hőgyész | 46.48 | 18.40 | 33 | 77 | 443 | 38 |
| Kapuvár | 47.69 | 17.01 | 15 | 206 | 73 | 7 |
| Kishuta | 48.45 | 21.46 | 4 | 51 | 68 | 27 |
| Püspökladány | 47.34 | 21.09 | 5 | 303 | 248 | 46 |
| Répáshuta | 48.07 | 20.56 | 8 | 153 | 540 | 56 |
| Sasrét | 46.20 | 17.90 | 35 | 158 | 295 | 29 |
| Sopron | 47.66 | 16.55 | 15 | 36 | 34 | 19 |
| Sumony | 45.95 | 17.91 | 50 | 332 | 288 | 138 |
| Szalafő | 46.86 | 16.38 | 27 | 96 | 32 | 12 |
| Szentendre | 47.69 | 19.0 | 419 | 71 | ||
| Szentpéterfölde | 46.61 | 16.75 | 19 | 102 | 105 | 37 |
| Tolna | 46.42 | 18.80 | 4 | 43 | 240 | 24 |
| Vámosatya | 48.19 | 22.40 | 223 | 333 | ||
| Várgesztes | 47.47 | 18.40 | 90 | 227 | 351 | 30 |
| Total | 925 | 21,415 | 9317 | 1288 |
| Date | Class | 2003 | 2004 | 2005 | 2006 |
|---|---|---|---|---|---|
| 14 April | severe damage | 0.22 | 0.13 | 6.78 | 1.28 |
| damage | 29.94 | 8.17 | 22.89 | 21.65 | |
| neutral state | 67.09 | 68.34 | 52.82 | 60.70 | |
| regeneration | 2.70 | 22.42 | 16.27 | 15.88 | |
| strong regeneration | 0.05 | 0.93 | 1.23 | 0.49 | |
| 30 April | severe damage | 3.50 | 10.85 | 6.78 | 9.71 |
| damage | 23.19 | 34.24 | 22.89 | 67.87 | |
| neutral state | 37.76 | 35.01 | 52.82 | 6.09 | |
| regeneration | 32.18 | 18.02 | 16.27 | 14.97 | |
| strong regeneration | 3.37 | 1.88 | 1.23 | 1.36 | |
| 16 May | severe damage | 10.25 | 4.66 | 6.27 | 4.95 |
| damage | 14.71 | 14.43 | 18.04 | 14.35 | |
| neutral state | 26.38 | 41.90 | 36.68 | 3.41 | |
| regeneration | 39.15 | 35.88 | 36.78 | 60.72 | |
| strong regeneration | 9.50 | 3.13 | 2.24 | 16.56 | |
| 31 May | severe damage | 3.86 | 7.24 | 5.01 | 4.67 |
| damage | 12.29 | 17.14 | 11.15 | 8.34 | |
| neutral state | 33.75 | 37.17 | 32.34 | 25.42 | |
| regeneration | 46.94 | 35.53 | 47.56 | 48.10 | |
| strong regeneration | 3.16 | 2.92 | 3.93 | 13.47 | |
| 16 June | severe damage | 3.84 | 4.69 | 6.68 | 3.92 |
| damage | 11.28 | 9.16 | 8.01 | 13.76 | |
| neutral state | 31.72 | 31.97 | 26.43 | 41.30 | |
| regeneration | 47.82 | 46.50 | 52.48 | 36.77 | |
| strong regeneration | 5.33 | 7.68 | 6.40 | 4.25 | |
| 2 July | severe damage | 5.42 | 4.65 | 7.27 | 2.58 |
| damage | 15.86 | 7.34 | 8.04 | 7.89 | |
| neutral state | 40.41 | 31.10 | 25.28 | 27.98 | |
| regeneration | 36.61 | 50.07 | 50.20 | 54.00 | |
| strong regeneration | 1.70 | 6.83 | 9.21 | 7.54 | |
| 18 July | severe damage | 6.71 | 9.44 | 3.19 | 0.15 |
| damage | 17.04 | 23.95 | 4.81 | 6.54 | |
| neutral state | 36.24 | 40.32 | 15.37 | 32.03 | |
| regeneration | 35.97 | 24.33 | 50.51 | 60.63 | |
| strong regeneration | 4.04 | 1.96 | 26.12 | 0.65 | |
| 3 August | severe damage | 4.98 | 2.85 | 3.17 | 7.68 |
| damage | 15.91 | 8.94 | 9.36 | 26.86 | |
| neutral state | 37.83 | 31.34 | 26.88 | 59.06 | |
| regeneration | 38.51 | 49.22 | 47.56 | 0.39 | |
| strong regeneration | 2.78 | 7.66 | 13.03 | 6.02 |
| Observation | OBS Pixel No. | MODIS Defoliated Pixels (Limit: −0.07) | MODIS Defoliated Pixels (Limit: −0.15) |
|---|---|---|---|
| extreme | 674 | 242 (39.5%) | 125 (46.5%) |
| strong | 742 | 270 (44%) | 112 (41.6%) |
| moderate | 386 | 37 (6%) | 14 (5.2%) |
| weak | 971 | 34 (5.5%) | 10 (3.7%) |
| negligible | 2899 | 30 (4.9%) | 8 (3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molnár, T.; Móricz, N.; Hirka, A.; Csóka, G.; Kern, A. Retrospective Analysis of a Large-Scale Gypsy Moth Outbreak in Hungary Combining Multi-Source Satellite and In Situ Data. Forests 2025, 16, 1472. https://doi.org/10.3390/f16091472
Molnár T, Móricz N, Hirka A, Csóka G, Kern A. Retrospective Analysis of a Large-Scale Gypsy Moth Outbreak in Hungary Combining Multi-Source Satellite and In Situ Data. Forests. 2025; 16(9):1472. https://doi.org/10.3390/f16091472
Chicago/Turabian StyleMolnár, Tamás, Norbert Móricz, Anikó Hirka, György Csóka, and Anikó Kern. 2025. "Retrospective Analysis of a Large-Scale Gypsy Moth Outbreak in Hungary Combining Multi-Source Satellite and In Situ Data" Forests 16, no. 9: 1472. https://doi.org/10.3390/f16091472
APA StyleMolnár, T., Móricz, N., Hirka, A., Csóka, G., & Kern, A. (2025). Retrospective Analysis of a Large-Scale Gypsy Moth Outbreak in Hungary Combining Multi-Source Satellite and In Situ Data. Forests, 16(9), 1472. https://doi.org/10.3390/f16091472








