Analysis of Volatile Organic Compounds in Cinnamomum camphora Leaves by Direct Thermal Desorption–Gas Chromatography/Mass Spectrometry (DTD-GC/MS)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Detection Instruments and Conditions
2.3. Statistical Analysis
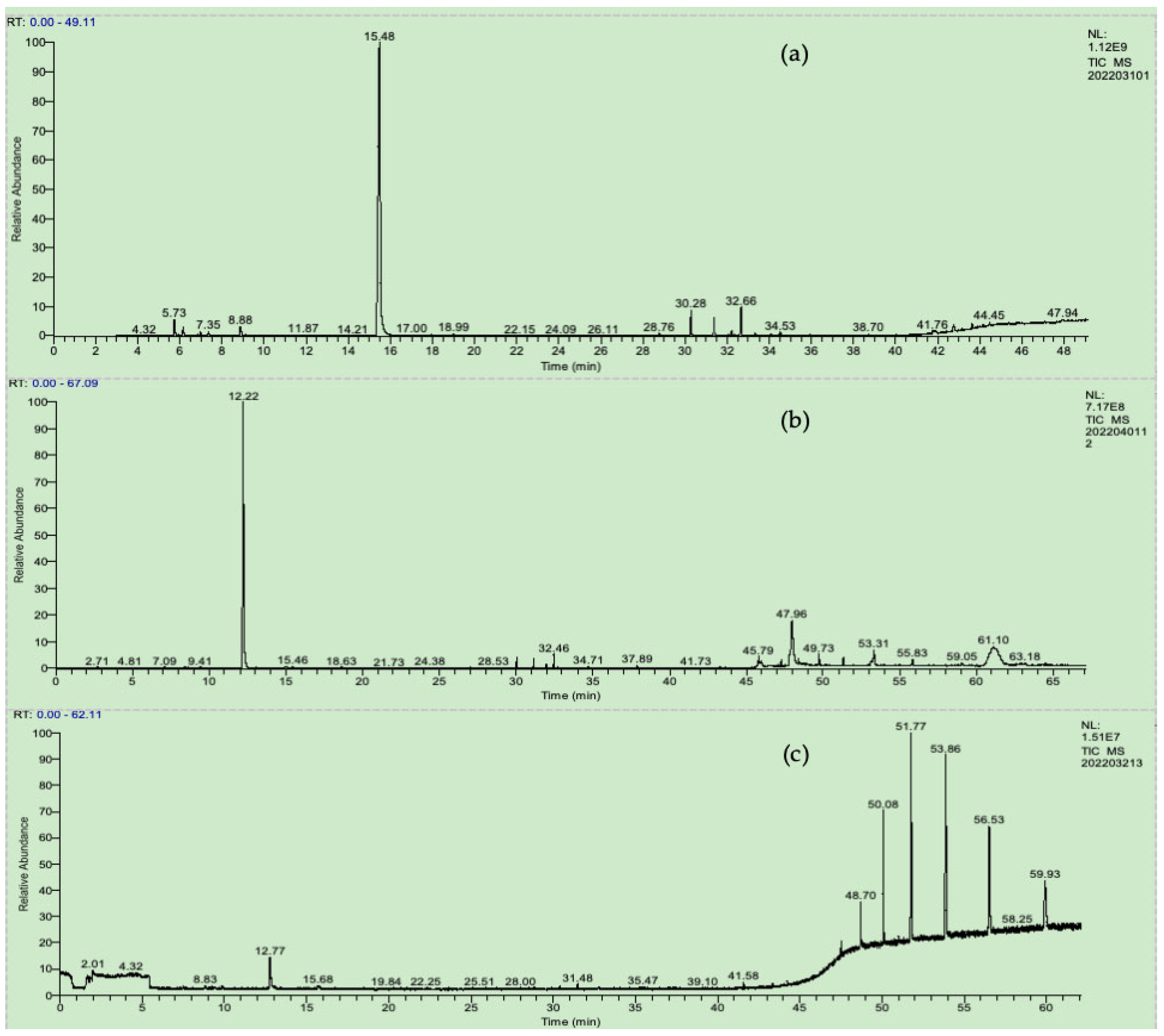
3. Results
3.1. Influence of Parameters
3.1.1. Influence of Loading Amount on the Peak Shape
3.1.2. Effects of Thermal Desorption Temperature on VOCs in Camphor Leaves
3.2. Main VOCs in Camphor Leaves
3.2.1. Main VOCs in Camphor-Type and Linalool-Type Camphor Leaves
3.2.2. Main VOCs in Mature and Young Linalool-Type Leaves
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Choi, Y.; Kim, G.; Park, S.; Lee, S.; Kim, S.; Kim, E. Statistical Evidence for Managing Forest Density in Consideration of Natural Volatile Organic Compounds. Atmosphere 2021, 12, 1113. [Google Scholar] [CrossRef]
- Levy, C.M.; Riederer, A.M.; Simpson, C.D.; Gassett, A.J.; Gilbert, A.J.; Paulsen, M.H.; Silva, L.K.; Bhandari, D.; Newman, C.A.; Blount, B.C.; et al. Effects of filtered vs. non-filtered forest air on stress-related psychophysiological outcomes: A randomized crossover trial. Environ. Res. 2025, 276, 121482. [Google Scholar] [CrossRef]
- Zhang, Z.; Ye, B. Forest Therapy in Germany, Japan, and China: Proposal, Development Status, and Future Prospects. Forests 2022, 13, 1289. [Google Scholar] [CrossRef]
- Chen, H.; Meng, Z.; Luo, J. Is forest bathing a panacea for mental health problems? A narrative review. Front. Public Health 2025, 13, 1454992. [Google Scholar] [CrossRef]
- Xu, R.Y.; Gong, D.C.; Meng, X.X.; Lin, Y.J.; Li, Z.; Chen, S.J.; Chen, X.; Chang, Q.H.; Liang, Y.; Wang, L.; et al. Spatio-temporal variations, influential factors and environmental health assessment of negative air ions (NAIs) in Hainan tropical rainforests. China Environ. Sci. 2025, 45, 657–670. [Google Scholar]
- Chen, X.; Gong, D.; Liu, S.; Meng, X.; Li, Z.; Lin, Y.; Li, Q.; Xu, R.; Chen, S.; Chang, Q.; et al. In-situ online investigation of biogenic volatile organic compounds emissions from tropical rainforests in Hainan, China. Sci. Total Environ. 2024, 954, 176668. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Q.; Xu, C.; Lun, X.; Wang, L.; Gao, Y.; Huang, L.; Zhang, Q.; Li, L.; Liu, B.; et al. Biogenic volatile organic compounds emitted by Pinus tabuliformis mitigate under-canopy ozone concentrations and their healthcare effects in forest therapy bases. Sci. Total Environ. 2024, 931, 172944. [Google Scholar] [CrossRef]
- Zhang, B.H.; Xiao, Z.F.; Wang, Y.B.; Zhang, H.Y.; Li, F.; Cheng, H.J.; Jin, Z.N. Review on Chemotypes and Essential Oil Accumulation of Cinnamomum camphora. For. Sci. 2020, 48, 623–630. [Google Scholar]
- Li, J.X.; Meng, B.B.; Zhu, K. Components and Antioxidant Activity of Camphor Leaves Essential Oil. Chem. Ind. For. Prod. 2020, 40, 84–90. [Google Scholar]
- Hu, W.J.; Gao, H.D.; Jiang, X.M.; Yang, H.K. Analysis on Constituents and Contents in Leaf Essential Oil from Three Chemical Types of Cinnamomum camphora. J. Cent. South Univ. For. Technol. 2012, 32, 186–194. [Google Scholar]
- Tao, G.F.; Deng, J.K.; Sun, H.D. The chemical constituents of the essential oil from leaves of Cinnamomum Longepaniculatum in Hubei. China J. Wuhan Bot. Res. 2010, 20, 75–77. [Google Scholar]
- Yang, N. Mechanism of Cinnamomum camphora essential oil for analgesia based on gas chromatography-mass spectrometry integrated network pharmacology. Asian J. Tradit. Med. 2023, 18, 87–97. [Google Scholar]
- Sharma, N. Folkare and modern pharmacology of camphor (Cinnamomum camphora Nees & Eberm): Review. Sch. Int. J. Tradit. Complement. Med. 2021, 4, 128–135. [Google Scholar]
- Alabadi, R.B.; Kolkar, K.P.; Meti, N.T. Camphor tree, Cinnamomum camphora (L.); Ethnobotany and pharmacological updates. Biomedicine 2021, 41, 181–184. [Google Scholar] [CrossRef]
- Shi, Y.Y.; He, W.; Wen, G. Clasification and composition of essential oils. J. Integr. Plant Biol. 1989, 47–52. [Google Scholar]
- Jiang, Y. Study on The Change Law of The Main Components in Essential Oil of Linalool—Type Cinnamomum camphora (L.) Presl Leaves. Guangxi Sci. Technol. 2018, 16, 1–12. [Google Scholar]
- Li, G.Z.; Liang, Z.Y.; Cai, L.; Chen, H.Y. Analysis on the Chemical Compositions of Oil from the Camphor Tree Leaves of Camphor Type. Bull. Bot. Res. 2017, 6, 6–11. [Google Scholar]
- Zhou, Q.; Wang, J.F.; Xu, Y.Q.; Xia, S.F.; Shen, F.Q.; Xu, L.Y.; Chen, Z.M. Seasonal and diurmal change laws of volatile organic compounds from leaves of Cimnamomum camphora. Guihaia 2020, 40, 1021–1032. [Google Scholar]
- Zhou, Q.; Wang, J.; Wu, Q.; Chen, Z.; Wang, G. Seasonal dynamics of VOCs released from Cinnamomum camphora forests and the associated adjuvant therapy for geriatric hypertension. Ind. Crops Prod. 2021, 174, 114131. [Google Scholar] [CrossRef]
- Perez-Coello, M.S.; Sanz, J.; Cabezudo, M.D. Analysis of volatile components of oak wood by solvent extraction and direct thermal desorption-gas chromatography-mass spectrometry. J. Chromatogr. A 1997, 778, 427–434. [Google Scholar] [CrossRef]
- García, M.A.; Sanz, J.; Cabezudo, M.D. Analysis of Origanum vulgarevolatiles by direct thermal desorption coupled to gas chromatography-mass spectrometry. J. Chromatogr. A 2001, 918, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.; Soria, A.C.; García-Vallejo, M.C. Analysis of volatile components of Lavandula luisieri L. by direct thermal desorption–gas chromatography–mass spectrometry. J. Chromatogr. A 2004, 1024, 139–146. [Google Scholar] [CrossRef]
- Esteban, J.L.; Martinez-Castro, I.; Morales, R.; Fabrellas, B.; Sanz, J. Rapid Identification of Volatile Compounds in Aromatic Plants by Automatic Thermal Desorption-GC-MS. Chromatographia 1996, 43, 63–72. [Google Scholar] [CrossRef]
- Hartman, T.G.; Eri, S.; Khoo, B.K.; Lech, J. Direct Thermal Desorption-Gas Chromatography and Gas Chromatography-Mass Spectrometry Profiling of Hop (Humulus lupulus L.) Essential Oils in Support of Varietal Characterization. J. Agric. Food Chem. 2000, 48, 1140–1149. [Google Scholar]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barbero, G.F. Application of Direct Thermal Desorption-Gas Chromatography-Mass Spectrometry for Determination of Volatile and Semi-Volatile Organosulfur Compounds in Onions: A Novel Analytical Approach. Pharmaceuticals 2023, 16, 715. [Google Scholar] [CrossRef]
- Wang, H.; Yao, L. Comparative Analysis of Components Obtained from Rose Petals by Direct Thermal Desorption and Water Distillation. J. Shanghai Jiaotong Univ. (Agric. Sci.) 2013, 31, 46–51. [Google Scholar]
- Özel, M.Z.; Göğüş, F.; Lewis, A.C. Comparison of direct thermal desorption with water distillation and superheated water extraction for the analysis of volatile components of Rosa damascena Mill using GCxGC-TOF/MS. Anal. Chim. Acta 2006, 566, 172–177. [Google Scholar] [CrossRef]
- Piryaei, M. Direct thermal desorption technique as a very fast, easy and low-cost method for analysis of volatile components compounds by gas chromatography with mass spectrometry. Sep. Sci. Plus 2019, 2, 416–421. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, N.; Cole, R.B. Analysis of the volatile organic compounds in Cinnamomum cassia bark by direct sample introduction thermal desorption gas chromatography-mass spectrometry. J. Essent. Oil Res. 2013, 25, 458–463. [Google Scholar] [CrossRef]
- Sun, L.-J.; Ke, X.; Wang, J.-J.; Chen, Y. Research progress on constituents and biological activities of phytoncides from forest. Chin. Tradit. Herb. Drugs 2021, 52, 7032–7043. [Google Scholar]
- Jian, Y.; Lin, J.; Li, J.; He, J.; Luo, Z. Temporal Dynamic Characteristics of Chemical Components and Relative Contents of Phytoncide in Five Forest Stands. J. Sichuan For. Sci. Technol. 2020, 41, 44–50. [Google Scholar]


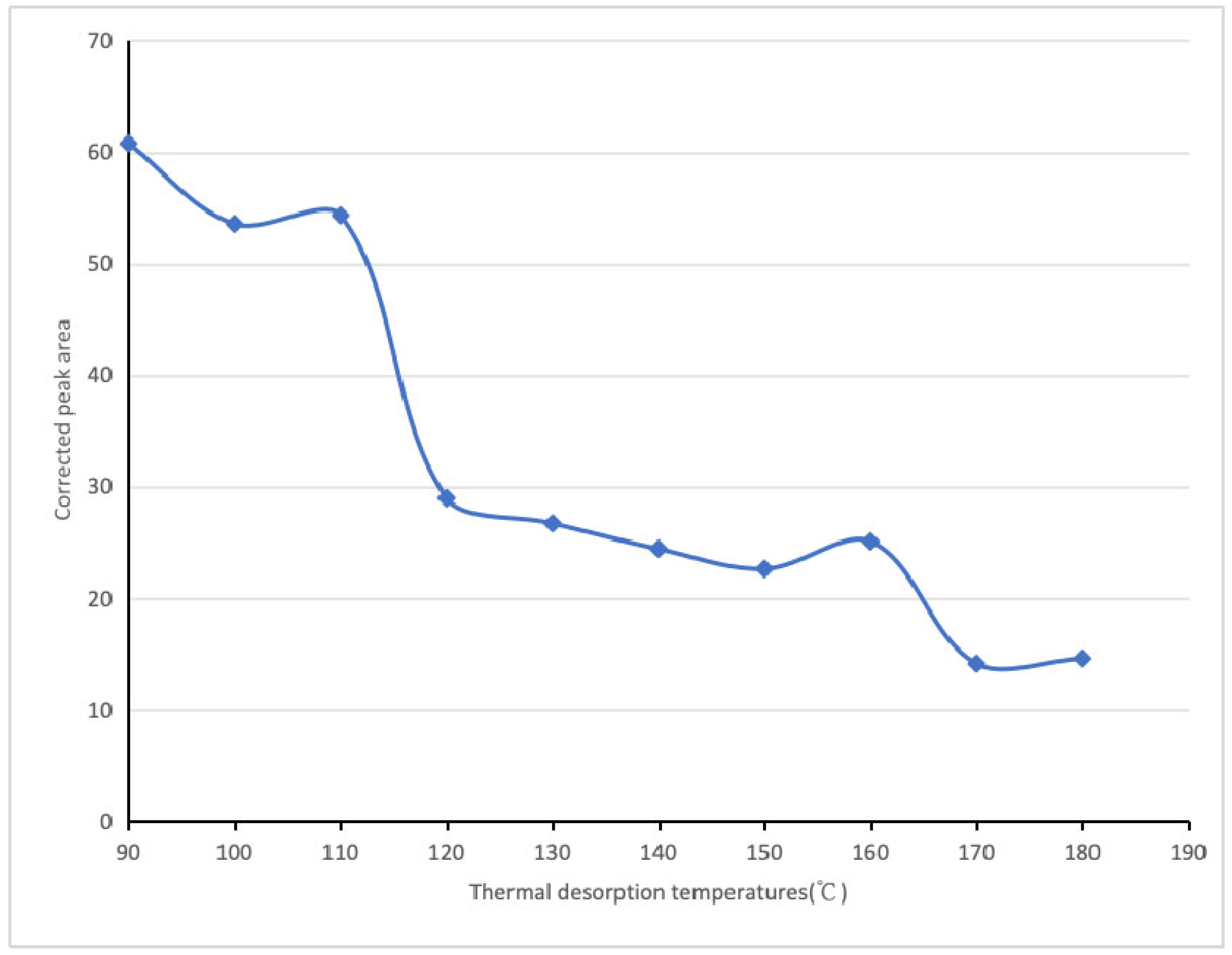
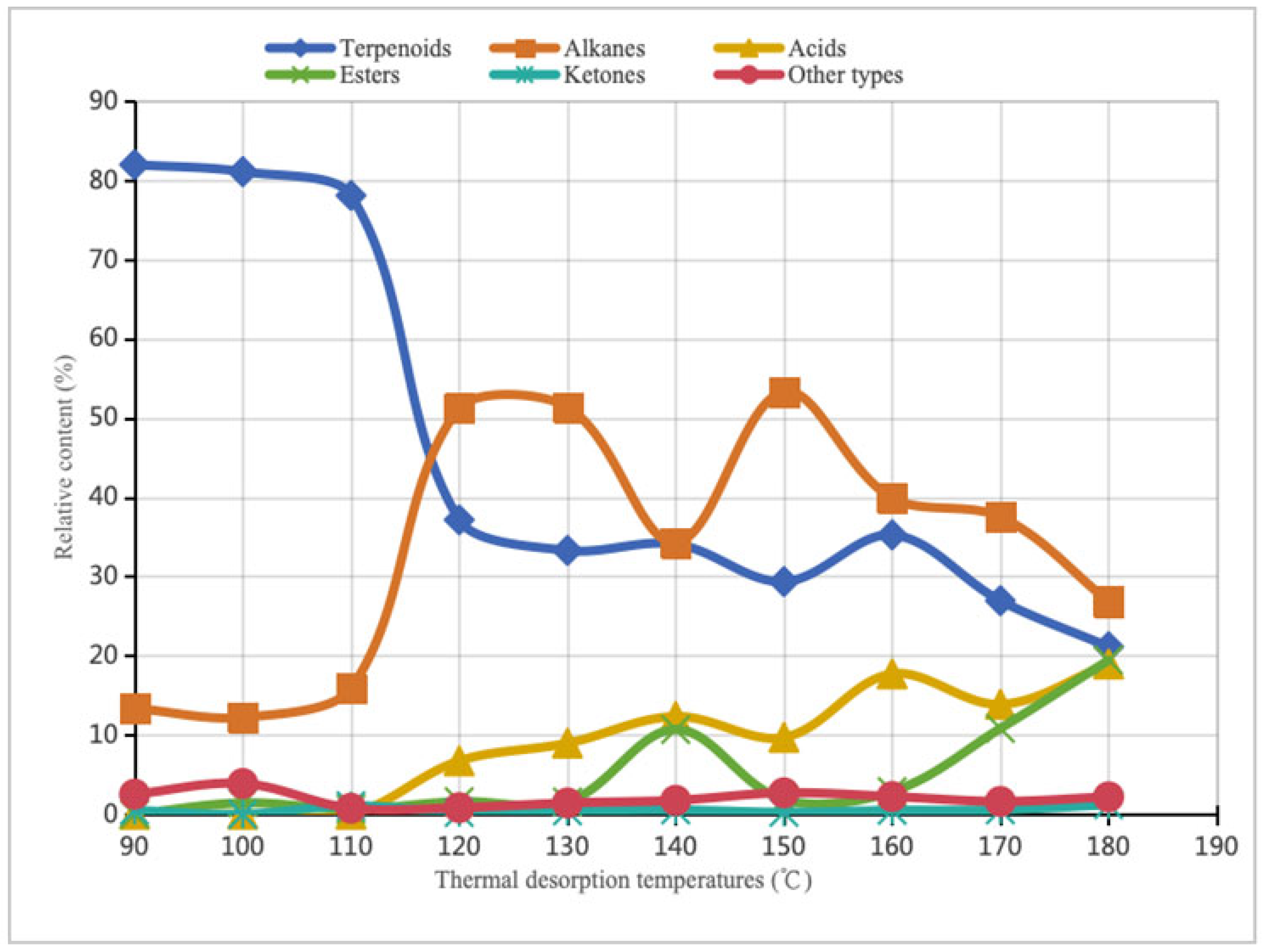
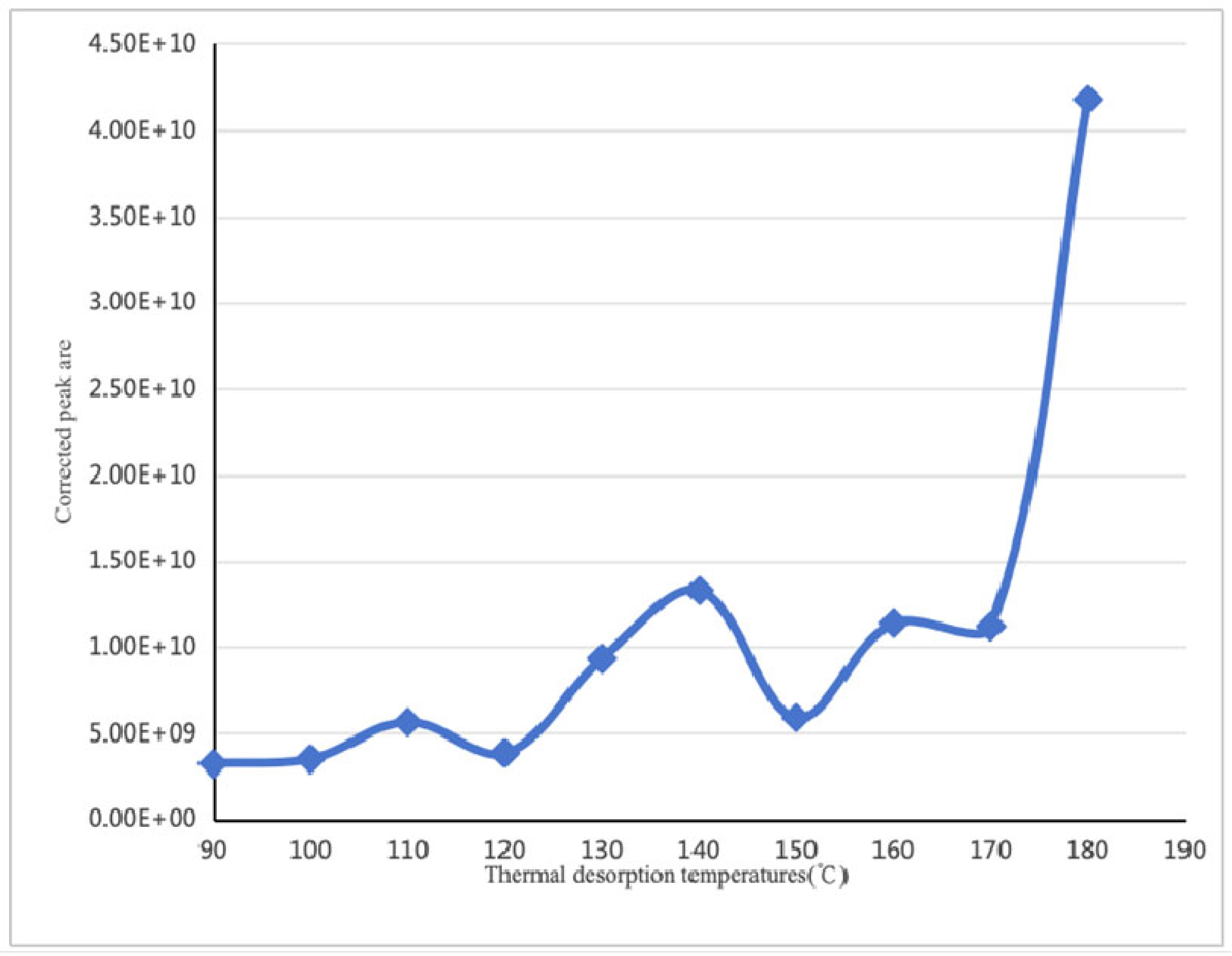
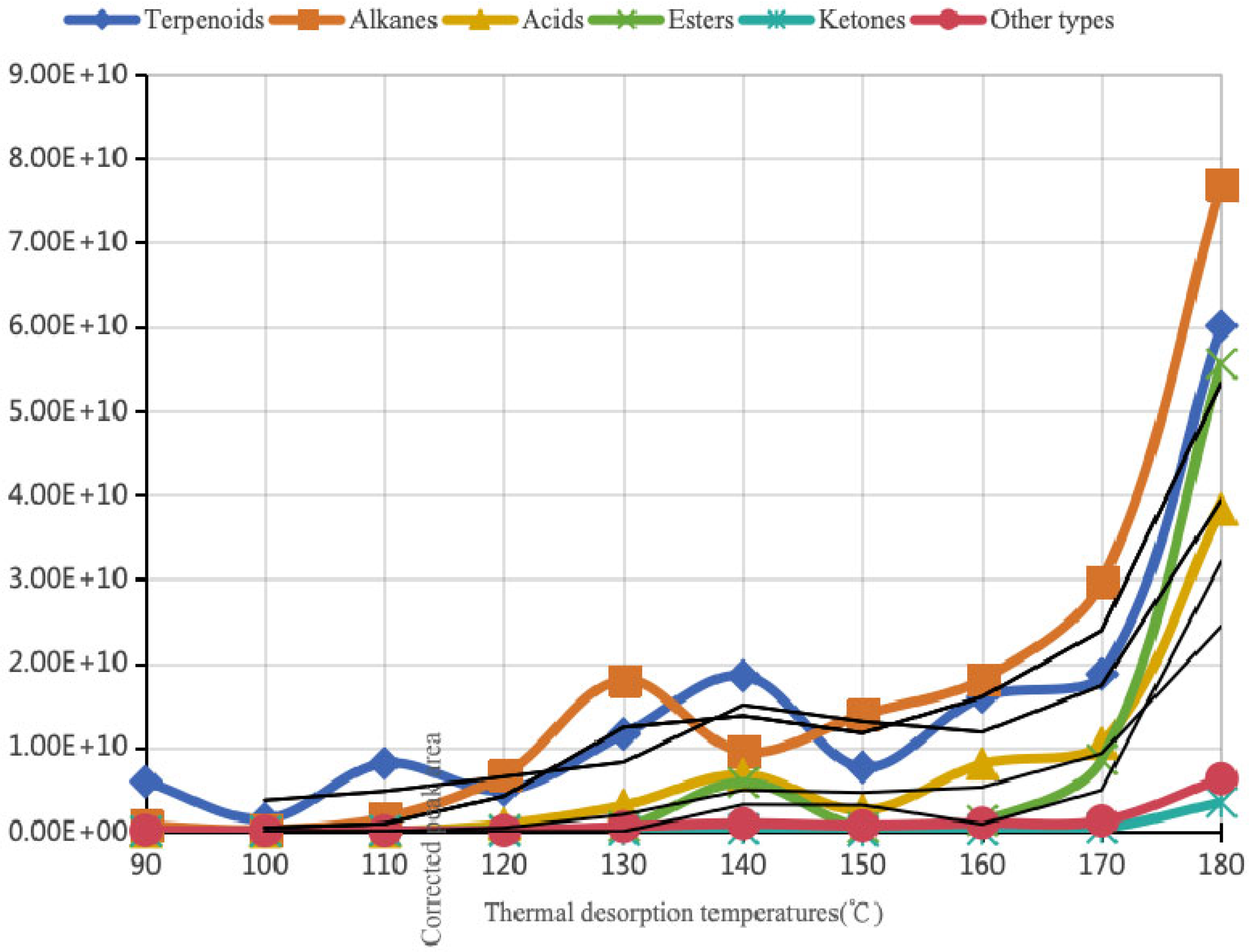
| Thermal Desorption Temperatures (°C) | 90 | 100 | 110 | 120 | 130 | 140 | 150 | 160 | 170 | 180 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Linalool | Corrected peak area | 3.23 × 109 | 1.01 × 109 | 5.64 × 109 | 3.84 × 109 | 9.33 × 109 | 1.33 × 1010 | 5.90 × 109 | 1.14 × 1010 | 1.12 × 1010 | 4.18 × 1010 |
| Relative content (%) | 60.76 | 53.54 | 54.31 | 28.95 | 26.68 | 24.4 | 22.6 | 25.06 | 14.11 | 14.58 | |
| Terpenoids | Corrected peak area | 5.94 × 109 | 1.51 × 109 | 8.11 × 109 | 4.92 × 109 | 1.16 × 1010 | 1.85 × 1010 | 7.65 × 109 | 1.60 × 1010 | 1.87 × 1010 | 6.01 × 1010 |
| Relative content (%) | 81.95 | 81.06 | 78.06 | 37.14 | 33.28 | 34.08 | 29.35 | 35.24 | 26.94 | 21.13 | |
| Alkanes | Corrected peak area | 6.98 × 108 | 2.16 × 108 | 1.65 × 109 | 6.79 × 109 | 1.80 × 1010 | 9.50 × 109 | 1.39 × 1010 | 1.81 × 1010 | 2.97 × 1010 | 7.69 × 1010 |
| Relative content (%) | 13.37 | 12.22 | 15.87 | 51.26 | 51.38 | 34.29 | 53.3 | 39.87 | 37.53 | 26.85 | |
| Acids | Corrected peak area | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | 8.92 × 108 | 3.13 × 109 | 6.75 × 109 | 2.53 × 109 | 8.04 × 109 | 1.05 × 1010 | 3.83 × 1010 |
| Relative content (%) | 0 | 0 | 0 | 6.73 | 8.96 | 12.33 | 9.69 | 17.71 | 13.87 | 18.95 | |
| Esters | Corrected peak area | 0.00 × 100 | 2.70 × 107 | 6.87 × 107 | 2.10 × 108 | 6.03 × 108 | 5.83 × 109 | 4.78 × 108 | 1.30 × 109 | 8.57 × 109 | 5.56 × 1010 |
| Relative content (%) | 0 | 1.44 | 0.65 | 1.6 | 1.55 | 10.69 | 1.83 | 2.86 | 10.83 | 19.44 | |
| Ketones | Corrected peak area | 2.59 × 107 | 0.00 × 100 | 1.12 × 108 | 2.82 × 107 | 1.45 × 108 | 3.00 × 108 | 3.66 × 107 | 2.23 × 108 | 4.34 × 108 | 3.47 × 109 |
| Relative content (%) | 0.49 | 0 | 1.08 | 0.21 | 0.42 | 0.55 | 0.24 | 0.49 | 0.55 | 1.2 | |
| Other types | Corrected peak area | 1.35 × 108 | 6.49 × 107 | 7.91 × 107 | 1.12 × 108 | 5.85 × 108 | 9.49 × 108 | 7.07 × 108 | 1.02 × 109 | 1.28 × 109 | 6.30 × 109 |
| Relative content (%) | 2.53 | 3.88 | 0.76 | 0.84 | 1.43 | 1.76 | 2.71 | 2.24 | 1.62 | 2.2 | |
| Thermal Desorption Temperatures (°C) | 90 | 100 | 110 | 120 | 130 | 140 | 150 | 160 | 170 | 180 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Nº CAS | Formula | Relative Contents (%) | |||||||||
| Linalool | 78-70-6 | C10H18O | 60.76 | 53.54 | 54.31 | 28.95 | 26.68 | 24.40 | 22.60 | 25.06 | 14.11 | 14.58 |
| dl-α-Tocopherol | 10191-41-0 | C29H50O2 | 13.26 | 3.26 | ||||||||
| γ-Elemene | 3242-08-8 | C15H24 | 2.3 | 4.97 | 7.28 | 2.03 | 1.64 | 2.09 | 1.74 | 2.51 | 1.6 | 1.77 |
| Caryophyllene | 87-44-5 | C15H24 | 1.83 | 3.95 | 5.34 | 1.52 | 1.33 | 1.9 | 1.35 | 2.06 | 1.32 | 1.54 |
| Humulene | 6753-98-6 | C15H24 | 1.49 | 3.56 | 4.84 | 1.44 | 1.2 | 1.66 | 1.35 | 1.86 | 1.23 | |
| Germacrene D | 23986-74-5 | C15H24 | 0.56 | 1 | 1.88 | 0.54 | 0.44 | 0.65 | 0.47 | 0.74 | 0.5 | 0.52 |
| Camphor | 464-48-2 | C10H16O | 0.36 | 0.97 | 0.33 | 0.22 | 0.06 | 0.17 | ||||
| (−)-Spathulenol | 77171-55-2 | C15H24O | 0.26 | 1.51 | 1.32 | 1.11 | 0.68 | 0.78 | 0.57 | 1.21 | 2.93 | 1.84 |
| 3,7-Octadiene-2,6-diol,2,6-dimethyl- | 13741-21-4 | C10H18O2 | 0.22 | 0.41 | 0.41 | 0.44 | 0.39 | 0.53 | 0.16 | 0.38 | 0.25 | |
| β-Pinene | 127-91-3 | C10H16 | 0.21 | 5.2 | ||||||||
| α-Ocimene | 13877-91-3 | C10H16 | 0.2 | 0.19 | 0.1 | |||||||
| α-Pinene | 80-56-8 | C10H16 | 0.1 | 1.44 | 0.11 | |||||||
| β-Elemene | 110823-68-2 | C15H24 | 0.05 | 0.17 | 0.18 | 0.06 | 0.12 | |||||
| γ-Elemene | 29873-99-2 | C15H24 | 0.35 | 0.09 | 0.13 | 0.09 | 0.12 | |||||
| α-Myrcene | 123-35-3 | C10H16 | 0.24 | 0.12 | ||||||||
| Globulol | 51371-47-2 | C15H26O | 0.23 | 0.08 | 0.15 | 0.23 | 0.47 | 0.37 | ||||
| Caryophyllene oxide | 1139-30-6 | C15H24O | 0.16 | 0.1 | 0.08 | |||||||
| Phytol | 150-86-7 | C20H40O | 0.6 | 0.48 | 1.54 | 0.54 | 0.95 | 1.52 | ||||
| Squalene | 111-02-4 | C30H50 | 0.19 | 0.07 | 0.25 | |||||||
| Tetracosane | 646-31-1 | C24H50 | 14.39 | 14.48 | ||||||||
| Eicosane | 112-95-8 | C20H42 | 20.93 | 0.93 | 22.53 | 4.43 | 23.59 | |||||
| Eicosane, 2-methyl- | 1560-84-5 | C21H44 | 10.28 | 8.25 | 14.54 | 50.53 | 29.97 | 18.81 | 30.47 | 24.89 | 19.62 | 3.26 |
| Tetratriacontane | 14167-59-0 | C34H70 | 12.31 | |||||||||
| Pentadecanoic acid | 1002-84-2 | C15H30O2 | 6.56 | 7.56 | 7.27 | |||||||
| α-Linolenic Acid | 463-40-1 | C18H30O2 | 0.17 | 9.36 | 3.26 | 8.78 | 11.43 | 11.59 | ||||
| Octadecanoic acid | 57-11-4 | C18H36O2 | 2.74 | 1.66 | 1.9 | 5.69 | ||||||
| l-(+)-Ascorbic acid 2,6-dihexadecanoate | 28474-90-0 | C38H68O8 | 8.36 | 7.94 | 17.3 | |||||||
| Butyl 9,12,15-octadecatrienoate | 38370-68-2 | C22H38O2 | 0.15 | 1.08 | 0.92 | 0.63 | 0.55 | |||||
| 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 28564-83-2 | C6H8O4 | 0.19 | 0.26 | 0.27 | |||||||
| 3-Octen-2-ol | 76649-14-4 | C8H16O | 0.37 | 0.25 | 0.6 | 0.29 | 0.36 | 0.24 | 0.49 | 0.27 | ||
| 9-Octadecenamide, (Z)- | 301-02-0 | C18H35NO | 1.23 | 1.47 | 0.88 | 0.91 | ||||||
| Types | Name | Nº CAS | Formula | Relative Contents (%) | ||
|---|---|---|---|---|---|---|
| Camphor-Type Leaves | Linalool-Type | |||||
| Old Leaves | Young Leaves | |||||
| Terpenoids | Humulene | 6753-98-6 | C15H24 | 4.84 | ||
| β-Pinene | 127-91-3 | C10H16 | 0.66 | 0.19 | ||
| Isocarvestrene | 13898-73-2 | C10H16 | 0.18 | |||
| α-Pinene | 80-56-8 | C10H16 | 6.05 | 0.11 | ||
| cis-Linalool oxide | 5989-33-3 | C10H18O2 | 0.12 | |||
| Linalool | 78-70-6 | C10H18O | 54.31 | 4.18 | ||
| L(−)-Camphor | 464-48-2 | C10H16O | 72.8 | 0.33 | ||
| 3,7-Octadiene-2,6-diol, 2,6-dimethyl- | 13741-21-4 | C10H18O2 | 0.41 | |||
| (−)-Isocaryophyllene | 118-65-0 | C15H24 | 0.31 | |||
| Isosativene | 24959-83-9 | C15H24 | 0.28 | |||
| Globulol | 51371-47-2 | C15H26O | 0.23 | |||
| (−)-β-Elemene | 110823-68-2 | C15H24 | 0.05 | 0.18 | ||
| Caryophyllene | 87-44-5 | C15H24 | 4.16 | 5.34 | ||
| γ-Elemene | 29873-99-2 | C15H24 | 0.14 | 0.35 | ||
| Germacrene D | 23986-74-5 | C15H24 | 0.72 | 1.88 | ||
| γ-Elemene | 3242-08-8 | C15H24 | 4.16 | 7.28 | ||
| (−)-Spathulenol | 77171-55-2 | C15H24O | 0.31 | 1.32 | ||
| Caryophyllene oxide | 1139-30-6 | C15H24O | 0.05 | 0.16 | ||
| Camphene | 79-92-5 | C10H16 | 1.47 | |||
| α-Myrcene | 123-35-3 | C10H16 | 0.63 | 0.24 | ||
| 2-Thujene | 28634-89-1 | C10H16 | 0.24 | |||
| D-Limonene | 5989-27-5 | C10H16 | 1.62 | |||
| trans-β-Terpineol | 7299-41-4 | C10H16O | 0.03 | |||
| Terpinolene | 586-62-9 | C10H18O | 0.07 | |||
| isoborneol | 10385-78-1 | C10H16 | 0.14 | |||
| (−)-Terpinen-4-ol | 20126-76-5 | C10H18O | 0.08 | |||
| α-Terpineol | 98-55-5 | C10H18O | 0.25 | |||
| Epi-B-Santalene | 25532-78-9 | C10H18O | 0.04 | |||
| Nerolidol | 7212-44-4 | C15H24 | 0.02 | |||
| Rhodopin | 105-92-0 | C15H26O | 0.02 | |||
| Alkanes | Eicosane, 2-methyl- | 1560-84-5 | C21H44 | 1.02 | 14.54 | 68.44 |
| Octadecane, 2-methyl- | 1560-88-9 | C19H40 | 1.53 | 1.33 | ||
| Octadecane | 55282-12-7 | C26H54 | 0.1 | |||
| 1-Iodo-2-methylundecane | 73105-67-6 | C12H25I | 8.19 | |||
| Heptadecane, 2,6-dimethyl- | 54105-67-8 | C19H40 | 13.22 | |||
| Nonadecane, 2-methyl- | 1560-86-7 | C20H42 | 3.85 | |||
| Esters | Dibutyl phthalate | 84-74-2 | C16H22O4 | 0.09 | 0.21 | |
| 2,5-Octadecadiynoic acid, methyl ester | 57156-91-9 | C19H30O2 | 0.05 | |||
| Diethyl Phthalate | 84-66-2 | C12H14O4 | 0.17 | |||
| Triethyl citrate | 77-93-0 | C12H20O7 | 0.11 | |||
| 9-Octadecenoic acid (Z)-, hexyl ester | 20290-84-0 | C24H26O2 | 0.16 | |||
| Ketones | Dodecanal | 112-54-9 | C12H24O | 0.08 | 0.55 | |
| 2-Pentadecanone, 6,10,14-trimethyl- | 502-69-2 | C18H36O | 0.02 | |||
| Acetoin | 513-86-0 | C4H8O2 | 0.36 | |||
| Methyl vinyl ketone | 78-94-4 | C4H6O | 0.17 | |||
| Others | o-Cymene | 527-84-4 | C10H14 | 0.08 | 0.16 | |
| Rhodoxanthin | 116-30-3 | C40H50O2 | 0.06 | |||
| 3-Octen-2-ol | 76649-14-4 | C8H16O | 0.60 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Cai, F.; Hu, J.; Hu, Y.; Wang, Y. Analysis of Volatile Organic Compounds in Cinnamomum camphora Leaves by Direct Thermal Desorption–Gas Chromatography/Mass Spectrometry (DTD-GC/MS). Forests 2025, 16, 1433. https://doi.org/10.3390/f16091433
Li G, Cai F, Hu J, Hu Y, Wang Y. Analysis of Volatile Organic Compounds in Cinnamomum camphora Leaves by Direct Thermal Desorption–Gas Chromatography/Mass Spectrometry (DTD-GC/MS). Forests. 2025; 16(9):1433. https://doi.org/10.3390/f16091433
Chicago/Turabian StyleLi, Guangrong, Fang Cai, Jiayang Hu, Ying’ao Hu, and Yixun Wang. 2025. "Analysis of Volatile Organic Compounds in Cinnamomum camphora Leaves by Direct Thermal Desorption–Gas Chromatography/Mass Spectrometry (DTD-GC/MS)" Forests 16, no. 9: 1433. https://doi.org/10.3390/f16091433
APA StyleLi, G., Cai, F., Hu, J., Hu, Y., & Wang, Y. (2025). Analysis of Volatile Organic Compounds in Cinnamomum camphora Leaves by Direct Thermal Desorption–Gas Chromatography/Mass Spectrometry (DTD-GC/MS). Forests, 16(9), 1433. https://doi.org/10.3390/f16091433





