Physiological and Biochemical Responses of Idesia polycarpa to Botryosphaeria dothidea Infection at Different Stages of Stem Canker Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.1.1. Experimental Plants
2.1.2. Pathogen Strain
2.2. Experimental Methods
2.2.1. Pathogen Inoculation
2.2.2. Sample Collection and Pretreatment
2.2.3. Indicator Measurement Methods
Pathogenicity Assessment
- Grade I: Callus formation without visible lesions;
- Grade II: Lesions with browning of the stem 1–3 cm around the inoculation site;
- Grade III: Browning exceeding 3 cm from the inoculation site;
- Grade IV: Browning extending along the main stem;
- Grade V: Browning of both the main stem and upper branches, with symptoms such as leaf wilting and bark exfoliation.
Determination of Chlorophyll and Carotenoid Contents
Determination of Lipid Peroxidation Products and Osmoregulatory Substances
Determination of Antioxidant Enzyme Activities
2.2.4. Data Analysis
3. Results
3.1. Disease Symptoms
3.2. Effects of B. dothidea Inoculation on Photosynthetic Pigments
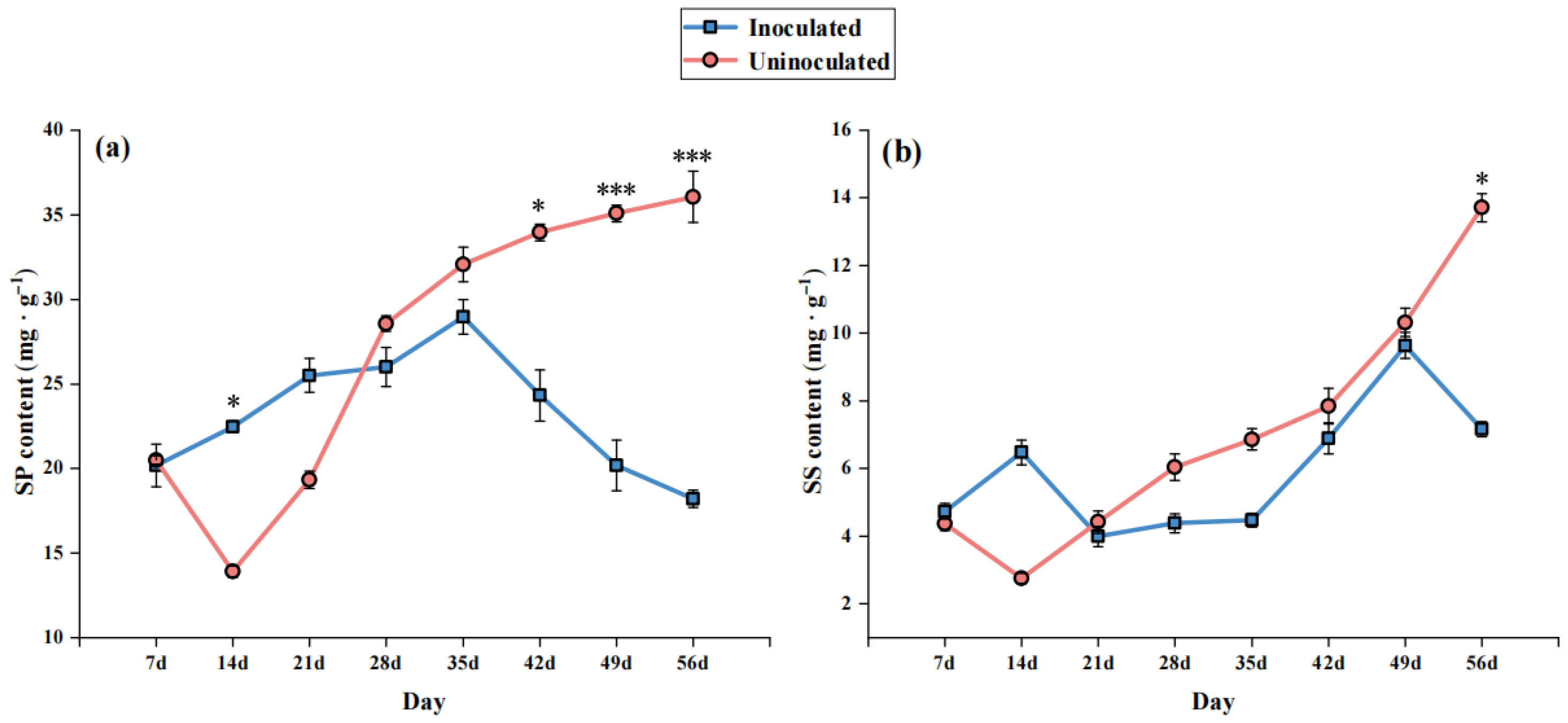
3.3. Effects of B. dothidea Inoculation on Lipid Peroxidation and Osmoregulatory Substances
3.4. Effect of B. dothidea Inoculation on Antioxidant Enzyme Activity
3.5. Correlation Analysis of Physiological and Biochemical Indicators
4. Discussion
4.1. Stage-Specific Physiological Response Mechanisms
4.2. Changes in Antioxidant Activity: Establishment and Decline of Early Defense
4.3. MDA Accumulation: Marker of Oxidative Damage and Membrane Disruption
4.4. Osmotic Adjustment Substances: Early Adaptive Response and Late Metabolic Disruption
4.5. Photosynthetic Pigments: Structural Damage and Physiological Decline
4.6. Coordinated Patterns Among Physiological Indicators: Consistency with a Systemic Stress Network
4.7. Novelty and Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, L.; Chang, J.; He, W.; Yang, Y.; Zhang, L.; Yang, X.; Xie, S. Preliminary study on the occurrence pattern of poplar canker disease in the field. Pract. For. Technol. 2012, 10, 38–40. [Google Scholar]
- Kovač, M.; Diminić, D.; Orlović, S.; Zlatković, M. Botryosphaeria dothidea and Neofusicoccum yunnanense Causing Canker and Die-Back of Sequoiadendron giganteum in Croatia. Forests 2021, 12, 695. [Google Scholar] [CrossRef]
- Kumar, A.M.; Sharma, R.M.; Dubey, A.K.; Awasthi, O.P.; Singh, D.; Dahuja, A.; Mitra, S.V.A.C.R.; Kumar, A. Effect of weather parameters and citrus genotypes on the occurrence of citrus canker incited by Xanthomonas citri pv. citri. Indian Phytopathol. 2023, 76, 605–613. [Google Scholar] [CrossRef]
- Lin, F.; Chen, Z.; Deng, J.; Wang, K. Estimation of nitrogen content in rice leaves using Fourier transform mid-infrared spectroscopy. Plant Nutr. Fertil. Sci. 2009, 15, 750–755. [Google Scholar]
- Luo, M.; Yao, Y.; Gong, J.; Feng, A.; Zhang, W.; Yang, W.; Wang, W.; Zhao, J.; Wang, L.; Zhang, L. Investigation of bitter compounds in Idesia polycarpa and identification of associated biosynthetic genes. Ind. Crops Prod. 2025, 232, 121285. [Google Scholar] [CrossRef]
- Luo, X.; Liu, Y.; Lei, Y.; He, Z.; Gong, X.; Ye, M.; Xiao, Q. Genetic Diversity Analysis and Polyploid Induction Identification of Idesia polycarpa. Plants 2024, 13, 3394. [Google Scholar] [CrossRef]
- Tian, X.; Fang, X.; Du, M. Study on nutritional quality and antioxidant capacity of oils from different parts of Idesia polycarpa fruit. J. Chin. Cereals Oils Assoc. 2020, 35, 91–95. [Google Scholar]
- Yang, Y.; Miao, C.; Yuan, Q.; Zhong, W.; Hu, Z.; Chen, C.; Liu, Z.; Wang, Y.; Geng, X.; Cai, Q.; et al. Changes in the quality of Idesia polycarpa Maxim fruits from different ecotypes during the growth process. Plants 2025, 14, 2324. [Google Scholar] [CrossRef]
- Chen, Z.; Ai, Q.J. A new “aerial oil depot” of China is emerging—Summary of the symposium on the oil quality, value, and market prospects of oil grape (Idesia polycarpa). China For. Ind. 2019, Z2, 63–67. [Google Scholar]
- China Meteorological Data Service Center (CMDC). Daily Climate Data for Zhengzhou (1981–2010). Available online: http://data.cma.cn (accessed on 15 August 2025).
- Fang, L.; Zheng, T.; Feng, J.; Zhi, W.; Wang, Y.; Li, Z.; Cai, Q.; Geng, X.; Liu, Z. Botryosphaeria dothidea causes stem canker of Idesia polycarpa in China. Plant Pathol. 2024, 73, 1372–1381. [Google Scholar] [CrossRef]
- Feng, J.; Yuan, Q.; Chen, X.; Fang, L.; Zhang, T.; Liu, Z.; Wang, Y.; Geng, X.; Cai, Q.; Li, Z. Identification and chemical control of stem canker pathogen of Idesia polycarpa. Plants 2025, 14, 1393. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Q.; Li, Y.; Lian, H.; Li, Y. The Relativity Investigation Among Frost Injury and Dry not of Pomegranate Pomegranate in Zhengzhou; Research Progress on Pomegranate in China(I); Preparatory Group of Pomegranate Branch of Chinese Horticultural Society, Henan Agricultural University: Zhengzhou, China, 2010. [Google Scholar]
- Wang, X. Principles and Techniques of Plant Physiology and Biochemistry Experiments; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Shi, J.; Tong, R.; Yao, J.; Wang, S.; Wang, S.; Li, J.; Song, C.; Zhang, K.; Jiao, J.; Wang, M.; et al. Controlled atmosphere storage with high CO2 concentration extends storage life of fresh pomegranate fruit by regulating antioxidant capacity and respiration metabolism. BMC Plant Biol. 2025, 25, 684. [Google Scholar] [CrossRef]
- Mhamdi, A.; Van Breusegem, F. Reactive oxygen species in plant development. Development 2012, 139, 3901–3904. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, J.; Hu, D.; Liu, G.; Chen, L.; Li, B.; Du, W.; Song, B. Characterization of Maize Germplasm Resistance to Common Smut and Analysis of Physiological Differences. Sci. Agric. Sin. 2025, 58, 2504–2521. [Google Scholar]
- Wang, Y.; Zhang, Y.; Fan, J.; Li, H.; Chen, Q.; Yin, H.; Qi, K.; Xie, Z.; Zhu, N.; Sun, X.; et al. Physiological and autophagy evaluation of different pear varieties (Pyrus spp.) in response to Botryosphaeria dothidea infection. Tree Physiol. 2024, 44, tpad139. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, Y.; Chen, G. Changes in antioxidative enzymes and membrane lipid peroxidation in grape leaves infected with Sclerotinia sclerotiorum. Hortic. Plant J. 2021, 7, 51–60. [Google Scholar]
- Kumar, S.; Shukla, V.; Tripathi, Y.N.; Aamir, M.; Divyanshu, K.; Yadav, M.; Upadhyay, R.S. Biochemical changes, antioxidative profile, and efficacy of the bio-stimulant in plant defense response against Sclerotinia sclerotiorum in common bean (Phasaeolus vulgaris L.). Heliyon 2024, 10, e23030. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, S.; Zeng, K. Exogenous nitric oxide-induced postharvest disease resistance in citrus fruit to Colletotrichum gloeosporioide. J. Sci. Food Agric. 2016, 96, 505–512. [Google Scholar] [CrossRef]
- Qi, A.; Liu, C.; Lü, D.; Chen, H.; Li, Y.; Wang, A.; Shi, R. Physiological responses and cold resistance evaluation of Sapindus mukorossi seedlings from four provenances under low temperature stress. J. Cent. South Univ. For. Technol. 2024, 44, 46–54. [Google Scholar]
- Li, S. Physiological Characteristics of Apple Flesh Affected by Moldy Core Disease and Its Nondestructive Detection Using Near-Infrared Spectroscopy. Ph.D. Thesis, Northwest A&F University, Shaanxi, China, 2012. [Google Scholar]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Kissoudis, C.; Sunarti, S.; Van De Wiel, C.; Bai, Y. Responses to combined abiotic and biotic stress in tomato are governed by stress intensity and resistance mechanism. J. Exp. Bot. 2016, 67, 5119–5132. [Google Scholar] [CrossRef]
- Xu, Z.; Zhu, X.; Wang, S.; Liu, Z.; Feng, J.; Li, Z. Research progress on major diseases and pests of Idesia polycarpa and their control technologies. Henan For. Sci. Technol. 2021, 41, 21–25+38. [Google Scholar]
- Xu, Q.; Zhu, K.Q. Research progress on the degradation mechanism of chlorophyll in plants. J. Plant Physiol. 2020, 56, 401–410. [Google Scholar]
- Ma, X.; Zheng, J.; Zhang, X.; Hu, Q.; Qian, R. Salicylic acid alleviates the adverse effects of salt stress on Dianthus superbus by activating photosynthesis, antioxidant system and osmotic adjustment. Sci. Rep. 2016, 6, 32392. [Google Scholar]
- Gao, F.; Wang, Z.; Zhu, J.; Li, W.; Wang, X.; Yang, X.; Hao, Y.; An, S.; Yin, X.; Liu, X. The characterization and antifungal activities of two new Trichoderma antagonistic fungi against four apple disease pathogens. Biol. Control 2025, 200, 105689. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Naz, S.; Altaf, M.M.; Khan, L.U.; Tiwari, R.K.; Lal, M.K.; Shahid, M.A.; Kumar, R.; et al. Melatonin improves drought stress tolerance of tomato by modulating plant growth, root architecture, photosynthesis, and antioxidant defense system. Antioxidants 2022, 11, 309. [Google Scholar] [CrossRef]
- Xia, T.; Yang, Y.; Zheng, H.; Han, X.; Jin, H.; Xiong, Z.; Qian, W.; Xia, L.; Ji, X.; Li, G.; et al. Efficient expression and function of a receptor-like kinase in wheat powdery mildew defense require an intron-located MYB binding site. Plant Biotechnol. J. 2021, 19, 897–909. [Google Scholar]





| Grade | Assigned Value | Description |
|---|---|---|
| I | 0 | No lesion. |
| II | 1 | very small lesions |
| III | 2 | Small scattered lesions |
| IV | 3 | Numerous lesions |
| V | 4 | Extensive lesions |
| Days After Inoculation | Disease Incidence (%) | Disease Index |
|---|---|---|
| 7 d | 0 | 0 |
| 14 d | 20.00 | 5.00 |
| 21 d | 45.00 | 15.00 |
| 28 d | 70.00 | 27.50 |
| 35 d | 85.00 | 36.25 |
| 42 d | 90.00 | 42.50 |
| 49 d | 95.00 | 56.25 |
| 56 d | 95.00 | 68.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Q.; Zhu, Y.; Yang, Y.; Miao, C.; Zhong, W.; Hu, Z.; Chen, C.; Liu, Z.; Wang, Y.; Geng, X.; et al. Physiological and Biochemical Responses of Idesia polycarpa to Botryosphaeria dothidea Infection at Different Stages of Stem Canker Disease. Forests 2025, 16, 1411. https://doi.org/10.3390/f16091411
Yuan Q, Zhu Y, Yang Y, Miao C, Zhong W, Hu Z, Chen C, Liu Z, Wang Y, Geng X, et al. Physiological and Biochemical Responses of Idesia polycarpa to Botryosphaeria dothidea Infection at Different Stages of Stem Canker Disease. Forests. 2025; 16(9):1411. https://doi.org/10.3390/f16091411
Chicago/Turabian StyleYuan, Qiupeng, Yigeng Zhu, Yi Yang, Chao Miao, Wenwen Zhong, Zuwei Hu, Chen Chen, Zhen Liu, Yanmei Wang, Xiaodong Geng, and et al. 2025. "Physiological and Biochemical Responses of Idesia polycarpa to Botryosphaeria dothidea Infection at Different Stages of Stem Canker Disease" Forests 16, no. 9: 1411. https://doi.org/10.3390/f16091411
APA StyleYuan, Q., Zhu, Y., Yang, Y., Miao, C., Zhong, W., Hu, Z., Chen, C., Liu, Z., Wang, Y., Geng, X., Cai, Q., Dai, L., Wang, J., Ren, Y., Liu, F., Zou, H., Yao, S., Zhong, T., & Li, Z. (2025). Physiological and Biochemical Responses of Idesia polycarpa to Botryosphaeria dothidea Infection at Different Stages of Stem Canker Disease. Forests, 16(9), 1411. https://doi.org/10.3390/f16091411






