Abstract
Urbanization is a driving force of landscape transformation. One of the ecosystems most vulnerable to urban expansion processes is montane forests located in high altitude mountainous regions. Despite their significance for biodiversity, regulation of the hydrological cycle, stability, prevention of soil erosion, and potential for organic carbon storage, these forest ecosystems show high vulnerability and risk due to the global urbanization process. We analyzed the potential variations produced by land cover change in some attributes related to soil organic matter in transitional forest fragments due to the expansion of a predominantly urban matrix landscape. We identified and characterized a fragment of a high montane evergreen forest in the Western Cordillera of the Northern Andes located in the urban limits of Quito. Then, we comparatively analyzed the variations in the attributes associated with soil organic carbon: soil organic matter, density, texture, nitrogen, phosphorus, and pH. We also considered the following soil coverages: forest, eucalyptus plantations, and grassland. We viewed the latter two as hinge coverages between forests and urban expansion. Finally, we estimated variations in soil organic carbon stock in the three analyzed coverages. For the montane forest fragment, we identified 253 individuals distributed among 18 species, corresponding to 10 families and 14 genera. We found significant variations in soil attributes associated with organic matter and an estimated 66% reduction in the carbon storage capacity of montane soils when they lose their natural cover and are replaced by Eucalyptus globulus plantations. Urban planning strategies should consider the conservation and restoration of natural and degraded peri-urban areas, ensuring sustainability and utilizing nature-based solutions for global climate change adaptation and mitigation. Peri-urban agroforestry systems represent an opportunity to replace and restore conventional forestry or crop plantation systems in peri-urban areas that affect the structure and function of ecosystems and, therefore, the goods and services derived from them.
1. Introduction
Urbanization is a driving force in landscape transformation [1,2,3]. Globally, the urbanization process has experienced a sharp increase in recent decades, driven by rapid population growth [4,5]. In the early 20th century, the urban population represented only 13% of the world’s population, a figure that escalated to more than four billion urban dwellers in 2018, equivalent to 55% of the total, and that continues to increase at a rate of approximately one million every 10 days [5,6]. This demographic growth explosion has generated an equally accelerated expansion of urban areas [7]. Thus, studies [3,8] have estimated that the world’s urban area doubled between 1992 and 2003 and continues to expand at an accelerated rate, with even more significant projections for 2030 [9,10].
Urbanized areas were frequently established on previously deforested or degraded land [11], including native forests cleared due to increasing pressure from rapidly growing cities [12,13,14]. One of the most vulnerable ecosystems to urban expansion processes is the world’s montane forests, located in high altitude mountainous regions. Despite the significance of these ecosystems to biodiversity [15], the regulation of the hydrological cycle [16], the stability and prevention of soil erosion [17], and their potential organic carbon stock, which contribute to climate change mitigation [18], these forests exhibit high vulnerability and risk.
The soil in which montane forests are established is characterized by its high heterogeneity and capacity to support rich biodiversity. It is usually deep with high organic matter content, which is crucial for nutrient and water retention [19]. Often acidic and limited in nitrogen (N) and phosphorus (P), this soil has a slow decomposition of organic matter (OM) due to low temperatures, accumulating humus that contributes to its fertility [20]. It also has a high potential as a carbon sink contributing to the mitigation and generation of potential nature-based strategies for climate change adaptation and mitigation [18].
Recent studies in montane and high-altitude ecosystems show that replacing native forests with grasslands or Eucalyptus globulus plantations significantly alters soil organic matter and attributes such as bulk density, texture, nitrogen, phosphorus, and pH [21,22,23]. In Andean soils, bulk density is negatively correlated with organic carbon, while pH—ranging from strongly to slightly acidic—also shows a negative relationship [24,25]. Montane grasslands often retain substantial carbon storage in deep root systems despite management practices [26,27,28]. Eucalyptus plantations often decrease nitrogen and phosphorus and increase bulk density over time [29].
Montane forest ecosystems and human populations in cities and communities that depend on their ecosystem services in the urban–rural–natural interface are affected by the dynamics of urbanization, which alters precipitation and temperature patterns [30,31,32], modifies biogeochemical cycles [33,34], increases greenhouse gas emissions [35,36], pollutes the air [37,38], enriches nutrient loading in aquatic ecosystems [39,40], alters water flow patterns [41,42], impacts the availability of quality water [43,44,45], and occupies [46] or enriches, contaminates, degrades, and desertifies forest soil [47,48,49].
An example of this dynamic is observed in the Ecuador capital, Metropolitan District of Quito (2580 m.a.s.l.), where urban growth of 13% was recorded between 2001 and 2010 [50], with a high probability of alteration of the montane forests surrounding the city [2]. This territorial transformation was characterized by poor planning, with a tendency to favor expansion toward peripheral areas [2,51]. The city developed a bulge-type expansive growth model characterized by external urban expansion in planned patches and unplanned patches adjacent to the urban periphery in different directions along a time scale [2,52]. This expansive process affects a green belt, located around the most urban slopes of the city, where the forest component prevails, characterized by eucalyptus plantations, grassland, and native forest [53,54].
At both local and global scales, research on the impact of the urban matrix on heterogeneous landscapes is relatively recent. This emerging body of work informs the development of climate risk adaptation and mitigation strategies within the context of sustainable urban planning [55]. This study aimed to analyze the potential variations produced by land cover change in some attributes related to soil organic matter in transitional forest fragments due to the expansion of landscapes with a predominantly urban matrix in the city of Quito. To achieve this objective, we analyzed the soil organic carbon (SOC) stock and assessed variations in specific attributes related to soil organic matter—such as bulk density, texture, organic carbon, nitrogen, phosphorus, and pH—across different land cover types within an urban matrix: native forest, eucalyptus plantations, and grasslands. Additionally, we conducted a forest inventory and structural characterization of the high montane evergreen forest in the Western Cordillera of the Northern Andes, within an urban–natural interface landscape. The hypothesis of this study is that eucalyptus plantations and grasslands present significantly different soil attributes compared to the montane forest, reflecting the impact of urban expansion on the region’s natural ecosystems.
2. Materials and Methods
2.1. Study Area: Quito
Located in a valley of the Northern Andes in South America, the Metropolitan District of Quito (MDQ) covers a wide altitudinal range that extends from 500 to 4500 m above sea level. The territory comprises 65 administrative units in an urban–rural–natural interface. The urban area is established in a valley located on the eastern flank of the Pichincha volcano, with approximate dimensions of 34.8 km from north to south and 13.5 km from east to west [2] and at an altitude of 2580 m above sea level (Figure 1). For historical and economic reasons, the shape of the city has evolved from a radial-concentric (1748–1904), to a longitudinal (1904–1960), to a longitudinal polynuclear (1960–1970), and, finally, to a metropolitan [56] model. The urban area shows a bulge expansion that could affect the district’s natural and peri-urban ecosystems [46].
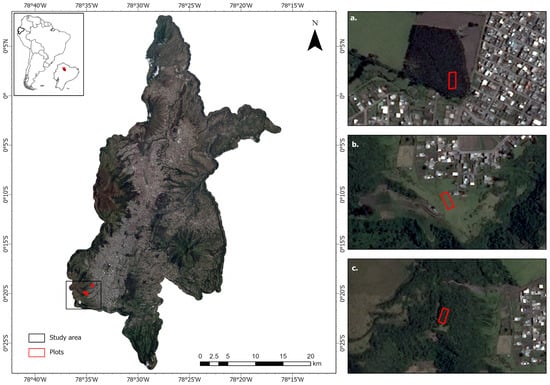
Figure 1.
Study Area: plots in the southern Quito area with potential for urban expansion. (a) Eucalyptus plantation, (b) grassland, (c) montane forest.
Our study area is located in a fragment of evergreen montane forest of the western mountain range of the Andes located in the urban south of the city in the foothills of the Atacazo volcano in the parish of La Ecuatoriana. This area is part of the city’s Protected Areas System and was categorized as the Pichincha–Atacazo Special Intervention and Recovery Area in 2013 [57,58] and is now vulnerable to the city’s expansion. The area has an average local temperature of 11 °C and an average annual precipitation of 1500–2000 mm [59]. In addition to its biodiversity, the conservation of this ecosystem plays a crucial role in risk reduction and the urban green network and is a significant source of the city’s water. However, the original vegetation has been destroyed over the past centuries and replaced by agricultural fields and extensive grasslands due to the expansion of agriculture, livestock farming, and urbanization [60]. Eucalyptus trees were introduced in the 20th century to reduce soil erosion and to improve the supply of timber and fuelwood due to the depletion of native forests, further contributing to the alteration of the landscape [61].
2.2. Methodological Stages
To analyze the interactions between urban expansion and montane forest fragments by quantifying the variations produced by land use change in some attributes related to soil organic matter, we developed a methodological protocol that considered two phases. (i) Estimation of soil organic carbon stock and variations in certain attributes associated with soil organic matter—density, texture, organic carbon, nitrogen, phosphorus, and pH—in the following land covers: native forest, eucalyptus plantations, and grasslands; (ii) forest inventory and structural characterization of the high montane evergreen forest of the Western Cordillera of the Northern Andes in an urban–natural interface landscape.
2.2.1. Soil Organic Carbon Stock and Variations in Soil Attributes
To analyze the potential variations in soil attributes, due to the effect of change in use in native forests, three plots were established in the same interface that included the following coverages: a monoculture of the Eucalyptus globulus plantation; grassland that corresponded to spaces with abandoned previously cultivated pastures; and native forest, one of the last fragments of a high montane forest in the Western Cordillera with a high probability of urbanization [46].
The native forest plots (3504 m.a.s.l.) and grasslands (3498 m.a.s.l.) were classified under the Pachic Melanundands soil subgroup, while the Eucalyptus globulus plots (3499 m.a.s.l.) were located within the Vitrandic Hapludolls subgroup. Both soil types exhibit andic characteristics and have developed on andesitic lava with a hard, slightly fractured aphanitic texture, as well as consolidated volcanic tuffs interspersed with moderately weathered pumice layers at the surface. These are deep, well-drained loamy soils, characterized by moderately acidic pH, high organic matter content, high base saturation, and medium cation exchange capacity [61,62].
In these plots, a grid sampling design was established, marking sampling points every 5 m in perpendicular directions. This design resulted in a grid of 55 soil samples per category, meaning that 165 soil samples were analyzed. Soil samples were taken at a depth of 0–15 cm. To obtain values of pH, organic matter (OM), nitrogen (N), and phosphorus (P) [63], the samples were analyzed at the Soil, Foliar, and Water Laboratory of the Ecuadorian Agro Agency with ISO 9001:2008 certification and parameters accredited under ISO/IEC 17025:2005 (Table 1). In addition, to obtain the bulk density attribute (BD), five more soil samples were taken from each plot using cylinders (250 cm3). The data obtained from these samples were used to construct a logistic regression (r2 = 0.33) relating OC concentrations to BD (g/cm3).

Table 1.
Parameters and methods used for soil analysis.
We calculated soil organic carbon (SOC) stock from the ratio of OM to the Van Bemmelen factor (1.724) [64,65] and bulk density (BD) estimation [47,66,67]. First, we applied the LOI (loss on ignition) method, widely used to estimate soil organic matter. A total of 20 g of each soil sample was dried in an oven for 24 h at 105 °C; the weight was recorded. Then, the samples were cooled in a desiccator and weighed with a range of ~5 g ± 0.5 g. These samples were added to porcelain capsules and allowed to ash for 3 h at 450 °C; the weight was again recorded. The calculation of OM was performed by weight difference at various temperatures according to the percentage of OM = (weight 105 °C − weight 450 °C)/weight 105 °C) × 100).
SOC stock kg/m2 = OC%/100BD kg/m3 0.15 m
2.2.2. Data Analysis
First, we obtained descriptive statistics for each soil attribute, pH, OM, N, P, and texture, with R Commander package of R software version 2.9-5 [68]. Then, the assumption of normality was verified for each variable. In cases in which the distribution was not normal, we applied the bcpower function of the R Car package [69,70], making the necessary adjustments to obtain a normal distribution of the data. Next, we applied an ANOVA to indicate significant changes (α = 0.05) in the soil’s physical and chemical attributes of the dynamics of land cover change [71].
In terms of spatial analysis, we ran and compared three interpolation methods to identify the dynamics of the spatial distribution of edaphic attributes in each of the analyzed cover categories: IDW, kriging, and cokriging [63,72,73]. The methods were undertaken using ArcMap 10.8.2 software. Cokriging (CK) and ordinary kriging (K), and experimental semi-variance adjusted to the following theoretical models was applied: spherical, circular, Gaussian, and linear. Two additional covariates were used in the CK method: altitude and slope, derived from a Digital Terrain Model with a 5 m resolution. In CK, the autocorrelation of Z1 as the main variable and the cross-correlation between Z1 and the other variables were used to make better predictions [74]. Once the models were applied, their statistical information was examined and plotted, and those with the lowest standard errors were selected.
2.2.3. Inventory and Forest Structure
To characterize the native forest, a plant inventory of individuals with a diameter at breast height ≥ 0.10 m was conducted. From the inventory, some structural parameters of the forest were obtained (Table 2): diameter structure, taxonomic richness, plant composition, relative density, relative dominance, relative diversity of family or genus, the Shannon–Wiener index, and the Simpson index [75,76,77].

Table 2.
Structural parameters of vegetation and species diversity.
3. Results
3.1. Soil Organic Carbon Stock and Soil Attribute Variations
Across the three land covers—montane forest, eucalyptus plantation, and grassland—soil pH values were in the moderately acidic range, with no significant differences among categories (Table 3). Soil texture was sandy loam in montane forest and loam in eucalyptus plantations and grasslands.

Table 3.
Mean, standard deviation of soil attributes associated with organic matter in different land covers.
Regarding spatial modeling, kriging and co-kriging methods provided the least biased predictions for OM, N, and P, as indicated by mean prediction errors close to zero and adequate standard error estimations (Table 4). These models allowed accurate representation of the spatial variability of soil attributes across land covers (Figure 2, Figure 3, Figure 4 and Figure 5).

Table 4.
Cross-validation statistics of CK and K interpolation for attributes OM, N, and P.
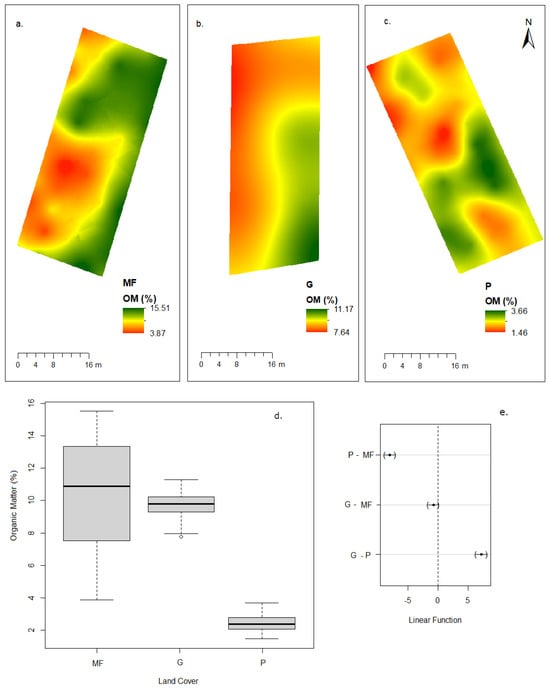
Figure 2.
Percentage concentrations of organic matter to land cover category. Spatial distribution; (a) montane forest, (b) grassland, (c) eucalyptus plantation (d) box plot, organic matter distribution by land cover category, and (e) differences in the mean levels of the groups (95% family-wise confidence level).
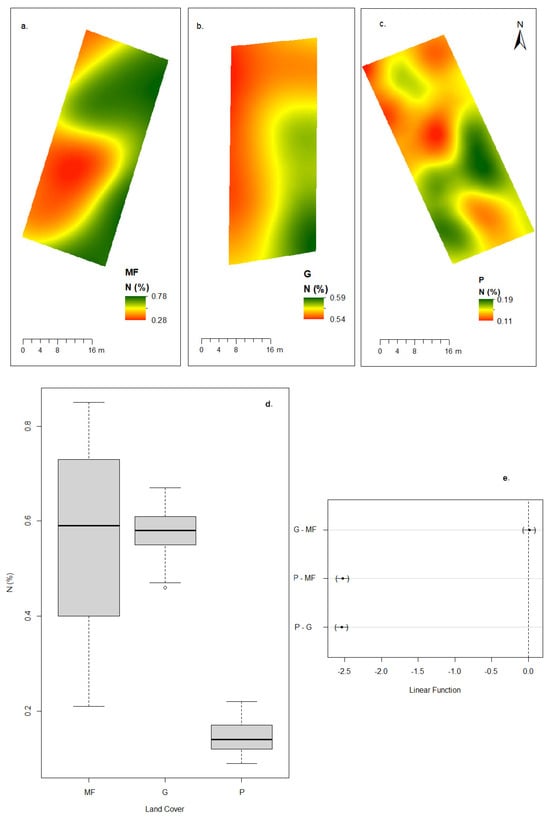
Figure 3.
N concentrations according to cover category. Spatial distribution: (a) montane forest, (b) grassland, (c) eucalyptus plantation, (d) box plot: nitrogen data distribution by land cover category, and (e) differences in mean levels of group (95% family-wise confidence level).
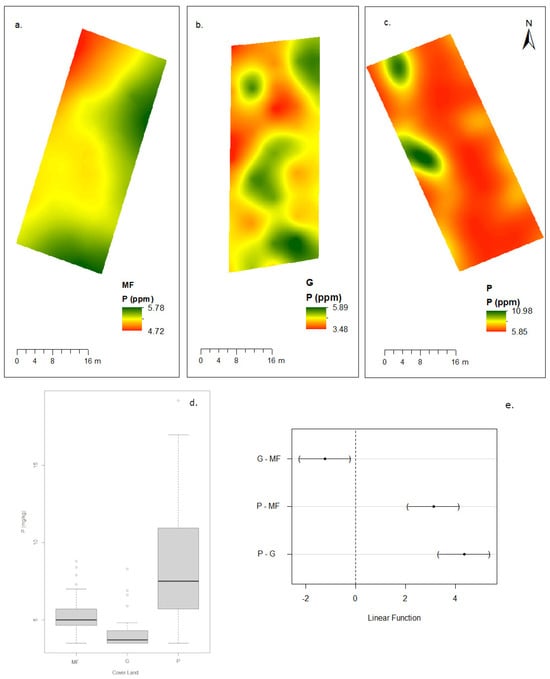
Figure 4.
Concentrations of P by land cover category. Spatial distribution: (a) montane forest, (b) grassland, (c) eucalyptus plantation, (d) box plot: phosphorus data distribution by land cover category, and (e) differences in the mean levels of the groups (95% family confidence level).
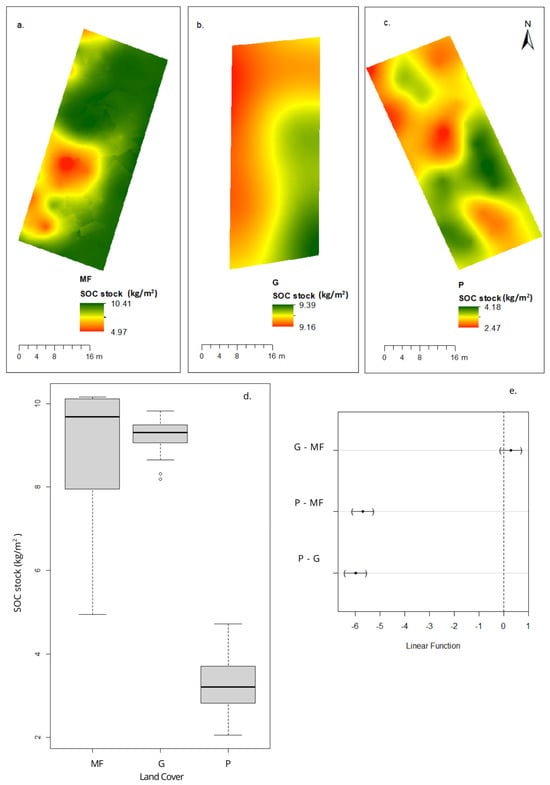
Figure 5.
Concentrations of soil organic carbon (SOC) stock by land cover category. Spatial distribution: (a) montane forest, (b) grassland, (c) eucalyptus plantation, (d) box plot: soil organic carbon stock distribution by land cover category, and (e) differences in the mean levels of the groups (95% family confidence level).
Significant differences were found in soil organic matter (OM), nitrogen (N), and phosphorus (P) contents, as well as in soil organic carbon (SOC) stock and bulk density (BD). Montane forest and grassland had similar OM levels (10.31% ± 1.94 and 9.64% ± 0.45, respectively), both significantly higher than eucalyptus plantations (2.42% ± 0.34). Nitrogen concentrations followed the same trend, with the highest values in grassland (0.58% ± 0.05) and montane forest (0.57% ± 0.18), and the lowest in eucalyptus plantations (0.14% ± 0.03). A strong positive relationship between OM and N was observed across covers, indicating that OM is the main source of N in these soils (Figure 2 and Figure 3).
Phosphorus showed an inverse pattern: eucalyptus plantations had the highest P content (8.42 ± 3.62 ppm) compared to montane forest (5.30 ± 1.08 ppm) and grassland (4.08 ± 0.93 ppm) (Figure 4). This difference is likely related to management practices and the chemical composition of litter. Bulk density was highest in eucalyptus plantations (1.20 ± 0.03 g/cm3), followed by grassland (0.81 ± 0.06 g/cm3) and montane forest (0.79 ± 0.09 g/cm3), with higher BD values corresponding to lower OM and SOC stock.
Soil organic carbon stock was highest in montane forest (9.69 ± 1.44 kg/m2), followed closely by grassland (9.31 ± 0.36 kg/m2), and was significantly reduced in eucalyptus plantations (3.27 ± 0.67 kg/m2) (Figure 5), representing a 66.3% decrease compared to native forest. This reduction is consistent with the combined effects of lower OM, reduced N, higher BD, and altered litter decomposition in eucalyptus monocultures.
3.2. Structure and Diversity of the Montane Forest
The montane forest plot contained 253 individuals belonging to 18 species, 14 genera, and 10 families (Table 5). Shrubs dominated the growth form composition (71.1%), with trees representing 28.9% of individuals. The diameter distribution followed an inverted “J” pattern (Figure 6), indicating active regeneration, with 60% of individuals having diameters < 12 cm.

Table 5.
Inventory of species located in the native forest plot.
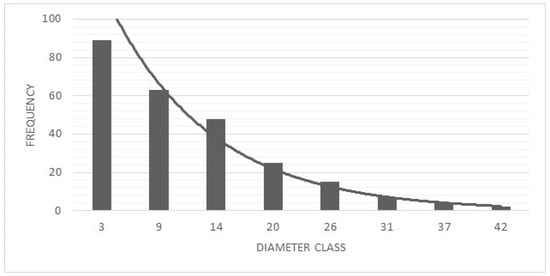
Figure 6.
Diametric structure of the montane forest fragment inventoried in the urban limits of southern Quito.
Asteraceae and Melastomataceae were the most species-rich families (four species each, 22.2%), followed by Campanulaceae and Myrtaceae (two species each, 11.1%). The most species-rich genus was Miconia (three species, 16.7%). The most abundant species were Brachyotum ledifolium (17.8%), Gynoxys campii (15%), and Baccharis latifolia (9.9%) (Table 5). In terms of dominance, Alnus acuminata contributed the largest basal area (27.3%), followed by Oreopanax ecuadorensis (8.8%) and Myrcianthes aff. discolor (8.4%).
Diversity indices reflected high species richness and evenness, with Shannon’s index (H′ = 2.51) and Simpson’s index (λ = 0.10). These values align with patterns reported for other Andean montane forests and confirm the conservation value of this fragment.
4. Discussion
4.1. Soil Organic Carbon Stock and Soil Attributes
The significant variation in SOC stock among the analyzed land covers is consistent with findings from other studies in the tropical Andes [2,48,55]. The substantial decrease in SOC stock observed when montane forest is replaced by Eucalyptus globulus plantations [78,79,80] is linked to reduced litter diversity, slower decomposition rates, and the emission of allelochemicals that inhibit germination and growth of other plant species, influencing organic inputs and nutrient mineralization [81]. In contrast, montane forests and grasslands maintained higher OM and N contents, supported by continuous organic matter inputs from diverse plant species, fine root turnover, and higher biological activity [82,83,84].
The higher bulk density in eucalyptus plantations indicates compaction and reduced porosity, leading to lower moisture retention [80,85], a pattern observed globally in monocultures [86,87,88]. The elevated P concentrations in eucalyptus plots are likely related to fertilization amendments [89] and the contribution of organic acids from eucalyptus litter [90,91], which can increase P availability.
From a management perspective, these results suggest that large-scale replacement of native montane forest with Eucalyptus globulus plantations is not advisable in the Andean context due to SOC stock losses (66.3%), increased BD, and declines in soil fertility. While eucalyptus can be used for reforestation in severely degraded areas with low natural regeneration potential [92,93], its high water demand and reduced contribution to soil quality make it unsuitable for high-biodiversity montane regions. Restoration of degraded peri-urban soils should prioritize native species or mixed-species plantations that maintain soil structure, fertility, and carbon storage capacity.
4.2. Montane Forest
The floristic composition and structure of the montane forest fragment align with studies from southern Ecuador [94,95] and global tropical montane forests [96,97,98], showing high biodiversity and ecosystem service potential. The inverted “J” diameter distribution [14,99,100] reflects regeneration dynamics typical of disturbed forests, with a high proportion of individuals in smaller diameter classes and fewer large trees, indicating ongoing recruitment and reflecting the high pressure or disturbance in the region by urban expansion.
Families such as Asteraceae, Melastomataceae, and Campanulaceae, which were the most species-rich in our study, are recognized for their wide altitudinal distribution and high number of endemic species in the Andes [101,102,103]. These families also contribute substantially to the flora of other Andean countries [100,104]. The detection of Gynoxys campii, classified as endangered [15], underscores the conservation value of this fragment and its potential role as a biodiversity reservoir within Quito’s green belt [105,106,107].
Given this ecological significance, conservation strategies should focus on protecting remaining montane forest fragments and restoring degraded areas within the urban–natural interface. Integrating these fragments into Quito’s urban planning framework would help preserve biodiversity, maintain ecological connectivity, and ensure the continued provision of ecosystem services.
4.3. Interrelationship Between Vegetation and Soil
The structural and compositional attributes of montane forests directly influence soil properties. Dense canopy cover, diverse litterfall, and extensive root systems enhance OM accumulation, N availability, and SOC stock, while maintaining low bulk density [108]. Grasslands maintain relatively high OM and N contents through rapid root turnover and soil cover that reduces erosion [83,84], but may not match the long-term carbon storage potential of intact forests [109,110,111].
In contrast, eucalyptus monocultures, with uniform canopy structure and chemically distinctive litter, reduce the quantity and diversity of organic inputs, leading to declines in OM, N, and SOC stock, and increasing BD [84,85,91]. This relationship between vegetation composition and soil attributes has been documented in other Andean montane ecosystems [112,113,114], reinforcing the importance of maintaining plant diversity for sustaining soil health.
To address these challenges, peri-urban agroforestry systems [115,116,117] and mixed-species plantations offer viable alternatives to conventional monocultures. These approaches can sustain both soil quality and vegetation diversity, while providing ecosystem services such as water regulation, carbon sequestration, and biodiversity conservation. In the Andean context, they represent a strategic opportunity to reconcile productive land use with ecological sustainability.
5. Conclusions
Land use and land cover change from montane forest fragments to other transitional land covers, due to the expansion of predominantly urban matrix landscapes, impacts the structure and function of significant natural montane ecosystems. This, in turn, affects the provision of ecosystem goods and services for cities. These changes also reduce the SOC stock potential of montane forest soils, thus limiting nature-based solution strategies for climate change adaptation and mitigation in urban mountain environments.
Our study determined that montane soils undergo a 66% reduction in carbon storage capacity when they lose their natural cover and are replaced by eucalyptus forest plantations. This finding elucidates the loss of soil’s ecological functions that affect biogeochemical cycles. Such a loss impacts the potential of these ecosystems to mitigate climate risk in Andean cities.
Accordingly, urban planning strategies in Andean cities should consider an urban–rural natural gradient approach. This approach should evaluate and address a sustainable city growth model on a case-by-case basis, integrating strategies for the conservation of peri-urban natural areas and the restoration of areas subject to land cover changes that do not enhance sustainability. Limiting such changes is essential to ensure the continued use of nature-based solutions to mitigate climate risks.
In this context, urban and peri-urban agroforestry systems based on ecosystem engineering represent an opportunity to replace and restore conventional forest plantation or cropping systems in the peri-urban areas of the Andes. These systems can support and improve the availability of ecosystem goods and services for urban dwellers, thereby contributing to their well-being and livelihood.
Author Contributions
Conceptualization, S.B.-B.; methodology, S.B.-B., K.U. and L.S.-C.; software, S.B.-B. and K.U.; validation, K.U. and L.S.-C.; formal analysis, S.B.-B. and K.U.; investigation, S.B.-B., L.S.-C. and J.R.M.; resources, S.B.-B., J.R.M. and G.F.S.; data curation, S.B.-B., K.U. and L.S.-C.; writing—original draft preparation, K.U. and S.B.-B.; writing—review and editing, K.U. and S.B.-B.; visualization, K.U. and S.B.-B.; supervision, S.B.-B., J.R.M. and G.F.S.; project administration, S.B.-B.; funding acquisition, S.B.-B., J.R.M. and G.F.S. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Universidad Indoamérica.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Angel, S.; Civco, D. The Persistent Decline in Urban Densities: Global and Historical Evidence of “Sprawl”. Lincoln Institute of Land Policy. 2010. Available online: https://www.lincolninst.edu/app/uploads/2024/04/1834_1085_angel_final_1.pdf (accessed on 25 March 2025).
- Bonilla-Bedoya, S.; Estrella, A.; Santos, F.; Herrera, M.Á. Forests and Urban Green Areas as Tools to Address the Challenges of Sustainability in Latin American Urban Socio-Ecological Systems. Appl. Geogr. 2020, 125, 102343. [Google Scholar] [CrossRef]
- Seto, K.C.; Fragkias, M.; Güneralp, B.; Reilly, M.K. A Meta-Analysis of Global Urban Land Expansion. PLoS ONE 2011, 6, e23777. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B. Urban Growth in Developing Countries: A Review of Current Trends and a Caution Regarding Existing Forecasts. World Dev. 2004, 32, 23–51. [Google Scholar] [CrossRef]
- United Nations. World Population Prospects 2019 Highlights; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Acuto, M.; Parnell, S.; Seto, K.C. Building a Global Urban Science. Nat. Sustain. 2018, 1, 2–4. [Google Scholar] [CrossRef]
- Angel, S.; Parent, J.; Civco, D.L.; Blei, A.; Potere, D. The Dimensions of Global Urban Expansion: Estimates and Projections for All Countries, 2000–2050. Prog. Plan. 2011, 75, 53–107. [Google Scholar] [CrossRef]
- Angel, S.; Sheppard, S.; Civco, D. The Dynamics of Global Urban Expansion; Transport and Urban Development Department, The World Bank: Washington, DC, USA, 2005. [Google Scholar]
- Seto, K.C.; Reenberg, A.; Boone, C.G.; Fragkias, M.; Haase, D.; Langanke, T.; Marcotullio, P.; Munroe, D.K.; Olah, B.; Simon, D. Urban Land Teleconnections and Sustainability. Proc. Natl. Acad. Sci. USA 2012, 109, 7687–7692. [Google Scholar] [CrossRef]
- UN-Habitat. Urbanization and Development—Emerging Futures. 2016. Available online: https://unhabitat.org/world-cities-report-2016 (accessed on 1 May 2022).
- Sist, P.; Mazzei, L.; Blanc, L.; Rutishauser, E. Large Trees as Key Elements of Carbon Storage and Dynamics after Selective Logging in the Eastern Amazon. For. Ecol. Manag. 2014, 318, 103–109. [Google Scholar] [CrossRef]
- Laurance, W.F.; Sayer, J.; Cassman, K.G. Agricultural Expansion and Its Impacts on Tropical Nature. Trends Ecol. Evol. 2014, 29, 107–116. [Google Scholar] [CrossRef]
- Achard, F.; Eva, H.D.; Stibig, H.-J.; Mayaux, P.; Gallego, J.; Richards, T.; Malingreau, J.-P. Determination of Deforestation Rates of the World’s Humid Tropical Forests. Science 2002, 297, 999–1002. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat Fragmentation and Its Lasting Impact on Earth’s Ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef]
- IUCN. World Conservation Congress 2020; IUCN: Gland, Switzerland, 2020. [Google Scholar] [CrossRef]
- Bruijnzeel, L.A. Hydrological Functions of Tropical Forests: Not Seeing the Soil for the Trees? Agric. Ecosyst. Environ. 2004, 104, 185–228. [Google Scholar] [CrossRef]
- Sidle, R.C.; Ziegler, A.D.; Negishi, J.N.; Nik, A.R.; Siew, R.; Turkelboom, F. Erosion Processes in Steep Terrain—Truths, Myths, and Uncertainties Related to Forest Management in Southeast Asia. For. Ecol. Manag. 2006, 224, 199–225. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The Vertical Distribution of Soil Organic Carbon and Its Relation to Climate and Vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Taušnerová, H.; Hanč, A.; Tlustoš, P. Stabilization of Different Starting Materials through Vermicomposting in a Continuous-Feeding System: Changes in Chemical and Biological Parameters. Waste Manag. 2017, 62, 33–42. [Google Scholar] [CrossRef]
- Bruijnzeel, L.A.; Kappelle, M.; Mulligan, M.; Scatena, F.N. Tropical Montane Cloud Forests: State of Knowledge and Sustainability Perspectives in a Changing World. In Tropical Montane Cloud Forests: Science for Conservation and Management; Cambridge University Press: Cambridge, UK, 2010; pp. 691–740. [Google Scholar] [CrossRef]
- Castilho-Balbinot, L.; Marques, R.; Tonello, K.C.; Berguetti, Á.L.P.; Larsen, J.G. Recent insights in soil nutrient cycling: Perspectives from forests of Pinus and Eucalyptus species. iForest 2024, 17, 394–404. [Google Scholar] [CrossRef]
- Carrión-Paladines, V.; Crespo, P.; Buytaert, W.; Célleri, R. Conversion of Andean montane forest to exotic forest plantation modifies soil physicochemical properties in the buffer zone of Ecuador’s Podocarpus National Park. Sci. Rep. 2022, 12, 20644. [Google Scholar] [CrossRef]
- Ortiz, J.; Panichini, M.; Neira, P.; Henríquez-Castillo, C.; Jara, R.E.G.; Rodriguez, R.; Mutis, A.; Ramos, C.; Espejo, W.; Puc-Kauil, R.; et al. How Natural Regeneration After Severe Disturbance Affects Ecosystem Services Provision of Andean Forest Soils at Contrasting Timescales. Forests 2025, 16, 456. [Google Scholar] [CrossRef]
- Berthrong, S.T.; Jobbágy, E.G.; Jackson, R.B. A global meta-analysis of soil exchangeable cations, pH, carbon, and nitrogen with afforestation. Ecol. Appl. 2009, 19, 2228–2241. [Google Scholar] [CrossRef] [PubMed]
- Marian, F.; Castillo, P.R.; Armijos, C.I.; Günter, S.; Maraun, M.; Scheu, S. Conversion of Andean montane forests into plantations: Effects on litter layer thickness, pH, water content, and C-to-N ratio. Biotropica 2020, 52, 1142–1154. [Google Scholar] [CrossRef]
- Wang, N.; Xia, L.; Goodale, C.L.; Butterbach-Bahl, K.; Kiese, R. Climate Change Can Accelerate Depletion of Montane Grassland Carbon. Glob. Biogeochem. Cycles 2021, 35, e2020GB006792. [Google Scholar] [CrossRef]
- Jiang, Z.; Ma, H.; Li, S.; Zheng, C.; Bai, E. Grasslands contain approximately 525 Pg C, which accounts for 16% to 19% of total organic carbon in terrestrial ecosystems. Ecol. Process. 2025, 14, 54. [Google Scholar] [CrossRef]
- Dube, T.; Chiduza, C.; Muchaonyerwa, P. Effect of management strategies on soil organic carbon fractions and stocks in grasslands: A review. J. Soils Sediments 2014, 14, 1581–1595. [Google Scholar]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Change Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Buyantuyev, A.; Wu, J. Urban heat islands and landscape heterogeneity: Linking spatiotemporal variations in surface temperatures to land-cover and socioeconomic patterns. Landsc. Ecol. 2010, 25, 17–33. [Google Scholar] [CrossRef]
- Li, G.; Cao, Y.; He, Z.; He, J.; Cao, Y.; Wang, J.; Fang, X. Understanding the Diversity of Urban–Rural Fringe Development in a Fast Urbanizing Region of China. Remote Sens. 2021, 13, 2373. [Google Scholar] [CrossRef]
- Santamouris, M. Regulating the damaged thermostat of the cities—Status, impacts and mitigation challenges. Energy Build. 2015, 91, 43–56. [Google Scholar] [CrossRef]
- Turner, R.K.; Daily, G.C. The Ecosystem Services Framework and Natural Capital Conservation. Environ. Resour. Econ. 2008, 39, 25–35. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Yuan, D.; Cui, J.; Li, Y.; Yang, J.; Cao, M. Source and Flux of Anthropogenically Enhanced Dissolved Inorganic Carbon: A Comparative Study of Urban and Forest Karst Catchments in Southwest China. Sci. Total Environ. 2020, 725, 138255. [Google Scholar] [CrossRef] [PubMed]
- Creutzig, F.; Agoston, P.; Minx, J.C.; Canadell, J.G.; Andrew, R.M.; le Quéré, C.; Peters, G.P.; Sharifi, A.; Yamagata, Y.; Dhakal, S. Urban Infrastructure Choices Structure Climate Solutions. Nat. Clim. Change 2016, 6, 1054–1056. [Google Scholar] [CrossRef]
- Kennedy, C.; Steinberger, J.; Gasson, B.; Hansen, Y.; Hillman, T.; Havránek, M.; Pataki, D.; Phdungsilp, A.; Ramaswami, A.; Mendez, G.V. Greenhouse Gas Emissions from Global Cities. Environ. Sci. Technol. 2009, 43, 7297–7302. [Google Scholar] [CrossRef]
- EEA. EMEP/EEA Air Pollutant Emission Inventory Guidebook 2019: Technical Guidance to Prepare National Emission Inventories; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Zhang, Q.; Zheng, Y.; Tong, D.; Shao, M.; Wang, S.; Zhang, Y.; Xu, X.; Wang, J.; He, H.; Liu, W.; et al. Drivers of Improved PM2.5 Air Quality in China from 2013 to 2017. Proc. Natl. Acad. Sci. USA 2019, 116, 24463–24469. [Google Scholar] [CrossRef]
- Liu, S.; Deng, Y.; Jiang, Z.; Wu, Y.; Huang, X.; Macreadie, P.I. Nutrient Loading Diminishes the Dissolved Organic Carbon Drawdown Capacity of Seagrass Ecosystems. Sci. Total Environ. 2020, 740, 140185. [Google Scholar] [CrossRef]
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.E.; Qin, B.; Scott, J.T. Mitigating Cyanobacterial Harmful Algal Blooms in Aquatic Ecosystems Impacted by Climate Change and Anthropogenic Nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef]
- Konrad, C.P.; Olden, J.D.; Lytle, D.A.; Melis, T.S.; Schmidt, J.C.; Bray, E.N.; Freeman, M.C.; Gido, K.B.; Hemphill, N.P.; Kennard, M.J.; et al. Large-Scale Flow Experiments for Managing River Systems. BioScience 2011, 61, 948–959. [Google Scholar] [CrossRef]
- Pickett, S.T.A.; Cadenasso, M.L.; Grove, M.; Groffman, P.M.; Band, L.E.; Boone, C.G.; Burch, W.R.; Grimmond, S.B.; Hom, J.; Jenkins, J.; et al. Beyond Urban Legends: An Emerging Framework of Urban Ecology, as Illustrated by the Baltimore Ecosystem Study. BioScience 2008, 58, 139–150. [Google Scholar] [CrossRef]
- Gao, Y.; Jia, Y.; Yu, G.; He, N.; Zhang, L.; Zhu, B.; Wang, Y. Anthropogenic Reactive Nitrogen Deposition and Associated Nutrient Limitation Effect on Gross Primary Productivity in Inland Water of China. J. Clean. Prod. 2019, 208, 530–540. [Google Scholar] [CrossRef]
- Ma, T.; Sun, S.; Fu, G.; Hall, J.W.; Ni, Y.; He, L.; Yi, J.; Zhao, N.; Du, Y.; Pei, T.; et al. Pollution Exacerbates China’s Water Scarcity and Its Regional Inequality. Nat. Commun. 2020, 11, 536. [Google Scholar] [CrossRef]
- Walsh, C.J.; Roy, A.H.; Feminella, J.W.; Cottingham, P.D.; Groffman, P.M.; Morgan, R.P. The Urban Stream Syndrome: Current Knowledge and the Search for a Cure. J. N. Am. Benthol. Soc. 2005, 24, 706–723. [Google Scholar] [CrossRef]
- Bonilla-Bedoya, S.; Mora, A.; Vaca, A.; Estrella, A.; Herrera, M.Á. Modelling the Relationship between Urban Expansion Processes and Urban Forest Characteristics: An Application to the Metropolitan District of Quito. Comput. Environ. Urban Syst. 2019, 79, 101420. [Google Scholar] [CrossRef]
- Bonilla-Bedoya, S.; Herrera, M.Á.; Vaca, A.; Salazar, L.; Zalakeviciute, R.; Mejía, D.; López-Ulloa, M. Urban Soil Management in the Strategies for Adaptation to Climate Change of Cities in the Tropical Andes. Geoderma 2022, 417, 115840. [Google Scholar] [CrossRef]
- Lal, R. Digging Deeper: A Holistic Perspective of Factors Affecting Soil Organic Carbon Sequestration in Agroecosystems. Glob. Change Biol. 2018, 24, 3285–3301. [Google Scholar] [CrossRef] [PubMed]
- Montanarella, L.; Pennock, D.J.; McKenzie, N.; Badraoui, M.; Chude, V.; Baptista, I.; Mamo, T.; Yemefack, M.; Aulakh, M.S.; Yagi, K.; et al. World’s Soils Are Under Threat. Soil 2016, 2, 79–82. [Google Scholar] [CrossRef]
- SHAH. Informe Nacional del Ecuador Tercera Conferencia de las Naciones Unidas sobre la Vivienda y el Desarrollo Urbano Sostenible Habitat III; Ministerio de Desarrollo Urbano y Vivienda: Quito, Ecuador, 2015.
- MIDUVI. Agenda Hábitat Sostenible 2036; Ministerio de Desarrollo Urbano y Vivienda: Quito, Ecuador, 2020.
- Forman, R.T.T. Urban Ecology; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Bonilla-Bedoya, S.; Estrella, A.; Vaca Yánez, A.; Herrera, M.Á. Urban Socio-Ecological Dynamics: Applying the Urban-Rural Gradient Approach in a High Andean City. Landsc. Res. 2020, 45, 327–345. [Google Scholar] [CrossRef]
- Bonilla-Bedoya, S.; Zalakeviciute, R.; Coronel, D.M.; Durango-Cordero, J.; Molina, J.R.; Macedo-Pezzopane, J.E.; Herrera, M.Á. Spatiotemporal Variation of Forest Cover and Its Relation to Air Quality in Urban Andean Socio-Ecological Systems. Urban For. Urban Green. 2021, 59, 127008. [Google Scholar] [CrossRef]
- McDonald, R.I.; Mansur, A.V.; Ascensão, F.; Colbert, M.; Crossman, K.; Elmqvist, T.; Gonzalez, A.; Güneralp, B.; Haase, D.; Hamann, M.; et al. Research gaps in knowledge of the impact of urban growth on biodiversity. Nat. Sustain. 2020, 3, 16–24. [Google Scholar] [CrossRef]
- Carrión, F.; Erazo Espinosa, J. La Forma Urbana de Quito: Una Historia de Centros y Periferias. Bulletin de l’Institut Français d’études Andines 2012, 41, 503–522. [Google Scholar] [CrossRef]
- Carrera, M.; Bustamante, M.; Sáenz, M. Las Áreas Protegidas del Distrito Metropolitano de Quito. 2016. Available online: https://condesan.org/wp-content/uploads/2017/07/Libro1.pdf (accessed on 1 July 2022).
- Secretaría de Ambiente. Plan Estratégico del Área de Intervención Especial y Recuperación del Pichincha-Atacazo y Bosque Protector Flanco Oriental del Volcán Pichincha; Secretaría de Ambiente: Quito, Ecuador, 2012. [Google Scholar]
- Instituto Nacional de Meteorología e Hidrología. Anuario Meteorológico; INAMHI: Quito, Ecuador, 2017. [Google Scholar]
- Sanchez, Z. Los Bosques del Ecuador. 2020. Available online: https://www.academia.edu/44163908/LOS_BOSQUES_DEL_ECUADOR (accessed on 23 January 2022).
- Ross, C.E.; Munro, N.T.; Barton, P.S.; Evans, M.J.; Gillen, J.; Macdonald, B.C.T.; McIntyre, S.; Cunningham, S.A.; Manning, A.D. Effects of Digging by a Native and Introduced Ecosystem Engineer on Soil Physical and Chemical Properties in Temperate Grassy Woodland. PeerJ 2019, 7, e7506. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; Agriculture Handbook No. 436; U.S. Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 1999.
- Bonilla-Bedoya, S.; López-Ulloa, M.; Vanwalleghem, T.; Herrera-Machuca, M.Á. Effects of Land Use Change on Soil Quality Indicators in Forest Landscapes of the Western Amazon. Soil Sci. 2017, 182, 128–136. [Google Scholar] [CrossRef]
- Dabadie, M.; Pérez, C.; Arturi, M.; Goya, J.; Sandoval, M. Calibración del Método de Pérdida de Peso por Ignición para la Estimación de Carbono Orgánico en Inceptisoles del NE de Entre Ríos. Rev. Fac. Agron. 2018, 117, 157–162. [Google Scholar]
- Heaton, L.; Fullen, M.A.; Bhattacharyya, R., II. Critical Analysis of the Van Bemmelen Conversion Factor Used to Convert Soil Organic Matter Data to Soil Organic Carbon Data: Comparative Analyses in a UK Loamy Sand Soil. Espaço Abierto 2016, 1, 35–44. [Google Scholar] [CrossRef]
- Barančíková, G.; Halás, J.; Gutteková, M.; Makovníková, J.; Nováková, M.; Skalský, R.; Tarasovičová, Z. Application of Roth C Model to Predict Soil Organic Carbon Stock on Agricultural Soils of Slovakia. Soil Water Res. 2010, 5, 1–9. [Google Scholar] [CrossRef]
- Dincă, L.C.; Dincă, M.; Vasile, D.; Spârchez, G.; Holonec, L. Calculating Organic Carbon Stock from Forest Soils. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 568–575. [Google Scholar] [CrossRef][Green Version]
- Fox, J.; Marquez, M.M.; Bouchet-Valat, M. Rcmdr: R Commander. R Package Version 2.9-5. 2024. Available online: https://github.com/RCmdr-Project/rcmdr (accessed on 27 May 2023).
- Fox, J.S.; Weisberg, D.; Adler, D.; Bates, G.; Boud-Bovy, S.; Ellison, M.; Friendly, M. R-Package “car”. Version 3.1-2. 2015. Available online: https://cran.r-project.org/web/packages/car/index.html (accessed on 27 May 2023).
- Yeo, I.; Johnson, R.A. A New Family of Power Transformations to Improve Normality or Symmetry. Biometrika 2000, 87, 949–959. Available online: http://biomet.oxfordjournals.org/ (accessed on 27 May 2023). [CrossRef]
- Bonilla-Bedoya, S.; Lugo-Salinas, L.; Mora-Garcés, A.; Villarreal, A.; Arends, E.; Herrera, M. Piaroa Shifting Cultivation: Temporal Variability of Soil Characteristics and Spatial Distribution of Crops in the Venezuelan Orinoco. Agrofor. Syst. 2013, 87, 1189–1199. [Google Scholar] [CrossRef]
- Mulla, D.J.; McBratney, A.B. Soil Spatial Variability; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Lozano, Z.; Bravo, C.; Ovalles, F.; Hernández, R.M.; Moreno, B.; Piñango, L.; Villanueva, J.G. Selección de un Diseño de Muestreo en Parcelas Experimentales a Partir del Estudio de la Variabilidad Espacial de los Suelos. Bioagro 2004, 16, 61–72. [Google Scholar]
- Rossiter, D. Technical Note: Co-kriging with the gstat Package of the R Environment for Statistical Computing. 2012. Available online: https://www.css.cornell.edu/faculty/dgr2/_static/files/R_PDF/CoKrigeR.pdf (accessed on 27 May 2023).
- Aguirre, Z.; Reyes, B.; Quizhpe, W.; Cabrera, A. Composición Florística, Estructura y Endemismo del Componente Leñoso de un Bosque Montano en el Sur del Ecuador. Arnaldoa 2017, 24, 543–556. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004. [Google Scholar]
- Chave, J. Measuring Wood Density for Tropical Forest Trees: A Field and Analysis Manual. Global Wood Density Database. 2005. Available online: https://afritron.org/upload/en/manuals/wood_density_english[1].pdf (accessed on 30 June 2025).
- Don, A.; Schumacher, J.; Freibauer, A. Impact of tropical land-use change on soil organic carbon stocks—A meta-analysis. Glob. Change Biol. 2011, 17, 1658–1670. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Carbon sequestration in agricultural soils via cultivation of cover crops—A meta-analysis. Agric. Ecosyst. Environ. 2015, 200, 33–41. [Google Scholar] [CrossRef]
- Chen, H.; Tian, D.; Liu, X.; Fang, J. Impacts of land-use change on soil organic carbon stocks: A global synthesis. Glob. Change Biol. 2022, 28, 4743–4756. [Google Scholar] [CrossRef]
- Hofstede, R.; Groenendijk, J.P.; Coppus, R.; Fehse, J.C.; Sevink, J. Impact of Pine Plantations on Soils and Vegetation in the Ecuadorian High Andes. Mt. Res. Dev. 2002, 22, 159–167. [Google Scholar] [CrossRef]
- Najera González, O.; Murray Núñez, R.M.; Orozco Benitez, M.G.; Bojorquez Serrano, J.I. Cambios en Carbono Orgánico en Suelos Cambisoles, Solonetz y Arenosoles. Rev. Iberoam. Cienc. Biol. Agropecu. 2015, 2007, 9990. [Google Scholar]
- Rosero, J.D.M.; Rincón, E.C.; Oviedo, F.H.; López, P.A.P.; Pastrana, Á.M.C. Cultivo y Ensilaje de Avena (Avena sativa L.) en el Trópico Alto del Departamento de Nariño; AGROSAVIA Editorial: Nariño, Colombia, 2022. [Google Scholar]
- Otero, J.D.; Figueroa, A.; Muñoz, F.A.; Peña, M.R. Loss of soil and nutrients by surface runoff in two agro-ecosystems within an Andean paramo area. Ecol. Eng. 2011, 37, 2035–2043. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Ma, J.; Chen, H.; Lai, R. Global patterns and drivers of soil organic carbon change following forest conversion to agriculture. Glob. Change Biol. 2023, 29, 2413–2428. [Google Scholar] [CrossRef]
- Quichimbo, P.; Tenorio, G.; Borja, P.; Cárdenas, I.; Crespo, P.; Célleri, R. Efectos sobre las propiedades físicas y químicas de los suelos por el cambio de la cobertura vegetal y uso del suelo: Páramo de Quimsacocha al sur del Ecuador. Suelos Ecuat. 2012, 42, 138–153. [Google Scholar]
- Vigo, C.; Oclocho, F. Influencia de las Plantaciones de Eucalipto (Eucalyptus globulus) en las Características del suelo a Diferentes Pisos Altitudinales, Distritos de Magdalena, Tingo y San Isidro del Maino, Amazonas; Universidad Nacional Toribio Rodríguez de Mendoza de Amazonas: Amazonas, Peru, 2017; Available online: https://repositorio.untrm.edu.pe/handle/20.500.14077/1195 (accessed on 30 May 2023).
- Hernández, A.; Vera, L.; Naveda, C.A.; Guzmán, Á.M.; Vivar, M.; Zambrano, T.; López Alava, G.A. Variaciones en Algunas Propiedades del Suelo por el Cambio de Uso de la Tierra, en las Partes Media y Baja de la Microcuenca Membrillo, Manabí, Ecuador. Cult. Trop. 2017, 38, 50–56. [Google Scholar]
- Cantera, B.; Ihlenfeld, S. Efecto de la Fertilización y Aplicación de Bioestimulantes en el Desarrollo Inicial de Plantaciones de Eucalyptus globulus Sobre Suelos de Lavalleja. 2014. Available online: https://www.colibri.udelar.edu.uy/jspui/handle/20.500.12008/8808 (accessed on 5 May 2023).
- Behan, M. Soil-Plant-Water Relationships; FACE Foundation: Arnhem, The Netherlands, 1992. [Google Scholar]
- Fox, T.R. The Influence of Low-Molecular-Weight Organic Acids on Properties and Processes in Forest Soils. In Carbon Forms and Functions in Forest Soils; Soil Science Society of America: Madison, WI, USA, 1995; pp. 43–62. [Google Scholar]
- Anón. Eucalypts: Curse or Cure? In The Impacts of Australia’s World Tree in Other Countries; Australian Centre for International Agricultural Research (ACIAR): Canberra, Australia, 1992. [Google Scholar]
- Cordero-Rivera, A.; Martínez Álvarez, A.; Álvarez, M. Eucalypt Plantations Reduce the Diversity of Macroinvertebrates in Small Forested Streams. 2017. Available online: https://museucienciesjournals.cat/en/abc/issue/40-1-2017-abc/eucalypt-plantations-reduce-the-diversity-of-macroinvertebrates-in-small-forested-streams?lang=en (accessed on 15 May 2023).
- Cuvi, M.; Caranqui, J. Estudio de la Diversidad Florística en Diferente Gradiente Altitudinal en el Bosque Montano Alto Llucud, Cantón Chambo, Provincia de Chimborazo. Bachelor’s Thesis, Escuela Superior Politécnica de Chimborazo, Facultad de Recursos Naturales, Escuela de Ingeniería Forestal, Riobamba, Ecuador, 2010. Available online: https://rraae.cedia.edu.ec/vufind/Record/ESPOCH_dc3abdbb60c64b91875ab8a9a482c4ea?sid=5967454 (accessed on 1 July 2023).
- Homeier, J.; Englert, F.; Leuschner, C.; Weigelt, P.; Unger, M. Factors Controlling the Abundance of Lianas along an Altitudinal Transect of Tropical Forests in Ecuador. Forest Ecol. Manag. 2010, 259, 1399–1405. [Google Scholar] [CrossRef]
- Rahbek, C.; Borregaard, M.K.; Colwell, R.K.; Dalsgaard, B.; Holt, B.G.; Morueta-Holme, N.; Nogues-Bravo, D.; Whittaker, R.J.; Fjeldså, J. Humboldt’s enigma: What causes global patterns of mountain biodiversity? Science 2019, 365, 1108–1113. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Grace, J.; Mitchard, E.T.A.; Gloor, E. Perturbations in the carbon budget of the tropics. Glob. Change Biol. 2014, 20, 3238–3255. [Google Scholar] [CrossRef]
- Arturi, M.F.; Grau, H.R.; Aceñolaza, P.G.; Brown, A.D. Estructura y sucesión en bosques montanos del Noroeste de Argentina. Rev. Biol. Trop. 1998, 46, 525–532. [Google Scholar] [CrossRef]
- Burga-Cieza, A.M.; Burga Cieza, J.; Iglesias-Osores, S.; Alcalde-Alfaro, V.W.; Martínez-Sovero, G.; Dávila-Estela, L.; Villena-Velásquez, J.J. Estructura, diversidad y endemismo de la flora del relicto Los Lanches del bosque montano Las Palmas, Cajamarca, Perú. Cienc. Amaz. (Iquitos) 2021, 9, 43–58. [Google Scholar] [CrossRef]
- León-Yánez, S.; Valencia, R.; Pitman, N.; Endara, L.; Ulloa, C.; Navarrete, H. Libro Rojo de las Plantas Endémicas del Ecuador, 2nd ed.; Herbario QCA, Pontificia Universidad Católica del Ecuador: Quito, Ecuador, 2011. [Google Scholar]
- Curipoma, S.; Cevallos, D.; Pérez, Á.J. Composición y estructura florística de dos remanentes de Bosque Andino Montano Alto en el volcán Ilaló, Ecuador. Rev. Ecuat. Med. Cienc. Biol. 2018, 39, 93–104. [Google Scholar] [CrossRef]
- Haro, M.S. Estudio de regeneración natural en tres sitios de bosque montano en la reserva geobotánica Pululahua, Pichincha, Ecuador. Rev. Ecol. Trop. 2018, 45, 123–145. [Google Scholar]
- Farfan-Rios, W.; Garcia-Cabrera, K.; Salinas, N.; Raurau-Quisiyupanqu, M.N.; Silman, M.R. An annotated checklist of trees and relatives in tropical montane forests from southeast Peru: The importance of continue collecting. Rev. Peru Biol. 2015, 22, 145–174. [Google Scholar] [CrossRef]
- Pinto, E.; Pérez, A.J.; Ulloa Ulloa, C.; Cuesta, F. Árboles Representativos de los Bosques Montanos del Noroccidente de Pichincha, Ecuador; Consorcio para el Desarrollo Sostenible de la Ecorregión Andina (CONDESAN): Quito, Ecuador, 2018. [Google Scholar]
- Vistín-Guamantaqui, D.; Espinoza-Castillo, D.D. Estructura y Diversidad de Especies Arbóreas del Bosque Siempreverde Montano Alto del Parque Nacional Sangay-Ecuador. Dominio Cienc. 2021, 7, 1406–1430. [Google Scholar]
- Burga-Cieza, A.M.; Burga-Cieza, J.J.; Alcalde-Alfaro, V.W.; Martínez-Sovero, G.; Iglesias-Osores, S.; Villena-Velásquez, J.J. Floristic Characterization of the Los Lanches Relict of the Montane Forest Las Palmas Chota, Peru. SciELO Prepr. 2020. [Google Scholar] [CrossRef]
- Tonneijck, F.H.; Jansen, B.; Nierop, K.G.J.; Verstraten, J.M.; Sevink, J.; de Lange, L. Towards understanding of carbon stocks and stabilization in volcanic ash soils in natural Andean ecosystems of northern Ecuador. Eur. J. Soil Sci. 2010, 61, 392–405. [Google Scholar] [CrossRef]
- Dilas-Jiménez, J.O.; Huamán Jiménez, A.O. Captura de carbono por un bosque montano de neblina del Perú. Alpha Centauri 2020, 1, 13–25. [Google Scholar] [CrossRef]
- Guallpa-Calva, M.Á.; Guadalupe-Arias, O.B.; Rosero-Haro, S.C.; Morocho-Lema, V.M. Carbono Almacenado en el Suelo de dos Sistemas de Uso de la Tierra de la Reserva Huayrapalte. 2019. Available online: https://dominiodelasciencias.com/ojs/index.php/es/article/view/1082 (accessed on 1 July 2023).
- Álvarez-Arteaga, G.; García Calderón, N.E.; Krasilnikov, P.; García-Oliva, F. Almacenes de carbono en bosques montanos de niebla de la Sierra Norte de Oaxaca. Agrociencia 2013, 47, 171–180. [Google Scholar]
- Jiménez, E. Composición y Estructura de una Hectárea de Bosques en la Cordillera del Paso Alto, San José de Minas, Pichincha-Ecuador. Cinchonia 2007, 8, 107–125. [Google Scholar]
- Rosero, G.A. Evaluación de Carbono Orgánico del Suelo en el Ecosistema de Páramo de la Microcuenca del río Chimborazo en Base a las Actividades Antrópicas. Bachelor’s Thesis, Escuela Superior Politécnica de Chimborazo, Riobamba, Ecuador, 2019. [Google Scholar]
- Cantú, I.; Yañez, M.I. Efecto del cambio de uso de suelo en el contenido de carbono orgánico y nitrógeno del suelo. Rev. Mex. Cienc. For. 2018, 9. [Google Scholar] [CrossRef]
- Costa de Mendonça, G.; Araújo Costa, R.C.; Parras, R.; Marianno de Oliveira, L.C.; Nogueira Abdo, M.T.V.; Leal Pacheco, F.A.; Tarlé Pissarra, T.C. Spatial indicator of priority areas for the implementation of agroforestry systems: An optimization strategy for agricultural landscapes restoration. Sci. Total Environ. 2022, 839, 156185. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.R.; Taylor-Lovell, S. Designing multifunctional urban agroforestry with people in mind. Urban Agric. Reg. Food Syst. 2021, 6, e20016. [Google Scholar] [CrossRef]
- FAO. Trees, Forests and Land Use in Drylands: The First Global Assessment; FAO Forestry Paper No. 184; FAO: Rome, Italy, 2022. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).