Abstract
The objective of this study was to evaluate the effect of fire on the recruitment of six woody species sown at two soil depths. The study area was the western Argentine Chaco region, South America. The selected species were Aspidosperma quebracho-blanco Schlecht, Schinopsis lorentzii (Griseb.) Engl., Sarcomphalus mistol (Griseb.) Hauenschild, Neltuma nigra (Griseb.) Hieron, Senegalia gilliesii (Steud.) Seigler & Ebinger, and Vachellia aroma (Gillies ex Hook. & Arn.) Seigler & Ebinger. Seedling emergence was monitored following experimental burns (EB) in 2017 and 2019 under similar environmental conditions. In 2017, seeds were placed on the litter layer in 2 × 2 m plots; in 2019, seeds were buried at 2 cm depth in 2.5 × 10 m plots. Field emergence was recorded, and viability of non-germinated seeds was tested in the lab. Recruitment was also assessed two years post-EB 2017 in 20 plots (2 × 5 m) under burnt and control conditions. No emergence occurred from seeds on the litter layer, but S. lorentzii and N. nigra germinated in the laboratory (<7%, significant difference, p < 0.0001). All species with buried seeds germinated in the field, with significant differences among species and compared to controls (p < 0.0027). Field recruitment revealed regeneration of A. quebracho-blanco, N. nigra, and S. gilliesii in the burnt area, with variations in seedling size and density.
1. Introduction
The dry tropical and subtropical forests are the world’s most threatened natural areas by anthropogenic perturbations and climate change [1]. Fire frequency is increasing across different continents, driven by persistent droughts, heat waves, and deforestation, with alarming changes in forest structure, biodiversity, and provision of ecosystem services [2,3]. The persistence of these valuable ecosystems requires integrative research that explores seed biology and the effects of fire on seed germination and recruitment of plant species to address conservation and sustainable management strategies [4,5].
Fire can induce seed mortality through heat shock [6] but also may promote germination by breaking physical dormancy [7] or through the release of compounds from burnt biomass, such as ash and smoke [8]. Plants develop different adaptive strategies to establish and maintain populations in fire-disturbed environments, such as thick bark to resist the heat shock on tissues and organs, soil seed bank (SSB) formation to recruit after fire, and delayed germination to reduce germination failure under adverse conditions [9]. Seed recruitment is a key ecological process to maintain genetic diversity, vegetation structure, and population resilience in native species [10]. The post-disturbance recruitment also depends on seed functional traits such as size, mass, dormancy, seed bank persistence, and tolerance of seedlings to growth in altered environments [5,11].
The soil seed bank (SSB) represents reservoirs of viable seeds for recruitment of new individuals [12,13], under natural conditions or after disturbance. The seed-to-seedling transition represents a highly selective bottleneck in the life cycle of native plant species. This feature is exacerbated in semiarid fire-prone environments due to high temperatures, water deficit, changes in irradiance levels, and increased predation or seed removal [14]. Plant species from arid and semiarid environments have seeds with morphophysiological traits such as physical dormancy and protective structures (e.g., hard seed coats, woody endocarps), which are adaptations to selective pressures such as predation, fire, drought, and seasonality [15,16,17]. These functional traits also enhance fire resistance through mechanisms such as insulation provided by indehiscent fruits [18] or thicker seed coats, which regulate physiological tolerance to environmental changes caused by fire [19].
Fire is a key factor in the plant communities’ dynamics from semiarid, arid, and temperate environments because of the pulses of fuel accumulation and desiccation [20], and its action can alter the SSB by direct combustion of their components. In addition to natural factors, land use patterns may also affect SSB formation and its persistence in changing environmental sceneries [21,22]. Like other biomes of the world, the Chaco region is experiencing climatic and land use changes, with increasing fires with extreme behavior [3]. Previous studies under laboratory conditions have shown that seeds of native woody species from the Chaco region differ in thermal shock responses under controlled conditions. For example, seeds of some species are considered heat-tolerant when thermal shock does not significantly affect the germination, and this behavior has been attributed to protective fruit structures or their hard seed coats, while others are categorized as sensitive to heat shock [16]. However, studies with post-fire recruitment evaluation under natural conditions are still scarce in the semiarid Chaco forests.
Experimental burns have been broadly used to understand the role of fire in plant regeneration, since this methodology allows for evaluating the short- to medium-term recruitment after this disturbance. These research lines have been conducted mainly in fire-adapted ecosystems, such as Mediterranean systems [23], and in fire-sensitive ecosystems, such as Brazil’s Cerrado [17,24], with remarkable differences among biomasses and species.
According to previous studies [25,26], post-fire regeneration of native species in the Central Chaco, Argentina, seems to depend mainly on sprouting strategies, since there is low similarity between the standing vegetation and SSB composition [27]. This trend has also been observed in other semiarid regions [13,28,29], suggesting that woody species differ markedly in their ability to form SSBs and to persist after fire. A previous study [11] assessed the aptitude of six native woody species from the Chaco region to form transient SSBs in native forest, suggesting that seed size and shape may influence burial capacity and protect seeds against heat shock. This aptitude could be critical in SSB persistence, and for recruitment under increasing fire frequency or intensity. Additionally, another study [22] found that combinations of different land management practices could affect the soil seed density of the most representative woody species. Such information is crucial for species conservation, restoration of burned areas, and development of sustainable forest management plans. However, despite increasing interest in fire ecology, detailed studies on seed germination and survival after fire in semi-arid tropical forests, such as the Chaco, remain notably scarce [13,29]. This knowledge gap could limit our understanding of recruitment mechanisms in ecosystems increasingly affected by natural and anthropogenic fires.
This study evaluated the post-fire germination and field recruitment of six forest species after experimental burns. All these species form transient SSBs in areas disturbed by fires and mechanical vegetation removal methods, and the SSB sizes have been evaluated in previous studies [11]. We considered the following hypotheses: (a) Species have seeds with different burial aptitudes, mainly determined by the seed mass and dispersal unit shape, which influence the maintenance of seed viability after fire and recruitment probabilities in burnt areas [29,30,31]. (b) Forest disturbances such as fire, logging, and roller chopping create unfavorable environments for recruitment due to causing more severe abiotic and biotic conditions [32]. We predicted that fire-tolerant species with seed traits that allow them to be buried in the soil would have greater recruitment in fire-disturbed forests, whereas fire-sensitive species with seed traits that prevent soil burial would face recruitment limitations [11].
2. Materials and Methods
2.1. Study Area
The study area was located in the Western Chaco, Argentina (28°03′ S, 64°15′ W), (Figure 1). The region presents a semiarid seasonal climate, defined as monsoonal [33], with cold and dry winters. Daily temperatures vary from −15 to −10 °C and 38 to 40 °C in spring and summer, respectively [33]. The annual precipitation is 574 mm, concentrated in the wet season from October to March [34]. The dry season extends from April to September. The fire season matches the dry season, accompanied by hot winds from the north and northeast, which promote the occurrence of fires [33]. More severe fires occur at the end of the dry season, coinciding with the increase in spring temperatures and the prolonged accumulation and drying of fuels [33]. Fire has been used in the Chaco region since pre-Columbian times to improve the quality of pastures for livestock, control the invasion of woody in cattle-raising areas, and reduce or control the shrub layer in silvopastoral systems [33]. The typical vegetation of the semiarid Chaco region is a seasonal xerophytic forest, which is generally characterized by three strata. The open canopy is dominated by Aspidosperma quebracho-blanco Schlecht and Schinopsis lorentzii (Griseb.) Engl. The intermediate tree layer includes Sarcomphalus mistol (Griseb.) Hauenschild and Neltuma nigra (Griseb.) Hieron, while the lower stratum is mainly composed of thorny shrubs and subshrubs such as Vachellia aroma (Gillies ex Hook. & Arn.) Seigler & Ebinger, Senegalia gilliesii (Steud.) Seigler & Ebinger, Condalia microphylla Cav., Castela coccinea Griseb., Monteverdia spinosa (Griseb.) Biral, Celtis ehrenbergiana (Klotzsch) Liebm., and Ximenia americana L., along with several non-thorny species such as Atamisquea emarginata Miers ex Hook. & Arn. and Justicia spp. [18]. The soil is classified as Entic Haplustoll, with a texture composed of 43% sand (2000–53 μm), 49% silt (53–2 μm), and 8% clay (<2 μm) [35].
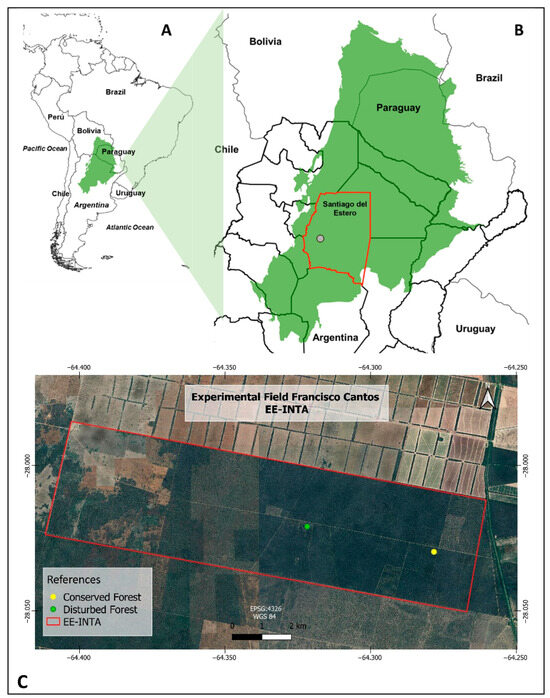
Figure 1.
(A) The Chaco biogeographic region is shown in green. (B) The province of Santiago del Estero, situated within the Chaco region, is indicated by a red outline. (C) The research area and sampling location are at the Francisco Cantos Experimental Station, part of the National Institute of Agricultural Technology (INTA).
2.2. Sampling Sites
To analyze the germination capability of seeds from six woody species under natural conditions, we selected two sampling sites located within the Campo Experimental Francisco Cantos (28°03′ S, 64°15′ W), belonging to the Instituto Nacional de Tecnología Agropecuaria (INTA), situated 28 km from the capital city of the province of Santiago del Estero. Within the experimental ranch, two forest sites with different land use histories were identified using satellite images, references, and field surveys [11]. The sampling sites were identified as follows: (a) disturbed forest—subjected to uncontrolled fires and mechanical treatments for partial reductions in the shrub layer (DF); (b) conserved forest (CF)—representing a reference condition, with very limited human intervention in the past four decades [36]. The distance between the two sites was approximately 4.4 km. For the experimental burns, the forest site with fire and rolling (DF) was selected because of the permits required to conduct these experiments in natural environments.
2.3. Species Selected and Seed Collection
Six woody species were selected for this study: A. quebracho-blanco, S. lorentzii, S. mistol, N. nigra, S. gilliesii, and V. aroma. These represent different forest strata of native forests in the Western Chaco [37] and show a significant variability in seed traits [11,38]. Fruits and seeds were collected in the CF area from at least ten individuals of each selected species [39,40] during the reproductive seasons of 2017 and 2019. Selection criteria to collect seeds from individual plants included mature individuals with a healthy appearance and no signs of pathogen attack [16]. The fruit collection was adjusted to the phenology calendar of each selected species [41]. The material was stored for between 1 and 7 months in paper bags under controlled conditions [18], free of impurities, and refrigerated at 12° ± 2 °C to prevent insect attacks [16] until the start of the experiments.
2.4. Seed Water Content (Cw) and Viability
The seed water content and viability were assessed prior to the trials. To determine the seed water content (Cw), two independent samples of 60 seeds for each species were weighed on a digital scale with a precision of ±0.001 g. Then, the samples were placed in a drying oven (103 ± 3 °C) for 24 h, after which the samples were allowed to cool and were weighed again [42]. The water content was calculated using Equation (1). The results obtained are expressed as percentages of moisture [6,43].
where Mf is the fresh mass (fresh weight) and Md is the dry mass (dry weight) of the seeds.
Cw = [(Mf − Md) (Mf)−1] × 100
The initial viability of the seed lots for each selected species was evaluated using the tetrazolium (TZ) test [42]. Four replicates of 25 seeds per species were tested. Previously, seeds were hydrated in distilled water for 24 h at 30 °C, then immersed in a 1% tetrazolium (TZ) solution and incubated in darkness at 30 °C for an additional 24 h [24,42]. After incubation, each seed was bisected with a scalpel to expose the embryo and assess the staining pattern. Embryos that stained red or pink were classified as viable, while unstained embryos were considered non-viable, based on topographic staining patterns [44,45].
2.5. Characterization of Experimental Burn
In October 2017 and 2019, two experiments using experimental burns (EBs) were conducted at the DF site under similar environmental conditions, during the fire season in the Chaco region, following local prescribed burning guidelines for this area [34]. The burns took place at the end of the dry season, when high temperatures and prolonged drought promote fuel accumulation and desiccation, and the fine fuel moisture content is typically low (<30%) [33]. The first one was in 2017, when six 2 m × 2 m plots (one for each species) were randomly established, and seeds were located in metallic mesh cells at litter level; the second one was in 2019, when a 2.5 m × 10 m plot was delimitated and excavated at 2 cm depth to metallic boxes containing soil and seeds of the studied species (4 replicates of each one), which were then covered with soil. Control replicates for each species were set in nearby unburned plots at the DF site to compare the effects of the burns. Before the EB, the fine fuel load for igniting and spreading surface fires was assessed. In plots with insufficient fuel load, an additional load of this fuel was added to each experimental plot to achieve ≅4000 kg/ha, considered the minimum required to ignite and sustain fire [34,46]. In both experiments, fire ignition was carried out with a drip torch, following strict regional protocols [33].
Before each EB, seeds of selected species were either disposed over the leaf litter (2017) or buried at a 2 cm soil depth (2019). In 2017, four replicates of 25 seeds or fruits (referred to as propagules) of each species were placed in 20 cells made of fine metal and distributed over the leaf litter lightly covered with mulch. These meshes prevented seed loss due to runoff and predation after the burns. For N. nigra, both isolated seeds and segments with endocarp were tested, whereas for S. mistol and S. lorentzii, endocarps and samaras were tested, respectively. For A. quebracho-blanco, closed capsules were used to assess heat-induced fruit opening and seed release. In 2019, EB was conducted to evaluate its effects on buried propagules. Four replicates of 25 propagules of each species were placed in heat-resistant trays, buried 2 cm deep, and covered with 2 cm of soil. A. quebracho-blanco was not included due to its inability to be buried under natural conditions. Unburned controls were set up for each species in plots similar to the burned ones.
The meteorological conditions during EB dates were monitored every 30 min (air temperature (°C), air relative humidity (%), wind speed (Km h−1), and wind direction for fire control) [34]. The variable used to characterize fire behavior [46] during the burns were:
Fire intensity (I): Defined as the energy released during the burn, estimated using flame length with the Formula (2) [47]:
where LL is the average flame length, visually estimated by two independent observers; greater LL indicates higher fire intensity [46].
I (kWm−1) = 259.83 × LL2.174
Residence time (II): Recorded as the time for the fire to traverse the entire plot (tt, minutes) and until the fuels were fully burned (tf, minutes); longer residence times correlate with more severe effects on vegetation and soil [48].
2.6. Experimental Evaluation of Fire Effects on Seed Germination at Two Soil Depths
Post-experimental burn (EB) evaluations were carried out in two phases, in both burned and unburned (controls) plots. In 2017, the first phase involved monitoring seedling emergence in the field every two weeks for nine months. After this period, ungerminated hard propagules were extracted from grids and transported to the lab for germination tests (second evaluation phase). In 2019, the evaluation period was extended for 12 months after EB, and the remaining propagules were extracted and transported to the lab for germination tests. Before this, the propagules were manually scarified and placed in Petri dishes on moistened filter paper for germination. These sequential analyses allowed us to determine the number of ungerminated but viable seeds. To accurately identify the emerging seedlings, preliminary trials were conducted for their identification, and references were consulted whenever necessary [49]. The purpose of this post-burn scarification treatment was to confirm whether the seeds were still viable. The Petri dishes were placed in germination chambers at 30 °C with a 12/12 h light cycle. Samples were evaluated every two days for 15 days [50].
2.7. Field Assessment Recruitment
Two years after the 2017 EB, the field recruitment of the six selected woody species was again evaluated within both the DF and CF plots as the controls, using 20 plots of 2 × 5 m randomly selected in each sampling site. Only the seedlings originated from propagules (without resprouting at the base of the plant), with a base diameter (BD) > 1 mm and a height < 50 cm, were identified and measured. These size thresholds correspond to established regeneration according to studies for the Chaco region [49,51]. These data allowed us to obtain the average seedling diameter, the average seedling height, and the density of seedlings per hectare.
To assess the possible effects of tree occupation on species regeneration, the total and species-specific basal area (BA) density (m2·ha−1) (K = 3) values were estimated for the CF and DF forests using Bitterlich’s angular count method [52]. This method is considered precise and unbiased across a range of spatial patterns and diameter distributions [53].
2.8. Data Analysis
The initial viability of the seeds from both years was compared through a one-way ANOVA with Tukey’s post hoc tests. Differences were considered significant at p < 0.05. Data about germination percentages (seedling emergence) in the field were analyzed considering four treatments: propagules placed on the leaf litter and exposed to fire (PL-F), propagules placed on the leaf litter without fire (control) (PL-C), propagules buried in the soil and exposed to fire (PI-F), and propagules buried in the soil without fire (control) (PI-C). General linear models (GLM) were used to assess post-fire germination percentages between species, under different treatments, with fire as the main fixed factor. Since zeros were not excessive, a standard GLM was adopted instead of zero-inflated models. This model was considered for heterogeneity of variances, and the model selection was based on Akaike’s information criterion (AIC). The intraspecific differences under the different treatments were assessed using the Kruskal–Wallis non-parametric test, due to the presence of zero germination, which makes using a general linear model difficult. Two models were performed, one for the seed germination percentage (seedling emergence) based on evaluation under field conditions (G1: germination one), and another for the germination percentage of seeds extracted from field samples with mechanical scarification applied under laboratory conditions (G2: germination two). In both models, the germination percentage of seeds was evaluated based on fire treatments and controls (PL-F, PL-C, PI-F, and PI-C).
Field recruitment data of the studied species in CF and DF were collected two years after the 2017 experimental burn (EB) (the relative abundance of the native woody species, basal area of trees per treatment, basal area of standing trees, height and density of seedlings, and proportion of each species based on total number of seedlings) were analyzed using a one-way ANOVA with Tukey’s post hoc tests. Differences were considered significant at p < 0.05. Statistical analyses were performed using InfoStat v.2017 [54].
3. Results
3.1. Seed Water Content and Viability
The seed water content (Cw) and seed viability values for the six species under study are shown in Table 1. The seed water contents varied from 5.8% in S. lorentzii to 11.2% in S. gilliesii. Only S. mistol and N. nigra showed significant differences in the seed viability percentages among years (p = 0.0047 and 0.0036, respectively). The percentages of seed viability for V. aroma and N. nigra were the highest among the studied species, but interannual variability in seed viability was significant only for N. nigra and S. mistol, without a clear tendency among years (Table 1).

Table 1.
Average seed water content (Cw; mean ± SD, 2017) and seed viability (mean ± SD) values of six native woody species from the semi-arid Chaco of Argentina, corresponding to different harvest years. Different letters indicate significant differences among means (Tukey test; α = 0.05).
3.2. Experimental Burns
The experimental burns (EB) conducted in 2017 and 2019 were of medium intensity, with average flame lengths (mean ± SD) of 1.51 ± 0.30 m and 1.6 m, respectively (Table 2). The meteorological conditions during the burns and data about fire behavior are shown in Table 2. The average fire duration across the experimental plots was 78 ± 32 s.

Table 2.
Meteorological conditions and flame lengths were recorded during the experimental burns conducted in the 2017 and 2019 fire seasons in the semi-arid Chaco region of Argentina.
After 2017 EB, we did not observe seedling emergence for any of the studied species, neither in the fire treatments (PL-F) nor in the controls (PL-C) (Phase 1) (Figure 2a). Seed losses were primarily due to both carbonization and post-fire seed predation. Seeds damaged by carbonization ranged from 28% for V. aroma to 100% for A. quebracho-blanco, and seeds damaged by predation ranged from 13% for S. mistol to 34% for N. nigra with endocarp (Appendix A). The results of germination in phase 2 are shown in Table 3 (PL-F; PL-C).
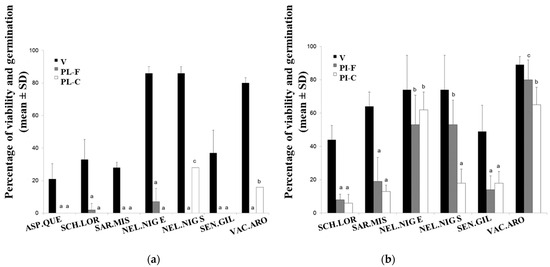
Figure 2.
Percentage of viability (V) and accumulated germination (mean ± SD) of seeds from six woody species native to the semi-arid Chaco of Argentina, subjected to experimental burning treatments: (a) experimental burn 2017, with propagules placed over the litter exposed to fire (PL-F) and their respective controls in unburnt plots (PL-C); (b) experimental burn 2019, with propagules buried in the soil and exposed to fire (PI-F) and their respective controls (PI-C). APS.QUE: Aspidosperma quebracho-blanco; SCH.LOR: Schinopsis lorentzii; SAR.MIS: Sarcomphalus mistol; NEL.NIG E (with endocarp).: Neltuma nigra with endocarp; NEL.NIG S (seed): N. nigra with seed; SEN.GIL: Senegalia gilliesii; VAC.ARO: Vachellia aroma. Different letters indicate significant differences among treatments within each species, as determined by a general linear model (GLM), followed by Fisher’s LSD test (α = 0.05).

Table 3.
Germination percentages (± standard deviation) under lab conditions of the seeds germinated under field conditions after EBs in 2017 and 2019. Seeds were scarified (G2 phase) to check viability by germination under lab conditions. An analysis was performed using the Kruskal–Wallis test (α = 0.05).
The accumulated post-fire germination percentages (considering both field germination and laboratory germination) varied significantly (p < 0.001) among species in the EB of 2017. Only two species with seeds protected by fruit structures and disposed on leaf litter showed post-fire germination—S. lorentzii and N. nigra—and only two species germinated under control conditions showed the same in phase 2—N. nigra and V. aroma. In all cases, the germination percentages were low considering the average viability of the seed lots, and delayed germination (approximately nine months after EB) was observed in S. lorentzii and N. nigra. Significant differences in germination percentages were observed for in N. nigra and V. aroma compared to controls (p < 0.0001; p = 0.0082; respectively) (Figure 2a).
During the EB of 2019, the temperatures above ground ranged between 115 °C and 320 °C, while the temperatures at 2 cm depth in the soil varied between 27 °C and 67 °C. The mulch was consumed superficially. In this EB, there was recruitment in the field for five species (phase 1). Only N. nigra and V. aroma showed germination in phase 2 (Table 3). For the accumulated germination as a percentage for each species under the different treatments, PI-F and PI-C showed significant differences (p = 0.0027) (Figure 2b). A. quebracho-blanco was not included in this trial, considering their seeds are not prone to be buried and always remain over the fallen litter (Figure 2b). V. aroma, N. nigra, S. lorentzii, and S. mistol showed higher accumulated germination in the burnt plot than control plots but differences were significant only in the two first species (80 and 53%, respectively) (p = 0.0249 and p = 0.0002). Neltuma nigra seeds included in endocarps and S. gilliesii showed the opposite tendency, with higher germination in the control plot than in the burnt plots (62 and 18%, respectively), but the differences were not significant. S. lorentzii and S. mistol presented the lowest germination percentages (<20%), with almost one-third of the average seed viability percentages determined in their seed plots.
The germination percentages of phase 1 after the EB of 2019 showed the responses for the propagules to fire under field conditions; higher germination percentages were observed for N. nigra and V. aroma in burnt plots than in control plots, although significant differences were found only for the first species. However, seeds of N. nigra within the endocarp showed higher germination in the control plot (Figure 3).
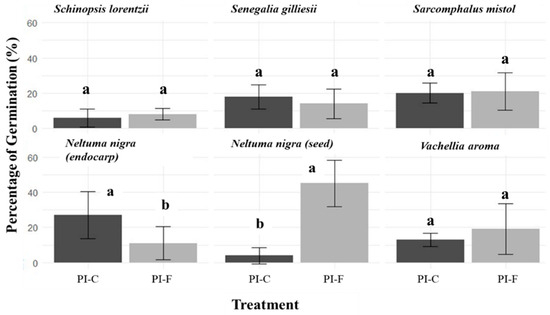
Figure 3.
Germination percentages of seeds from five native species of Chaco region forests, Argentina. The values correspond to phase 1 of field germination after EB 2019 with buried seeds. Ref.: PI-F: propagules incorporated into soil and exposed to fire; PI-C: propagules incorporated into soil without experimental burns. Different letters indicate significant differences among means according to the Kruskal–Wallis test (α = 0.05).
The results of germination in phase 2 corresponding to 2017 and 2019 EBs are shown in Table 3, indicating that S. lorentzii (2017), N. nigra (2017 and 2019), and V. aroma (2019) remained ungerminated yet viable for nine and twelve months, respectively, after fires in the burnt plots. The mechanical scarification stimulated germination in N. nigra (10.50 ± 10.13%) and V. aroma (14.75 ± 3.20%). In PI-C, seeds of N. nigra with endocarp and V. aroma exhibited lower germination than in PI-F, whereas seeds of N. nigra with the endocarp removed showed a higher germination percentage in PI-C (Table 3).
3.3. Field Recruitment Two Years After an Experimental Burn
Two years after the EB of 2017, a total of 346 seedlings of ten native woody species of the Chaco region forest, belonging to eight botanical families, were recorded in the field: 223 in CF and 123 in DF (Table 4). Only four of the six species selected for this study were registered in these field surveys: A. quebracho-blanco, S. gilliesii, S. lorentzii, and N. nigra (Figure 4). The first two species were recorded in both forest types, while the third was found only in CF, and the last one only in DF (Table 4). There was no recruitment of S. mistol or V. aroma in either forest type. Other native woody species recorded both CF and DF, namely Atamisquea emarginata, Condalia microphylla, Schinus fasciculatus, and Celtis ehrenbergiana, whereas Monteverdia spinosa and Ximenia americana were recorded only in CF (Table 4). The relative abundance levels of seedlings varied among forest types, with S. gilliessi and A. quebracho-blanco exhibiting the highest values in the DF forest. Regeneration in the disturbed forest was dominated by A. quebracho-blanco seedlings.

Table 4.
Relative abundance of seedlings of woody species in the semi-arid Chaco forests with different disturbance regimes, namely the conserved forest (CF) and forest with fire and rolling (DF). Ref: (*): study species; (**): recorded species common to both sites; (-): absences.

Figure 4.
Recruitment seedlings: (A) Senegalia gilliesii; (B) Aspidosperma quebracho-blanco.
3.4. Tree Canopy in Forest and Regeneration
The CF and DF did not show statistically significant differences in total basal area (m2/ha1) (Appendix B). The mature individuals of canopy species, A. quebracho-blanco and S. lorentzii, and intermediate forest stratum species, S. mistol and N. nigra, were present in both forest types, with no significant differences in the basal areas among types of forests. Other characteristic species of the Chaco forests, such as Opuntia sp. and Parkinsonia praecox, were found only in CF (Appendix B).
Height (H), average basal diameter (ABD), and seedling density (SD) values of A. quebracho-blanco differed significantly between forest types, with a higher, lower-sized seedling density in the DF than in the CF forest. S. gilliesii seedlings were recorded in both forest types (CF and DF), with a higher seedling density in the CF (p = 0.0064) (Table 5) (Figure 3). On other hand, S. lorentzii seedlings were absent in the DF, but N. nigra seedlings were only present in this forest type, with a high seedling density (Table 5). Other studied species did not show significant differences in these seedling variables (Table 5).

Table 5.
Height, diameter at the base, and density of seedlings of native woody species from Chaco forests, under different treatments. CF: conserved forest (without recent disturbances, considered as control); DF: disturbed forest with rolling chopping and experimental burns; H: seedling height (cm); ABD: average basal diameter of seedlings (mm); SD: seedling density (individuals/ha). Average values ± standard error. Different letters indicate significant differences among means (Tukey test; α = 0.05). Fisher’s F-statistic values and corresponding error probability (p) are indicated. Dots (.) indicate missing data or measurements not available.
4. Discussion
Our results represent a significant improvement in understanding of the germination behavior and post-fire recruitment of native woody species in the semi-arid Chaco of Argentina. The relative position of propagules in the substrate (soil or litter) significantly influenced both seed viability and seedling emergence. Two years after the EB, recruitment of some studied species was still observed in the field. The results suggest that morphological traits of dispersal units, their burial potential in the soil, and seed quality could regulate the sexual regeneration in burned areas.
The absence of recruitment after the EB of 2017 for all studied species with propagules placed over the litter indicated their low tolerance to the high temperatures generated during a fire, and also the potential effects of seed predation and pathogen attacks on their recruitment potential. Despite flame lengths recorded during the EBs of 2017 suggesting fires of intermediate intensity [46], carbonization of seeds and fruits was observed in the litter. The maintenance of viability observed later under lab conditions (phase 2) for a low number of ungerminated seeds of S. lorentzii and N. nigra could be considered negligible. Except for N. nigra and V. aroma, the absence of viability observed among the ungerminated seeds of most studied species in the controls also suggests that the impacts of low seed quality or drastic desiccation in litter on the potential recruitment of these species (Table 3). These species exhibited significant differences in seed viability between the 2017 and 2019 harvests, consistent with results reported by [55] for native woody species from the Argentine Chaco region. These authors attribute these differences to particular environmental conditions such as desiccant winds or abnormal rains or temperatures during reproductive phases. The lack of viable seeds for A. quebracho-blanco, S. mistol, and S. gilliesii in phase 2 of the 2017 EB could indicate additive effects of low initial viability of their seed lots, and unfavorable environmental conditions in post-fire environments (e.g., wide thermal amplitudes and high radiation levels, soil desiccation) could affect seed viability under field conditions [5]. On the other hand, the low germination percentage (<7%) observed for propagules of S. lorentzii and N. nigra over the litter in phase 2 could be attributed to their protective pericarps or hard seed coats. Both traits have been related to physical dormancy, which may likely enhance tolerance to dehydration during flame exposure or seedling establishment [24]. A previous study [18] established physical dormancy in these species attributed to ligneous fruit walls and hard seed coats, respectively, by experiments with thermal shock in the laboratory. The absence of germination in A. quebracho-blanco, S. lorentzii, S. mistol, and N. nigra endocarps in control plots suggests that recruitment of these species under field conditions may be limited by the harder physical conditions [10]. The interannual variation in seed viability observed could be attributed to climate variability and plague or pathogen incidence during the reproductive phases. In addition to thermal tolerance, studies have shown that fire-induced changes in abiotic and biotic factors affect recruitment in burned areas due to biomass loss and predation [5,56]. Environmental factors post-disturbance, such as high desiccation risk [57], low water availability for imbibition [58], and increased predation rates [50], may limit recruitment.
The recruitment percentages observed for most of the studied species after the 2019 EB indicate that the soil protected the propagules from the high temperatures reached during the fire [24]. Temperatures recorded in the soil during combustion, as recorded in our study, were markedly lower than temperatures registered at the litter level; these findings aligned with results obtained in other studies, such as in the Brazilian Cerrado [17,59], suggesting that burial could help the maintenance of seed viability in areas subjected to fire. Heat tolerance thresholds for plant tissues typically sit around 60 °C but depend on the tissue type, hydration level, genetics, and duration of heat exposure [34]. Seeds of several woody species do not tolerate temperatures exceeding 100 °C, as was observed in lab heat shock tests [16,60]. Species with lower heat tolerance experience higher seed mortality and reduced seed bank viability post-fire [61]. The high accumulated germination percentages in N. nigra and V. aroma in burned plots suggest some level of germination stimulation within the recorded temperature range, since they were significantly higher compared to their respective controls (Figure 2b). This germinative behavior observed in the field after EBs exhibits some differences compared to propagule responses to heat shock in the laboratory. Based on their responses to different experimental heat shock temperatures, Ref. [18] assigned the category “sensitive” to N. nigra and “tolerant” to V. aroma. However, in our study, both species showed a positive response to fire when seeds were buried in soil.
Seed distribution within the soil profile is related to seed traits influencing burial potential [11]. Species with larger seeds or softer seed coats are more susceptible to fire due to limited burial [14]. According to [11], species such as A. quebracho-blanco, S. lorentzii, and S. gilliessi have shape indexes that can limit their burial, making them more sensitive to heat shocks in fire-prone areas. Conversely, some Fabaceae species such as N. nigra and V. aroma have the potential to be buried in the soil, increasing their potential for fire tolerance. Results obtained in this study corroborate the important role of seed burial for the fire tolerance of seeds and fruits. Our results support the hypothesis that propagule distribution within soil profiles significantly influences post-fire seedling survival and establishment.
Field Recruitment Two Years After an Experimental Burn
Recruitment studies of woody species in forests with different disturbance histories are mandatory for designing forest management plans [62]. In our study, the recruitment observed for four of the six species analyzed in the CF and DF reflects the challenges of seed-based establishment in semi-arid environments [29]. Basal area values observed in the species selected for this study were twice as high as those reported for recovering forests [37]. However, recruitment patterns varied according to disturbance history. This variation could be attributed to biomass removal due to roller chopping and accidental fires (DF), as well as the recent impact of the 2017 EB [63].
S. lorentzii, the canopy tree species of greatest conservation interest, showed no natural seed regeneration in the DF, suggesting specific germination requirements and seed sensitivity to disturbances. The woody pericarp arrests seed imbibition [18], which may cause require high moisture levels for germination and successful recruitment [5,36]. The exclusion of disturbances has been identified as a key factor for its regeneration [64,65], which is in line with its low frequency of occurrence in the study area and the size of its SSB in the CF [11].
In contrast, A. quebracho-blanco was the only species of the canopy that showed seed-based regeneration in the DF. This species exhibited a higher density of seedlings in the DF, although smaller in size (H and ABD). Its seeds can germinate under very low moisture levels, and this process may be facilitated by the potential moisture retention provided by the litter [29]. Efficient dispersal from surrounding areas and rapid response to low-intensity fires [3] may explain its predominance in open sites with recurrent disturbances. However, previous studies reported higher recruitment in protected areas compared to disturbed areas [21,66]. On the other hand, Ref. [66] reported a rapid positive population response of A. quebracho-blanco to low-intensity fires, provided it is accompanied by grazing exclusion.
The absence of recruitment for S. mistol and V. aroma in both forest types could be due to the irregular distribution of their propagules in the SSB, short-term seed viability loss [67], and physical dormancy [68,69]. Additionally, the sensitivity of S. mistol to heat shock [17] may explain its absence in the DF. The lack of dispersers and seed predation could be additional factors limiting recruitment in these species [70].
The lack of recruitment of V. aroma does not align with what was expected. This species has the largest SSB size among the studied species, together with N. nigra, and its seeds exhibit a considerable tolerance to heat shock [11,18], especially when its propagules are buried. These results support previous findings that highlight the dissimilarity between the species composition of standing vegetation and that of the SSB [27,29]. Therefore, the availability of propagules is not the only bottleneck for the recruitment of native species.
The recruitment of N. nigra exclusively in DF highlights its behavior as a pioneer species and its ability to colonize disturbed environments, which is similar to other pioneer species of Fabaceae [71,72]. The high predation rates (>30%) observed within N. nigra seeds suggest that propagule predation (such as observed after EB 2017) may have prevented recruitment in CF, despite the presence of adult individuals in both forest types [50,63]. While N. nigra and V. aroma propagules have shown relative tolerance to heat shock (80–110 °C), recruitment levels were lower than expected [18]. The smaller SSB size in the DF than CF [12] suggests that their recruitment is hindered by several constraints, likely influenced by unfavorable environmental conditions following disturbances.
The presence of S. gilliesii seedlings in both forest types aligns with previous findings on SSBs [11], which confirmed the presence of propagules of this species in both sites. The similar seedling structures (height and diameter) in both forest types suggest seedling establishment likely occurred during the same period, with higher recruitment success in the CF. S. gilliesii is dominant in undisturbed sites and regenerates successfully under such conditions [58]. However, the seedling density in the DF seems to indicate high tolerance to post-disturbance conditions, as this species can regenerate under varying light conditions and low soil moisture levels, thereby favoring germination even in more stressful environments [58].
The results obtained in the field recruitment experiments post-EB with seeds in different soil depths seem to indicate the combined effect of interannual seed viability and fire resistance, since all species showed higher viability in the seed plots collected in 2019, but the differences were significant only in two of the species studied. Both the seed viability and the recruitment observed after the 2019 EB support the hypothesis regarding the effect of burial capacity on the maintenance of seed viability, since all species recruited differentially after fire. The increase in average germination observed in N. nigra, V. aroma, and S. mistol in 2019 burnt plots suggests a stimulation by heat shock, despite the temperature range in the soil not exceeding 100 °C, and the differences were significant only in the first two species.
Half of the studied species showed no recruitment in the DF, suggesting that environmental and biological factors influence their seed-based regeneration. When the propagules do not have the aptitude to bury in the soil, their availability from adjacent, undisturbed sites and the presence of dispersers become crucial for the recruitment of these woody species in Chaco forests [29,58,63]. Both dominant tree species from these forests depend on this strategy to recolonize burnt areas, highlighting the demand for conservation areas with mature trees to ensure the seed-based regeneration. Other studied species exhibited aptitude to bury into the soil and to recruit in forests disturbed by fires and roller chopping, allowing for the recovery of biomass, protecting soil from erosion, and promoting nutrient cycling with restrictions related to the physical changes generated by disturbances.
Our results allowed us to generate recommendations for the native forest management plans, which should consider the importance of soil structure conservation as a reservoir of native species seeds. The litter has a significant role in seed storage [22], but in this soil fractions are exposed to high mortality during fires, becoming highly susceptible. The canopy species, S. lorentzii and A. quebracho-blanco, do not have the aptitude to burrow in the soil; therefore, they are dependent on mature trees as seed sources to recruit in fire-disturbed areas. The intermediate forest stratum species, such as N. nigra, S. mistol, and V. aroma, have a relative aptitude to incorporate into the soil [11], providing them the opportunity to germinate after fires. N. nigra and V. aroma showed higher germination rates after fires when propagules were buried. However, the seedlings’ establishment seems to be another bottleneck in seed regeneration, demonstrated by only four of the studied species recruiting in disturbed forests. A. quebracho-blanco is the only forest canopy species that recruits in disturbed forest, probably from seed rain after fires. On the other hand, V. aroma showed the highest germination rate after EB, but it was absent in the forest disturbed by roller chopping and fires, suggesting particular environmental requirements for the growth and establishment of seedlings. The monitoring of environmental conditions over time in disturbed forests and the knowledge of the physical and biological requirements of species for seedling growth are indispensable for the conservation of the Chaco region’s native forests. This represents an important knowledge gap that could contribute to a better interpretation of different forest degradation patterns. This understanding will contribute to the restoration activities, since the studied species revealed differences in their regeneration potential in disturbed forests. More studies including high numbers of native forest species, their responses to other fire severities, and the monitoring of environmental changes in burnt forest areas are desirable to assess the issues analyzed in this work. In particular, integrating post-fire climatic monitoring, such as for rainfall and soil temperature, could clarify additional constraints on seedling recruitment and survival.
5. Conclusions
The results of our study indicate different post-fire recruitment patterns among the selected native woody species. The distribution of propagules within the soil profile (soil or litter) was a key factor for seed survival and successful field recruitment after medium- to low-intensity wildfire events (EBs). Seeds located in the litter can be lethally affected by fire. However, when incorporated into the soil, their chances of recruitment increased due to insulation from surface temperatures. Species such as A. quebracho-blanco, whose seeds cannot burrow, seem to depend on the seed rain that allows them to recruit in burnt or disturbed areas. The higher the viability and heat shock tolerance of propagules, the greater their recruitment potential, as demonstrated in N. nigra. The field recruitment rates two years after the EB differed among the studied species, indicating that the species with the highest seedling establishment success were A. quebracho-blanco, N. nigra, and S. gilliesii. These species exhibit higher tolerance for germination and seedling establishment under the environmental conditions of the disturbed forests. The absence of S. lorentzii recruitment in disturbed forests affected by rolling and fire highlights the need for differentiated forest management strategies to facilitate the sexual regeneration of this valuable canopy species. Our results will contribute to forest land management and restoration activities in the Chaco region’s native forests, as demonstrated by the differential susceptibility of the studied species to fires affecting seed viability. These results also highlight the need to monitor environmental changes generated by this disturbance that can limit the growth and establishment of seedlings, affecting the natural regeneration dynamic.
Author Contributions
A.V.I.M.: Conceptualization, investigation, methodology, formal analysis, writing—original draft, writing—review and editing. F.B.: Methodology, investigation, formal analysis, writing—review and editing, visualization. L.G.: Methodology, investigation, formal analysis, writing—review and editing, visualization. J.M.C.: Investigation, formal analysis, data curation. S.J.B.: Conceptualization, investigation, methodology, writing—original draft, writing—review and editing, visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Secretaría de Ciencia y Técnica, Universidad Nacional de Santiago del Estero, under the project Estudios ecológicos en bancos de semillas de especies leñosas nativas del Chaco y su relación con disturbios antrópicos (2016–2020), code: 23/B133. Additionally, A.V.I.M. acknowledges the fellowship granted by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and F.B. acknowledges funding from Fundação de Apoio à Pesquisa do Distrito Federal (FAPDF, Projeto 00193.00001818/2023-98) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, projeto 407132/2023-6).
Data Availability Statement
Data will be made available on request.
Acknowledgments
We express our gratitude to the Facultad de Cs. Forestales (FCF) at Universidad Nacional de Santiago del Estero (UNSE), the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and the authorities and field staff of the INTA Experimental Station in Santiago del Estero Province for their valuable support. We also appreciate the assistance of the field personnel and our colleagues from the Chair of General Botany and the Forest Species Germplasm Bank (BAGEFOR), FCF, and UNSE, for their collaboration during both fieldwork and laboratory activities, as well as Dante Loto, who provided important comments on this manuscript.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A

Table A1.
Post-fire seed fates of six native woody species from Chaco forests. Percentages of carbonized and predated seeds (mean ± SD, n = 100).
Table A1.
Post-fire seed fates of six native woody species from Chaco forests. Percentages of carbonized and predated seeds (mean ± SD, n = 100).
| Species | Carbonized Seeds (%) | Predated Seeds (%) |
|---|---|---|
| Aspidosperma quebracho-blanco | 100 ± 0 | 0 ± 0 |
| Schinopsis lorentzii | 33.0 ± 27.3 | 25.0 ± 6.8 |
| Sarcomphalus mistol | 56.0 ± 30.4 | 13.0 ± 17.7 |
| Neltuma nigra with endocarp | 32.0 ± 26.0 | 34.0 ± 13.2 |
| Neltuma nigra seed | 59.0 ± 6.8 | 30.0 ± 20.0 |
| Vachellia aroma | 28.0 ± 24.8 | 32.0 ± 11.3 |
| Senegalia gilliessi | 45.0 ± 34.0 | 26.0 ± 36.5 |
Appendix B

Table A2.
Species compositions in Chaco forests with different land uses: conserved forest (CF) and forest disturbed by rolling chopping and experimental burns (DF). Average values ± standard error of basal area (m2/ha−1). Fisher’s F-statistic values and corresponding error probability (p) are indicated.
Table A2.
Species compositions in Chaco forests with different land uses: conserved forest (CF) and forest disturbed by rolling chopping and experimental burns (DF). Average values ± standard error of basal area (m2/ha−1). Fisher’s F-statistic values and corresponding error probability (p) are indicated.
| Species | Basal Area | f | p | |
|---|---|---|---|---|
| CF | DF | |||
| Aspidosperma quebracho-blanco | 5.89 ± 0.93 | 4.62 ± 0.58 | 1.34 | 0.2549 |
| Schinopsis lorentzii | 3.18 ± 0.76 | 2.55 ± 0.63 | 0.41 | 0.52 |
| Neltuma nigra | 0.47 ± 0.26 | 1.11 ± 0.53 | 1.16 | 0.28 |
| Sarcomphalus mistol | 2.71 ± 1.04 | 2.07 ± 0.81 | 0.23 | 0.63 |
| Opuntia sp. | 0.31 ± 0.21 | 0 | 2.11 | 0.15 |
| Parkinsonia praecox | 0.15 ± 0.15 | 0 | 1 | 0.32 |
| total | 12.75 ± 1.24 | 10.36 ± 1.26 | 1.82 | 0.1859 |
References
- Rivas, C.A.; Navarro-Cerrillo, R.M. Forest fragmentation and connectivity in South American dry forests. Biodivers. Conserv. 2024, 33, 3015–3037. [Google Scholar] [CrossRef]
- San Martin, R.; Ottlé, C.; Sörensson, A. Fires in the South American Chaco, from dry forests to wetlands: Response to climate depends on land cover. Fire Ecol. 2023, 19, 57. [Google Scholar] [CrossRef]
- Vidal-Riveros, C.; Currey, B.; McWethy, D.B.; Bieng, M.A.N.; Souza-Alonso, P. Spatiotemporal analysis of wildfires and their relationship with climate and land use in the Gran Chaco and Pantanal ecoregions. Sci. Total Environ. 2024, 955, 176823. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Salgado, J.C.; Pizano, C. Effect of temperatures that simulate fire on seed germination in a tropical dry forest. Colomb. For. 2019, 22, 55–66. [Google Scholar]
- Ocampo-Zuleta, K.; Bravo, S. Recruitment of woody species in tropical forests exposed to wildland fires: An overview. Ecosistemas 2019, 28, 106–117. [Google Scholar] [CrossRef]
- Ribeiro, L.; Borghetti, F. Comparative effects of desiccation, heat shock and high temperaturas on seed germination of savanna and forest tree species. Austral. Ecol. 2013, 39, 267–278. [Google Scholar] [CrossRef]
- Silveira, F.A.O.; Fernandes, G.W. Effect of light, temperature and scarification on the germination of Mimosa foliolosa (Leguminosae) seeds. Seed Sci. Technol. 2006, 34, 585–592. [Google Scholar] [CrossRef]
- Ghebrehiwot, H.M.; Kulkarni, M.G.; Light, M.E.; Kirkman, K.P.; Van Staden, J. Germination activity of smoke residues in soils following a fire. South Afr. J. Bot. 2011, 77, 718–724. [Google Scholar] [CrossRef]
- Macedo, M.A.; Pinhate, S.B.; Bowen, E.C.; Musso, C.; Miranda, H.S. Constraints on tree seedling establishment after fires: Passing the germination bottlenecks. Plant Biol. 2021, 24, 176–184. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Elsevier/Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Ibañez Moro, A.V.; Borghetti, F.; Galetto, L.; Cellini, J.M.; Bravo, S.J. The influence of seed functional traits and anthropogenic disturbances on persistence and size of the soil seed bank from dry subtropical forest species. For. Ecol. Manag. 2024, 551, 121524. [Google Scholar]
- Ghorbani, J.; Le Duc, M.G.; Mcallister, H.A.; Pakeman, R.J.; Marrs, R.H. Effects of the litter layer of Pteridium aquilinum on seed banks under experimental restoration. Appl. Veg. Sci. 2006, 9, 127–136. [Google Scholar] [CrossRef]
- Fortunato, V. Retención de Semillas en la Broza y Reclutamiento de Especies Leñosas Dominantes, en Distintas Intensidades de Uso del Suelo Actual e Histórico del Bosque Chaqueño del Oeste de Córdoba. Bachelor’s Thesis, Facultad de Ciencias Exactas, Físicas y Naturales, Universidad Nacional de Córdoba, Córdoba, Argentina, 2017. [Google Scholar]
- Ooi, M.K.; Tangney, R.; Auld, T.D. Fire and regeneration from seeds in a warming world, with emphasis on Australia. In Plant Regeneration from Seeds; Academic Press: Cambridge, MA, USA, 2022; pp. 229–242. [Google Scholar]
- Díaz, S.; Cabido, M.; Zak, M.; Martínez Carretero, E.; Araníbar, J. Plant functional traits, ecosystem structure and land-use history along a climatic gradient in central-western Argentina. J. Veg. Sci. 1999, 10, 651–660. [Google Scholar] [CrossRef]
- Jaureguiberry, P.; Díaz, S. Post-burning regeneration of the Chaco seasonally dry forest: Germination response of dominant species to experimental heat shock. Oecologia 2015, 177, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Daibes, L.F.; Zupo, T.; Silveira, F.A.; Fidelis, A. A field perspective on effects of fire and temperature fluctuation on Cerrado legume seeds. Seed Sci. Res. 2017, 27, 74–83. [Google Scholar] [CrossRef]
- Ibañez Moro, A.V.; Bravo, S.J.; Abdala, N.R.; Borghetti, F.; Chaib, A.M.; Galetto, L. Heat shock effects on germination and seed survival of five woody species from the Chaco region. Flora 2021, 275, 151751. [Google Scholar] [CrossRef]
- Rodrigo, A.; Arnan, X.; Retana, J. Relevance of soil seed bank and seed rain to immediate seed supply after a large wildfire. Int. J. Wildland Fire 2012, 21, 449–458. [Google Scholar] [CrossRef]
- Pausas, J.G. Incendios Forestales; Catarata-CSIC: Madrid, Spain, 2012; 119p. [Google Scholar]
- Lipoma, M.L.; Cuchietti, C.D.A.; Enrico, L.; Gorné, L.D.; Díaz, S. Low resilience at the early stage of recovery of the semi-arid Chaco Forest—Evidence from a field experiment. J. Ecol. 2021, 109, 3246–3259. [Google Scholar] [CrossRef]
- Bravo, S.; Abdala, R.; Ibañez Moro, A.V. Soil seed banks of dry tropical forests under different land management. Forests 2023, 14, 3. [Google Scholar] [CrossRef]
- Fajardo-Cantos, Á.; Peña, E.; Plaza-Álvarez, P.; González-Romero, J.; Moya, D.; González-Camuñas, H.; Díaz, A.; Botella, R.; Lucas-Borja, M.E.; De Las Heras, J. Soil response in a Mediterranean forest ecosystem of Southeast Spain following early prescribed burning. Heliyon 2024, 10, e37948. [Google Scholar] [CrossRef]
- Soares, V.C.; Scremin-Dias, E.; Daibes, L.F.; Damasceno-Junior, G.A.; Pott, A.; de Lima, L.B. Fire has little to no effect on the enhancement of germination, but buried seeds may survive in a Neotropical wetland. Flora 2021, 278, 151801. [Google Scholar] [CrossRef]
- Torres, R.C.; Giorgis, M.A.; Trillo, C.; Volkmann, L.; Demaio, P.; Heredia, J.; Renison, D. Post-fire recovery occurs overwhelmingly by resprouting in the Chaco Serrano forest of Central Argentina. Austral Ecol. 2014, 39, 346–354. [Google Scholar] [CrossRef]
- Lipoma, M.L.; Funes, G.; Díaz, S. Fire effects on the soil seed bank and post-fire resilience of a semi-arid shrubland in central Argentina. Austral Ecol. 2017, 43, 46–55. [Google Scholar] [CrossRef]
- Abdala, N.R. Banco de Semillas del Suelo de Especies Leñosas de un Bosque Nativo del Chaco Semiárido. Master’s Thesis, Maestría en Desarrollo de Zonas Áridas y Semiáridas Facultad de Agronomía y Agroindustrias. Universidad Nacional de Santiago del Estero, Santiago del Estero, Argentina, 2016. [Google Scholar]
- Tessema, Z.K.; Ejigu, B.; Nigatu, L. Tree species determine soil seed bank composition and its similarity with understory vegetation in a semi-arid African savanna. Ecol. Process. 2017, 6, 9. [Google Scholar] [CrossRef][Green Version]
- Lipoma, M.L.; Cuchietti, A.; Gorne, L.D.; Díaz, S. Not gone with the wind: Vegetation complexity increases seed retention during windy periods in the Argentine Semiarid Chaco. J. Veg. Sci. 2019, 30, 542–552. [Google Scholar] [CrossRef]
- Kwiatkowska-Falińska, A.; Jankowska-Błaszczuk, M.; Jaroszewicz, B. Post-fire changes of soil seed banks in the early successional stage of pine forest. Pol. J. Ecol. 2014, 62, 455–466. [Google Scholar] [CrossRef]
- Brasil Mendes, L.; Andrade da Silva, K.; Melo dos Santos, D.; Falcao Fraga dos Santos, J.M.; Albuquerque, U.P.; Lima Araújo, E. What happens to the soil seed bank 17 years after clear cutting of vegetations? Rev. Biol. Trop. 2015, 63, 321–332. [Google Scholar] [CrossRef]
- Paula, S.; Pausas, J.G. Burning seeds: Germinative response to heat treatments in relation to resprouting ability. J. Ecol. 2008, 96, 543–552. [Google Scholar] [CrossRef]
- Kunst, C.; Bravo, S. Ecología y régimen de fuego en la región chaqueña argentina. In El Fuego en los Ecosistemas Argentinos; Kunst, C., Bravo, S., Panigatti, J.L., Eds.; Ediciones INTA; Sociedad Argentina de Botánica: Buenos Aires, Argentina, 2003; Chapter 10; pp. 109–118. [Google Scholar]
- Santacruz García, A.C.; Bravo, S.; Del Corro, F.; Ojeda, F. A comparative assessment of plant flammability through a functional approach: The case of woody species from Argentine Chaco region. Austral Ecol. 2019, 44, 1416–1429. [Google Scholar] [CrossRef]
- Silberman, J.E.; Anriquez, A.L.; Dominguez Nuñez, J.A.; Kunst, C.G.; Albanesi, A.S. La cobertura arbórea en un sistema silvopastoril del Chaco y su contribución diferencial al suelo. Cienc. Suelo 2015, 33, 19–29. [Google Scholar]
- Navall, M. Aporte de hojarasca en un quebrachal semiárido santiagueño bajo manejo silvopastoril. In Proceedings of the Actas del II Congreso Nacional de Sistemas Silvopastoriles, Santiago del Estero, Argentina, 9–11 May 2012; pp. 1–6. [Google Scholar]
- Araujo, P.; Iturre, M.C.; Acosta, V.H.; Renolfi, R.F. Estructura del bosque de La María EEA INTA Santiago del Estero. Rev. Quebracho 2008, 16, 5–19. [Google Scholar]
- Tangney, R.; Merritt, D.J.; Callow, J.N.; Fontaine, J.B.; Miller, B.P.; Seymour, C. Seed traits determine species’ responses to fire under varying soil heating scenarios. Funct. Ecol. 2020, 34, 1967–1978. [Google Scholar] [CrossRef]
- Cornelissen, J.H.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Ter Steege, H.; Morgan, H.D.; Van Der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Pérez Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- de Noir, A.; Bravo, S. Frutos de Leñosas Nativas de Especies Leñosas Nativas de Argentina, 1st ed.; Editorial Universidad Nacional de Santiago del Estero—UNSE Facultad de Ciencias Forestales: Santiago del Estero, Argentina, 2014. [Google Scholar]
- ISTA. International Rules for Seed Testing; ISTA: Bassersdorf, Switzerland, 2015; ISSN 2310-3655. [Google Scholar]
- Souza, M.T.; Souza, M.T.; Panobianco, M. Morphological characterization of fruit, seed and seedling, and seed germination test of Campomanesia guazumifolia. J. Seed Sci. 2018, 40, 75–81. [Google Scholar] [CrossRef]
- Craviotto, R.M.; Arango, M.R.; Gallo, C. Prueba Topografica por Tetrazolio en Soja. In Revista Análisis de Semillas; Suplemento Especial: Santa Fe, Argentina, 2008; 96p, ISSN 1851-9615. N°1. [Google Scholar]
- Alzugaray, C.; Carnevale, N.J.; Salinas, A.R.; Pioli, R. Quality of Aspidosperma quebracho-blanco Schlecht, Seeds. Rev. Quebracho 2006, 13, 26–35. [Google Scholar]
- Ledesma, R.; Kunst, C.; Bravo, S.; Leiva, M.; Lorea, L.; Godoy, J.; Navarrete, V. Developing a prescription for brush control in the Chaco region, effects of combined treatments on the canopy of three native shrub species. Arid Land Res. Manage. 2018, 32, 351–366. [Google Scholar] [CrossRef]
- Alexander, M.E. Calculating and interpreting forest fire intensities. Can. J. Bot. 1982, 60, 349–357. [Google Scholar] [CrossRef]
- Stoof, C.; Moore, D.; Fernandes, P.; Stoorvogel, J. Hot fire, cool soil. Geophys. Lett. 2013, 40, 1534–1539. [Google Scholar] [CrossRef]
- Marino, G.D.; Mas, M.V.; Orlandoni, M.J. Morfología and reconocimiento de las principales especies leñosas nativas de la provincia de Santa Fe, Argentina, en el estado de plántula. Boletín Soc. Argent. Botánica 2008, 43, 67–81. [Google Scholar]
- Ferreras, A.E.; Funes, G.; Galetto, L. The role of seed germination in the invasión process of Honey locust (Gleditsia triacanthos L., Fabaceae): Comparison with a native confamilial. Plant Species Biol. 2015, 30, 126–136. [Google Scholar] [CrossRef]
- Barchuk, A.H.; Campos, E.B.; Oviedo, C.; Díaz, M.D.P. Supervivencia y crecimiento de plántulas de especies leñosas del Chaco Árido sometidas a remoción de la biomasa aérea. Ecol. Austral 2006, 16, 47–61. [Google Scholar]
- Bitterlich, W. The Relascope Idea; Relative Measurements in Forestry; Commonwealth Agricultural Bureaux: Slough, UK, 1984; 242p. [Google Scholar]
- Arturi, M. Evaluación del muestreo por recuento angular de Bitterlich en distintas distribuciones espaciales y diamétricas generadas por simulación. Bosque 2016, 37, 431–437. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat, Version 2017; Grupo InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2017. Available online: http://www.infostat.com.ar (accessed on 14 February 2022).
- Alzugaray, C.; Carnevale, N.J.; Salinas, A.R. Crecimiento en plántulas de Schinopsis balansae Engl. Ecotrópica 2008, 14, 27–35. [Google Scholar]
- Pausas, J.G.; Keeley, J.E. Wildfires as an ecosystem service. Front. Ecol. Environ. 2019, 17, 289–295. [Google Scholar] [CrossRef]
- Giorgis, M.A.; Zeballos, S.R.; Carbone, L.; Zimmermann, H.; von Wehrden, H.; Aguilar, R.; Ferreras, A.E.; Tecco, P.A.; Kowaljow, E.; Barri, F.; et al. A review of fire effects across South American ecosystems: The role of climate and time since fire. Fire Ecol. 2021, 17, 11. [Google Scholar]
- Venier, P.; Cabido, M.; Funes, G. Germination characteristics of five coexisting neotropical species of Acacia in seasonally dry Chaco forests in Argentina. Plant Species Biol. 2017, 32, 134–146. [Google Scholar] [CrossRef]
- Miranda, A.C.; Miranda, H.S.; Dias, I.F.O.; Dias, B.F.S. Soil and air temperatures during prescribed Cerrado fires in Central Brazil. J. Trop. Ecol. 1993, 9, 313–320. [Google Scholar] [CrossRef]
- Moreira, B.; Pausas, J.G. Tanned or burned: The role of fire in shaping physical seed dormancy. PLoS ONE 2012, 7, e51523. [Google Scholar] [CrossRef] [PubMed]
- Auld, T.D.; Denham, A.J. How much seed remains in the soil after a fire? Plant Ecol. 2006, 187, 15–24. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Plaza-Álvarez, P.A.; Gonzalez-Romero, J.; Sagra, J.; Alfaro-Sánchez, R.; Zema, D.A.; Moya, D.; de las Heras, J. Short-term effects of prescribed burning in Mediterranean pine plantations on surface runoff, soil erosion and water quality of runoff. Sci. Total Environ. 2019, 674, 615–622. [Google Scholar] [CrossRef]
- Trucco, C.E.; Caziani, S.M. Remoción de semillas en un borde inducido por un incendio forestal en el Chaco semiárido argentino. Ecosistemas 2008, 17, 123–133. [Google Scholar]
- Tálamo, A.; Barchuk, A.H.; Garibaldi, L.A.; Trucco, C.E.; Cardozo, S.; Mohr, F. Disentangling the effects of shrubs and herbivores on tree regeneration in a dry Chaco forest (Argentina). Oecologia 2015, 178, 847–854. [Google Scholar] [CrossRef]
- Trigo, C.B. Efecto de la Exclusión de Ganado Doméstico Sobre la Estructura y Funcionalidad de Una Comunidad Vegetal en el Bosque Chaqueño Semiárido. Ph.D. Thesis, Facultad de Ciencias Exactas Físicas y Naturales, Universidad Nacional de Córdoba, Córdoba, Argentina, 2018. [Google Scholar]
- Barchuk, A.H.; del Pilar Díaz, M. Regeneration and structure of Aspidosperma quebracho-blanco Schl. in the Arid Chaco (Córdoba, Argentina). For. Ecol. Manag. 1999, 118, 31–36. [Google Scholar] [CrossRef]
- Araoz, S.D.; Del Longo, O.; Karlin, O. Seed Germination of Zizyphus Mistol Grisebach. Parametric Correlations of Size and Weight of Drupes, Endocarps and Seeds With Germination and Vigor. Multequina 2004, 13, 45–50. [Google Scholar]
- Araoz, S.D.; Del Longo, O. Pregerminative treatments to break the physical dormancy imposed for the endocarps in Ziziphus mistol Grisebach. Quebracho Rev. Cienc. For. 2006, 13, 56–65. [Google Scholar]
- Bertuzzi, T. Conservación Ex Situ de Especies Leñosas Con Valor de Uso del Chaco Catamarqueño. Ph.D. Thesis, Facultad de Ciencias Naturales, Universidad Nacional de Salta, Salta, Argentina, 2024. [Google Scholar]
- Daibes, L.F.; Pausas, J.G.; Bonani, N.; Nunes, J.; Silveira, F.A.O.; Fidelis, A. Fire and legume germination in a tropical savanna: Ecological and historical factors. Ann. Bot. 2019, 123, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Casillo, J.; Kunst, C.; Semmartin, M. Effects of fire and water availability on the emergence and recruitment of grasses, forbs and woody species in a semiarid Chaco savanna. Austral Ecol. 2012, 37, 452–459. [Google Scholar] [CrossRef]
- Carbone, L.M.; Aguirre-Acosta, N.; Tavella, J.; Aguilar, R. Cambios florísticos inducidos por la frecuencia de fuego en el Chaco Serrano. Boletín Soc. Argent. Botánica 2017, 52, 753–778. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).