Ecological, Anatomical, and Genomic Insights into the Rare Tree Species Fraxinus sogdiana, Celtis caucasica, and Betula jarmolenkoana from the Northern Tien Shan
Abstract
1. Introduction
2. Materials and Methods
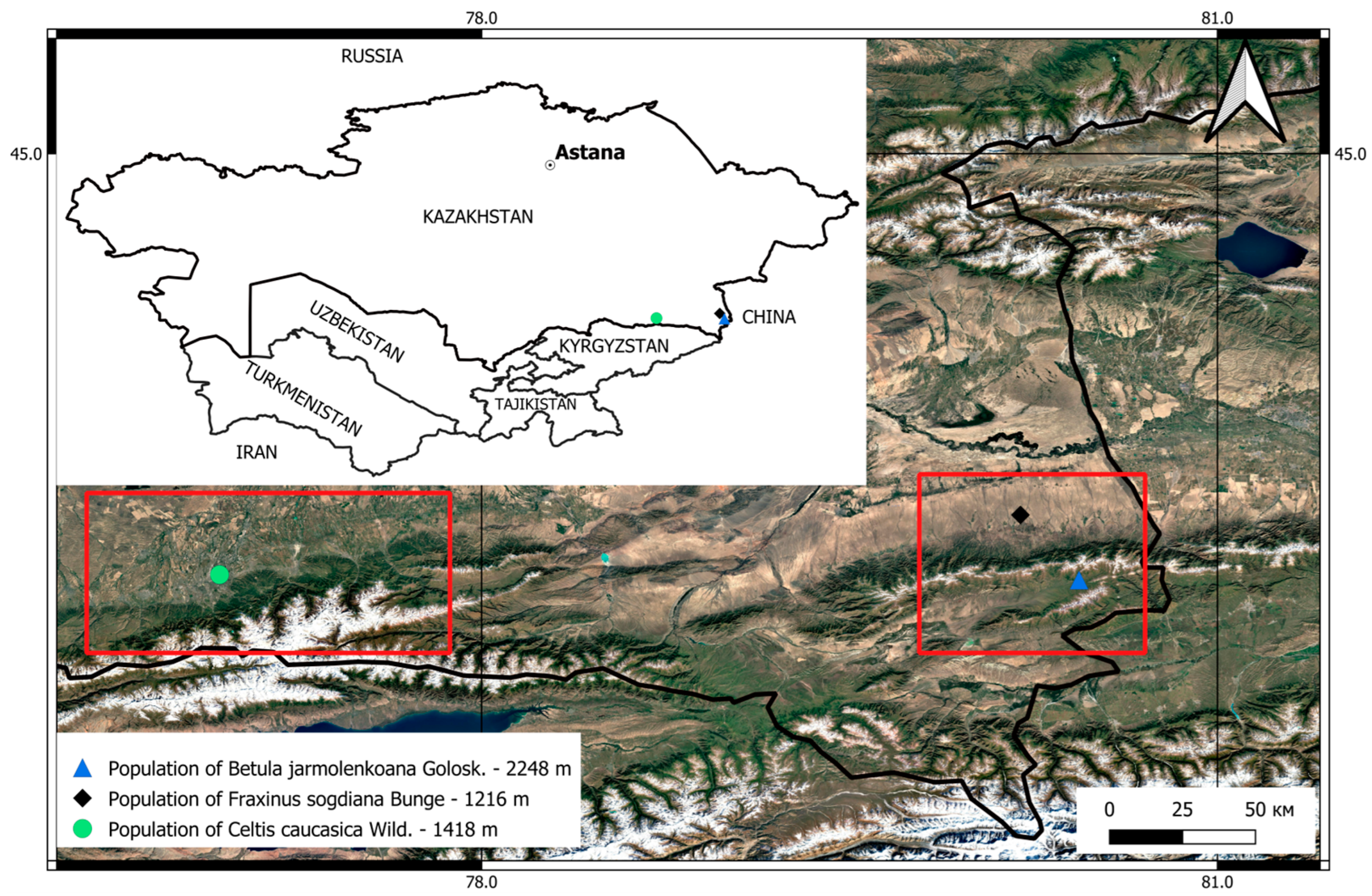
2.1. Plant Material, Geobotanical Survey, and Population Assessment
2.2. Morphological and Anatomical Analysis
2.3. DNA Extraction and Chloroplast Genome Assembly
3. Results
3.1. Bioecological Characteristics of the Studied Species
3.1.1. Population of F. sogdiana
3.1.2. Population of C. caucasica
3.1.3. B. jarmolenkoana Population
3.2. Anatomical Structure
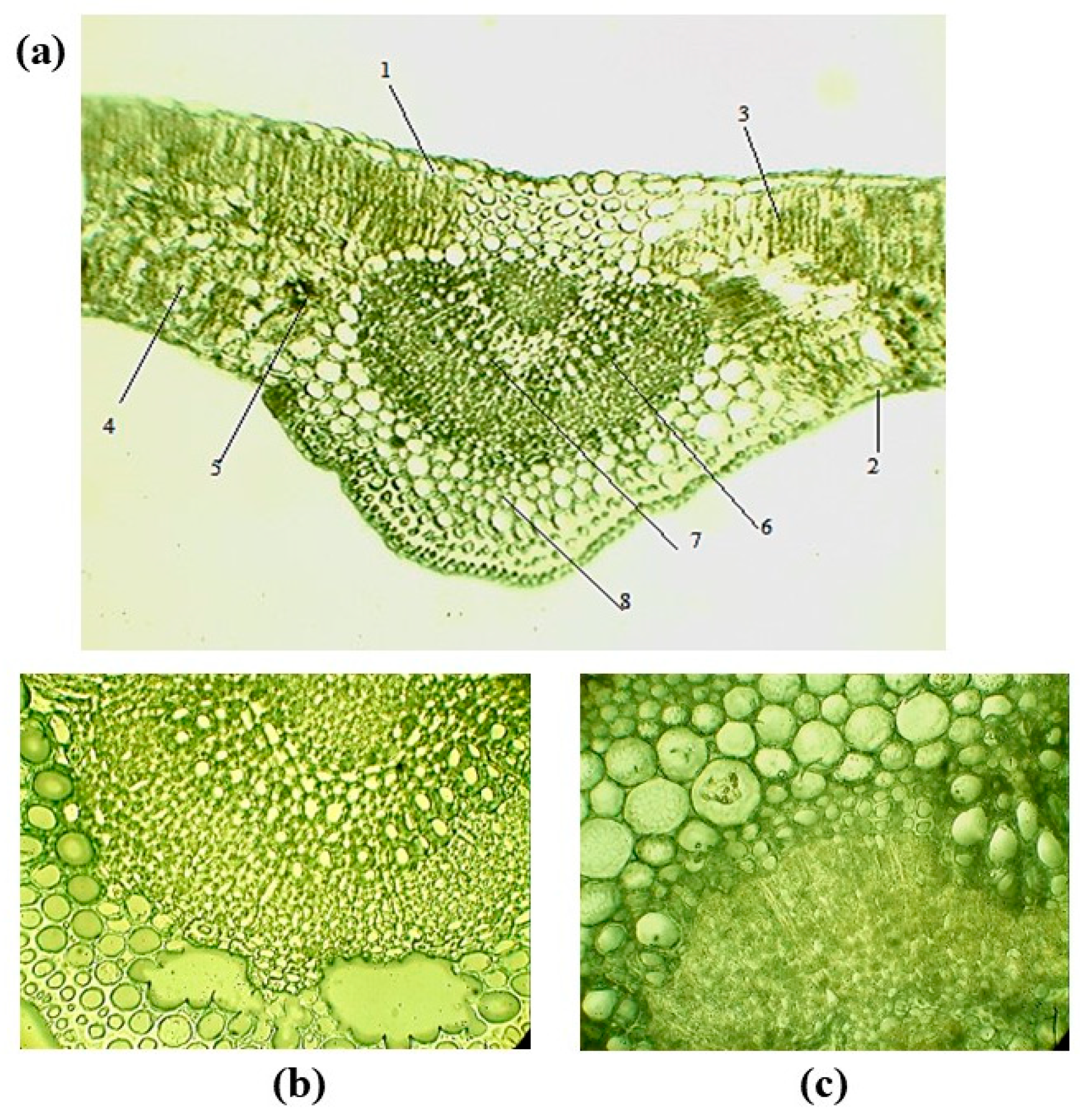
3.2.1. Leaf Anatomy of F. sogdiana
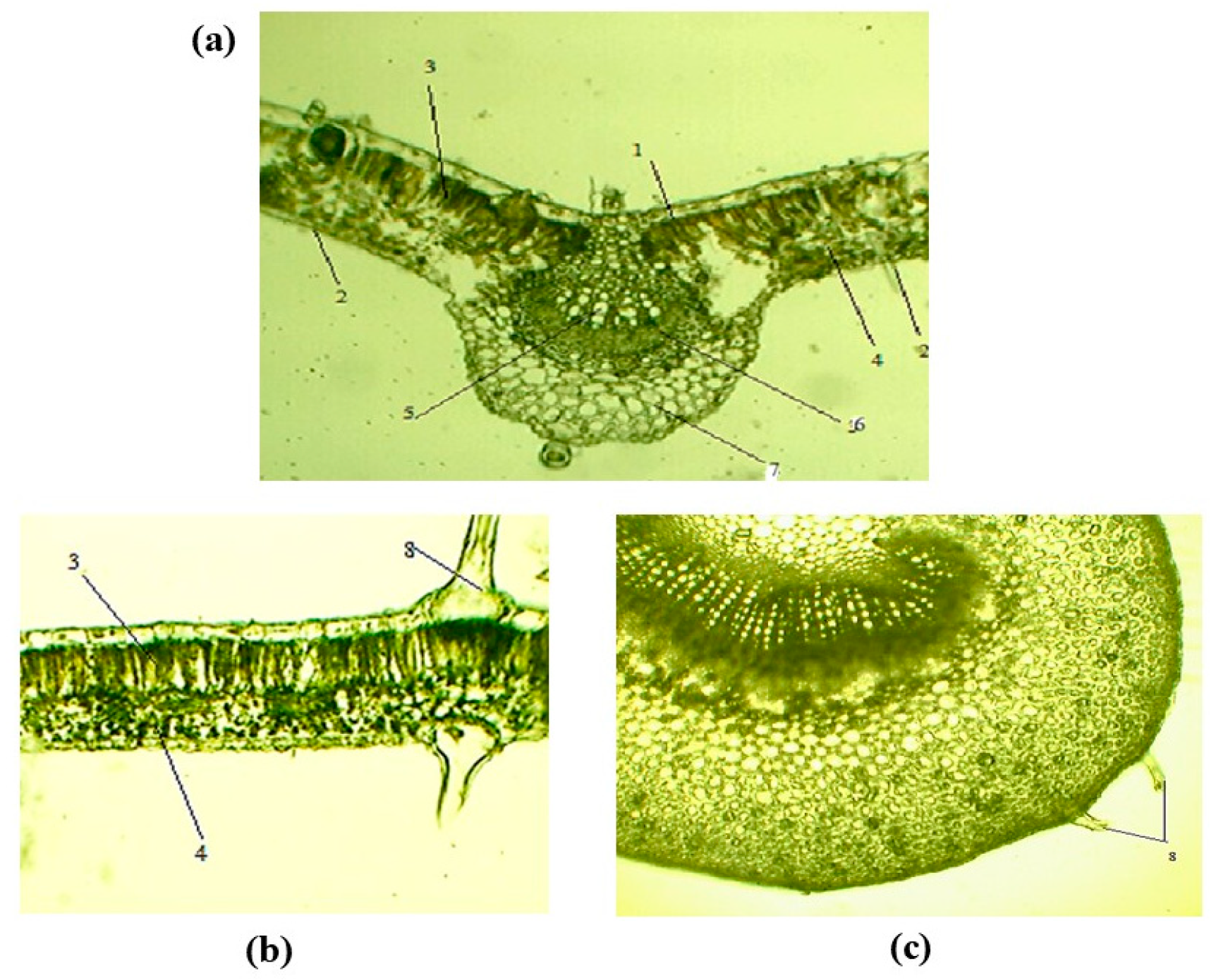
3.2.2. Leaf Anatomy of C. caucasica
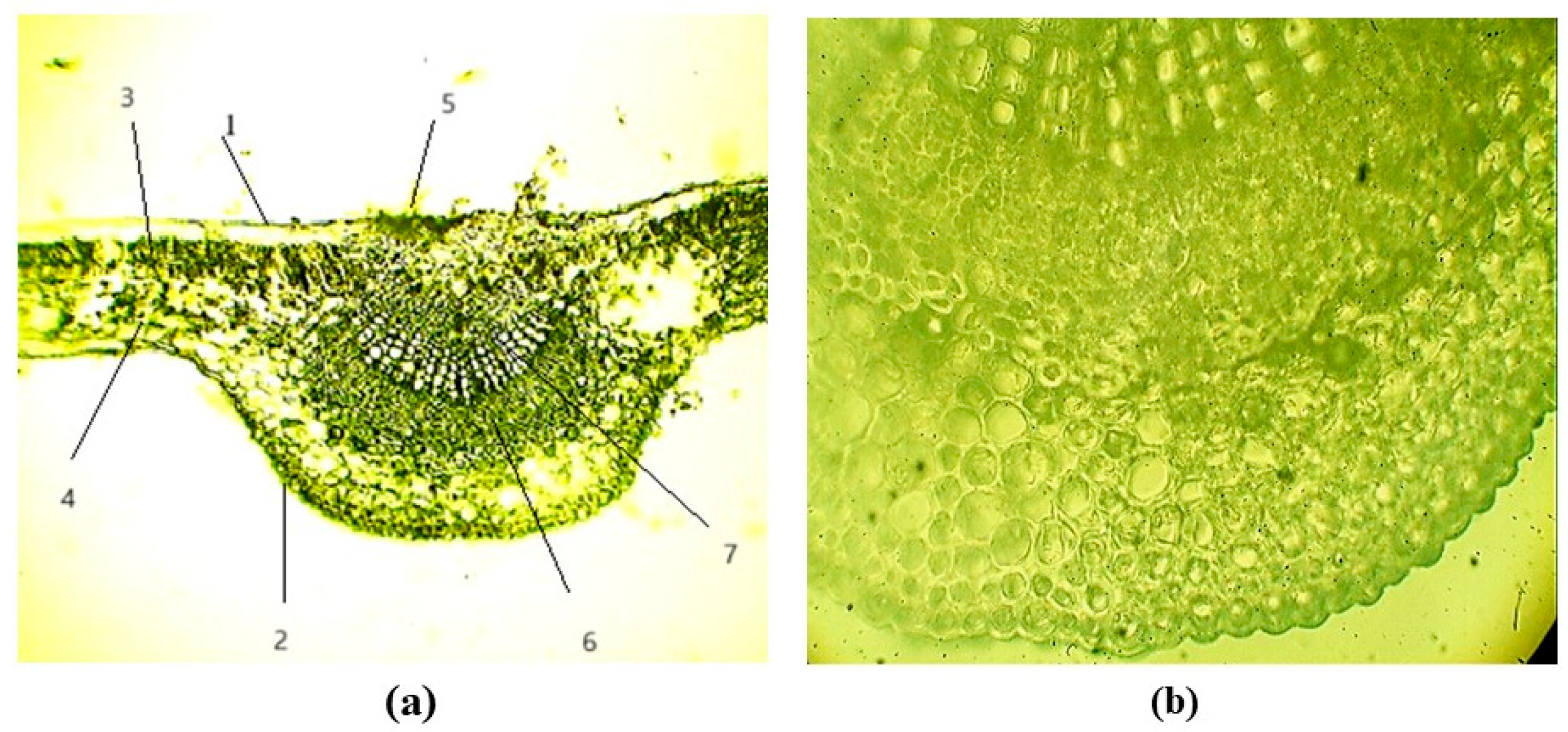
3.2.3. Leaf Anatomy of B. jarmolenkoana
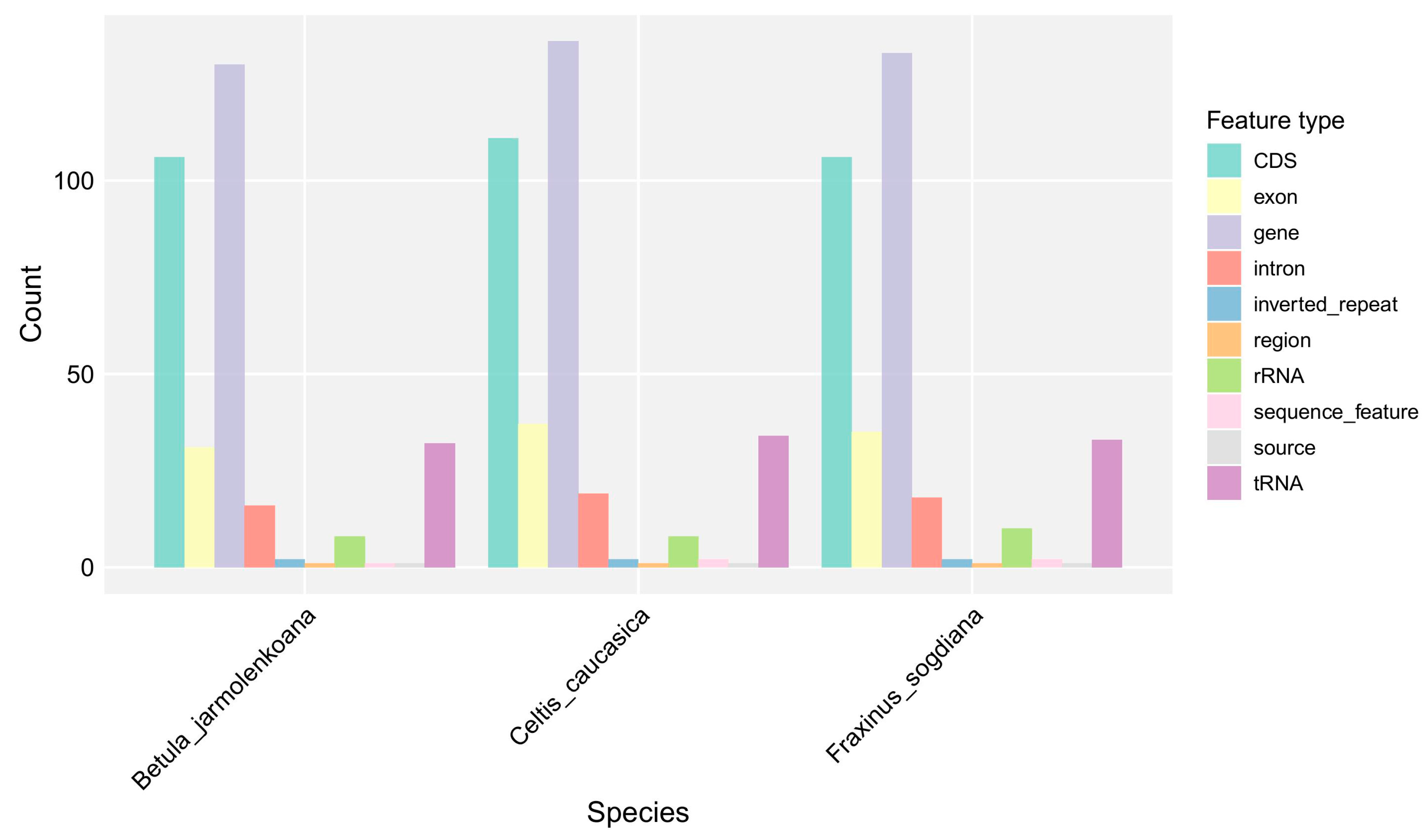
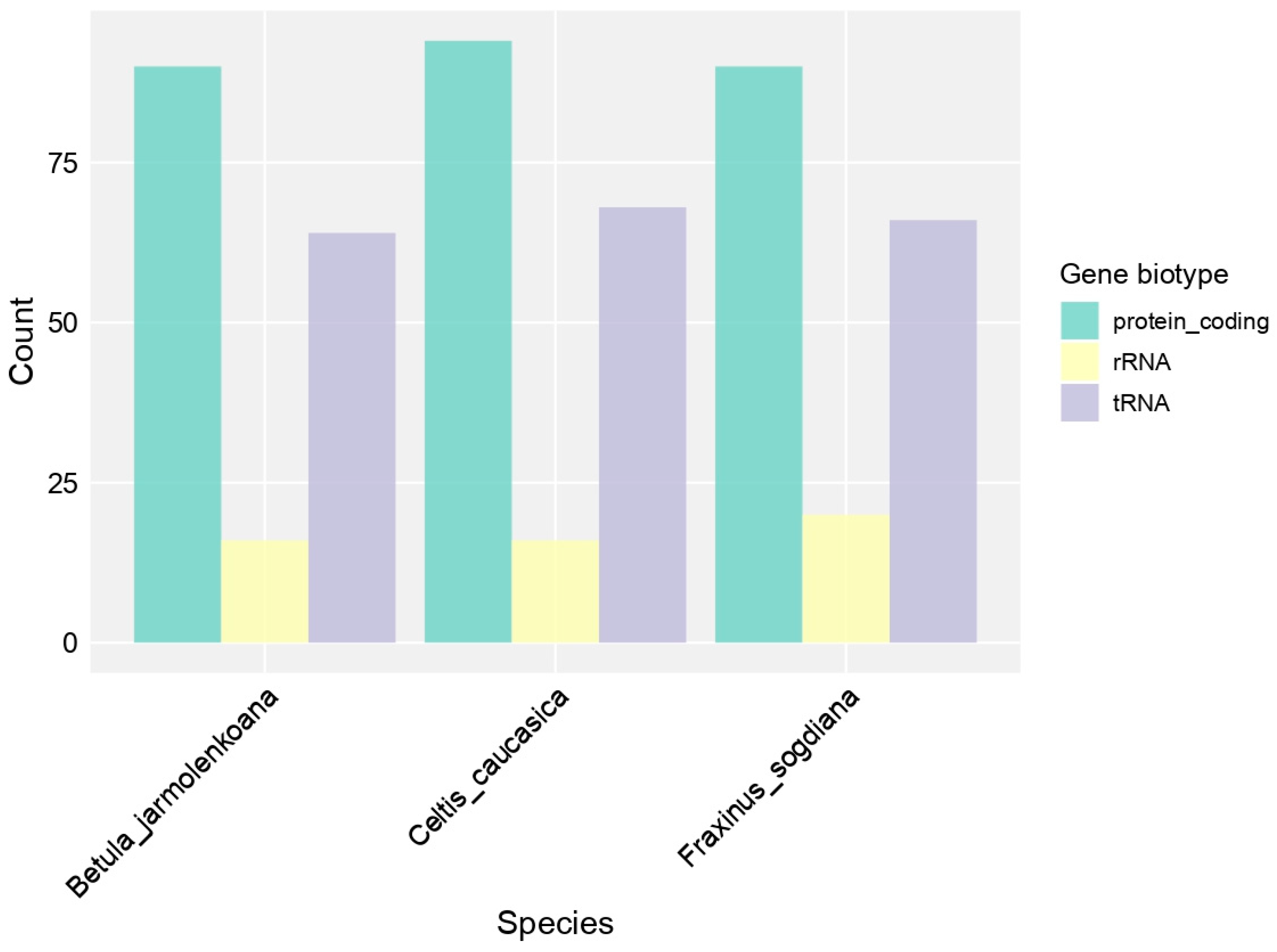
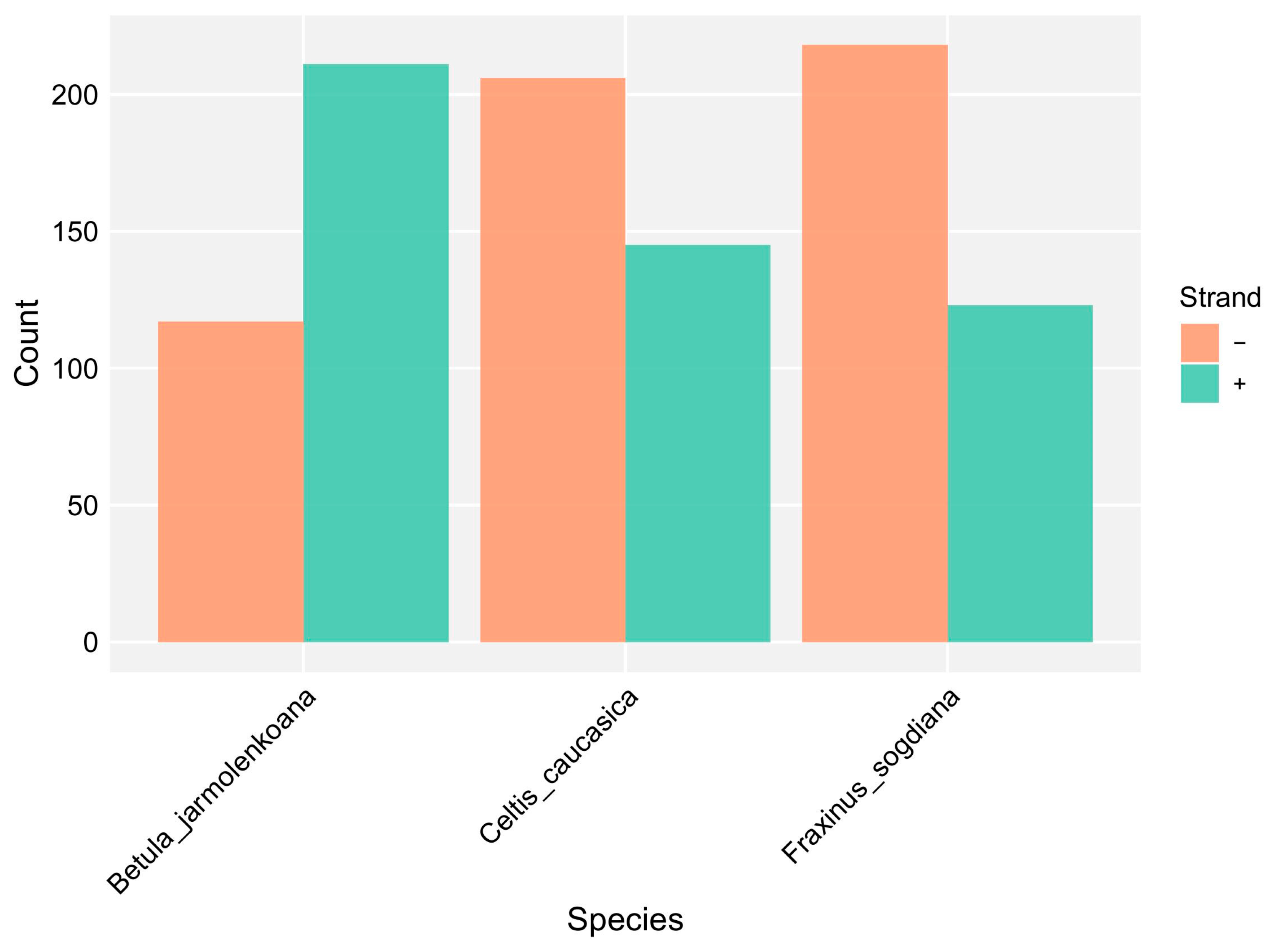
3.3. Chloroplast Genome Structure and Comparative Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kubentayev, S.A.; Alibekov, D.T.; Perezhogin, Y.V.; Lazkov, G.A.; Kupriyanov, A.N.; Ebel, A.L.; Izbastina, K.S.; Borodulina, O.V.; Kubentayeva, B.B. Revised Checklist of Endemic Vascular Plants of Kazakhstan. PhytoKeys 2024, 238, 241–279. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D.; Li, W.; Li, Y.; Zhang, C.; Guan, K.; Pan, B. Characteristics and Utilization of Plant Diversity and Resources in Central Asia. Reg. Sustain. 2020, 1, 1–10. [Google Scholar] [CrossRef]
- Sorg, A.; Bolch, T.; Stoffel, M.; Solomina, O.; Beniston, M. Climate Change Impacts on Glaciers and Runoff in Tien Shan (Central Asia). Nat. Clim. Change 2012, 2, 725–731. [Google Scholar] [CrossRef]
- Bolch, T. Climate Change and Glacier Retreat in Northern Tien Shan (Kazakhstan/Kyrgyzstan) Using Remote Sensing Data. Glob. Planet. Change 2007, 56, 1–12. [Google Scholar] [CrossRef]
- Talipova, E.; Shrestha, S.; Alimkulov, S.; Nyssanbayeva, A.; Tursunova, A.; Isakan, G. Influence of Climate Change and Anthropogenic Factors on the Ile River Basin Streamflow, Kazakhstan. Arab. J. Geosci. 2021, 14, 1756. [Google Scholar] [CrossRef]
- Salnikov, V.; Turulina, G.; Polyakova, S.; Petrova, Y.; Skakova, A. Climate Change in Kazakhstan during the Past 70 Years. Quat. Int. 2015, 358, 77–82. [Google Scholar] [CrossRef]
- Chen, J.; Wan, S.; Henebry, G.; Qi, J.; Gutman, G.; Sun, G.; Kappas, M. Dryland East Asia: Land Dynamics amid Social and Climate Change; Walter de Gruyter: Berlin, Germany, 2013; ISBN 3-11-028791-9. [Google Scholar]
- Baitulin, I. The Red Book of Kazakhstan (Plants); Art Print XXI: Astana, Kazakhstan, 2014; Volume 2. [Google Scholar]
- Shynybekov, M.K.; Abayeva, K.T.; Rakymbekov, Z.K.; Serikbayea, A.T.; Toktasinova, F.A. Study of Natural Regeneration of Sogdian Ash (Fraxinus sogdiana Bunge) and Silvicultural Measures to Promote It in the Sharyn River Floodplain of Almaty Region. Evergreen 2023, 10, 820–829. [Google Scholar] [CrossRef]
- Drenkhan, R.; Adamson, K.; Hanso, M. Fraxinus sogdiana, a Central Asian Ash Species, Is Susceptible to Hymenoscyphus fraxineus. Plant Prot. Sci. 2015, 51, 150–152. [Google Scholar] [CrossRef]
- Aldibekova, A.; Kurmanbayeva, M.; Aksoy, A.; Permitina, V.; Dimeyeva, L.; Zverev, N. Anatomical Structure and Phytochemical Composition of a Rare Species Fraxinus sogdiana Bunge (Oleaceae) Growing in Different Soils in Kazakhstan. Diversity 2023, 15, 769. [Google Scholar] [CrossRef]
- Salmurzauly, R.; Myrzagaliyeva, A.; Irsaliyev, S.; Samarkhanov, T.; Orazov, A.; Nurtazin, S.; Altynbek, M.; Sagynysh, B. Fraxinus sogdiana Bunge Forests in Charyn Canyon, Kazakhstan. Casp. J. Environ. Sci. 2024, 22, 1117–1131. [Google Scholar]
- Markhabat, K.; Gulnara, S. Sequencing Conserved Region of Endangered Species Celtis Caucasica Willd; EDP Sciences: Kemerovo, Russia, 2021; Volume 31, p. 9. [Google Scholar]
- Batsatsashvili, K.; Mehdiyeva, N.P.; Kikvidze, Z.; Khutsishvili, M.; Maisaia, I.; Sikharulidze, S.; Tchelidze, D.; Alizade, V.M.; Zambrana, N.Y.P.; Bussmann, R.W. Celtis caucasica Willd. Cannabaceae. In Ethnobotany of the Caucasus; Springer: Berlin/Heidelberg, Germany, 2017; pp. 183–186. ISBN 3-319-49412-0. [Google Scholar]
- Sepahvand, T.; Etemad, V.; Matinizade, M.; Shirvany, A. Symbiosis of AMF with Growth Modulation and Antioxidant Capacity of Caucasian Hackberry (Celtis caucasica L.) Seedlings under Drought Stress. Cent. Asian J. Environ. Sci. Technol. Innov. 2021, 2, 20–35. [Google Scholar]
- Eastwood, A.; Lazkov, G.; Newton, A.C. The Red List of Trees of Central Asia; Fauna & Flora International Cambridge: Cambridge, UK, 2009; ISBN 1-903703-27-1. [Google Scholar]
- Ashburner, K.; McAllister, H.A. The Genus Betula: A Taxonomic Revision of Birches. Reprinted with Corrections, 2016; Kew Publishing: Richmond, UK, 2016; ISBN 1-84246-141-9. [Google Scholar]
- Sadyrova, G.; Shimshikov, B.; Tynybekov, B.; Nurmakhanova, A.; Orazbekova, K.; Tastybay, M.; Imanaliyeva, M.; Bekbossyn, N. Ecological and Biological Features of Some Rare and Endemic Plant Species of South-East Kazakhstan. Bull. LN Gumilyov Eurasian Natl. Univ. Chem. Geogr. Ecol. Ser. 2024, 149, 166–184. [Google Scholar] [CrossRef]
- Shaw, K.; Stritch, L.; Rivers, M.; Roy, S.; Wilson, B.; Govaerts, R. Betulaceae. In Flowering Plants Dicotyledons; Botanic Gardens Conservation International: Richmond, UK, 2014. [Google Scholar]
- Rakymbekov, Z.; Abayeva, K.; Dosmanbetov, D.; Akhmetov, R.; Shynybekov, M. Relationship between Laboratory Germination and Biometric Parameters of Betula jarmolenkoana Golosk. Aglets. OnLine J. Biol. Sci. 2023, 23, 117–123. [Google Scholar] [CrossRef]
- Flora of Kazakhstan; Academy of Sciences of the Kazakh SSR: Almaty, Kazakhstan, 1956; Volume 1–9.
- Rastenii, O.; Azii, S. Key to Identification of the Plants of Central Asia; Fan: Tashkent, Uzbekistan, 1968; Volume 1–10. [Google Scholar]
- Illustrated Identifier of Plants of Kazakhstan; Academy of Sciences of the Kazakh SSR: Alma-Ata, Kazakhstan, 1962; Volume 1–2.
- Abdulina, S.A. Spisok Sosudistyh Rastenij Kazahstana (List of Vascular Plants of Kazakhstan); Steka: Almaty, Kazakhstan, 1998. [Google Scholar]
- Cherepanov, S.K. Vascular Plants of Russia and Neighboring States (Within the Former USSR); Mir I Semya: St. Petersburg, Russia, 1995. [Google Scholar]
- Rabotnov, T.A. Life Cycle of Perennial Herbaceous Plants in Meadow Cenoses. Proc. Komar. Bot. Inst. Acad. Sci. USSR 1950, 3, 7–204. [Google Scholar]
- Gatsuk, L.; Smirnova, O.; Vorontzova, L.; Zaugolnova, L.; Zhukova, L. Age States of Plants of Various Growth Forms: A Review. J. Ecol. 1980, 68, 675–696. [Google Scholar] [CrossRef]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Being the Collected Papers of C. Raunkear; Oxford Clarendon Press: Oxford, UK, 1934. [Google Scholar]
- Serebryakov, I.G. Ecological Morphology of Plants; Vyssh. Shk.: Moscow, Russia, 1962. [Google Scholar]
- Prozina, M.N. Botanical Microtechnics; Moscow State University: Moscow, Russia, 1960. [Google Scholar]
- Barykina, R.; Veselova, T.; Devyatov, A. Spravochnik Po Botanicheskoy Mikrotekhnike [Handbook of Botanical Microtechnology] (Fundamentals and Methods)—M.; Moscow State University: Moscow, Russia, 2004; 313p. [Google Scholar]
- Jin, J.-J.; Yu, W.-B.; Yang, J.-B.; Song, Y.; DePamphilis, C.W.; Yi, T.-S.; Li, D.-Z. GetOrganelle: A Fast and Versatile Toolkit for Accurate de Novo Assembly of Organelle Genomes. Genome Biol. 2020, 21, 241. [Google Scholar] [CrossRef]
- Tillich, M.; Lehwark, P.; Pellizzer, T.; Ulbricht-Jones, E.S.; Fischer, A.; Bock, R.; Greiner, S. GeSeq–Versatile and Accurate Annotation of Organelle Genomes. Nucleic Acids Res. 2017, 45, W6–W11. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.P.; Lowe, T.M. tRNAscan-SE: Searching for tRNA Genes in Genomic Sequences; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 1-4939-9172-8. [Google Scholar]
- Greiner, S.; Lehwark, P.; Bock, R. OrganellarGenomeDRAW (OGDRAW) Version 1.3. 1: Expanded Toolkit for the Graphical Visualization of Organellar Genomes. Nucleic Acids Res. 2019, 47, W59–W64. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing. Agric. Sci. 2021, 12, 12.
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2; Use R!; Springer International Publishing: Cham, Germany, 2016; ISBN 978-3-319-24275-0. [Google Scholar]
- Garnier, S.; Ross, N.; Rudis, R.; Camargo, A.; Sciaini, M.; Scherer, C. Viridis (Lite)—Colorblind-Friendly Color Maps for R. Viridis Package Version 0.6.5. Zenodo10 2023, 5281. [Google Scholar] [CrossRef]
- Sadyrova, G.; Taskuzhina, A.; Pozharskiy, A.; Orazbekova, K.; Yanin, K.; Kerimbek, N.; Zhamilova, S.; Kamiyeva, G.; Tanybaeva, A.; Gritsenko, D. Insights into Biological and Ecological Features of Four Rare and Endemic Plants from the Northern Tian Shan (Kazakhstan). Plants 2025, 14, 2305. [Google Scholar] [CrossRef]
- Alves, F.; Banks, S.C.; Edworthy, M.; Stojanovic, D.; Langmore, N.E.; Heinsohn, R. Using Conservation Genetics to Prioritise Management Options for an Endangered Songbird. Heredity 2023, 130, 289–301. [Google Scholar] [CrossRef]
- Huy, N.; Hung, H.; Buckney, R.; De Filippis, L. Molecular Biodiversity Convergence with Biogeography and Ethnobotany of Rare and Endangered Medicinal Plants from Northern Vietnam. In Plant and Human Health, Volume 1: Ethnobotany and Physiology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–52. [Google Scholar]
- Zhen, J.-H.; Liu, G.-H. Research Advance in Rare and Endemic Plant Tetraena mongolica Maxim. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2008, 19, 433–440. [Google Scholar]
- Dongyan, X. Conservation of Rare and Endangered Plants in Dabashan Nature Reserve. J. Anhui Agric. Univ. 2004, 31, 469–474. [Google Scholar]
- Daniell, H.; Lin, C.-S.; Yu, M.; Chang, W.-J. Chloroplast Genomes: Diversity, Evolution, and Applications in Genetic Engineering. Genome Biol. 2016, 17, 134. [Google Scholar] [CrossRef]
- Shang, C.; Li, E.; Yu, Z.; Lian, M.; Chen, Z.; Liu, K.; Xu, L.; Tong, Z.; Wang, M.; Dong, W. Chloroplast Genomic Resources and Genetic Divergence of Endangered Species Bretschneidera sinensis (Bretschneideraceae). Front. Ecol. Evol. 2022, 10, 873100. [Google Scholar] [CrossRef]
- Adhikari, B.; Parajuli, S.; Nepal, M.P. Reporting Complete Chloroplast Genome of Endangered Red Mulberry, Useful for Understanding Hybridization and Phylogenetic Relationships. Sci. Rep. 2025, 15, 13403. [Google Scholar] [CrossRef]
- Liu, H.; Jia, Y.; Xie, X.; Yang, F.; Zhang, W.; Yang, Y. Cespitose Population Structure and Dynamics of Rare Fraxinus sogdiana in the Yili River Valley, China. Forests 2025, 16, 567. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, D.; Chen, S.; Chen, S. Transcriptome Sequencing Analysis of Birch (Betula platyphylla Sukaczev) under Low-Temperature Stress. Forests 2020, 11, 970. [Google Scholar] [CrossRef]
- Luo, Y.; Cheng, J.; Yan, X.; Zhang, J.; Zhang, J. Germination of Seeds Subjected to Temperature and Water Availability: Implications for Ecological Restoration. Forests 2022, 13, 1854. [Google Scholar] [CrossRef]
- KIEW, R.; Ibrahim, C.S. Comparative Study of Leaf Anatomy of Malaysian Species of Chionanthus and Olea (Oleaceae) with Special Reference to Foliar Sclereids. Bot. J. Linn. Soc. 1982, 84, 79–102. [Google Scholar] [CrossRef]
- Abdinazarov, S.K.; Duschanova, G.M. Structural Features of the Leaf and Fruit of the Emerald Variety Olea europaea L., Growing under the Introduction Conditions of Surkhandarya. Am. J. Plant Sci. 2020, 11, 554–563. [Google Scholar] [CrossRef]
- Kolb, K.L.; Gomes, S.M.; Lombardi, J.A. Leaf Anatomy as a Taxonomy Tool for the Identification of Brazilian Native Species of Chionanthus (Oleaceae). Flora 2020, 266, 151590. [Google Scholar] [CrossRef]
- Zamengo, H.B.; Ferreira, D.A.S.; Leme, F.M.; Arruda, R.C.O. Leaf Anatomy of Celtis iguanaea (Cannabaceae): A Contribution to the Classification and Taxonomy of the Species. Rodriguésia 2025, 76, e00712024. [Google Scholar] [CrossRef]
- Ashton, P.M.S.; Olander, L.P.; Berlyn, G.P.; Thadani, R.; Cameron, I.R. Changes in Leaf Structure in Relation to Crown Position and Tree Size of Betula papyrifera within Fire-Origin Stands of Interior Cedar-Hemlock. Can. J. Bot. 1998, 76, 1180–1187. [Google Scholar] [PubMed]
- Franco Ortega, S.; Bedford, J.A.; James, S.R.; Newling, K.; Ashton, P.D.; Boshier, D.H.; Clark, J.; Hartley, S.E.; Harper, A.L. Fraxinus Excelsior Updated Long-Read Genome Reveals the Importance of MADS-Box Genes in Tolerance Mechanisms against Ash Dieback. G3: Genes Genomes Genet. 2025, 15, jkaf053. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Chen, Y.; Luo, M.; Liang, Z.; Nong, X. The Chloroplast Genome Characteristics, Comparative Genomics and Gene Resource Mining of Celtis sinensis(Persoon, 1805). Mitochondrial DNA Part B 2022, 7, 698–704. [Google Scholar] [CrossRef]
- Wang, S.; Yang, C.; Zhao, X.; Chen, S.; Qu, G.-Z. Complete Chloroplast Genome Sequence of Betula platyphylla: Gene Organization, RNA Editing, and Comparative and Phylogenetic Analyses. BMC Genom. 2018, 19, 950. [Google Scholar] [CrossRef] [PubMed]



| Age Group | Number of Individuals | Leaf Length (cm) ± SD | Plant Height (cm) ± SD |
|---|---|---|---|
| Juvenile (j) | 15 | 4.00 ± 1.00 | 30.0 ± 5.0 |
| Immature (i) | 20 | 7.67 ± 0.58 | 66.67 ± 15.28 |
| Virginile (v) | 34 | 16.00 ± 3.61 | 316.67 ± 47.26 |
| Generative (g) | 70 | 17.33 ± 2.52 | 533.33 ± 76.38 |
| Age Group | Number of Individuals | Leaf Length (cm) ± SD | Plant Height (cm) ± SD |
|---|---|---|---|
| Juvenile (j) | 3 | 2.33 ± 0.58 | 25.00 ± 5.00 |
| Immature (i) | 5 | 3.50 ± 0.71 | 70.00 ± 10.00 |
| Virginile (v) | 4 | 6.00 ± 1.41 | 270.00 ± 20.00 |
| Generative (g) | 5 | 8.00 ± 1.00 | 346.67 ± 25.17 |
| Age Group | Number of Individuals | Leaf Length (cm) ± SD | Plant Height (cm) ± SD |
|---|---|---|---|
| Juvenile (j) | 50 | 1.00 ± 0.27 | 66.67 ± 15.28 |
| Immature (i) | 39 | 2.00 ± 0.35 | 113.33 ± 35.12 |
| Virginile (v) | 73 | 1.92 ± 0.53 | 256.67 ± 90.18 |
| Generative (g) | 113 | 3.00 ± 0.46 | 356.67 ± 60.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadyrova, G.; Taskuzhina, A.; Yanin, K.; Kerimbek, N.; Nurmakhanova, A.; Shaganbek, K.; Bekenova, N.; Orazbekova, K.; Gritsenko, D. Ecological, Anatomical, and Genomic Insights into the Rare Tree Species Fraxinus sogdiana, Celtis caucasica, and Betula jarmolenkoana from the Northern Tien Shan. Forests 2025, 16, 1340. https://doi.org/10.3390/f16081340
Sadyrova G, Taskuzhina A, Yanin K, Kerimbek N, Nurmakhanova A, Shaganbek K, Bekenova N, Orazbekova K, Gritsenko D. Ecological, Anatomical, and Genomic Insights into the Rare Tree Species Fraxinus sogdiana, Celtis caucasica, and Betula jarmolenkoana from the Northern Tien Shan. Forests. 2025; 16(8):1340. https://doi.org/10.3390/f16081340
Chicago/Turabian StyleSadyrova, Gulbanu, Aisha Taskuzhina, Kirill Yanin, Nazym Kerimbek, Akmaral Nurmakhanova, Kusaev Shaganbek, Nazym Bekenova, Kuralai Orazbekova, and Dilyara Gritsenko. 2025. "Ecological, Anatomical, and Genomic Insights into the Rare Tree Species Fraxinus sogdiana, Celtis caucasica, and Betula jarmolenkoana from the Northern Tien Shan" Forests 16, no. 8: 1340. https://doi.org/10.3390/f16081340
APA StyleSadyrova, G., Taskuzhina, A., Yanin, K., Kerimbek, N., Nurmakhanova, A., Shaganbek, K., Bekenova, N., Orazbekova, K., & Gritsenko, D. (2025). Ecological, Anatomical, and Genomic Insights into the Rare Tree Species Fraxinus sogdiana, Celtis caucasica, and Betula jarmolenkoana from the Northern Tien Shan. Forests, 16(8), 1340. https://doi.org/10.3390/f16081340





