Distinguishing the Mechanisms Driving Community Structure Across Different Growth Stages in Quercus Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experiment Design and Surveys
2.3. Statistical Analysis
3. Results
3.1. Results of Phylogenetic Signals for Functional Traits
3.2. Community Phylogenetic and Functional Structure Across Growth Stages
3.3. Variation in Phylogenetic and Functional Structure Along Environmental Gradients
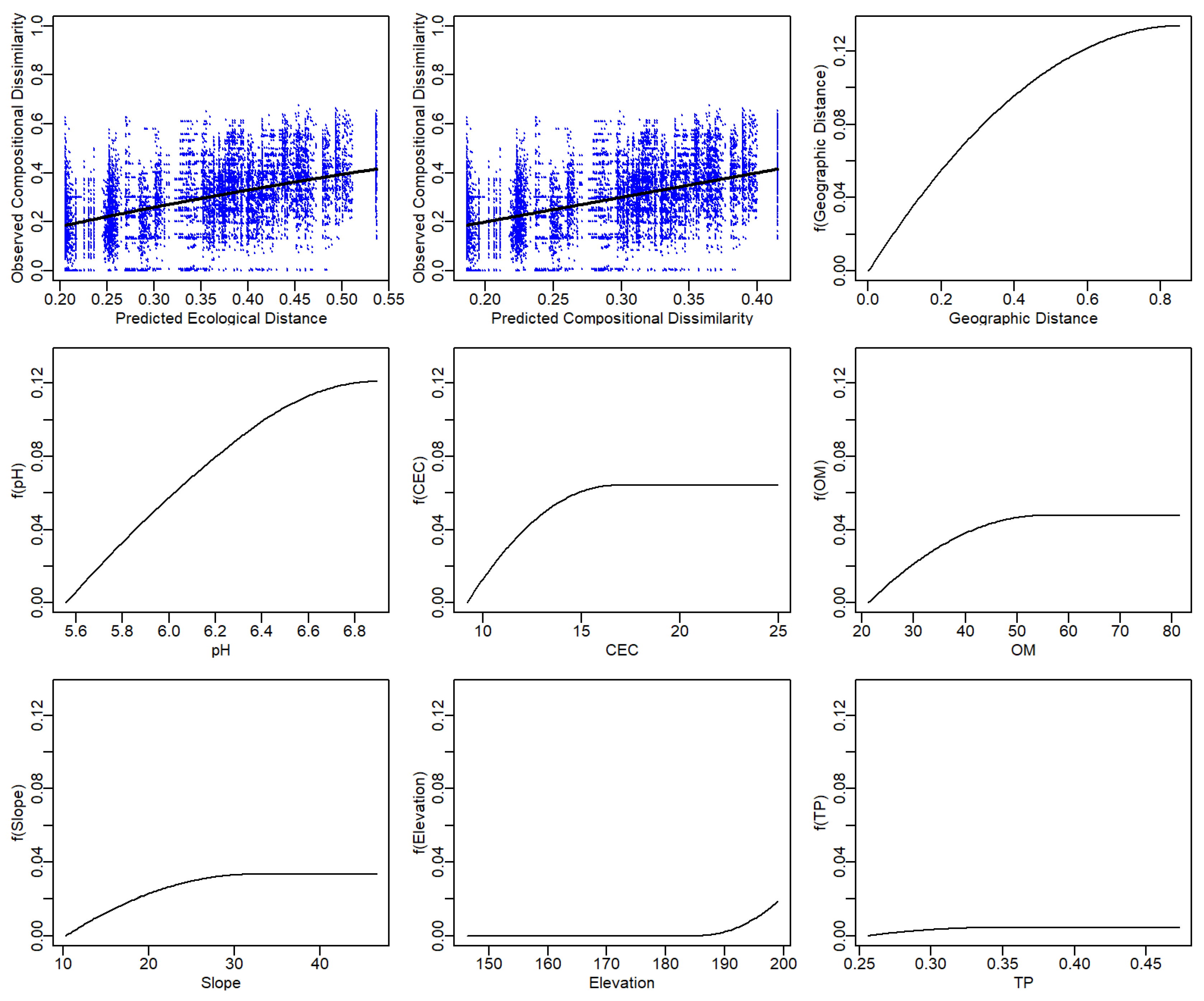
3.4. Drivers of Functional and Phylogenetic β-Diversity by Geographic Distance and Environmental Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Chesson, P. Updates on mechanisms of maintenance of species diversity. J. Ecol. 2018, 106, 1773–1794. [Google Scholar] [CrossRef]
- Mutshinda, C.M.; O’hara, R.B. Integrating the niche and neutral perspectives on community structure and dynamics. Oecologia 2011, 166, 241–251. [Google Scholar] [CrossRef]
- Furniss, T.J.; Larson, A.J.; Lutz, J.A. Reconciling niches and neutrality in a subalpine temperate forest. Ecosphere 2017, 8, e01847. [Google Scholar] [CrossRef]
- Scheffer, M.; Van Nes, E.; Vergnon, R. Toward a unifying theory of biodiversity. Proc. Natl. Acad. Sci. USA 2018, 115, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Kraft, N.J.B.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M. Community assembly, coexistence and the envi-ronmental filtering metaphor. Funct. Ecol. 2015, 29, 592–599. [Google Scholar] [CrossRef]
- Weiher, E.; Clarke, G.D.P.; Keddy, P.A. Community assembly rules, morphological dispersion, and the coexistence of plant species. Oikos 1998, 81, 309–322. [Google Scholar] [CrossRef]
- Hubbell, S.P. The Unified Neutral Theory of Biodiversity and Biogeography; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Bonsall, M.; Hastings, A. Demographic and environmental stochasticity in predator-prey metapopulations. J. Anim. Ecol. 2004, 73, 1043–1055. [Google Scholar] [CrossRef]
- Long, J.; Li, W.; Liao, N.; Qin, Y.; Pan, W.; Yang, T.; Tang, Y.; Liang, S. Study on community assembly mechanism of seasonal rainfor-est in fangcheng based on functional traits and phylogeny. J. Guangxi Acad. Sci. 2024, 40, 126–134. [Google Scholar] [CrossRef]
- Prinzing, A.; Durka, W.; Klotz, S.; Brandl, R. The niche of higher plants: Evidence for phylogenetic conservatism. Proc. Biol. Sci. 2001, 268, 2383–2389. [Google Scholar] [CrossRef]
- Wiens, J.; Graham, C. Niche conservatism: Integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 519–539. [Google Scholar] [CrossRef]
- Večeřa, M.; Axmanová, I.; Chytrý, M.; Divíšek, J.; Ndiribe, C.; Mones, G.V.; Čeplová, N.; Aćić, S.; Bahn, M.; Bergamini, A.; et al. Decoupled phylogenetic and functional diversity in European grasslands. Preslia 2023, 95, 413–445. [Google Scholar] [CrossRef]
- Huang, J.; Yu, R.; Ding, Y.; Xu, Y.; Yao, J.; Zang, R. The relationship between trait-based functional niche hypervolume and community phylogenetic structures of typical forests across different climatic zones in China. Forests 2024, 15, 954. [Google Scholar] [CrossRef]
- Zhao, L.; Xiang, W.; Li, J.; Liu, W.; Hu, Y.; Wu, H.; Zhang, Y.; Cheng, X.; Wang, W.; Wang, W.; et al. “Realistic strategies” and neutral processes drive the community assembly based on leaf functional traits in a subtropical evergreen broad-leaved forest. Ecol. Evol. 2022, 12, e9323. [Google Scholar] [CrossRef] [PubMed]
- Molina Venegas, R.; Ottaviani, G.; Campetella, G.; Canullo, R.; Chelli, S. Biogeographic deconstruction of phylogenetic and functional diversity provides insights into the formation of regional assemblages. Ecography 2022, 2022, e06140. [Google Scholar] [CrossRef]
- Liang, L.; Seibold, S.; Cadotte, M.; Zou, J.Y.; Song, J.; Mo, Z.; Tan, S.; Ye, L.; Zheng, W.; Burgess, K.; et al. Niche convergence and biogeographic history shape elevational tree community assembly in a subtropical mountain forest. Sci. Total Environ. 2024, 935, 173343. [Google Scholar]
- Baldeck, C.; Kembel, S.; Harms, K.; Yavitt, J.; John, R.; Turner, B.; Madawala, S.; Gunatilleke, N.; Gunatilleke, S.; Bunyavejchewin, S.; et al. Phylogenetic turnover along local environmental gradients in tropical forest communities. Oecologia 2016, 182, 547–557. [Google Scholar] [CrossRef]
- González Caro, S.; Umaña, M.; Álvarez, E.; Stevenson, P.; Swenson, N. Phylogenetic alpha and beta diversity in tropical tree assemblages along regional-scale environmental gradients in northwest south America. J. Plant Ecol. 2014, 7, 145–153. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, J.; Li, F.; Niu, J.; Zhang, Q. Taxonomic, functional, and phylogenetic beta diversity in the Inner Mongolia grassland. Glob. Ecol. Conserv. 2021, 28, e01634. [Google Scholar]
- Han, T.; Ren, H.; Hui, D.; Zhu, Y.; Lu, H.; Guo, Q.; Wang, J. Dominant ecological processes and plant functional strategies change during the succession of a subtropical forest. Ecol. Indic. 2023, 146, 109885. [Google Scholar] [CrossRef]
- Poorter, L.; Rozendaal, D.; Bongers, F.; De Almeida-Cortez, J.; Zambrano, A.A.; Álvarez, F.; Andrade, J.; Villa, L.F.A.; Balvanera, P.; Becknell, J.; et al. Wet and dry tropical forests show opposite successional pathways in wood density but converge over time. Nat. Ecol. Evol. 2019, 3, 928–934. [Google Scholar] [CrossRef]
- Luo, Y.H.; Cadotte, M.; Burgess, K.; Liu, J.; Tan, S.; Xu, K.; Li, D.Z.; Gao, L. Forest community assembly is driven by different strata-dependent mechanisms along an elevational gradient. J. Biogeogr. 2019, 46, 2174–2187. [Google Scholar] [CrossRef]
- Shi, N.; Han, Y.; Wang, Q.; Xiao, N.; Quan, Z. Spatial and temporal characteristics of fractional vegetation cover and its response to urbanization in Beijing. Environ. Sci. 2024, 45, 5318–5328. [Google Scholar] [CrossRef]
- Lu, B.; Xing, S.; Cui, G.; Liu, Q. Comparison of species diversity of plant communities in mountains of Beijing. J. Beijing For. Univ. 2010, 32, 36–44. [Google Scholar]
- Yang, J.; Wang, X.; Carmona, C.P.; Wang, X.; Shen, G. Inverse relationship between species competitiveness and intraspecific trait variability may enable species coexistence in experimental seedling communities. Nat. Commun. 2024, 15, 2895. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.D. Soil Agricultural Chemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000; pp. 30–107. [Google Scholar]
- Losos, J.B. Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol Lett. 2008, 11, 995–1003. [Google Scholar] [CrossRef]
- Laiolo, P.; Pato, J.; Jiménez Alfaro, B.; Obeso, J. Evolutionary conservation of within-family biodiversity patterns. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef]
- Blomberg, S.; Garland, T.; Ives, A. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution 2003, 57, 717–745. [Google Scholar] [CrossRef]
- Qian, H.; Jin, Y. An updated megaphylogeny of plants, a tool for generating plant phylogenies and an analysis of phylogenetic community structure. J. Plant Ecol. 2016, 9, 233–239. [Google Scholar] [CrossRef]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and community ecology. J. Annu. Rev. Ecol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Liu, X.; Swenson, N.G.; Zhang, J.; Ma, K. The environment and space, not phylogeny, determine trait dispersion in a subtropical forest. Funct. Ecol. 2013, 27, 264–272. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, D.; Si, X. The application of phylogenetic methods in community ecology. Bio. Protoc. 2021, e1010670. [Google Scholar] [CrossRef]
- Ferrier, S.; Manion, G.; Elith, J.; Richardson, K. Using generalized dissimilarity modelling to analyse and predict patterns of beta diversity in regional biodiversity assessment. Divers. Distrib. 2007, 13, 252–264. [Google Scholar] [CrossRef]
- Fitzpatrick, M.C.; Mokany, K.; Manion, G.; Nieto-Lugilde, D.; Ferrier, S. R Package, Version 1.6; gdm: Generalized Dissimilarity Modeling; 2024. Available online: https://cran.r-project.org/ (accessed on 18 September 2024).
- Asfaw, Z.; Wondimu, M.T.; Yusuf, M.M. Linking process to pattern in community assembly in dry evergreen afromontane forest of hararghe highland, southeast Ethiopia. Eur. J. Ecol. 2023, 9. [Google Scholar] [CrossRef]
- Lebrija Trejos, E.; Pérez-García, E.; Meave, J.; Bongers, F.; Poorter, L. Functional traits and environmental filtering drive community assembly in a species-rich tropical system. Ecology 2010, 91, 386–398. [Google Scholar] [CrossRef]
- Kermavnar, J.; Kutnar, L. Patterns of understory community assembly and plant trait-environment relationships in temperate se European forests. Diversity 2020, 12, 91. [Google Scholar] [CrossRef]
- Ramachandran, A.; Huxley, J.; McFaul, S.; Schauer, L.; Diez, J.; Boone, R.; Madsen Hepp, T.; McCann, E.; Franklin, J.; Logan, D.; et al. Integrating ontogeny and ontogenetic dependency into community assembly. J. Ecol. 2023, 111, 1561–1574. [Google Scholar] [CrossRef]
- Swenson, N.G.; Stegen, J.C.; Davies, S.J.; Erickson, D.L.; Forero-Montaña, J.; Hurlbert, A.H.; Kress, W.J.; Thompson, J.; Uriarte, M.; Wright, S.J.; et al. Temporal turnover in the composition of tropical tree communities: Functional determinism and phylogenetic stochasticity. Ecology 2012, 93, 490–499. [Google Scholar] [CrossRef]
- Singer, D.; Kosakyan, A.; Seppey, C.; Pillonel, A.; Fernández, L.; Fontaneto, D.; Mitchell, E.; Lara, E. Environmental filtering and phylogenetic clustering correlate with the distribution patterns of cryptic protist species. Ecology 2018, 99, 904–914. [Google Scholar] [CrossRef]
- Hai, N.; Erfanifard, Y.; Bui, V.B.; Mai, T.; Petritan, A.; Petrițan, I. Topographic effects on the spatial species associations in diverse heterogeneous tropical evergreen forests. Sustainability 2021, 13, 2468. [Google Scholar] [CrossRef]
- Rodrigues, A.; Villa, P.; Ali, A.; Ferreira-Júnior, W.; Neri, A. Fine-scale habitat differentiation shapes the composition, structure and aboveground biomass but not species richness of a tropical atlantic forest. J. For. Res. 2020, 31, 1599–1611. [Google Scholar] [CrossRef]
- Xu, W.; Ci, X.; Song, C.; He, T.; Zhang, W.; Li, Q.; Li, J. Soil phosphorus heterogeneity promotes tree species diversity and phylogenetic clustering in a tropical seasonal rainforest. Ecol. Evol. 2016, 6, 8719–8726. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Z.; Lin, H.; Deng, R.; Liang, Z.; Li, Y.; Liang, S. Trait-based community assembly and functional strategies across three subtropical karst forests, southwestern China. Front. Plant Sci. 2024, 15, 1451981. [Google Scholar] [CrossRef]
- Li, Y.; Bin, Y.; Xu, H.; Ni, Y.; Zhang, R.; Ye, W.; Lian, J. Understanding community assembly based on functional traits, ontogenetic stages, habitat types and spatial scales in a subtropical forest. Forests 2019, 10, 1055. [Google Scholar] [CrossRef]
- Wei, S.; Li, L.; Lian, J.; Nielsen, S.E.; Wang, Z.; Mao, L.; Ouyang, X.; Cao, H.; Ye, W. Role of the dominant species on the distributions of neighbor species in a subtropical forest. Forests 2020, 11, 352. [Google Scholar] [CrossRef]
- Wang, X.; Wiegand, T.; Anderson-Teixeira, K.J.; Bourg, N.A.; Hao, Z.; Howe, R.; Jin, G.; Orwig, D.A.; Spasojevic, M.J.; Wang, S.; et al. Ecological drivers of spatial community dissimilarity, species replacement and species nestedness across temperate forests. Glob. Ecol. Biogeogr. 2018, 27, 581–592. [Google Scholar] [CrossRef]
- Tian, L.; Letcher, S.G.; Ding, Y.; Zang, R. A ten-year record reveals the importance of tree species’ habitat specialization in driving successional trajectories on Hainan Island, China. For. Ecol. Manag. 2022, 507, 120027. [Google Scholar] [CrossRef]
- Fraaije, R.; Braak, C.; Verduyn, B.; Breeman, L.; Verhoeven, J.; Soons, M. Early plant recruitment stages set the template for the development of vegetation patterns along a hydrological gradient. Funct. Ecol. 2015, 29, 971–980. [Google Scholar] [CrossRef]
- Myers, J.; Harms, K. Seed arrival and ecological filters interact to assemble high-diversity plant communities. Ecology 2011, 92, 676–686. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, J.; Zhang, B.; Deng, G.; Maimaiti, A.; Guo, Z. Patterns and drivers of tree species diversity in a coniferous forest of northwest China. Front. For. Glob. Change 2024, 7, 1333232. [Google Scholar] [CrossRef]
- Ni, Y.; Yang, T.; Ma, Y.; Zhang, K.; Soltis, P.; Soltis, D.; Gilbert, J.; Zhao, Y.; Fu, C.; Chu, H. Soil ph determines bacterial distribution and assembly processes in natural mountain forests of eastern China. Glob. Ecol. Biogeogr. 2021, 30, 2164–2177. [Google Scholar] [CrossRef]
- Liu, X.; Ma, K. Plant functional traits—Concepts, applications and future directions. Sci. Sin. Vitae 2015, 45, 325–339. [Google Scholar] [CrossRef]
- Cui, E.; Lu, R.; Xu, X.; Sun, H.; Qiao, Y.; Ping, J.; Qiu, S.; Lin, Y.; Bao, J.; Yong, Y.; et al. Soil phosphorus drives plant trait variations in a mature subtropical forest. Glob. Change Biol. 2022, 28, 3310–3320. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, X.; Chang, S. Meta-analysis shows that plant mixtures increase soil phosphorus availability and plant productivity in diverse ecosystems. Nat. Ecol. Evol. 2022, 6, 1112–1121. [Google Scholar] [CrossRef]
- Klauschies, T.; Vasseur, D.A.; Gaedke, U. Trait adaptation promotes species coexistence in diverse predator and prey communities. Ecol. Evol. 2016, 6, 4141–4159. [Google Scholar] [CrossRef]
- Måren, I.; Kapfer, J.; Aarrestad, P.; Grytnes, J.; Vandvik, V. Changing contributions of stochastic and deterministic processes in community assembly over a successional gradient. Ecology 2018, 99, 148–157. [Google Scholar] [CrossRef]






| Sits | Plot | Altitude (m) | Slope (°) | Aspects | OM (g/kg) | TN (g/kg) | TP (g/kg) | pH | CEC (cmol/kg) | NRD (n/ha) | ES (n/ha) | LS (n/ha) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YJS | 1 | 173.65 | 46.59 | S | 81.40 | 4.27 | 0.44 | 6.89 | 24.97 | 2100 | 100 | 2000 |
| 2 | 154.01 | 24.45 | S | 55.24 | 2.65 | 0.34 | 6.34 | 17.81 | 13,975 | 2550 | 11,425 | |
| 3 | 150.33 | 34.29 | S | 77.24 | 3.87 | 0.43 | 6.42 | 24.85 | 7300 | 1425 | 5875 | |
| 4 | 154.85 | 33.36 | S | 58.88 | 2.93 | 0.47 | 6.14 | 16.63 | 3900 | 625 | 3275 | |
| WZL | 5 | 186.99 | 30.53 | SW | 33.89 | 1.66 | 0.25 | 6.11 | 9.85 | 1575 | 125 | 1450 |
| 6 | 190.3 | 30.61 | NE | 37.49 | 2.16 | 0.32 | 5.55 | 14.45 | 975 | 200 | 775 | |
| 7 | 195.25 | 29.24 | S | 36.1 | 2.0 | 0.37 | 6.14 | 15.42 | 800 | 250 | 550 | |
| 8 | 175.25 | 35.74 | SE | 21.20 | 1.14 | 0.30 | 5.94 | 9.18 | 2025 | 100 | 1925 | |
| XS | 9 | 190.18 | 33.26 | SE | 23.555 | 1.318 | 0.30 | 6.55 | 14.03 | 14,100 | 1650 | 12,450 |
| 10 | 193.93 | 21.24 | SE | 74.828 | 2.787 | 0.41 | 6.29 | 15.48 | 10,950 | 1675 | 9275 | |
| 11 | 185.17 | 32.23 | SE | 38.982 | 1.622 | 0.32 | 6.44 | 18.21 | 7700 | 1250 | 6450 | |
| 12 | 186.29 | 10.26 | N | 55.150 | 2.249 | 0.33 | 6.42 | 20.57 | 4875 | 575 | 4300 |
| Functional Traits | Mean ± SD | K Value | p Value |
|---|---|---|---|
| Basal Diameter (mm) | 12.63 ± 12.65 | 0.087 | 0.213 |
| Height (cm) | 108.80 ± 90.00 | 0.126 | 0.116 |
| Leaf Area (cm2) | 106.49 ± 116.39 | 0.034 | 0.453 |
| Specific Leaf Area (cm2/g) | 1207.65 ± 5358.35 | 0.390 | 0.003 |
| NRI (ES) | NRI (LS) | SES.FD_MPD (ES) | SES.FD_MPD (LS) | |
|---|---|---|---|---|
| Intercept | −16.227 (8.318) | 0.319 (0.088) ** | 21.512 (7.689) ** | 4.928 (1.536) ** |
| Elevation | −0.078 (0.016) *** | — | −0.015(0.007) | −0.029 (0.006) ** |
| Slope | −0.096 (0.027) ** | — | 0.068 (0.027) * | 0.041 (0.011) ** |
| Aspect | — | — | −2.286 (0.863) * | — |
| OM | — | — | — | — |
| TP | — | — | — | — |
| pH | 6.028 (1.643) *** | — | −2.961 (1.188) * | — |
| CEC | −0.314 (0.067) *** | — | — | −0.060 (0.023) * |
| R2c | 0.428 | 0.192 | 0.215 | 0.530 |
| R2m | 0.428 | 0 | 0.215 | 0.451 |
| ESβpd | LSβpd | ESβfd | LSβfd | |
|---|---|---|---|---|
| Percent deviance explained (%) | 7.36 | 20.86 | 1.73 | 7.62 |
| Predictor | Importance | |||
| Geographic | 0.485 | 36.462 *** | 74.599 | 0.625 |
| Elevation | 2.621 | 0.262 | 174.150 | 9.246 |
| Slope | 6.641 | 1.431 | — | — |
| Aspect | 0.340 | — | — | |
| OM | — | 2.062 | — | 3.697 |
| TP | 10.409 | 0.181 | — | 48.720 * |
| pH | 2.145 | 6.894 * | — | 0.778 |
| CEC | 5.704 | 2.425 | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, Z.; Jin, Y.; Hu, X.; Liu, Y.; Li, F.; Liang, F.; Wang, Y.; Li, Z.; Wang, J.; Chen, H. Distinguishing the Mechanisms Driving Community Structure Across Different Growth Stages in Quercus Forests. Forests 2025, 16, 1332. https://doi.org/10.3390/f16081332
Lian Z, Jin Y, Hu X, Liu Y, Li F, Liang F, Wang Y, Li Z, Wang J, Chen H. Distinguishing the Mechanisms Driving Community Structure Across Different Growth Stages in Quercus Forests. Forests. 2025; 16(8):1332. https://doi.org/10.3390/f16081332
Chicago/Turabian StyleLian, Zhenghua, Yingshan Jin, Xuefan Hu, Yanhong Liu, Fang Li, Fang Liang, Yuerong Wang, Zuzheng Li, Jiahui Wang, and Hongfei Chen. 2025. "Distinguishing the Mechanisms Driving Community Structure Across Different Growth Stages in Quercus Forests" Forests 16, no. 8: 1332. https://doi.org/10.3390/f16081332
APA StyleLian, Z., Jin, Y., Hu, X., Liu, Y., Li, F., Liang, F., Wang, Y., Li, Z., Wang, J., & Chen, H. (2025). Distinguishing the Mechanisms Driving Community Structure Across Different Growth Stages in Quercus Forests. Forests, 16(8), 1332. https://doi.org/10.3390/f16081332






