Edge Effects in the Amazon Rainforest in Brazil’s Roraima State
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
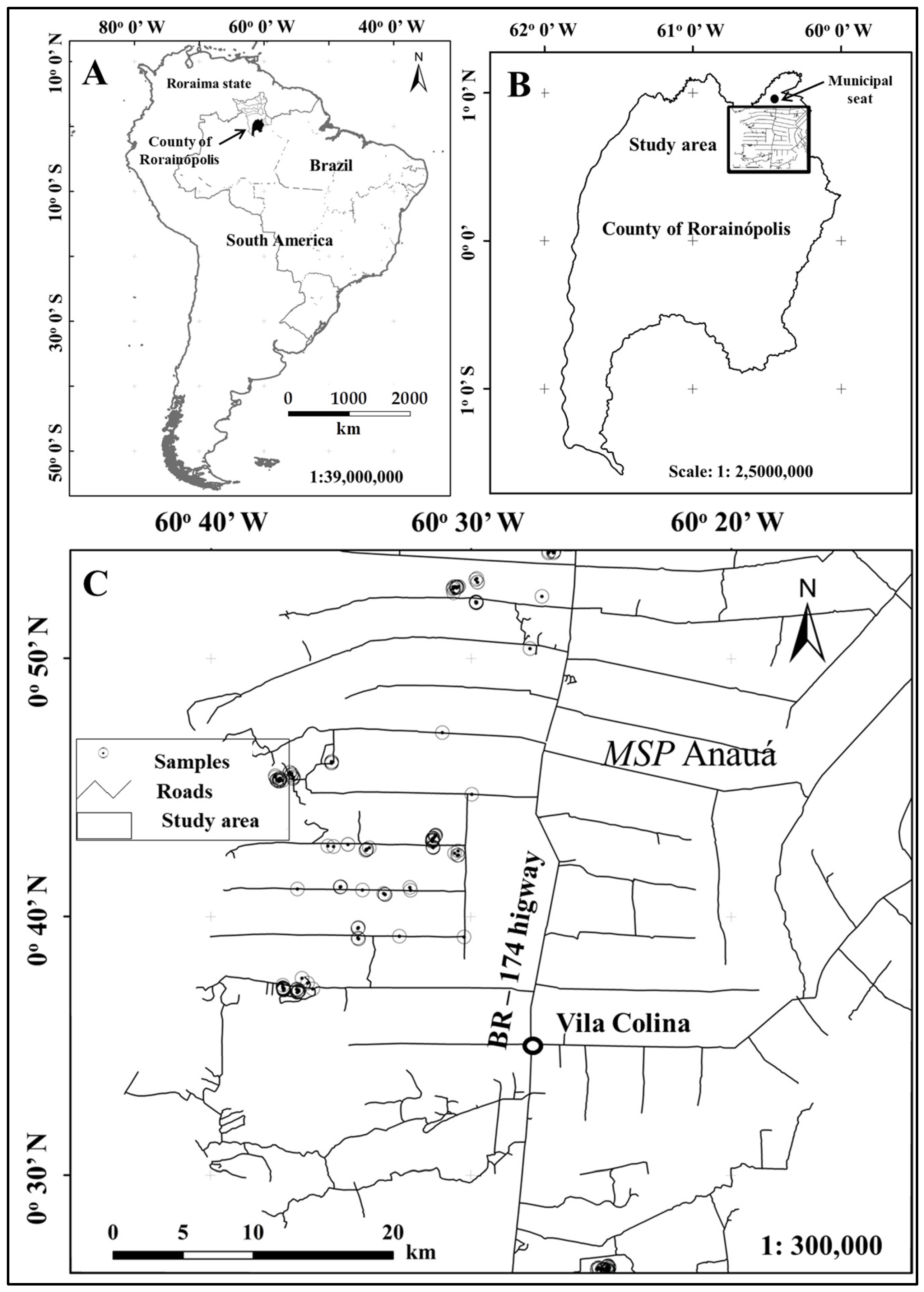
2.1.1. Study Area
2.1.2. Field Data
2.1.3. Geographic Database
- (1)
- Point observations of the presence or absence of SL and of fire traces (n = 92) in a georeferenced file (shapefile file); data were collected at the edge of the forest through forest inventories and field visits (Table S1 in the Supplementary Materials).
- (2)
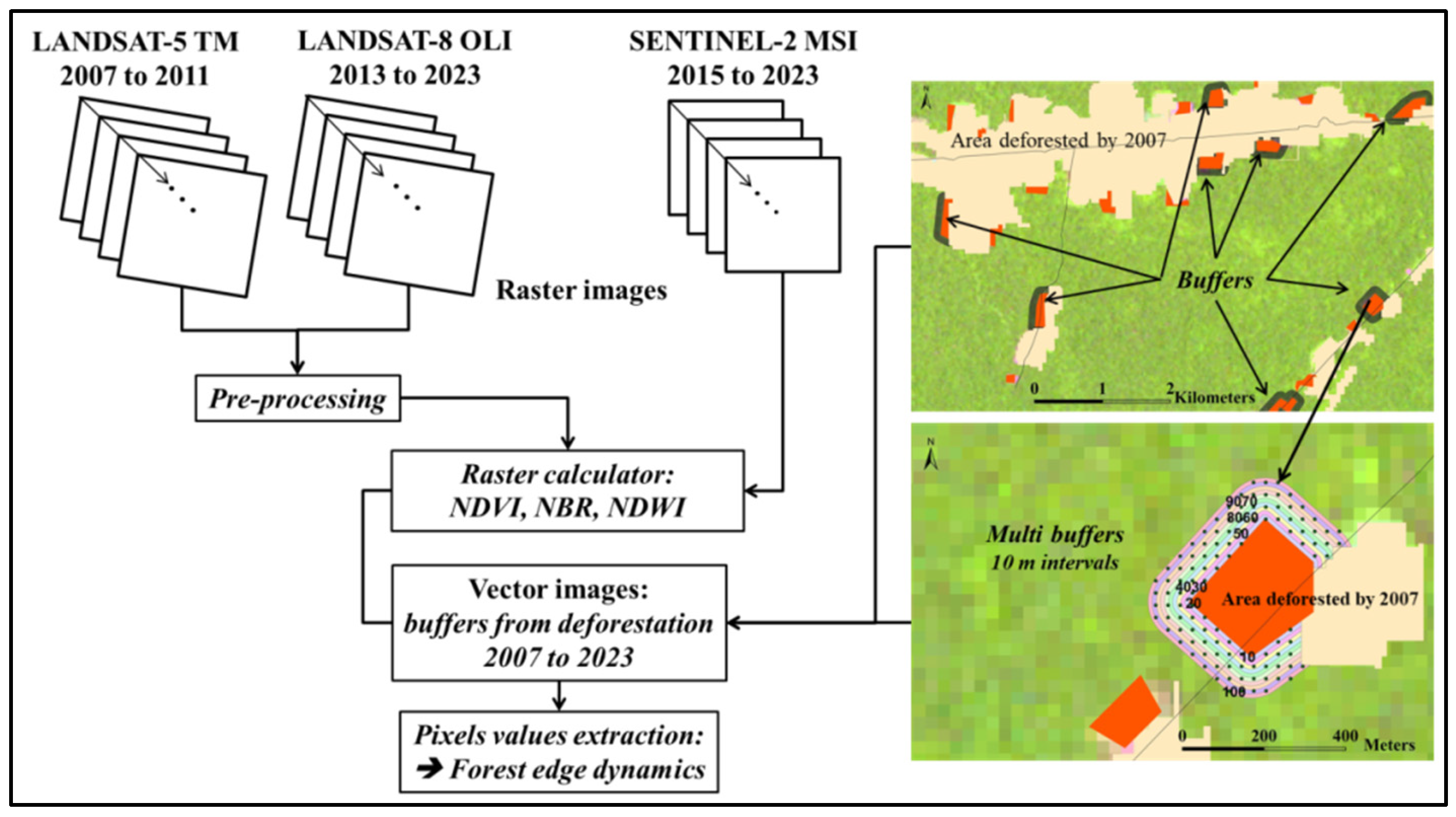
- From Barni et al. [2], data were obtained from multi-temporal satellite images: (a) Landsat-5 TM (2006 to 2011), (b) Landsat-8 OLI (2013 to 2023) (collection 2, level 1, with a 30 m spatial resolution) at path 231/row 60 (https://earthexplorer.usgs.gov/, accessed on 9 August 2025) and (c) Sentinel-2 MSI, scene 20NQF, with 10 m spatial resolution (2015 to 2023) (https://dataspace.copernicus.eu/, accessed on 9 August 2025)) (Table S2). These images covered the analysis period from 2007 to 2023, except for 2012, which lacked usable images [2]. All Landsat 5 and 8 images were pre-processed for atmospheric correction by subtracting “dark objects” (DOS1) (e.g., [63]) using the “Semi-Automatic Classification Plugin” (SCP) [64]. The Sentinel-2 (L2A) images were obtained with atmospheric correction already carried out by the image provider, using the Bottom of the Atmosphere (BOA) Reflectance Algorithm, which filters out or attenuates interference in the reflectance of the lower part of the atmosphere (https://docs.sentinel-hub.com/api/latest/data/sentinel-2-l2a/, accessed on 9 August 2025)). Vegetation indices were then calculated by extracting the values of pixels overlapping the forest edges.
- (3)
- Annual deforestation maps (shapefiles) were cut out for the study area, based on the PRODES deforestation data available for the entire Brazilian Amazon [4]. These data were used to define the edges and analyze their annual dynamics.
- (4)
- Shapefile maps of the great fire of 2015–2016 and SL areas (2007 to 2015), cut out for the study area, were obtained from Barni et al. [2].
- (5)
- The SL maps from 2016 to 2023 were recorded by manually editing polygons (≥1 hectare (ha)) from the annual Landsat-8 and Sentinel-2 satellite images These data (fire area and SL) were used in the analysis by systematically cross-referencing them with the annual forest edge data.
- (6)
- Data on the spatial distribution of forest biomass (Mg ha−1) were obtained from the study by Barni et al. [22]. These data were used for estimates of biomass exposed to deforestation and the edge effect, as well as the losses due to these processes.
- (7)
- Precipitation data from 1990 to 2023 for the point 0.7000 north latitude and −60.4500 west latitude (the center of the study area) were acquired from the “Nasa/Power Ceres/Merra2” portal (https://power.larc.nasa.gov/beta/data-access-viewer/, accessed on 9 August 2025)). These data were used to indicate the degree of humidity in the month/year when the images were acquired during the analysis period (Table S2).
2.2. Methods
2.2.1. Edge Dynamics
2.2.2. Forest Edge Degradation Levels
2.2.3. Calculating Vegetation Indices
2.2.4. Estimates of Biomass Loss
2.2.5. Evaluation of Error Propagation in Biomass Estimation
2.3. Statistical Analysis
Hypothesis Testing
3. Results
3.1. Quantification of Deforestation, SL, and Fire by Land Cover
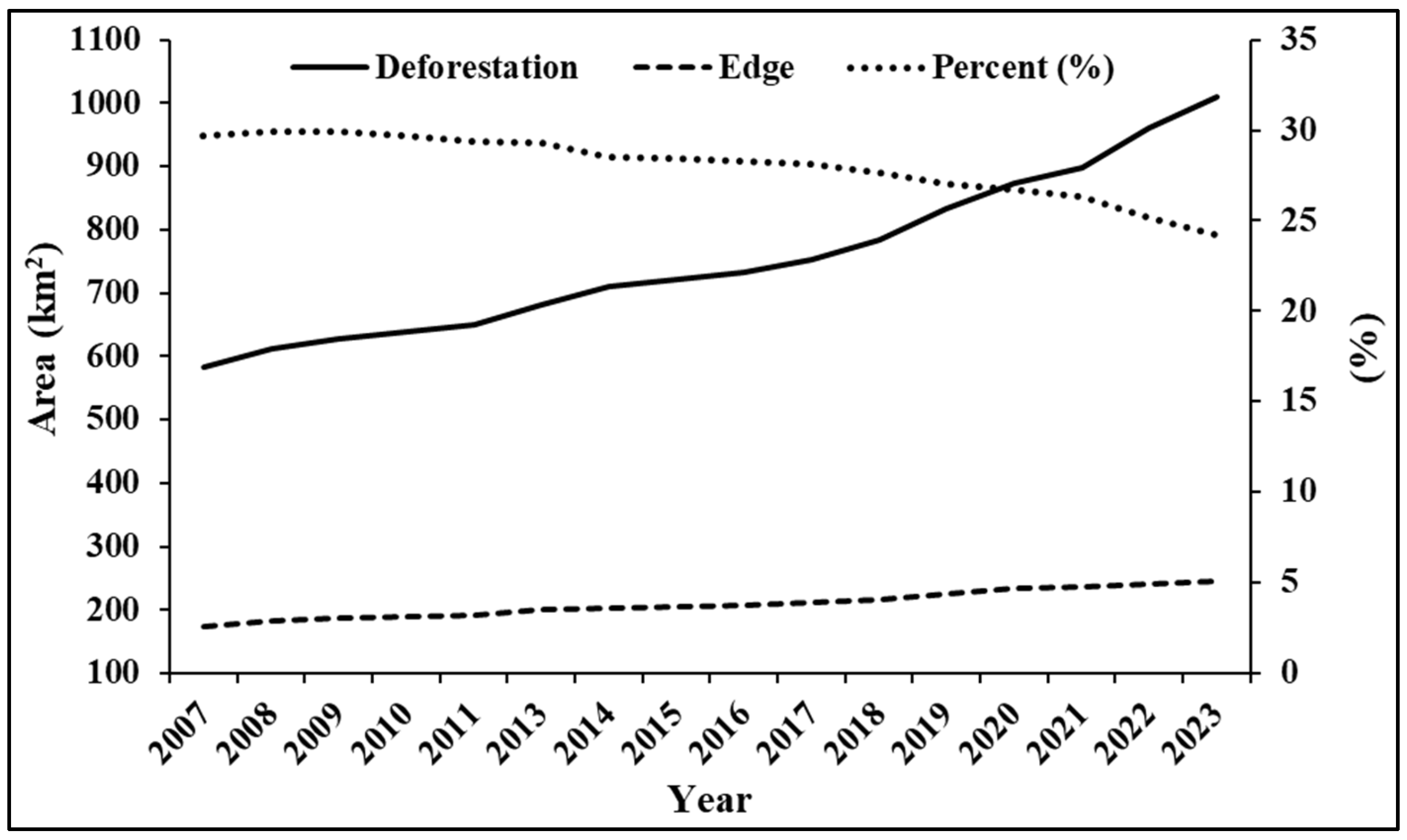
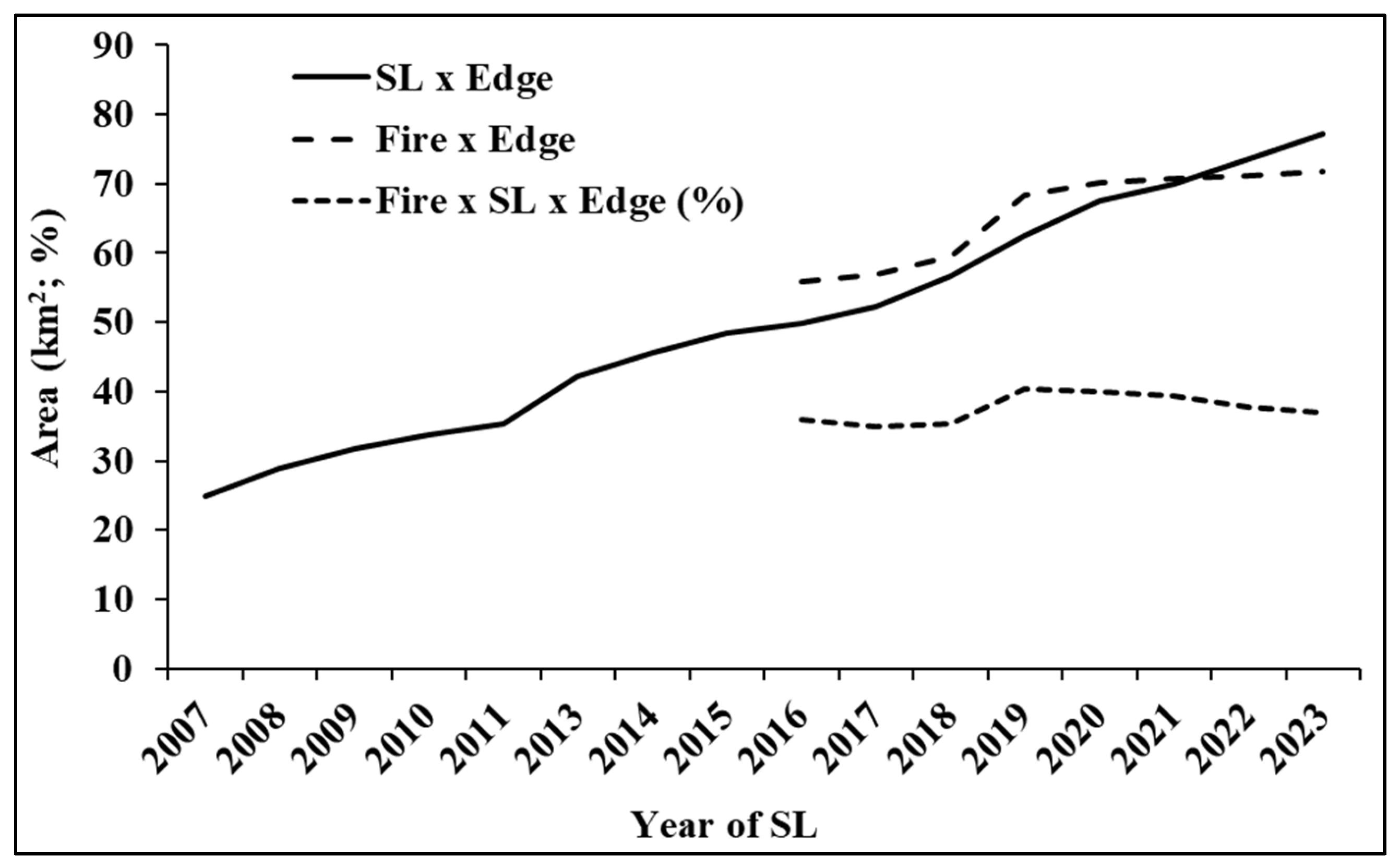
3.2. Dynamics of Deforestation and Edge Growth
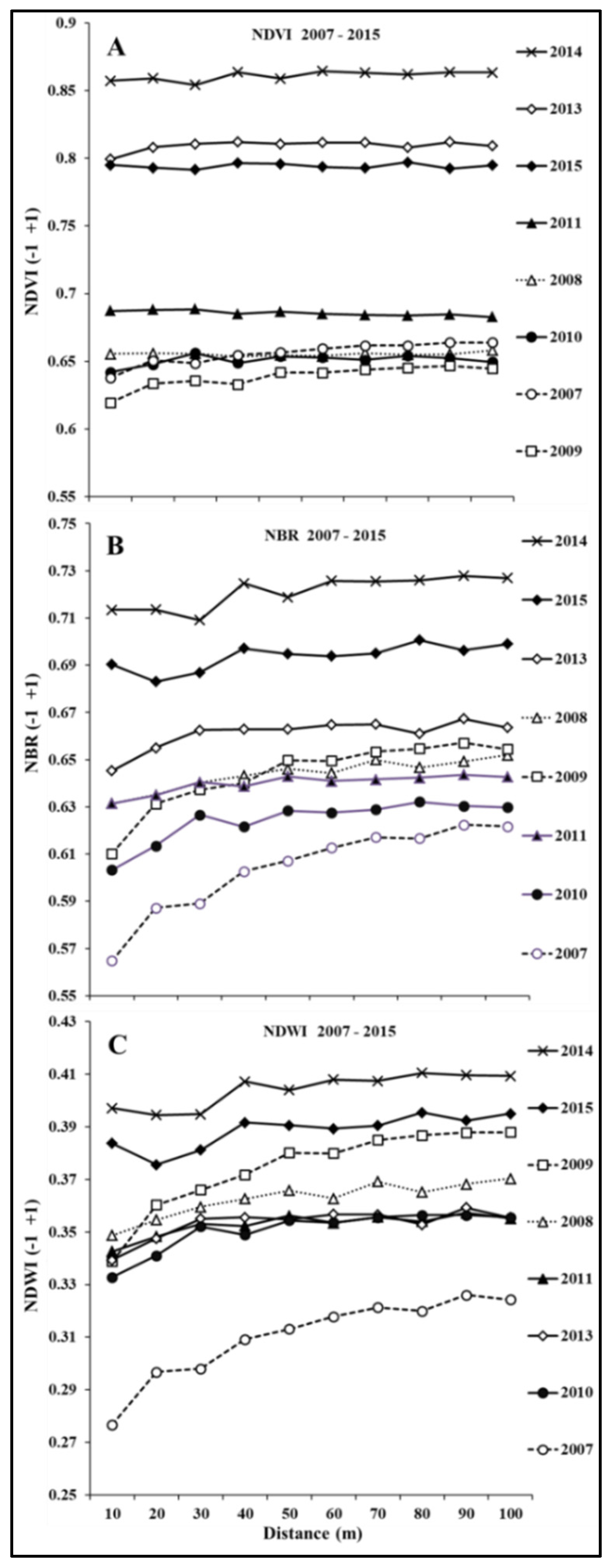
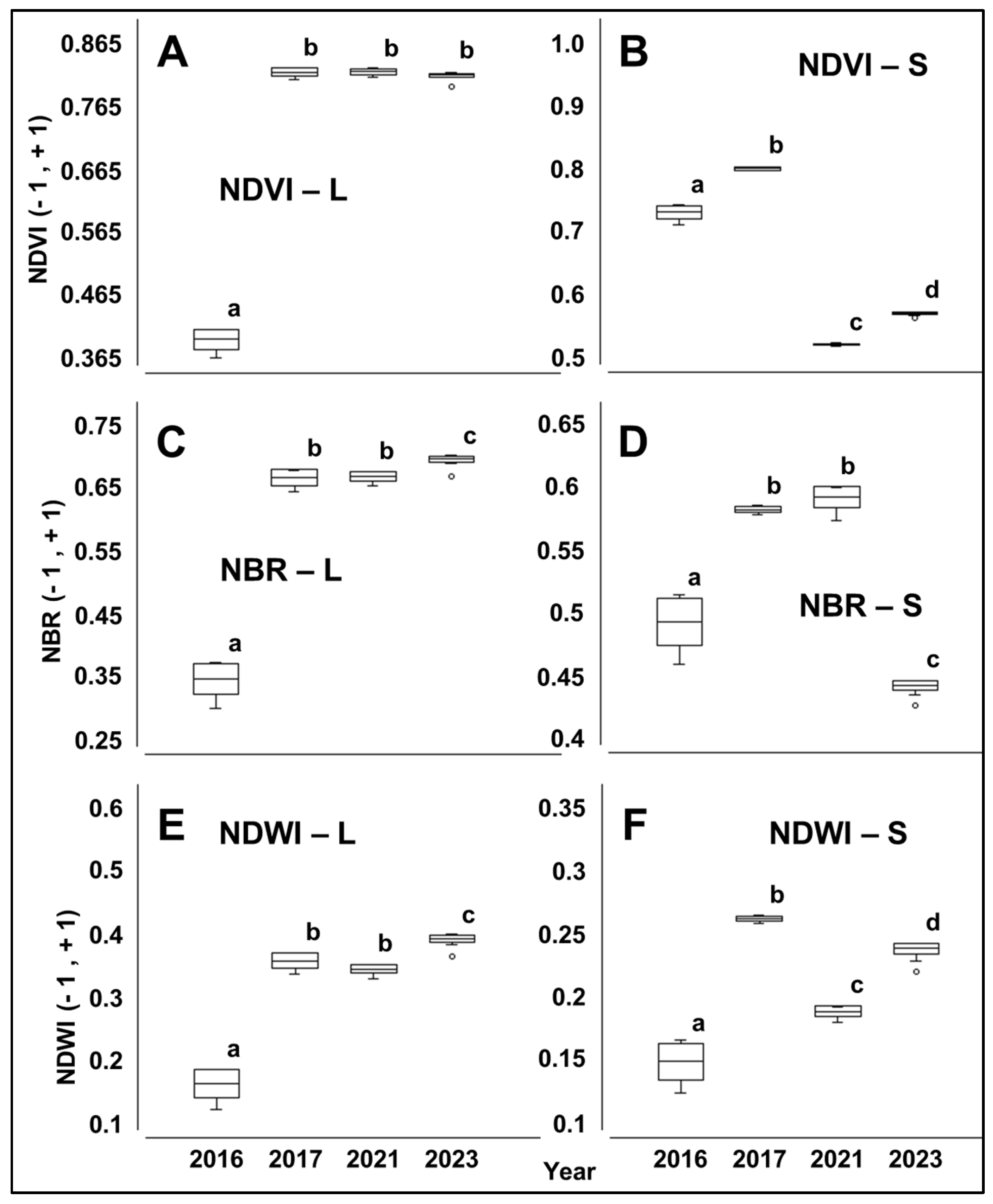
3.3. Spectral Behavior of Pixels at the Forest Edge from 2007 to 2015
3.4. Spectral Behavior of Pixels at the Forest Edge from 2015 to 2023
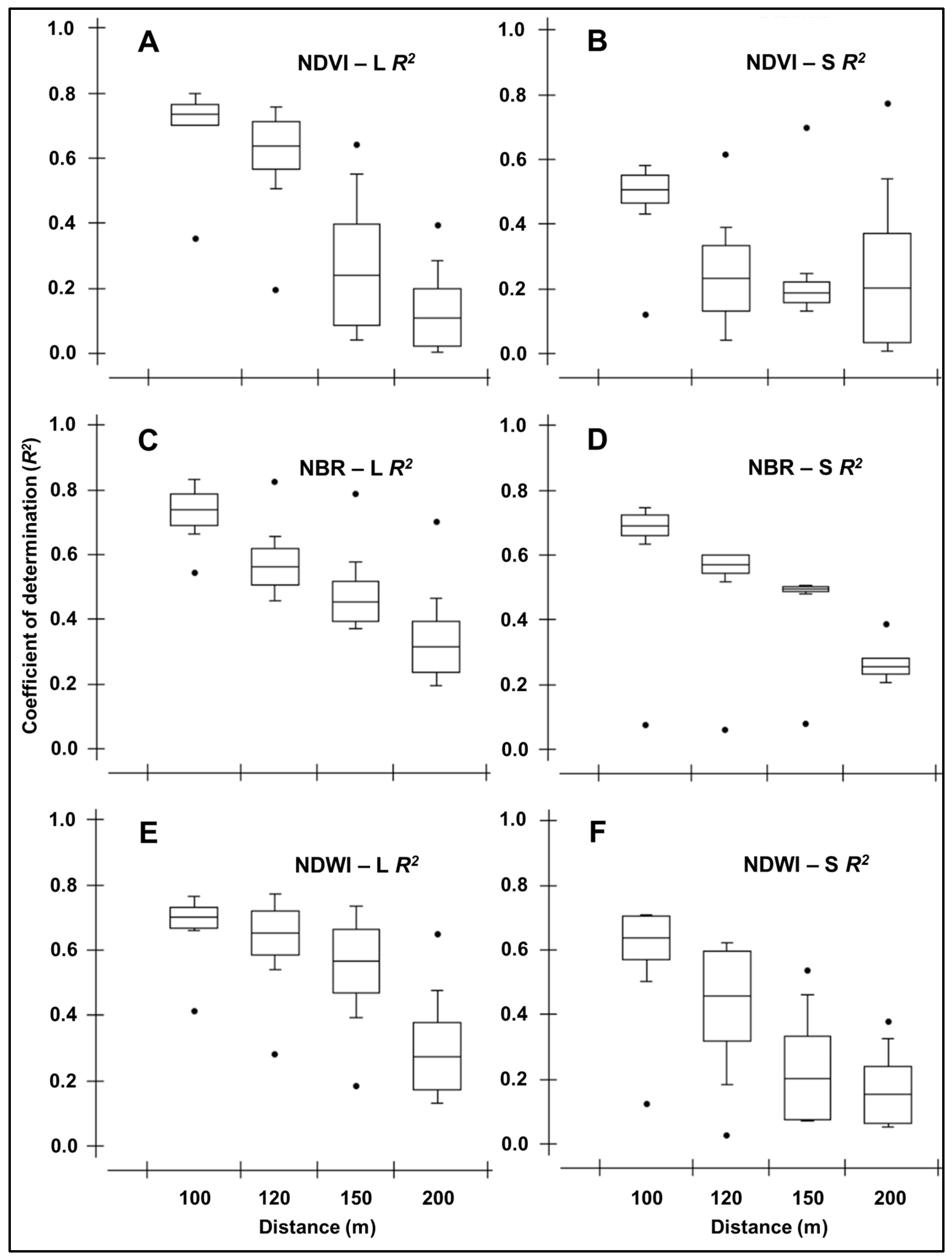
3.5. Spectral Behavior of Pixels Beyond the 100 m Edge
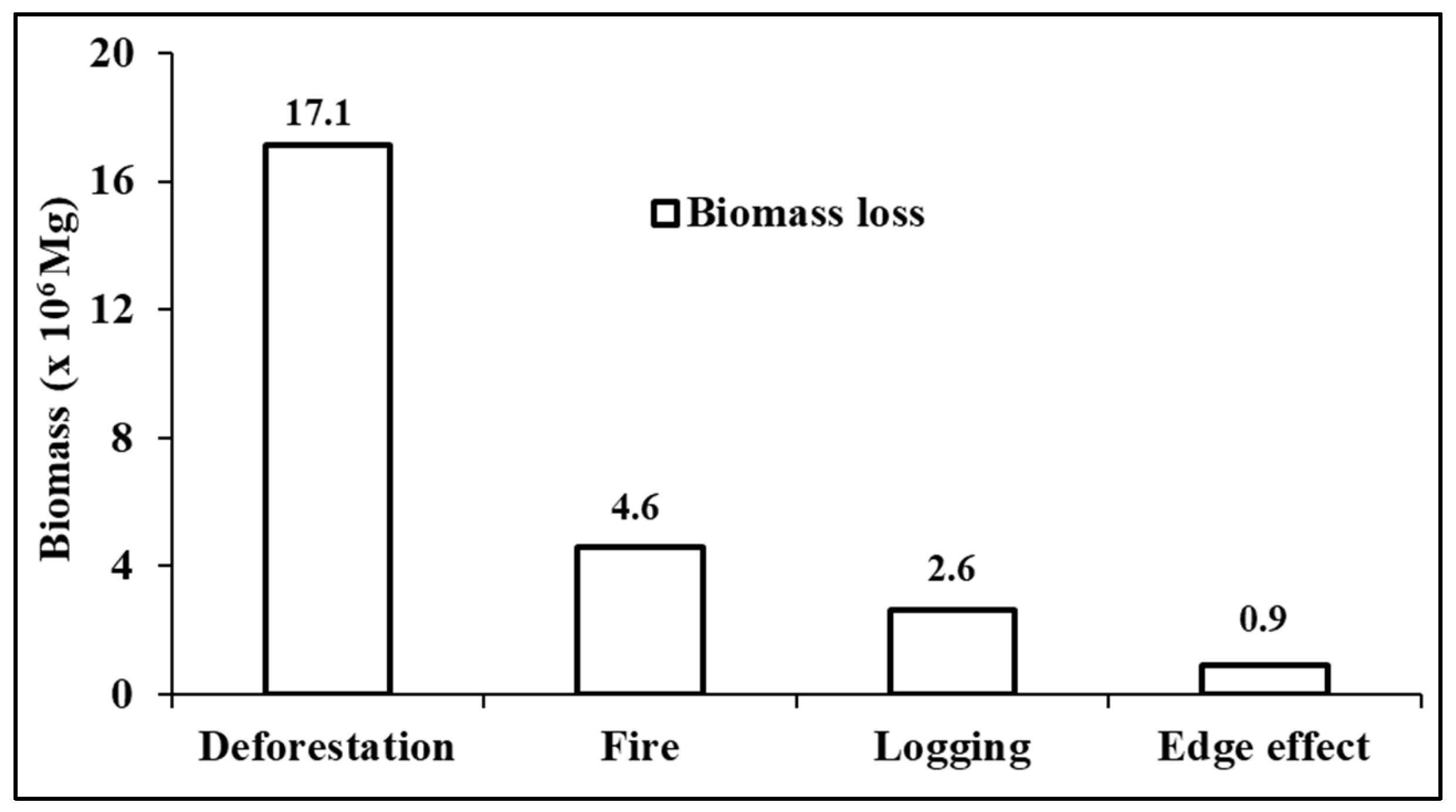
3.6. Biomass Impact and Biomass Loss
3.6.1. Biomass Loss Due to Deforestation
3.6.2. Biomass Loss Due to the Edge Effect
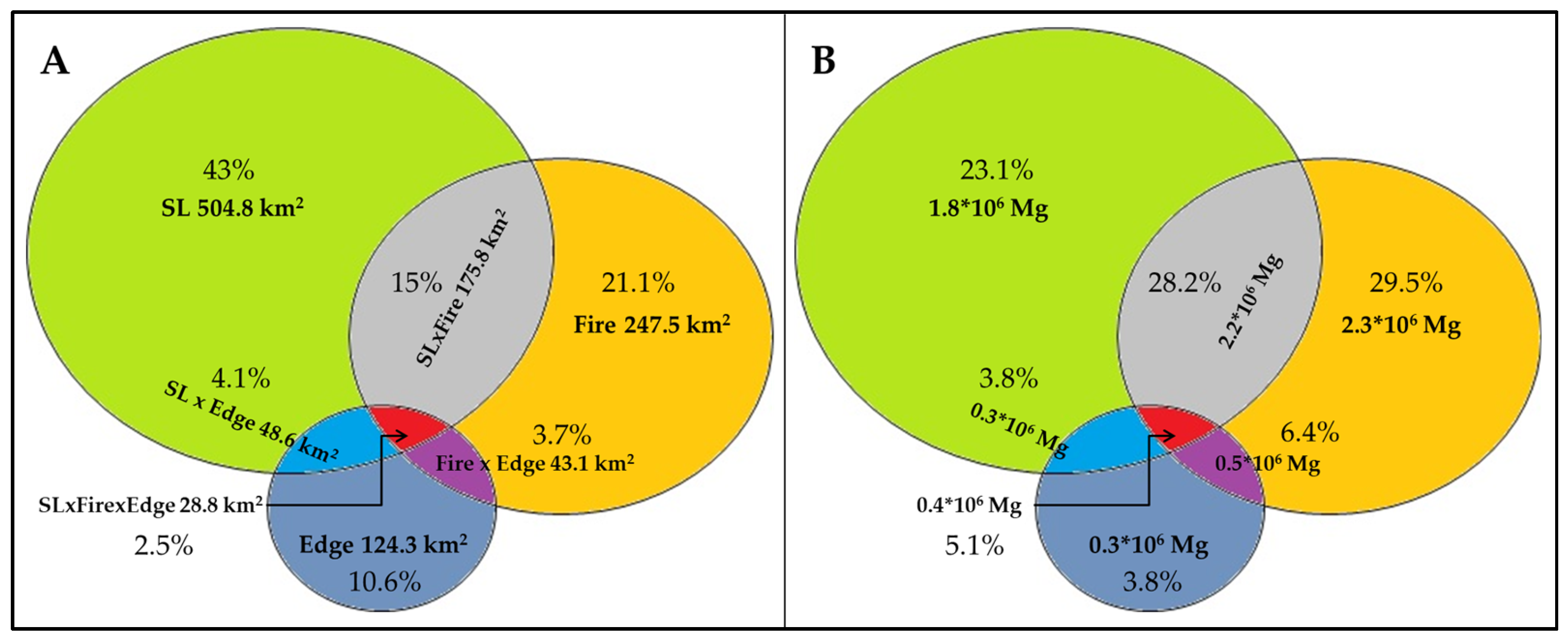
3.6.3. Biomass Loss in 2016 and 2023 Due to Fire and SL in Edge Areas
3.6.4. Biomass Loss in Overlapping Areas
4. Discussion
4.1. Forest Degradation in the Study Area
4.2. Deforestation and Edge Growth Dynamics
4.3. Interaction Among the Forest Edge, SL, and Fire
4.4. Spectral Behavior of Pixels at the Forest Edge
4.5. Estimation of Biomass Loss
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Condé, T.M.; Higuchi, N.; Lima, A.J.N. Illegal selective logging and forest fires in the northern Brazilian Amazon. Forests 2019, 10, 61. [Google Scholar] [CrossRef]
- Barni, P.E.; Rego, A.C.M.; Silva, F.C.F.; Lopes, R.A.S.; Xaud, H.A.M.; Xaud, M.R.; Barbosa, R.I.; Fearnside, P.M. Logging Amazon forest increased the severity and spread of fires during the 2015-2016 El Niño. For. Ecol. Manag. 2021, 500, 119652. [Google Scholar] [CrossRef]
- Vidal, E.; West, T.A.P.; Lentini, M.; Souza, S.E.X.F.; Klauberg, C.; Waldhoff, P. Sustainable forest management (SFM) of tropical moist forests: The case of the Brazilian Amazon. In Achieving Sustainable Management of Tropical Forests; Blaser, J., Hardcastle, P.D., Eds.; Burleigh Dodds: Cambridge, UK, 2021; Chapter 24. [Google Scholar] [CrossRef]
- Brazil, INPE (Instituto Nacional de Pesquisas Espaciais). Projeto de Desmatamento, Incrementos de desmatamento—Terra Brasilis: Amazônia Legal—Estados. Available online: https://terrabrasilis.dpi.inpe.br/app/dashboard/deforestation/biomes/legal_amazon/rates (accessed on 17 May 2024).
- Gerson, J.R.; Szponar, N.; Zambrano, A.A.; Bergquist, B.; Broadbent, E.; Driscoll, C.T.; Erkenswick, G.; Evers, D.C.; Fernandez, L.E.; Hsu-Kim, H.; et al. Amazon forests capture high levels of atmospheric mercury pollution from artisanal gold mining. Nat. Commun. 2022, 13, 559. [Google Scholar] [CrossRef]
- Vasconcellos, A.C.S.; Ferreira, S.R.B.; Sousa, C.C.; Oliveira, M.W.; Oliveira Lima, M.; Basta, P.C. Health risk assessment attributed to consumption of fish contaminated with mercury in the Rio Branco Basin, Roraima, Amazon, Brazil. Toxics 2022, 10, 516. [Google Scholar] [CrossRef]
- Fearnside, P.M. Amazon forest maintenance as a source of environmental services. An. Acad. Bras. Ciências 2008, 80, 101–114. [Google Scholar] [CrossRef]
- Silva Junior, C.H.L.; Aragão, L.E.O.C.; Anderson, L.O.; Fonseca, M.G.; Shimabukuro, O.E.; Vancutsem, C.; Achard, F.; Beuchle, R.; Numata, I.; Silva, C.A.; et al. Persistent collapse of biomass in Amazonian forest edges following deforestation leads to unaccounted carbon losses. Sci. Adv. 2020, 6, 40. [Google Scholar] [CrossRef]
- Chagas, V.B.P.; Chaffe, P.L.B.; Blöschl, G. Climate and land management accelerate the Brazilian water cycle. Nat. Commun. 2022, 13, 5136. [Google Scholar] [CrossRef]
- Broggio, I.S.; Silva-Junior, C.H.L.; Nascimento, M.T.; Villela, D.M.; Aragão, L.E.O.C. Quantifying landscape fragmentation and forest carbon dynamics over 35 years in the Brazilian Atlantic Forest. Environ. Res. Lett. 2024, 19, 034047. [Google Scholar] [CrossRef]
- Staal, A.; Tuinenburg, O.A.; Bosmans, J.H.C.; Holmgren, M.; van Nes, E.H.; Scheffer, M.; Zemp, D.C.; Dekker, S.C. Forest-rainfall cascades buffer against drought across the Amazon. Nat. Clim. Change 2018, 8, 539–543. [Google Scholar] [CrossRef]
- Anderson, L.; Dutra, D.; Jones, C.; Mataveli, G.; Ferreira, I.; Leão, H.; Cabral, B.; Fearnside, P.; Graça, P.; Yanai, A.; et al. Prediction of forest degradation as a subsidy for mitigating actions to preventing fires and wildfires in a new Amazonian frontier. In Proceedings of the EGU General Assembly 2024, Vienna, Austria, 14–19 April 2024. EGU24–6369. [Google Scholar] [CrossRef]
- Silva Junior, C.H.L.; Aragão, L.E.O.C.; Anderson, L.O.; Carvalho, N.S.; Pessôa, A.C.M.; Reis, J.B.C.; Dalagnol, R.; Wagner, F.; Saatchi, S.S. 2023 Emissions from forest degradation counteracted more than half of the Brazilian Amazon deforestation Redd+ Results. In Proceedings of the Anais do XX Simpósio Brasileiro de Sensoriamento Remoto, Florianópolis, Brazil, 2–5 April 2023; I.N.P.E.: São José dos Campos, Brazil, 2023. Available online: https://proceedings.science/sbsr-2023/trabalhos/emissions-from-forest-degradation-counteracted-more-than-half-of-the-brazilian-a (accessed on 22 April 2023).
- Nunes, M.H.; Vaz, M.C.; Camargo, J.L.C.; Laurance, W.F.; de Andrade, A.; Vicentini, A.; Laurance, S.; Raumonen, P.; Jackson, T.; Zuquim, G.; et al. Edge effects on tree architecture exacerbate bio-mass loss of fragmented Amazonian forests. Nat. Commun. 2023, 14, 8129. [Google Scholar] [CrossRef]
- Ray, D.; Nepstad, D.C.; Moutinho, P. Micrometeorological and canopy controls of flammability in mature and disturbed forests in an east-central Amazon landscape. Ecol. Appl. 2005, 15, 1664–1678. [Google Scholar] [CrossRef]
- Balch, J.K.; Nepstad, D.C.; Curran, L.M.; Brando, P.M.; Portela, O.; Guilherme, P.; Reuning-Scherer, J.D.; de Carvalho, O., Jr. Size, species, and fire behavior predict tree and liana mortality from experimental burns in the Brazilian Amazon. For. Ecol. Manag. 2011, 261, 68–77. [Google Scholar] [CrossRef]
- Nunes, M.H.; Camargo, J.L.C.; Vincent, G.; Calders, K.; Oliveira, R.S.; Huete, A.; de Moura, Y.M.; Nelson, B.; Smith, M.N.; Stark, S.C.; et al. Forest fragmentation impacts the seasonality of Amazonian evergreen canopies. Nat. Commun. 2022, 13, 917. [Google Scholar] [CrossRef] [PubMed]
- Cawson, J.G.; Collins, L.; Parks, S.A.; Nolan, R.H.; Penman, T.D. Atmospheric dryness removes barriers to the development of large forest fires. Agric. For. Meteorol. 2024, 350, 109990. [Google Scholar] [CrossRef]
- Alencar, A.A.; Brando, P.M.; Asner, G.P.; Putz, F.E. Landscape fragmentation, severe drought and the new Amazon forest fire regime. Ecol. Appl. 2015, 25, 1493–1505. [Google Scholar] [CrossRef]
- Alencar, A.A.C.; Arruda, V.L.S.; da Silva, W.V.; Conciani, D.E.; Costa, D.P.; Crusco, N.; Duverger, S.G.; Ferreira, N.C.; Franca-Rocha, W.; Hasenack, H.; et al. Long-term Landsat-based monthly burned area dataset for the Brazilian biomes using deep learning. Remote Sens. 2022, 14, 2510. [Google Scholar] [CrossRef]
- Tyukavina, A.; Hansen, M.C.; Potapov, P.V.; Stehman, S.V.; Smith-Rodriguez, K.; Okpa, C.; Aguilar, R. Types and rates of forest disturbance in Brazilian Legal Amazon, 2000–2013. Sci. Adv. 2017, 3, e1601047. [Google Scholar] [CrossRef]
- Barni, P.E.; Manzi, A.O.; Condé, T.M.; Barbosa, R.I.; Fearnside, P.M. Spatial distribution of forest biomass in Brazil’s state of Roraima, northern Amazonia. For. Ecol. Manag. 2016, 377, 170–181. [Google Scholar] [CrossRef]
- Barni, P.E.; Pereira, V.B.; Manzi, A.O.; Barbosa, R.I. Deforestation and forest fires in Roraima and their relationship with phytoclimatic regions in the northern Brazilian Amazon. Environ. Manag. 2015, 55, 1124–1138. [Google Scholar] [CrossRef]
- Brazil, IBGE (Instituto Brasileiro de Geografia e Estatística). População de Roraima. Available online: https://cidades.ibge.gov.br/brasil/rr (accessed on 21 April 2023).
- Barbosa, R.I.; Fearnside, P.M. Incêndios na Amazônia: Estimativa da emissão de gases de efeito estufa pela queima de diferentes ecossistemas de Roraima na passagem do evento El-Niño (1997/1998). Acta Amaz. 1999, 29, 513–534. [Google Scholar] [CrossRef]
- Barbosa, R.I.; Xaud, M.R.; Silva, G.N.F.; Cattâneo, A.C. Forest fires in Roraima, Brazilian Amazonia. Int. For. Fire News (IFFN) 2003, 28, 51–56. [Google Scholar]
- Barni, P.E.; Fearnside, P.M.; de Alencastro Graça, P.M.L. Simulating deforestation and carbon loss in Amazonia: Impacts in Brazil’s Roraima state from reconstructing Highway BR-319 (Manaus-Porto Velho). Environ. Manag. 2015, 55, 259–278. [Google Scholar] [CrossRef] [PubMed]
- Adeney, J.M.; Christensen, N.L.; Vicentini, A.; Cohn-Haft, M. White-sand ecosystems in Amazonia. Biotropica 2016, 48, 7–23. [Google Scholar] [CrossRef]
- Flores, W.M.; França, I.; Santos, G.G.A.; Miranda, I.S.; Moraes, E.F.S.; Sánchez, G.H.; Silva, S.D.B.D.; Hernández-Ruz, E.J. Diametric growth of a forest under reduced-impact logging in the eastern region of the Brazilian Amazon. Land 2023, 12, 704. [Google Scholar] [CrossRef]
- Pivello, V.R.; Vieira, I.; Christianini, A.V.; Ribeiro, D.B.; Menezes, L.S.; Berlinck, C.N.; Melo, F.P.L.; Marengo, J.A.; Tornquist, C.G.; Tomas, W.M.; et al. Understanding Brazil’s catastrophic fires: Causes, consequences and policy needed to prevent future tragedies. Perspect. Ecol. Conserv. 2021, 19, 233–255. [Google Scholar] [CrossRef]
- Fonseca, M.G.; Anderson, L.O.; Arai, E.; Shimabukuro, Y.E.; Xaud, H.A.M.; Xaud, M.R.; Madani, N.; Wagner, F.H.; Aragão, L.E.O.C. Climatic and anthropogenic drivers of northern Amazon fires during the 2015-2016 El Niño event. Ecol. Appl. 2017, 27, 2514–2527. [Google Scholar] [CrossRef]
- Barni, P.E.; Silva, E.B.R.; Silva, F.C.F. Incêndios florestais de sub-bosque na zona de florestas úmidas do sul de Roraima: Área atingida e biomassa morta. In Anais do Simpósio Brasileiro de Sensoriamento Remoto 2017 Campinas; Galoá: São Paulo, Brazil, 2017; pp. 6280–6287. Available online: https://bityl.co/5JeV (accessed on 17 May 2024).
- Soares-Filho, B.S.; Nepstad, D.C.; Curran, L.M.; Cerqueira, G.C.; Garcia, R.A.; Ramos, C.A.; Voll, E.; McDonald, A.; Lefebvre, P.; Schlesinger, P. Modelling conservation in the Amazon basin. Nature 2006, 440, 520–523. [Google Scholar] [CrossRef]
- Skole, D.L.; Tucker, C.J. Tropical deforestation and habitat fragmentation in the Amazon: Satellite data from 1978 to 1988. Science 1993, 260, 1905–1910. [Google Scholar] [CrossRef]
- Souza, C.M., Jr.; Siqueira, J.V.; Sales, M.H.; Fonseca, A.V.; Ribeiro, J.G.; Numata, I.; Cochrane, M.A.; Barber, C.P.; Roberts, D.A.; Barlow, J. Ten-year Landsat classification of deforestation and forest degradation in the Brazilian Amazon. Remote Sens. 2013, 5, 5493–5513. [Google Scholar] [CrossRef]
- Buras, A.; Rammig, A.; Zang, C.S. The European forest condition monitor: Using remotely sensed forest greenness to identify hot spots of forest decline. Front. Plant Sci. 2021, 12, 1–19. [Google Scholar] [CrossRef]
- Laurance, W.F.; Laurance, S.G.; Ferreira, L.V.; Rankin-de-Merona, J.M.; Gascon, C.; Lovejoy, T.E. Biomass collapse in Amazonian forest fragments. Science 1997, 278, 1117–1118. [Google Scholar] [CrossRef]
- Vedovato, L.B.; Fonseca, M.G.; Arai, E.; Anderson, L.O.; Aragão, L.E.O.C. The extent of 2014 forest fragmentation in the Brazilian Amazon. Reg. Environ. Change 2016, 16, 2485–2490. [Google Scholar] [CrossRef]
- Laurance, W.F.; Laurance, S.G.; Delamonica, P. Tropical forest fragmentation and greenhouse gas emissions. For. Ecol. Manag. 1998, 110, 173–180. [Google Scholar] [CrossRef]
- Brinck, K.; Fischer, R.; Groeneveld, J.; Lehmann, S.; de Paula, M.D.; Pütz, S.; Sexton, J.O.; Song, D.; Huth, A. High resolution analysis of tropical forest fragmentation and its impact on the global carbon cycle. Nat. Commun. 2017, 8, 14855. [Google Scholar] [CrossRef]
- Numata, I.; Silva, S.S.; Cochrane, M.A.; d’Oliveira, M.V.N. Fire and edge effects in a fragmented tropical forest landscape in the southwestern Amazon. For. Ecol. Manag. 2017, 401, 135–146. [Google Scholar] [CrossRef]
- Igari, A.; Brites, A.; Valdiones, A.P.; Junior, B.; Salgado, B.; Vello, B.G.; Pinto, E.; Martins Neto, F.L.; Prioste, F.G.; Sousa, F.C.; et al. Código Florestal Avaliação 2017|2020; Observatório do Código Florestal e Instituto de Pesquisas da Amazônia (IPAM): Brasília, Brazil, 2021; p. 80. Available online: https://observatorioflorestal.org.br/avaliacao-do-codigo-florestal-2017-2020/ (accessed on 14 December 2023).
- Barona, E.; Ramankutty, N.; Hyman, G.; Coomes, O.T. The role of pasture and soybean in deforestation of the Brazilian Amazon. Environ. Res. Lett. 2010, 5, 024002. [Google Scholar] [CrossRef]
- Aragão, L.E.O.C.; Shimabukuro, Y.E. The incidence of fire in Amazonian forests with implications for REDD. Science 2010, 328, 1275–1278. [Google Scholar] [CrossRef]
- Morton, D.C.; Defries, R.S.; Nagol, J.; Souza, C.M., Jr.; Kasischke, E.S.; Hurtt, G.C.; Dubayah, R. Mapping canopy damage from understory fires in Amazon forests using annual time series of Landsat and MODIS data. Remote Sens. Environ. 2011, 115, 1706–1720. [Google Scholar] [CrossRef]
- Silva, C.V.J.; Aragão, L.E.O.C.; Barlow, J.; Fernando Espírito-Santo, F.; Young, P.J.; Anderson, L.A.; Berenguer, E.; Brasil, I.; Brown, I.F.; Castro, B.; et al. Drought-induced Amazonian wildfires instigate a decadal-scale disruption of forest carbon dynamics. Philos. Trans. R. Soc. B 2018, 373, 20180043. [Google Scholar] [CrossRef]
- Balch, J.; Schoennagel, T.; Williams, A.; Abatzoglou, J.; Cattau, M.; Mietkiewicz, N.; Denis, L. Switching on the big burn of 2017. Fire 2018, 1, 17. [Google Scholar] [CrossRef]
- Walker, X.J.; Rogers, B.M.; Baltzer, J.L.; Cumming, S.G.; Day, N.J.; Goetz, S.J.; Johnstone, J.F.; Schuur, E.A.G.; Turetsky, M.R.; Mack, M.C. Cross-scale controls on carbon emissions from boreal forest megafires. Glob. Change Biol. 2018, 24, 4251–4265. [Google Scholar] [CrossRef]
- Brando, P.M.; Paolucci, L.; Ummenhofer, C.C.; Ordway, E.M.; Hartmann, H.; Cattau, M.E.; Rattis, L.; Medjibe, B.; Coe, M.T.; Balch, J. Droughts, wildfires, and forest carbon cycling: A pantropical synthesis. Annu. Rev. Earth Planet. Sci. 2019, 47, 555–581. [Google Scholar] [CrossRef]
- Miller, R.G.; Tangney, R.; Enright, N.J.; Fontaine, J.B.; Merritt, D.J.; Ooi, M.K.J.; Ruthrof, K.X.; Miller, B.P. Mechanisms of fire seasonality effects on plant populations. Trends Ecol. Evol. 2019, 34, 1104–1117. [Google Scholar] [CrossRef]
- Dalagnol, R.; Phillips, O.L.; Gloor, E.; Galvão, L.S.; Wagner, F.H.; Locks, C.J.; Aragão, L.E.O.C. Quantifying canopy tree loss and gap recovery in tropical forests under low-intensity logging using VHR satellite imagery and airborne LiDAR. Remote Sens. 2019, 11, 817. [Google Scholar] [CrossRef]
- Dalagnol, R.; Wagner, F.H.; Galvão, L.S.; Braga, D.; Osborn, F.; Sagang, L.B.; Bispo, P.C.; Payne, M.; Silva Junior, C.; Favrichon, S.; et al. Mapping tropical forest degradation with deep learning and Planet NICFI data. Remote Sens. Environ. 2023, 298, 113798. [Google Scholar] [CrossRef]
- Chuvieco, E.; Martín, M.P.; Palacios, A. Assessment of different spectral indices in the red-near infrared spectral domain of burned land discrimination. Int. J. Remote Sens. 2002, 23, 5103–5110. [Google Scholar] [CrossRef]
- Bastarrika, A.; Chuvieco, E.; Martín, M.P. Automatic burned land mapping from MODIS time series images: Assessment in Mediterranean ecosystems. IEEE Trans. Geosci. Remote Sens. 2011, 49, 3401–3413. [Google Scholar] [CrossRef]
- Bullock, E.L.; Woodcock, C.E.; Olofsson, P. Monitoring tropical forest degradation using spectral unmixing and Landsat time series analysis. Remote Sens. Environ. 2020, 238, 110968. [Google Scholar] [CrossRef]
- Condé, T.M.; Higuchi, N.; Lima, A.J.N.; Campos, M.A.A.; Condé, J.D.; de Oliveira, A.C.; de Miranda, D.L.C. Spectral patterns of pixels and objects of the forest phytophysiognomies in the Anauá National Forest, Roraima State, Brazil. Ecologies 2023, 4, 686–703. [Google Scholar] [CrossRef]
- Cordeiro, A.P.A.; Berlato, M.A.; Fontana, D.C.; de Melo, R.W.; Shimabukuro, Y.E.; Fior, C.S. Regiões homogêneas de vegetação utilizando a variabilidade do NDVI. Ciência Florest. 2017, 27, 883–896. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Jensen, J.R. Sensoriamento Remoto do Ambiente: Uma Perspectiva em Recursos Terrestres, 2nd ed.; Prentice Hall/Parêntesis: São Paulo, Brazil, 2009. [Google Scholar]
- Alcaras, E.; Costantino, D.; Guastaferro, F.; Parente, C.; Pepe, M. Normalized Burn Ratio Plus (NBR+): A new index for Sentinel-2 imagery. Remote Sens. 2022, 14, 1727. [Google Scholar] [CrossRef]
- Simes, T.; Pádua, L.; Moutinho, A. Wildfire burnt area severity classification from UAV-based RGB and multispectral imagery. Remote Sens. 2024, 16, 30. [Google Scholar] [CrossRef]
- Barni, P.E.; Barbosa, R.I.; Xaud, H.A.M.; Xaud, M.R.; Fearnside, P.M. Precipitation in northern Amazonia: Spatial distribution in Brazil’s state of Roraima. Soc. Nat. 2020, 32, 439–456. [Google Scholar] [CrossRef]
- Song, C.; Woodcock, C.E.; Seto, K.C.; Lenney, M.P.; Macomber, S.A. Classification and change detection using landsat TM data. Remote Sens. Environ. 2001, 75, 230–244. [Google Scholar] [CrossRef]
- Congedo, L. Semi-automatic classification plugin: A Python tool for the download and processing of remote sensing images in QGIS. J. Open Source Softw. 2021, 6, 3172. [Google Scholar] [CrossRef]
- Broadbent, E.N.; Asner, G.P.; Keller, M.; Knapp, D.E.; Oliveira, P.J.C.; Silva, J.N. Forest fragmentation and edge effects from deforestation and selective logging in the Brazilian Amazon. Biol. Conserv. 2008, 141, 1745–1757. [Google Scholar] [CrossRef]
- Chen, X.; Vogelmann, J.; Rollins, M.; Ohlen, D.; Key, C.; Yang, L.; Shi, H. Detecting postfire burn severity and vegetation recovery using multitemporal remote sensing spectral indices and field-collected composite burn index data in a ponderosa pine forest. Int. J. Remote Sens. 2011, 32, 7905–7927. [Google Scholar] [CrossRef]
- Jiang, Z.; Huete, A.R.; Didan, K.; Miura, T. Development of a two-band enhanced vegetation index without a blue band. Remote Sens. Environ. 2008, 112, 3833–3845. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, X.; Ban, Y.; Nascetti, A.; Gong, M. Assessing Sentinel-2, Sentinel-1, and ALOS-2 PALSAR-2 Data for large-scale wildfire-burned area mapping: Insights from the 2017–2019 Canada wildfires. Remote Sens. 2024, 16, 556. [Google Scholar] [CrossRef]
- Fearnside, P.M. Global warming and tropical land-use change: Greenhouse gas emissions from biomass burning, decomposition and soils in forest conversion, shifting cultivation and secondary vegetation. Clim. Change 2000, 46, 115–158. [Google Scholar] [CrossRef]
- Brazil, IBGE (Instituto Brasileiro de Geografia e Estatística). Manual Técnico da Vegetação Brasileira—Manuais Técnicos em Geociências no 1. 2ª Edição revista e ampliada; Fundação Instituto Brasileiro de Geografia e Estatística: Rio de Janeiro, Brazil, 2012; p. 267. Available online: https://uc.socioambiental.org/sites/uc/files/2019-12/liv63011.pdf (accessed on 17 January 2024).
- Laurance, W.F.; Camargo, J.L.C.; Fearnside, P.M.; Lovejoy, T.E.; Williamson, G.B.; Mesquita, R.C.G.; Meyer, C.F.J.; Bobrowiec, P.E.D.; Laurance, S.G.W. An Amazonian rainforest and its fragments as a laboratory of global change. Biol. Rev. 2018, 93, 223–247. [Google Scholar] [CrossRef] [PubMed]
- Correia, C. Governo Publica Redução da Reserva Legal em Terras Rurais de Roraima. Folha de Boa Vista, 4 November 2022. Available online: https://www.folhabv.com.br/economia/governo-publica-reducao-da-reserva-legal-em-terras-rurais-de-roraima/ (accessed on 17 May 2024).
- Lopes, C.L.; Minsky, E. Implementação do código florestal em Roraima: Redução de reserva legal de 80% para 50% pode acelerar o desmatamento no Estado. Climate Policy Initiative, Rio de Janeiro, RJ, Brazil. 2023. Available online: https://www.climatepolicyinitiative.org/pt-br/publication/implementacao-do-codigo-florestal-em-roraima-reducao-de-reserva-legal-de-80-para-50-pode-acelerar-o-desmatamento-no-estado/ (accessed on 29 January 2024).
- Roraima. Diário Oficial do Estado—DOE. Diário Oficial. Decreto Nº 33.467-E, de 31 de Outubro de 2022. 2022. Available online: https://www.imprensaoficial.rr.gov.br/app/_visualizar-mes/?ano=2022&mes=10 (accessed on 3 February 2025).
- Ferrante, L.; Fearnside, P.M. Brazil’s new president and ‘ruralists’ threaten Amazonia’s environment, traditional peoples and the global climate. Environ. Conserv. 2019, 46, 261–263. [Google Scholar] [CrossRef]
- Oliveira, S. Rorainópolis, em RR, Entra na Lista de Municípios Com Prioridade no Controle ao Desmatamento na Amazônia. G1, 13 January 2021. Available online: https://bityl.co/5Jdh (accessed on 29 January 2024).
- Laurance, W.F.; Nascimento, H.E.M.; Laurance, S.G.; Andrade, A.C.; Fearnside, P.M.; Ribeiro, J.E.L.; Capretz, R.L. Rain forest fragmentation and the proliferation of successional trees. Ecology 2006, 87, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A. Mais da Metade do Desmatamento na Amazônia Ocorre em Terras Públicas. O Globo, 15 February 2022. Available online: https://oglobo.globo.com/brasil/mais-da-metade-do-desmatamento-na-amazonia-ocorre-em-terras-publicas-25395036 (accessed on 2 January 2024).
- Fararoda, R.; Reddy, R.S.; Rajashekar, G.; Chand, T.R.K.; Jha, C.S.; Dadhwal, V.K. Improving forest above ground biomass estimates over Indian forests using multi source data sets with machine learning algorithm. Ecol. Inform. 2021, 5, 1013922021. [Google Scholar] [CrossRef]
- Guo, Q.; Du, S.; Jiang, J.; Guo, W.; Zhao, H.; Yan, X.; Zhao, Y.; Xiao, W. Combining GEDI and sentinel data to estimate forest canopy mean height and aboveground biomass. Ecol. Inform. 2023, 78, 102348. [Google Scholar] [CrossRef]
- MapBiomas (Mapeamento dos Biomas Brasileiros). Alertas Consolidados em 2022: Dados por Estados. Available online: https://lookerstudio.google.com/u/0/reporting/11f468f3-fcf2-4957-865a-e47f1d7cfdc5/page/NUYRD (accessed on 21 March 2024).
- Aguiar, A., Jr.; Barbosa, R.I.; Barbosa, J.B.F.; Mourão, M., Jr. Invasion of Acacia mangium in Amazonian savannas following planting for forestry. Plant Ecol. Divers. 2013, 7, 359–369. [Google Scholar] [CrossRef]
- Souza, A.O.; Chaves, M.P.S.R.; Barbosa, R.I.; Clement, C.R. Spatial distribution and abundance of Acacia mangium on indigenous lands in the Serra da Lua region, Roraima state, Brazil. Human. Ecol. 2019, 47, 303–310. [Google Scholar] [CrossRef]
- Ionova, A. New Palm Oil Frontier Sparks Scramble for Land in the Brazilian Amazon. Mongabay Series: Forest Trackers, Global Palm Oil. Available online: https://news.mongabay.com/2021/04/new-palm-oil-frontier-sparks-scramble-for-land-in-the-brazilian-amazon/ (accessed on 2 May 2024).
- Lapola, D.M.; Pinho, P.; Barlow, J.; Aragão, L.E.O.C.; Berenguer, E.; Carmenta, R.; Liddy, H.M.; Seixas, H.; Silva, C.V.J.; Silva-Junior, C.H.L.; et al. The drivers and impacts of Amazon forest degradation. Science 2023, 379, eabp8622. [Google Scholar] [CrossRef]
- Brazil, MCTI (Ministério da Ciência, Tecnologia e Inovação). Estimativas Anuais de Emissões de Gases de Efeito Estufa no Brasil. Sexta Edição; MCTI: Brasília, Brazil, 2024; p. 137. Available online: http://gov.br/mcti/pt-br/acompanhe-o-mcti/sirene/publicacoes/estimativas-anuais-de-emissoes-gee/arquivos/6a-ed-estimativas-anuais.pdf (accessed on 30 April 2024).

| Sensor/Index | NDVI | NBR | NDWI |
|---|---|---|---|
| Landsat-5 TM | |||
| Landsat-8 OLI | |||
| Sentinel-2 SMI |
| Land Cover | * IBGE Code | Area | Deforestation | SL | Fire_1 | Fire_2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (km2) | (%) | (km2) | (%) | (km2) | (%) | (km2) | (%) | (km2) | (%) | ||
| Water | 7.8 | 0.3 | |||||||||
| Campina | La | 70.8 | 2.3 | ||||||||
| Campinarana | Ld | 601.4 | 19.4 | 59.2 | 5.9 | 79.3 | 10.5 | 108.3 | 22.1 | 7.5 | 35.8 |
| Ecotone | LO | 1.3 | 0 | 0.5 | 0.1 | 0.8 | 0.1 | 0.03 | 0.01 | ||
| Open Ombrophilous forest | As | 33.7 | 1.1 | 3.1 | 0.3 | 0.8 | 0.1 | ||||
| Dense Ombrophilous forest | Ds | 2385.4 | 76.9 | 947.3 | 94.1 | 678.8 | 89.4 | 381.5 | 77.9 | 13.5 | 64.2 |
| TOTAL | 3103.4 | 100 | 1010.1 | 100 | 759.3 | 100 | 489.7 | 100 | 21 | 100 | |
| Year | NDVI-R2 | NBR-R2 | NDWI-R2 |
|---|---|---|---|
| 2007 | 0.8402 *** | 0.8536 *** | 0.8323 *** |
| 2008 | −0.0221 | 0.8530 *** | 0.7898 *** |
| 2009 | 0.8765 *** | 0.7626 *** | 0.7983 *** |
| 2010 | 0.0969 | 0.6222 ** | 0.6455 ** |
| 2011 | 0.7476 ** | 0.6550 ** | 0.5727 ** |
| 2013 | 0.1506 | 0.4575 * | 0.4301 * |
| 2014 | 0.4226 * | 0.6783 ** | 0.7162 ** |
| 2015 | −0.1156 | 0.5539 ** | 0.6331 ** |
| Year | NDVI-R2 | NBR-R2 | NDWI-R2 | |
|---|---|---|---|---|
| Landsat-8 | 2015 | 0.638 ** | 0.6716 ** | 0.678 ** |
| 2016 | 0.8094 *** | 0.8105 *** | 0.8131 *** | |
| 2017 | 0.8700 *** | 0.8852 *** | 0.8397 *** | |
| 2018 | −0.0022 | 0.5211 * | 0.4432 * | |
| 2019 | 0.6992 ** | 0.7902 *** | 0.6417 ** | |
| 2020 | 0.5153 * | 0.7148 ** | 0.6338 ** | |
| 2021 | 0.2814 | 0.6095 ** | 0.4004 * | |
| 2022 | 0.2938 | 0.5164 * | 0.4742 * | |
| 2023 | 0.5000 * | 0.6413 ** | 0.6678 ** | |
| Sentinel-2 | 2015 | 0.5261 * | 0.6472 ** | 0.6284 ** |
| 2016 | 0.6535 ** | 0.7502 ** | 0.7257 ** | |
| 2017 | 0.6637 ** | 0.0789 | 0.0135 | |
| 2018 | 0.0076 | 0.1716 | −0.1087 | |
| 2019 | 0.0224 | 0.8589 *** | 0.5191 * | |
| 2020 | 0.1794 | 0.4363 * | −0.1239 | |
| 2021 | −0.0258 | 0.3867 * | 0.0468 | |
| 2022 | −0.0537 | 0.3627 * | 0.4465 * | |
| 2023 | 0.1712 | 0.4623 * | 0.5366 ** |
| Distance (m) | Area (ha) | Biomass (Mg) | % | Mean (Mg ha−1) | Sd | CV% |
|---|---|---|---|---|---|---|
| 10 | 40.2 | 1490.0 | 7.8 | 37.1 | 5.5 | 14.9 |
| 20 | 43.3 | 1620.4 | 8.5 | 37.4 | 5.0 | 13.3 |
| 30 | 45.3 | 1668.5 | 8.7 | 36.8 | 5.8 | 15.7 |
| 40 | 47.4 | 1775.2 | 9.3 | 37.4 | 5.0 | 13.4 |
| 50 | 49.7 | 1882.2 | 9.8 | 37.9 | 4.2 | 11.2 |
| 60 | 52.2 | 1917.6 | 10 | 36.7 | 5.8 | 15.8 |
| 70 | 54.7 | 2046.4 | 10.7 | 37.4 | 5.0 | 13.5 |
| 80 | 57.3 | 2162.5 | 11.3 | 37.7 | 4.6 | 12.2 |
| 90 | 60.3 | 2217.2 | 11.6 | 36.8 | 5.8 | 15.8 |
| 100 | 63.3 | 2362.3 | 12.3 | 37.3 | 5.1 | 13.6 |
| Total | 513.6 | 19,142.4 | 100.0 | 37.3 | 5.2 | 13.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barni, P.E.; Anderson, L.O.; de Aragão, L.E.O.e.C.; Citó, A.C.; Barbosa, R.I.; Xaud, H.A.M.; Xaud, M.R.; Fearnside, P.M. Edge Effects in the Amazon Rainforest in Brazil’s Roraima State. Forests 2025, 16, 1322. https://doi.org/10.3390/f16081322
Barni PE, Anderson LO, de Aragão LEOeC, Citó AC, Barbosa RI, Xaud HAM, Xaud MR, Fearnside PM. Edge Effects in the Amazon Rainforest in Brazil’s Roraima State. Forests. 2025; 16(8):1322. https://doi.org/10.3390/f16081322
Chicago/Turabian StyleBarni, Paulo Eduardo, Liana Oighenstein Anderson, Luiz Eduardo Oliveira e Cruz de Aragão, Arthur Camurça Citó, Reinaldo Imbrozio Barbosa, Haron Abrahim Magalhães Xaud, Maristela Ramalho Xaud, and Philip Martin Fearnside. 2025. "Edge Effects in the Amazon Rainforest in Brazil’s Roraima State" Forests 16, no. 8: 1322. https://doi.org/10.3390/f16081322
APA StyleBarni, P. E., Anderson, L. O., de Aragão, L. E. O. e. C., Citó, A. C., Barbosa, R. I., Xaud, H. A. M., Xaud, M. R., & Fearnside, P. M. (2025). Edge Effects in the Amazon Rainforest in Brazil’s Roraima State. Forests, 16(8), 1322. https://doi.org/10.3390/f16081322








