Tree-Ring-Based Analysis of Populus euphratica Radial Growth Response to Extreme Drought Across Lower Tarim River Sections, Xinjiang, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Sample Plot Design and Sampling Methods
2.3. Establishment of a Tree-Ring Chronology
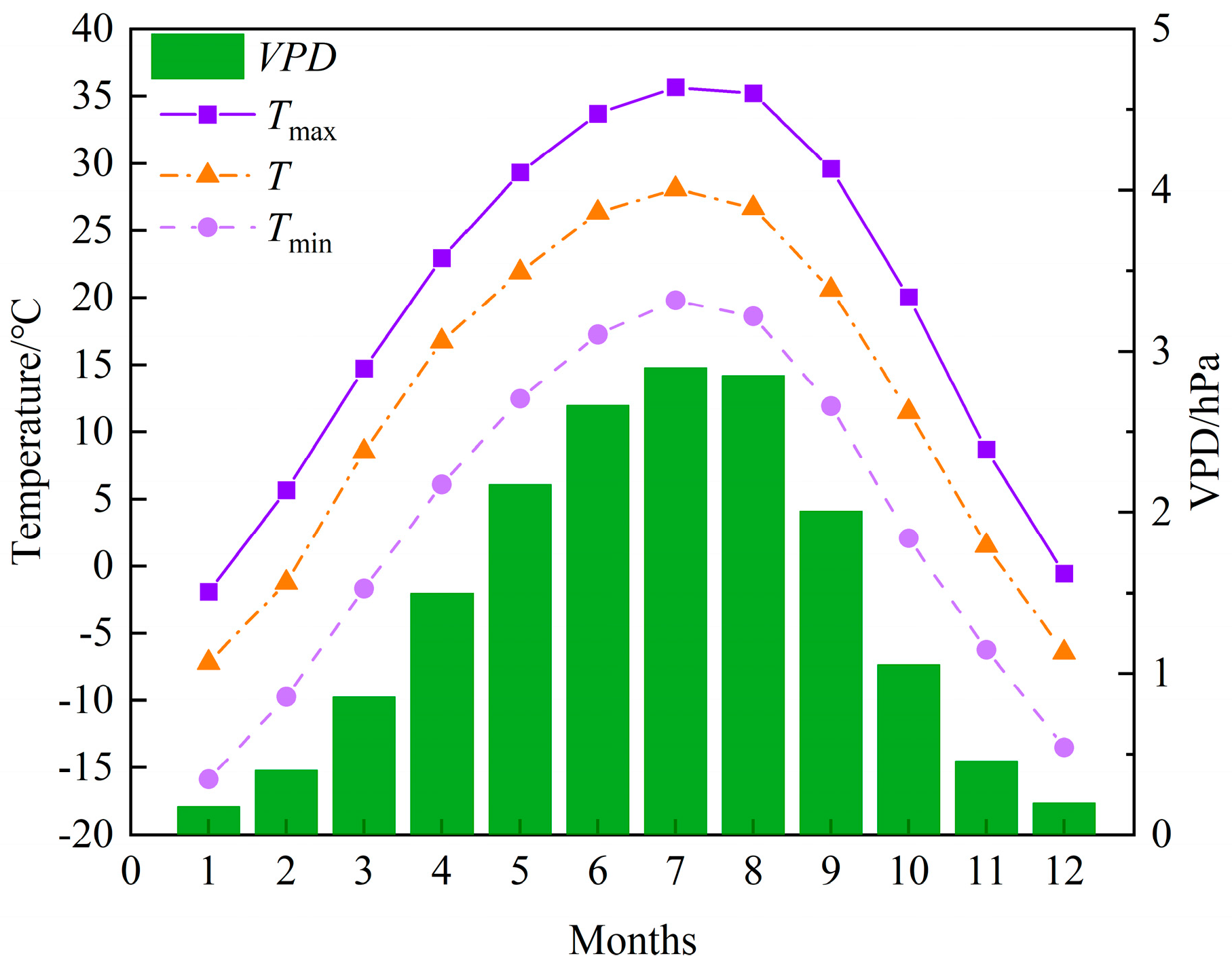
2.4. Meteorological Information
2.5. Resistance and Resilience Measurements
3. Results
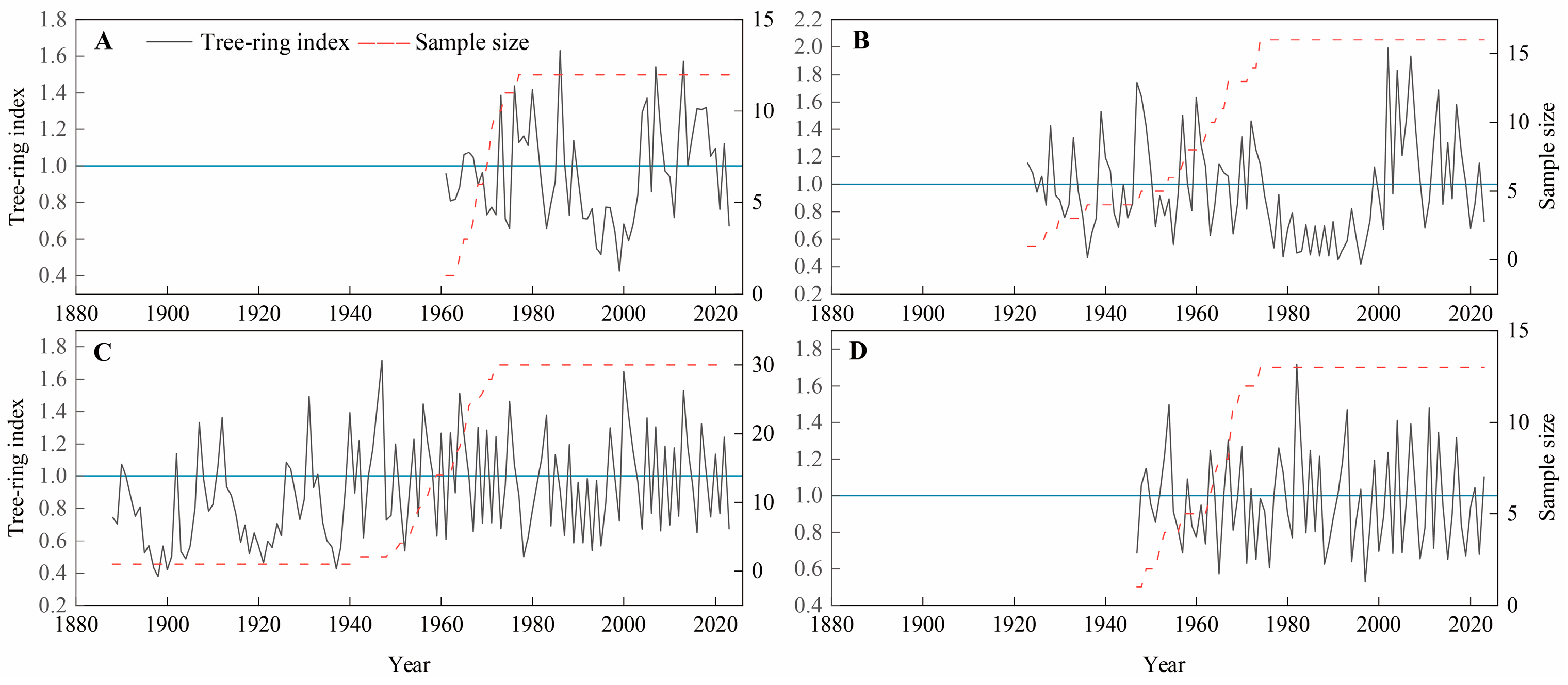
3.1. Chronological Characteristics
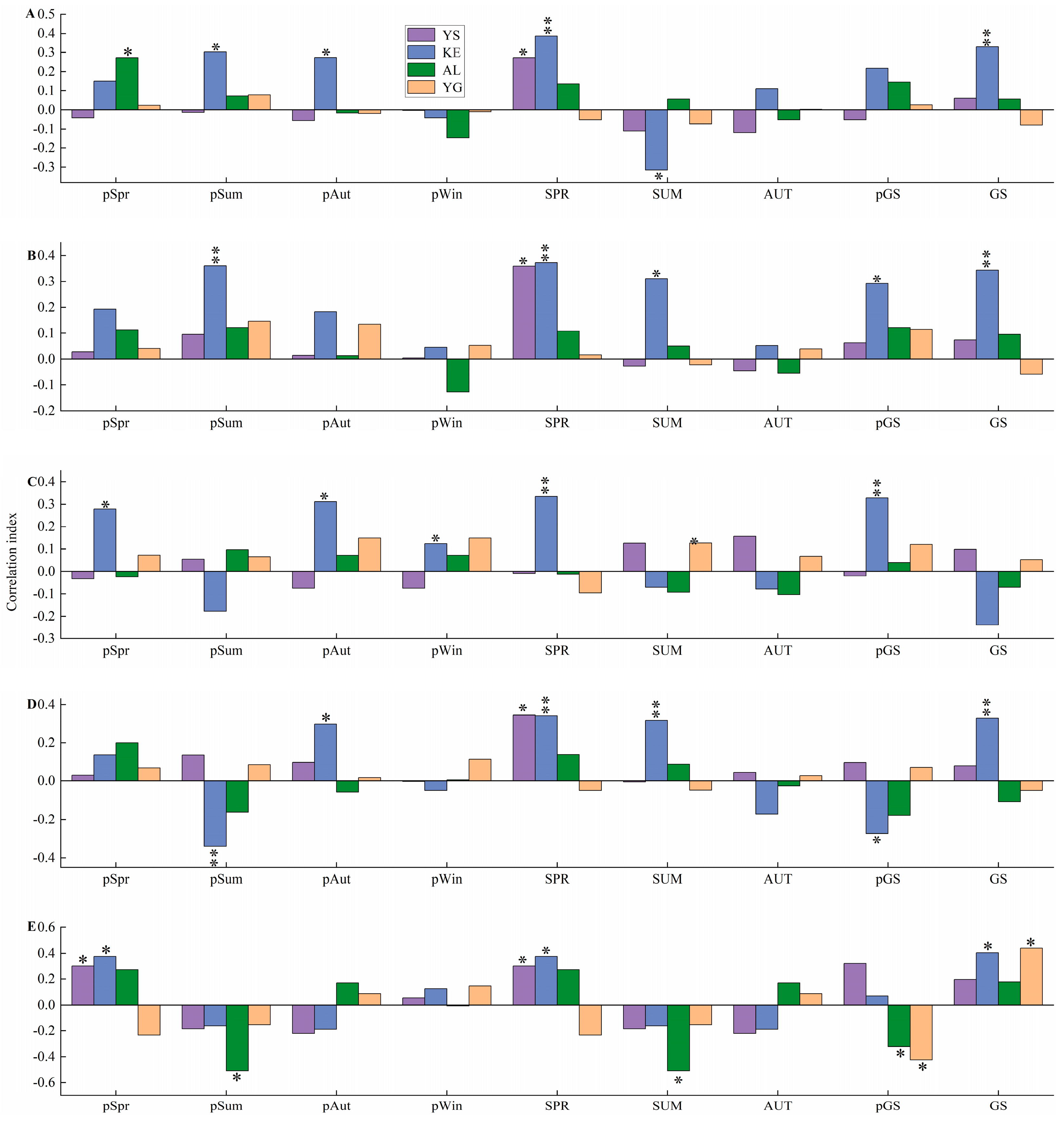
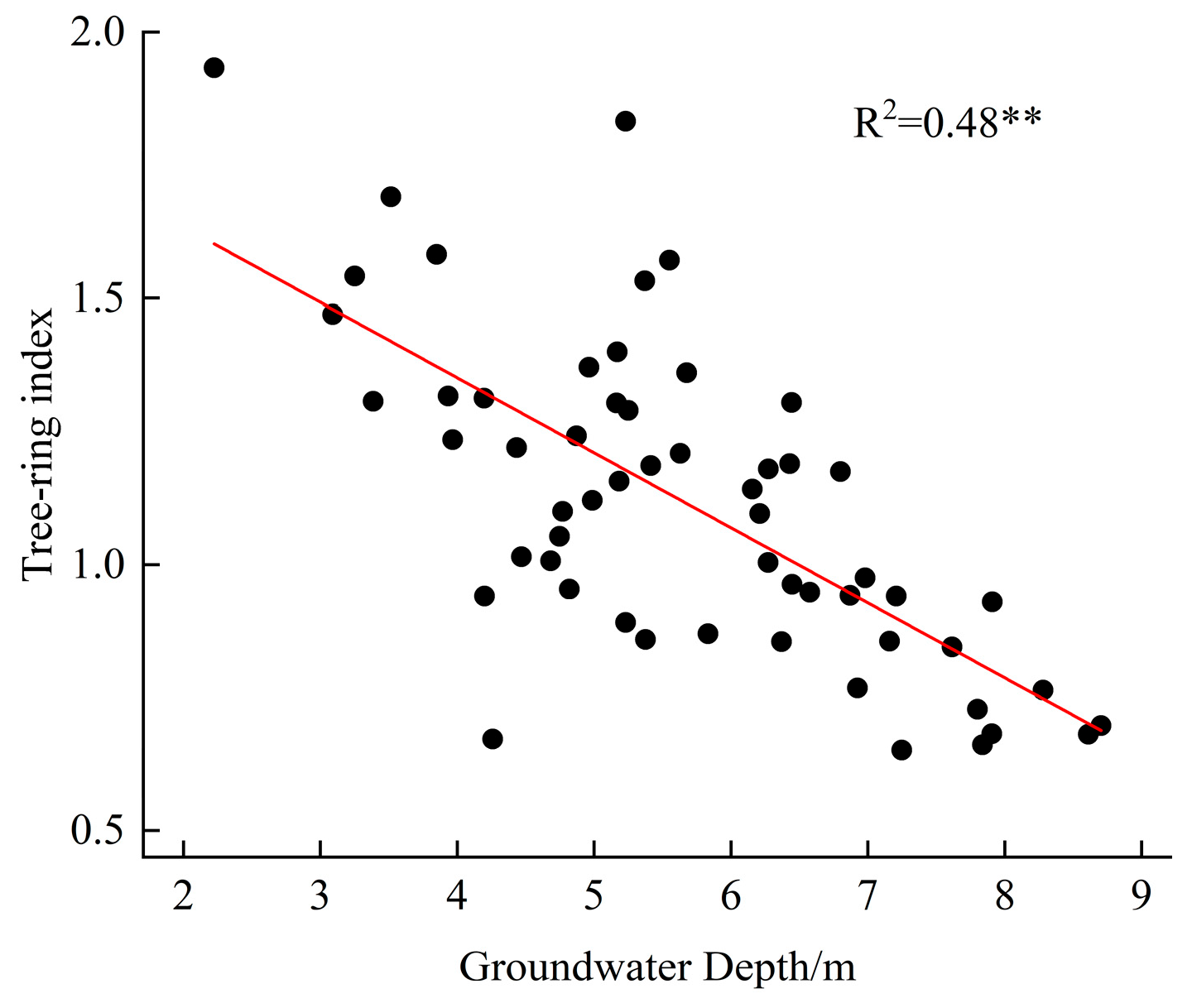
3.2. Correlation Between Radial Growth and Hydrothermal Factors
3.3. Status of and Factors Affecting Populus euphratica Resilience
4. Discussion
4.1. Differences in the Response of Populus euphratica Radial Growth to Hydrothermal Factors in Different Sample Sites
4.2. Response of Populus euphratica Radial Growth to Extreme Drought Events in Different Sample Sites
4.3. Water Management Plays an Important Regulatory Role in the Growth of Populus euphratica
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Trumbore, S.; Brando, P.; Hartmann, H. Forest health and global change. Science 2015, 349, 814–818. [Google Scholar] [CrossRef]
- Colangelo, M.; Camarero, J.J.; Borghetti, M.; Gazol, A.; Gentilesca, T.; Ripullone, F. Size matters a lot: Drought-affected Italian oaks are smaller and show lower growth prior to tree death. Front. Plant Sci. 2017, 8, 135. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Leuzinger, S.; Philipson, C.D.; Tay, J.; Hector, A. Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nat. Clim. Change 2014, 4, 710–714. [Google Scholar] [CrossRef]
- Ludwig, D.; Walker, B.; Holling, C.S. Sustainability, stability, and resilience. Ecol. Soc. 1997, 1, 7. [Google Scholar] [CrossRef]
- Beedlow, P.A.; Lee, E.H.; Tingey, D.T.; Waschmann, R.S.; Burdick, C.A. The importance of seasonal temperature and moisture patterns on growth of Douglas-fir in western Oregon, USA. Agric. For. Meteorol. 2013, 169, 174–185. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Kane, J.M.; Anderegg, L.D.L. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Change 2012, 3, 30–36. [Google Scholar] [CrossRef]
- Cole, L.E.S.; Bhagwat, S.A.; Willis, K.J. Recovery and resilience of tropical forests after disturbance. Nat. Commun. 2014, 5, 3906. [Google Scholar] [CrossRef]
- Shen, J.Y.; Li, S.F.; Huang, X.B.; Wang, S.W.; Su, J.R. Ecological Resilience and Growth Degradation of Pinus yunnanensis at Different Altitudes in Jinsha River Basin. Linye Kexue 2020, 56, 1–11. [Google Scholar]
- Helman, D.; Osem, Y.; Yakir, D.; Lensky, I.M. Relationships between climate, topography, water use and productivity in two key Mediterranean forest types with different water-use strategies. Agric. For. Meteorol. 2017, 232, 319–330. [Google Scholar] [CrossRef]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of tree resilience: Effects of successive low-growth episodes in old ponderosa pine forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- Li, X.; Piao, S.; Wang, K.; Wang, X.; Wang, T.; Ciais, P.; Chen, A.; Lian, X.; Peng, S.; Peñuelas, J. Temporal trade-off between gymnosperm resistance and resilience increases forest sensitivity to extreme drought. Nat. Ecol. Evol. 2020, 4, 1075–1083. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Camarero, J.J.; Rozas, V.; Génova, M.; Olano, J.M.; Arzac, A.; Gazol, A.; Caminero, L.; Tejedor, E.; de Luis, M.; et al. Resist, recover or both? Growth plasticity in response to drought is geographically structured and linked to intraspecific variability in Pinus pinaster. J. Biogeogr. 2018, 45, 1126–1139. [Google Scholar] [CrossRef]
- Sun, S.J.; Lei, S.; Jia, H.S.; Li, C.; Zhang, J.; Meng, P. Tree-ring analysis reveals density-dependent vulnerability to drought in planted Mongolian pines. Forests 2020, 11, 98. [Google Scholar] [CrossRef]
- Lu, K.; Chen, N.; Zhang, C.; Dong, X.; Zhao, C. Drought enhances the role of competition in mediating the relationship between tree growth and climate in semi-arid areas of Northwest China. Forests 2019, 10, 804. [Google Scholar] [CrossRef]
- DeSoto, L.; Cailleret, M.; Sterck, F.; Jansen, S.; Kramer, K.; Robert, E.M.; Aakala, T.; Amoroso, M.M.; Bigler, C.; Camarero, J.J.; et al. Low growth resilience to drought is related to future mortality risk in trees. Nat. Commun. 2020, 11, 545. [Google Scholar] [CrossRef]
- Homeier, J.; Kurzatkowski, D.; Leuschner, C. Stand dynamics of the drought-affected floodplain forests of Araguaia River, Brazilian Amazon. For. Ecosyst 2017, 4, 1–10. [Google Scholar] [CrossRef]
- Zhong, Y.; Zheng, J.C.; Qiu, H.Y.; Lv, L.X. Differences in response of radial growth to extreme droughts for the mainconstructive tree species on sunny and shady slopes in eastern Tibett. Acta Ecol. Sin. 2024, 44, 1–10. [Google Scholar]
- Zhang, Q.B.; Fang, O.Y.; Lv, X.L. Study on tree-ring ecology on the Tibetan Plateau. In Ecological Studies on the Annual Rings of Trees on the Tibetan Plateau, 1st ed.; Li, D., Ed.; Science Publishing House: Beijing, China, 2019; pp. 58–63. [Google Scholar]
- Lucash, M.S.; Scheller, R.M.J.; Gustafson, E.; Sturtevant, B.R. Spatial resilience of forested landscapes under climate change and management. Landscape Ecol. 2017, 32, 953–969. [Google Scholar] [CrossRef]
- Serra-Maluquer, X.; Mencuccini, M.; Martínez-Vilalta, J. Changes in tree resistance, recovery and resilience across three successive extreme droughts in the northeast Iberian Peninsula. Oecologia 2018, 187, 343–354. [Google Scholar] [CrossRef]
- Willis, K.J.; Jeffers, E.S.; Tovar, C. What makes a terrestrial ecosystem resilient? Science 2018, 359, 988–989. [Google Scholar] [CrossRef]
- Calagari, M.; Ghasemi, R.A.; Bagheri, R. Growth comparison of Populus euphratica Oliv. provenances in research station of Karadj, Iran. Iran. J. For. Poplar Res. 2010, 18, 69–76. [Google Scholar]
- Qisen, L.; Qi, F.; Luxin, Z. Study of the height growth dynamic based on tree-ring data in Populus euphratica from the lower reach of the Heihe River, China. Dendrochronologia 2010, 28, 49–64. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Chen, Y.; Zhang, N.; Li, W.H. Degradation of Populus euphratica community in the lower reaches of the Tarim River, xinjiang, China. J. Environ. Sci. 2005, 17, 740–747. [Google Scholar]
- Aishan, T.; Halik, Ü.; Betz, F.; Tiyip, T.; Ding, J.; Nuermaimaiti, Y.; Teyip, T. Stand structure and height-diameter relationship of a degraded Populus euphratica forest in the lower reaches of the Tarim River, Northwest China. J. Arid Land 2015, 7, 544–554. [Google Scholar] [CrossRef]
- Misson, L.; Rocheteau, A.; Rambal, S.; Ourcival, J.; Limousin, J.; Rodriguez, R. Functional changes in the control of carbon fluxes after 3 years of increased drought in a Mediterranean evergreen forest? Glob. Change Biol. 2010, 16, 2461–2475. [Google Scholar] [CrossRef]
- Xu, H.L.; Chen, Y.N.; Yang, G. Effect of Translating Water on Vegetation at the Lower Reaches of TarimRiver. Acta Ecologiga Sin. 2003, 4, 18–22. [Google Scholar]
- WuBuyer, B.; Jiang, T.A.; Halik, M.; Wang, H.J.; Wang, N.; Jiang, W. Carbon storage and allocation characteristics of natural Populus euphratica forestsin the lower Tarim River. J. Forest. Environ. 2023, 43, 363–370. [Google Scholar]
- Chen, Y.N.; Wumaierjiang, W.; Aikeremu, A.; Cheng, Y.; Chen, Y.P. Monitoring and analysis of ecological benefits of water conveyancein the lower reaches of Tarim River in recent 20 years. Arid. Zone Geo. 2021, 44, 605–611. [Google Scholar]
- Wang, S.D.; Gao, Q.Z.; Pulati, S.; Xu, M.; Mao, W.Y.; Sheng, Y.P. Maintaining and concerning the life health of Tarim River:Connotation and Diagnoses for the Life Health of Tarim River. J. Arid. Land. 2008, 4, 594–603. [Google Scholar]
- Stokes, M.A.; Smiley, T.L. An Introduction to Tree-Ring Dating; University of Arizona Press: Tucson, AZ, USA, 1968. [Google Scholar]
- Zhang, Y.; Ye, M. Sensitivity Analysis of P, euphratica Radial Growth to Groundwater Changes in the Different Transects of the Lower Reaches of Tarim River. Acta Bot. Boreali-Occident. Sin. 2016, 36, 818–824. [Google Scholar]
- Grissino-Mayer, H.D. Evaluating crossdating accuracy: A manual and tutorial for the computer program COFECHA. Tree-Ring Res. 2001, 52, 205–221. [Google Scholar]
- Cook, E.R.; Krusic, P.J. Program ARSTAN: A Tree-Ring Standardization Program Based on Detrending and Autoregressive Time Series Modeling, with Interactive Graphics; Lamont-Doherty Earth Observatory, Columbia University: Palisades, NY, USA, 2005. [Google Scholar]
- Gruber, A.; Strobl, S.; Veit, B.; Oberhuber, W. Impact of drought on the temporal dynamics of wood formation in Pinus sylvestris. Tree Physiol. 2010, 30, 490–501. [Google Scholar] [CrossRef]
- Bi, Y.; Xu, J.; Gebrekirstos, A.; Guo, L.; Zhao, M.; Liang, E.; Yang, X. Assessing drought variability since 1650 AD from tree-rings on the Jade Dragon Snow Mountain, southwest China. Int. J. Climatol. 2015, 35, 4057–4065. [Google Scholar] [CrossRef]
- Qi, Y.Y.; Baierdang, K.Y.M.; Li, Z.S.; Zeng, F.J. Radial growth response of Populus euphratica to climate change in the Cele desert oasis ecotone, China. J. Appl. Ecol. 2024, 35, 1187–1195. [Google Scholar]
- Yang, W.Q.; Fan, Z.X.; Li, Z.S.; Wen, Q.Z. Radial growth of Pinus yunnanensis at different elevations and their responses toclimatic factors in the Yulong Snow Mountain, Northwest Yunnan, China. Acta Ecol. Sin 2018, 38, 8983–8991. [Google Scholar]
- He, Q.Z.; Ye, M.; Pan, X.T.; Zhao, F.F.; Zhang, K.L. Xylem formation of Populus euphratica and its response to water heat factors in the lower reaches of Tarim Riyer, China. J. Appl. Ecol. 2023, 34, 1244–1252. [Google Scholar]
- Li, T.; He, X.Y.; Chen, Z.J. Tree-ring growth responses of Mongolian oak (Quercus mongolica) to climate change in southern Northeast: A case study in Qianshan Mountains. J. Appl. Ecol. 2014, 25, 1841–1844. [Google Scholar]
- Han, J.S.; Zhao, H.Y.; Zhu, L.D.; Zhang, Y.D.; Wang, X.C. Comparing the responses of radial growth between Quercus mongolica and Phellodendron amurense to climate change in Xiaoxing’an Mountains, China. J. Appl. Ecol. 2019, 30, 2218–2230. [Google Scholar]
- Fan, Z.X.; Bräuning, A.; Cao, K.F.; Zhu, S.D. Growth–climate responses of high-elevation conifers in the central Hengduan Mountains, southwestern China. For. Ecol. Manag. 2009, 258, 306–313. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J.; Vicente-Serrano, S.M.; Sánchez-Salguero, R.; Gutiérrez, E.; de Luis, M.; Sangüesa-Barreda, G.; Novak, K.; Rozas, V.; Tíscar, P.A.; et al. Forest resilience to drought varies across biomes. Glob. Chang. Biol. 2018, 24, 2143–2158. [Google Scholar] [CrossRef]
- Millar, C.I.; Stephenson, N.L.; Stephens, S.L. Climate change and forests of the future: Managing in the face of uncertainty. Ecol. Appl. 2007, 17, 2145–2151. [Google Scholar] [CrossRef]
- Qiu, T.; Sharma, S.; Woodall, C.W.; Clark, J.S. Niche shifts from trees to fecundity to recruitment that determine species response to climate change. Front. Ecol. Evol. 2021, 9, 719141. [Google Scholar] [CrossRef]
- Hanbury-Brown, A.R.; Powell, T.L.; Muller-Landau, H.C.; Wright, S.J.; Kueppers, L.M. Simulating environmentally-sensitive tree recruitment in vegetation demographic models. New Phytol. 2022, 235, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Song, F.S.; Fang, O.Y. Research on history of Juniperus tibetica growth declinein Three-River-Source National Park. J. Forest. Environ. 2019, 39, 386–392. [Google Scholar]
- Adams, H.D.; Germino, M.J.; Breshears, D.D.; Barron-Gafford, G.A.; Guardiola-Claramonte, M.; Zou, C.B.; Huxman, T.E. Nonstructural leaf carbohydrate dynamics of P inus edulis during drought-induced tree mortality reveal role for carbon metabolism in mortality mechanism. New Phytol. 2013, 197, 1142–1151. [Google Scholar] [CrossRef]
- Bond, W. The tortoise and the hare: Ecology of angiosperm dominance and gymnosperm persistenc. Biol. J. Linn. Soc. Lond. 1989, 36, 227–249. [Google Scholar] [CrossRef]
- Johnston, D.B.; Cooper, D.J.; Hobbs, N.T. Relationships between groundwater use, water table, and recovery of willow on Yellowstone’s northern range. Ecosphere 2011, 2, 1–11. [Google Scholar] [CrossRef]


| Cross-Sectional | Longitude | Latitude | Average Chest Diameter/cm | Monitoring Well | Number of Cores |
|---|---|---|---|---|---|
| YS | 87°56′ E | 40°25′ N | 42.41 ± 13.09 | F1, F2, F10 | 34 |
| KE | 88°10′ E | 40°22′ N | 39.07 ± 8.79 | G1, G5 | 42 |
| AL | 88°20′ E | 40°8′ N | 37.33 ± 12.46 | H1-H5 | 62 |
| YG | 88°22′ E | 39°47′ N | 34.27 ± 4.63 | I1, I2 | 36 |
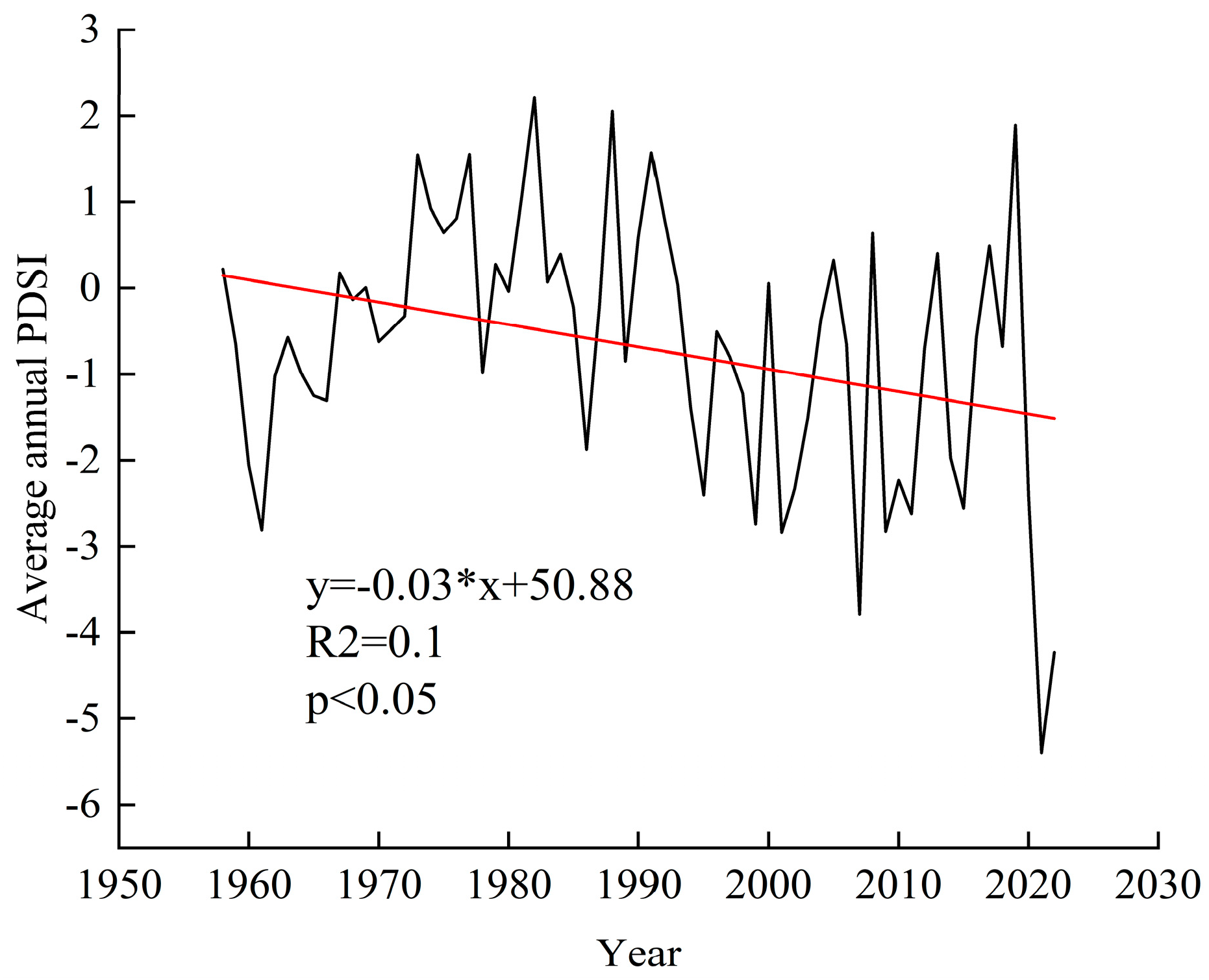
| Year | Degree of Drought | PDSI Last Year | PDSI for That Year’s Growing Season |
|---|---|---|---|
| 1984 | mild drought | −0.72 | −0.89 |
| 1994 | mild drought | −2.23 | −1.39 |
| 1998 | moderate drought | −1.79 | −2.23 |
| 2001 | moderate drought | −3.63 | −2.84 |
| 2010 | moderate drought | −2.22 | −2.26 |
| 2015 | mild drought | −1.23 | −1.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Chen, W.; Pan, X.; Wang, T.; Che, J.; Lv, Y.; Ye, M. Tree-Ring-Based Analysis of Populus euphratica Radial Growth Response to Extreme Drought Across Lower Tarim River Sections, Xinjiang, China. Forests 2025, 16, 1311. https://doi.org/10.3390/f16081311
Xie X, Chen W, Pan X, Wang T, Che J, Lv Y, Ye M. Tree-Ring-Based Analysis of Populus euphratica Radial Growth Response to Extreme Drought Across Lower Tarim River Sections, Xinjiang, China. Forests. 2025; 16(8):1311. https://doi.org/10.3390/f16081311
Chicago/Turabian StyleXie, Xiaodong, Weilong Chen, Xiaoting Pan, Tongxin Wang, Jing Che, Yexin Lv, and Mao Ye. 2025. "Tree-Ring-Based Analysis of Populus euphratica Radial Growth Response to Extreme Drought Across Lower Tarim River Sections, Xinjiang, China" Forests 16, no. 8: 1311. https://doi.org/10.3390/f16081311
APA StyleXie, X., Chen, W., Pan, X., Wang, T., Che, J., Lv, Y., & Ye, M. (2025). Tree-Ring-Based Analysis of Populus euphratica Radial Growth Response to Extreme Drought Across Lower Tarim River Sections, Xinjiang, China. Forests, 16(8), 1311. https://doi.org/10.3390/f16081311





