Understory Plant Diversity in Cunninghamia lanceolata (Lamb.) Hook. Plantations Under Different Mixed Planting Patterns
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Plot Establishment
2.3. Survey and Calculation of Understory Vegetation Diversity and Biomass
2.4. Soil Sample Collection
2.5. Determination of Soil Nutrient Indices
2.6. Data Processing
3. Results
3.1. Understory Species Composition and Importance Values Across Different Mixed Patterns
3.2. Understory Species Diversity and Biomass Across Different Mixed Patterns
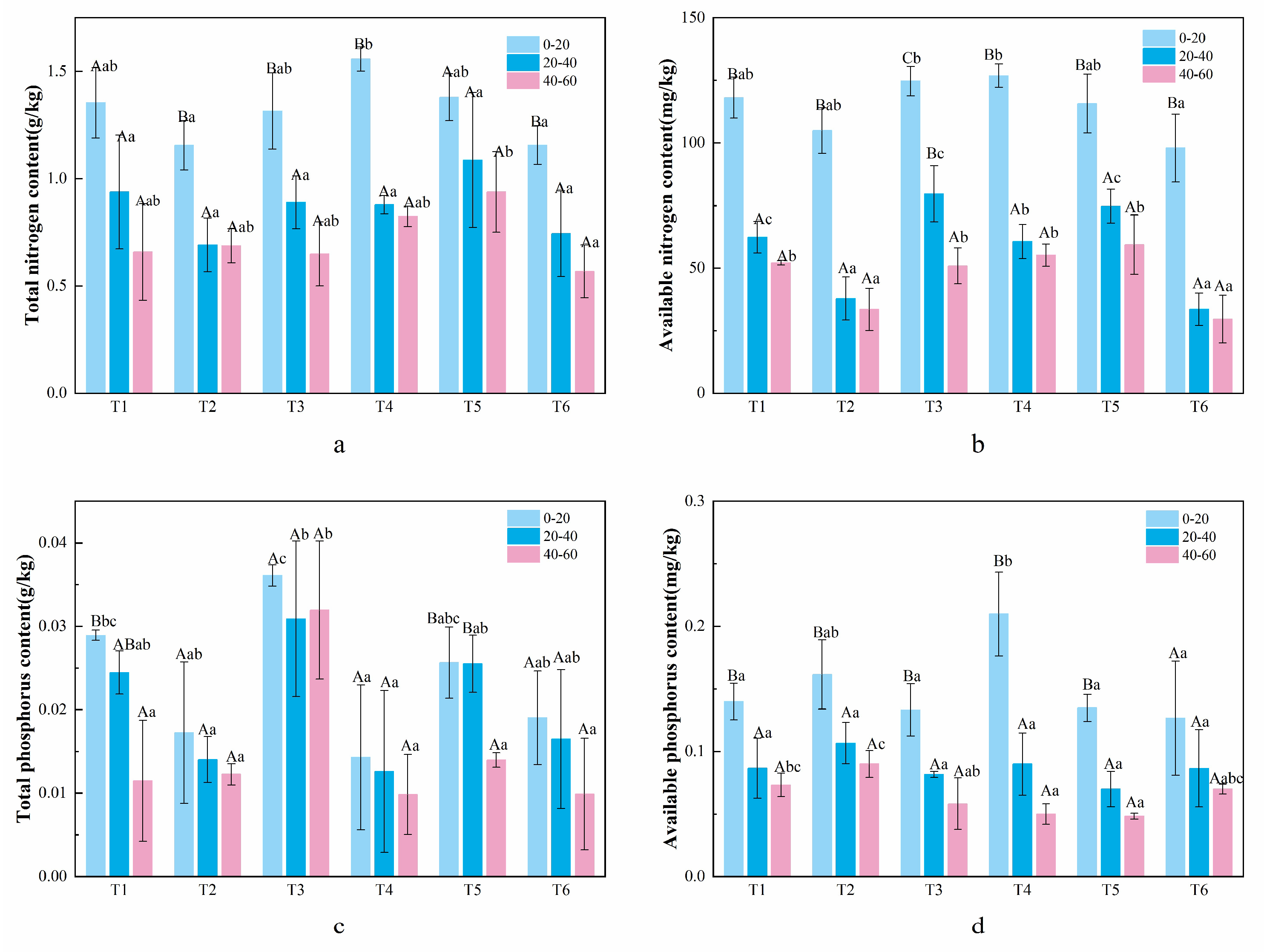
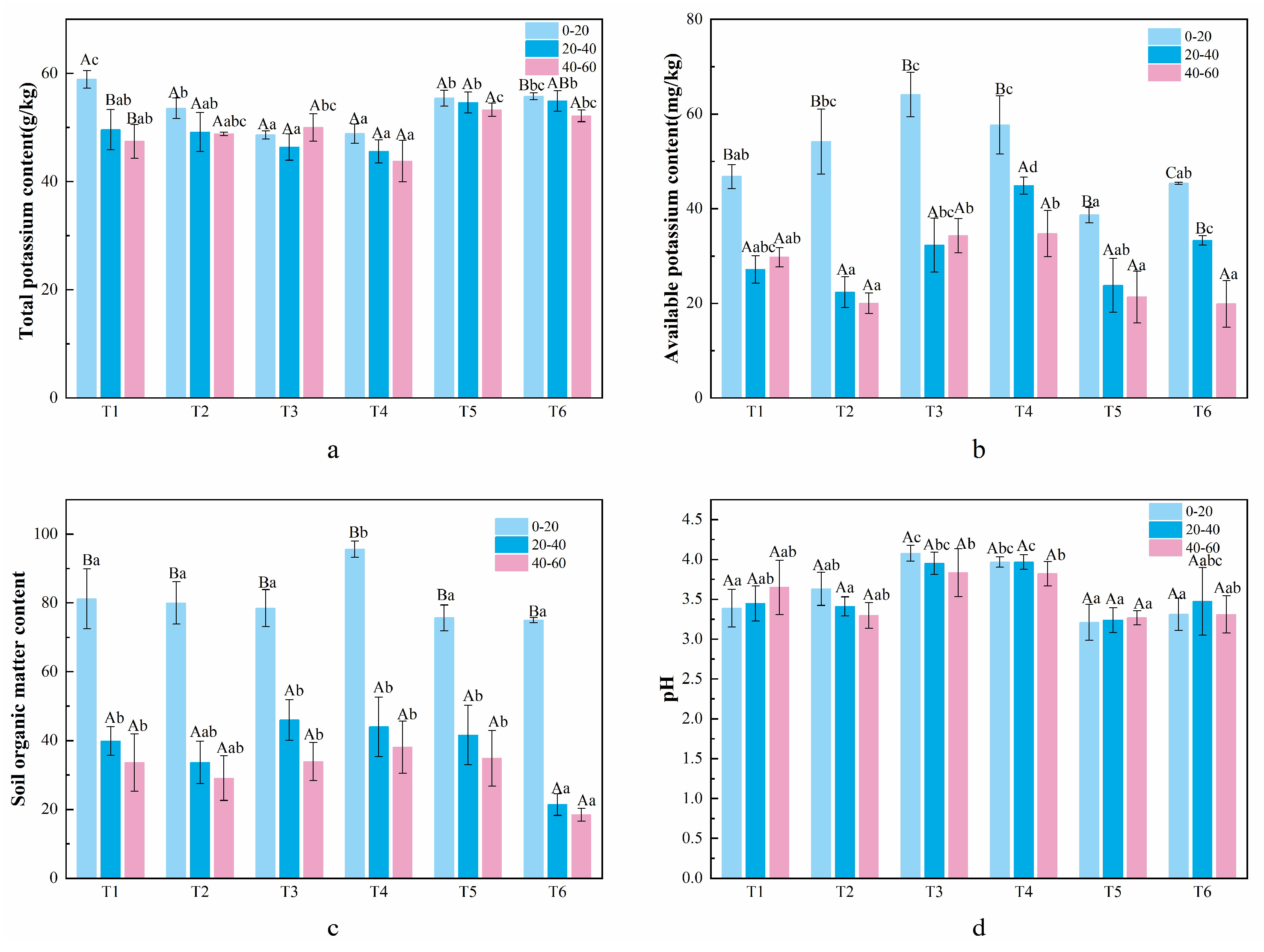
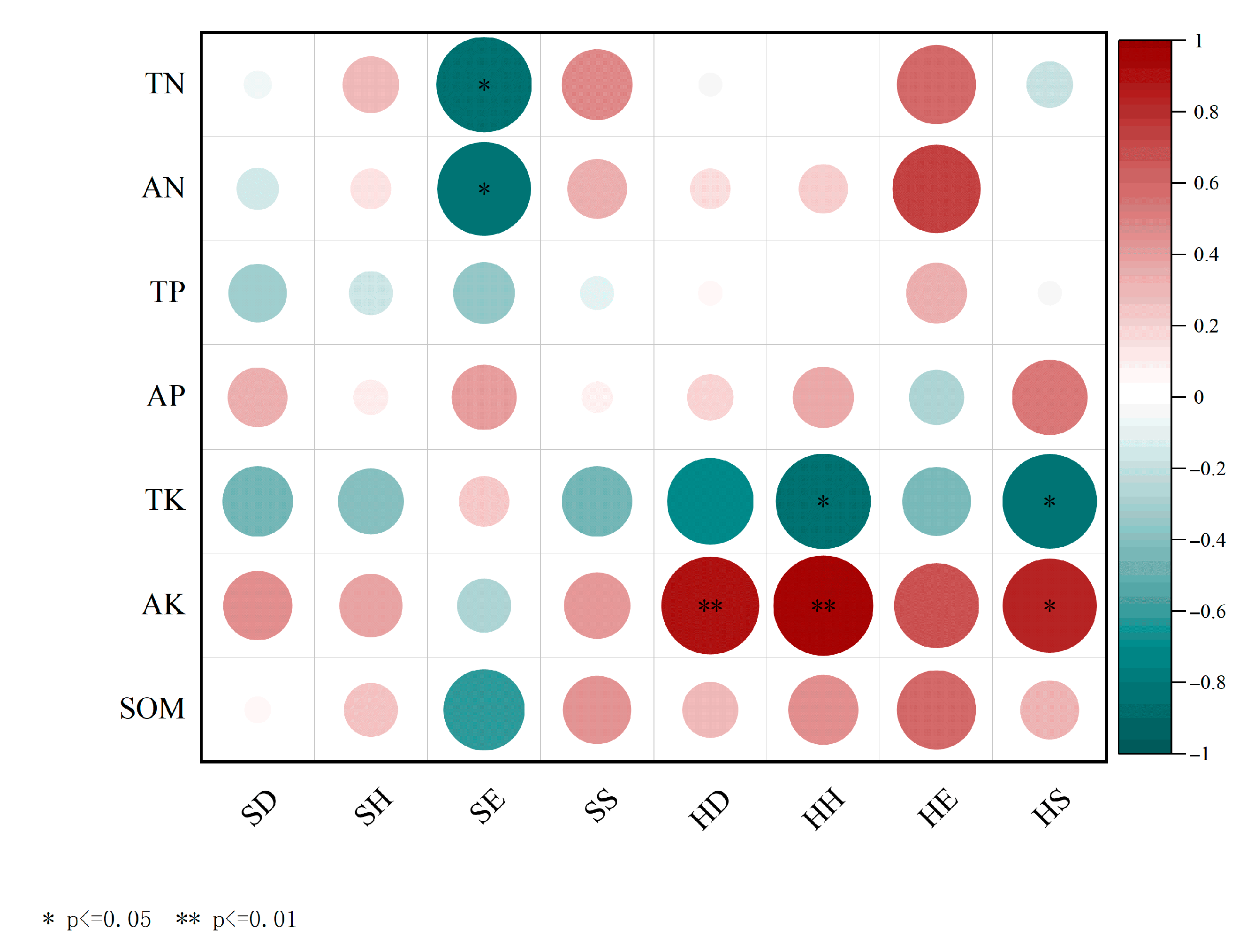
3.3. Understory Plant Diversity and Soil Physicochemical Properties in C. lanceolata Plantations with Different Mixed Patterns
4. Discussion
4.1. The Impact of Different Mixed Patterns on Understory Plant Diversity
4.2. Correlation Between Soil Physicochemical Properties and Mixed Patterns
4.3. The Effect of Mixed Patterns on Soil Nutrient Content
4.4. The Superiority of the C. lanceolata, P. bournei, and Symplocos Mixed Pattern
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, R.S.; Kang, W.X.; Zhou, Y.Q.; Tian, D.L.; Xiang, W.H. Changes in nutrient cycling with age in a Cunninghamia lanceolata plantation forest. J. Plant Ecol. 2018, 42, 173–184. [Google Scholar] [CrossRef][Green Version]
- Zhang, C.S.; Li, K. Research Status and Advances of Nutrient Cycling of Plantation. World For. Res. 2005, 18, 35–39. [Google Scholar] [CrossRef]
- Zhang, H.D.; Kang, X.R.; Shao, W.H.; Yang, X.; Zhang, J.F.; Liu, X.Q.; Chen, G.C. Characteristics of herbaceous plant biodiversity in Cunninghamia lanceolate plantations with different community structures. Acta Ecol. Sin. 2021, 41, 2118–2128. [Google Scholar] [CrossRef]
- Qu, Y.C.; Jiang, Y.H.; Chen, H.Y.; Zhang, J.G.; Zhang, X.Q. Dynamic change of stand leaf area for Chinese fir plantation. Acta Ecol. Sin. 2024, 44, 5609–5620. [Google Scholar] [CrossRef]
- Kang, X.R.; Li, X.G.; Zhang, H.D.; Liu, X.Q.; Chen, G.C. Community stability characteristics of Cunninghamia lanceolata plantations with different mixing measures. Chin. J. Ecol. 2020, 39, 2912–2920. [Google Scholar] [CrossRef]
- Chen, H.; Guo, H.T.; Chen, R.; Xue, G.H.; Wang, L.Y.; Jiang, J. Understory Plant Diversity of Cunninghamia lanceolata Plantations and Its Short-Term Environmental Response to Different Thinning Intensities. Acta Ecol. Sin. 2023, 43, 10274–10284. [Google Scholar] [CrossRef]
- Peng, Y.; Shi, Z.T.; He, L.; Shen, R.; Xu, R. Temporal and Spatial Changes of Natural Forest Loss in Xishuangbanna and its Landscape Ecologica Response Based on Landsat Data. For. Resour. Manag. 2022, 64, 73–80. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, X.Z.; Jia, H.Y.; Ming, A.G.; Chen, B.B.; Lu, Y.C. Effects of Stand Density on Understory Species Diversity and Soil Physicochemical Properties Following Close-to-Nature Transformation of Chinese Fir Plantations. J. Beijing For. Univ. 2019, 41, 170–177. [Google Scholar] [CrossRef]
- Yu, X.T. On the Regression of Chinese Fir Plantation—Discussion on the Sustainable Management of Chinese Fir in Terms of the Cause and Effect of Soil Degradation. World For. Res. 1999, 12, 15–19. [Google Scholar] [CrossRef]
- Xu, M.; Luo, Z.R.; Yu, M.J.; Ding, B.Y.; Wu, Y.G. Floristic composition and community structure of mid-montane evergreen broad-leaved forest in north slope of Baishanzu Mountain. J. Zhejiang Univ. (Agric. Life Sci.) 2007, 33, 450–457. [Google Scholar]
- Gong, H.D.; Zhang, Y.P.; Liu, Y.H.; Yang, G.P.; Lu, Z.Y.; Lu, H.Z. Nterception capability in an evergreen broad-leaved forest of Ailaoshan, Yunnan Province. J. Zhejiang A&F Univ. 2008, 25, 469–474. [Google Scholar] [CrossRef]
- Yu, D.J.; Zheng, Y.G.; Sun, C.H.; Zhao, C.; Jin, Y.H.; Zhang, L.J.; Li, J.G.; Liu, L.J. Investigation and Factors Analysis of Dendrolimus superansOutbreaks in Changbai Mountain National Nature Reserve. For. Res. 2022, 35, 103–111. [Google Scholar] [CrossRef]
- Xu, J.J.; Chen, X.; Liu, Z.Y.; Pang, C.M.; Yu, S.Y. Relationship between Spatial Heterogeneity of Soil Nutrients and Plant Composition in the Evergreen Broad-leaved Forest of Tianmu Mountain. Zhejiang For. Sci. Technol. 2022, 42, 33–41. [Google Scholar] [CrossRef]
- Hu, E.C.; Wang, Z.; Li, Z.H.; Li, Z.F.; Dong, D.W.; Bao, H.; Gao, R.H. Understory Plant Diversity and Its Relationship with Soil Physicochemical Properties in Different Plantations in Mu Us Sandy Land. For. Res. 2024, 37, 174–181. [Google Scholar] [CrossRef]
- Wang, M.H.; Cui, F.K.; Wu, Z.P.; Wang, M.H.; Cui, F.K.; Wu, Z.P.; Xu, X.Y.; Ou, S.K.; Chen, J.H.; Ma, S.J.; et al. The Pattern of Understory Plant Diversity and Soil Physicochemical Properties in the Severely Burned Areas of Forests. For. Environ. Sci. 2024, 40, 1–8. [Google Scholar] [CrossRef]
- Hu, G.D.; Zhou, J.Q.; Da, J.S.; Lei, L.J.; Zhao, C.X.; Li, C.N.; Zhou, Z.Q.; Liu, J.R. Relationship Between Understory Plant Diversity and Soil Properties of Different Forests in Sandy Land of Jinta. Acta Agrestia Sin. 2023, 31, 1834–1841. [Google Scholar]
- Gao, C.; Fu, R.X.; Mu, W.J.; Chen, L.X.; Wu, W.R.; Yu, Y.C. Effects of Mixed Forests of Chinese Fir and Broad-leaved Trees and Understory Planting on Soil Nitrogen Mineralization. For. Res. 2024, 37, 23–32. [Google Scholar] [CrossRef]
- Zou, X.C.; Wang, X.M.; Zuo, Y.F.; Zhang, Z.X.; He, K.N. Characteristics of herbaceous diversity and environmental interpretation of Picea crassifolia at different succession stages. Acta Ecol. Sin. 2023, 43, 10285–10294. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, R.S. Comprehensive Evaluation of Soil Nutrients after Mixed Transformation of Pinus Sylvestris var. Mngolica Plantation. For. Resour. Manag. 2021, 1, 132–139. [Google Scholar] [CrossRef]
- Cao, X.Y.; Li, J.P.; Hu, Y.J. Storage of Soil Microelements of Cunninghamia lanceolata in Fushou Forest Farm. J. Northwest For. Univ. 2016, 31, 55–59. [Google Scholar]
- Lu, N.N.; Gao, Z.X.; Zhang, P.; Xu, X.P.; Wang, X.J. Path Analysis between Soil Properties and Undergrowth Vegetation in Pure Chinese Fir Forest. J. Northeast For. Univ. 2015, 43, 73–77. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.T.; Yang, Q.J.; He, Z.M.; Cao, G.Q.; Chen, A.L. Differences of Soil Physicochemical Properties and Undergrowth Vegetation Diversity of 100-Year-Old Chinese Fir Plantations in Different Terrain. J. Southwest For. Univ. 2021, 41, 60–70. [Google Scholar] [CrossRef]
- Guo, H.T.; Ji, X.F.; Wang, C.; Zou, H.L.; Wang, L.Y.; Jiang, J. Spatial variability and influencing factors of plant diversity in the shrub layers of artificial forests in China. J. Nanjing For. Univ. 2022, 46, 144–152. [Google Scholar] [CrossRef]
- Shi, H.N.; Li, W.P.; Li, Z.H.; Wang, Y.G.; Yang, X.Z. Effects of fir and broad-leaved mixed forest with different mixing proportions on the species diversity and soil nutrients under the forest. J. Cent. South Univ. For. Technol. 2022, 42, 34–41. [Google Scholar] [CrossRef]
- Lai, A.H.; Wu, Z.L.; Zhou, X.N.; Zhou, C.J.; Wang, K.X. Influence of Selective Intensity on Stand Spatial Structure of Cunninghamia lanceolata-Broadleaved Mixed Plantation. J. Beihua Univ. 2016, 17, 109–115. [Google Scholar] [CrossRef]
- Yan, S.; Deng, H.Y.; Hu, D.H.; Wang, R.H.; Wei, R.P.; Zheng, H.Q.; Zou, Y.H.; Chen, X.W. Comprehensive Quality Evaluation of the Pure and Mixed Cunninghamia lanceolata Stands of the Ecological Public-Welfare Forests in Northern Guangdong. For. Resour. Manag. 2022, 5, 69–75. [Google Scholar] [CrossRef]
- Kang, Y.; Xin, X.B.; Pei, S.X.; Guo, H.; Fa, L.; Wu, S.; Ma, S.M.; Wu, D. Undergrowth Species Diversity characteristics of Larix principis-rupprechti plantation. J. Northwest For. Univ. 2025, 40, 23–31, 82. [Google Scholar] [CrossRef]
- Bai, Y.X.; Liu, R.H.; Su, J.H.; Shen, W.J. Effects of tree species mixing on bulk and rhizosphere soil microbial resource limitation in stands of Pinus massoniana and Castanopsis hystrix. Acta Ecol. Sin. 2024, 44, 10770–10781. [Google Scholar] [CrossRef]
- Yang, Q.; Pu, H.M.; Zhao, X.C.; Wang, Z.W.; Cheng, H.; Dong, R.; Chen, Y.L.; Jin, B.C. Comparison of different plant cover investigation methods for three artificial grasslands. Appl. Environ. Biol. 2021, 27, 220–227. [Google Scholar] [CrossRef]
- Hou, X.L.; Han, H.; Tigabu, M.; Cai, L.P.; Meng, F.R.; Liu, A.Q.; Ma, X.Q. Changes in soil physico-chemical properties following vegetation restoration mediate bacterial community composition and diversity in Changting, China. Ecol. Eng. 2019, 138, 171–179. [Google Scholar] [CrossRef]
- Buysse, J.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods of Soil Agrochemistry; Chinese Agriculture Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Fan, R.; Xu, H.F.; Li, C.; Zhang, H.; Zhang, H.; Chen, L.; Wang, N.L. Changes in plant diversity and water use efficiency during the recovery process of subtropical forest lands. Geogr. Res. 2024, 43, 776–790. [Google Scholar] [CrossRef]
- Feng, Y.H.; Schmid, B.; Loreau, M.; Forrester, D.I.; Fei, S.L.; Zhu, J.X.; Tang, Z.Y.; Zhu, J.L.; Hong, P.B.; Ji, C.J.; et al. Multispecies forest plantations outyield monocultures across a broad range of conditions. Science 2022, 376, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Kneeshaw, D.; Peng, C.; Wu, Y.; Feng, L.; Qu, X.; Wang, W.; Pan, C.; Feng, H. Positive effects of species mixing on biodiversity of understory plant communities and soil health in forest plantations. Proc. Natl. Acad. Sci. USA 2025, 122, e2418090122. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Gao, G.N.; Huang, H.M.; Li, J.J.; Xiao, N.; Liao, S.S.; Huang, X.M.; Zhao, L.J.; You, Y.M. Effects of Multi-layer Mixed-age Modification on Soil Phosphorus Components and Transformation in Cunninghamia lanceolata Plantation. Guangxi Sci. 2024, 31, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Ling, G.C.; Shen, H.; Fan, R.D.; Zhang, N.J.; Xia, C.C.; Hu, W.M. Growth and Stoichiometry Characteristics of C, N and P in Soil under Mixed Plantation of Different Aged Cunninghamia lanceolata and Phoebe bournei. J. Zhejiang For. Sci. Technol. 2022, 42, 14–20. [Google Scholar] [CrossRef]
- Meng, T.T.; Ni, J.; Wang, G.H. Plant Funcitional Traits, Environmental And Ecosystem Functioning. Chin. J. Plant Ecol. 2007, 31, 150–165. [Google Scholar] [CrossRef]
- Zhalnina, K.; Dias, R.; de Quadros, P.D.; Dörr de Quadros, P.; Davis-Richardson, A.; Camargo, F.A.O.; Clark, I.M.; McGrath, S.P.; Hirsch, P.R.; Triplett, E.W. Soil pH Determines Microbial Diversity and Composition in the Park Grass Experiment. Microb. Ecol. 2015, 69, 395–406. [Google Scholar] [CrossRef]
- Zou, G.H.; Zhao, F.L.; Lan, X.C.; Wu, T.H. Effects of Coconut Shell Biochar on Enzyme Activities and Microbial Community in Tropical Paddy Soil Under Different Water Conditions. Soils 2024, 56, 525–532. [Google Scholar] [CrossRef]
- She, T.; Tian, Y. Effects of litter diversity on decomposition process and soil microbial characteristics in forest ecosystems. Ecol. Sci. 2020, 39, 213–223. [Google Scholar] [CrossRef]
- Huang, H.M.; Yan, J.L.; Li, J.J.; Xiang, M.Z.; Li, C.H.; Liao, S.S.; Huang, X.M.; You, Y.M. Effects of Introducing Other Tree Species of Different Ages into Pinus massoniana Plantation on Activities and Stoichiometric Ratios of Enzymes Associated with Soil Aggregates. Guangxi Sci. 2024, 31, 427–438. [Google Scholar] [CrossRef]
- Dong, X.D.; Gao, P.; Li, T.; Zhang, J.C.; Dong, J.W.; Xu, J.W.; Dun, X.J. Effects of soil microbial community on the litter decomposition in mixed Quercus acutissima Carruth. and Robinia pseudoacacia L. forest. Acta Ecol. Sin. 2021, 41, 2315–2325. [Google Scholar] [CrossRef]
- Sun, C.C.; Wang, Z.Q.; Pan, C.; Song, Y.Q.; Yu, Y.C. Effects of Cunninghamia lanceolata and Schima superba Mixed Forest on Soil Nutrients and Enzyme Activities. J. Jiangxi Agric. Univ. 2023, 45, 517–525. [Google Scholar] [CrossRef]
- Su, S.; Jin, N.; Wei, X. Effects of thinning on the understory light environment of different stands and the photosynthetic performance and growth of the reforestation species Phoebe bournei. J. For. Res. 2024, 35, 6. [Google Scholar] [CrossRef]
- Pan, Y.Y. Preliminary study on the growth amount and soil fertility of Cunninghamia and broad-leaved mixed forest. Green Technol. 2016, 15, 8–10. [Google Scholar] [CrossRef]
- Maccherini, S.; Salerni, E.; Mocali, S.; Bianchetto, E.; Landi, S.; De Meo, I.; Di Salvatore, U.; Marchi, M.; Bacaro, G.; Tordoni, E. Silvicultural management does not affect biotic communities in conifer plantations in the short-term: A multi-taxon assessment using a BACI approach. For. Ecol. Manag. 2021, 493, 119257. [Google Scholar] [CrossRef]
- Tian, X.R.; Shu, L.F. The Application and Besearch of Fire Break Forest Belts. World For. Res. 2000, 13, 20–26. [Google Scholar] [CrossRef]
- Liang, H.; Wang, L.; Wang, Y.; Quan, X.; Li, X.; Xiao, Y.; Yan, X. Root Development in Cunninghamia lanceolata and Schima superba Seedlings Expresses Contrasting Preferences to Nitrogen Forms. Forests 2022, 13, 2085. [Google Scholar] [CrossRef]
- Yang, L.; Mao, M.Y.; Guo, J.L.; Yang, H.; Zhang, X.Y.; Li, S.B. Hydraulic architecture and photosynthetic characteristics of Chinese fir at different canopies heights. J. For. Environ. 2024, 44, 468–475. [Google Scholar] [CrossRef]
- Jia, X.R.; Su, Z.Y.; Ou, Y.D.; Xie, D.D. Canopy structural parameters and understory light regimes of 3 artificial forest stands in South China. Guangxi Plants 2011, 31, 473–478, 544. [Google Scholar] [CrossRef]
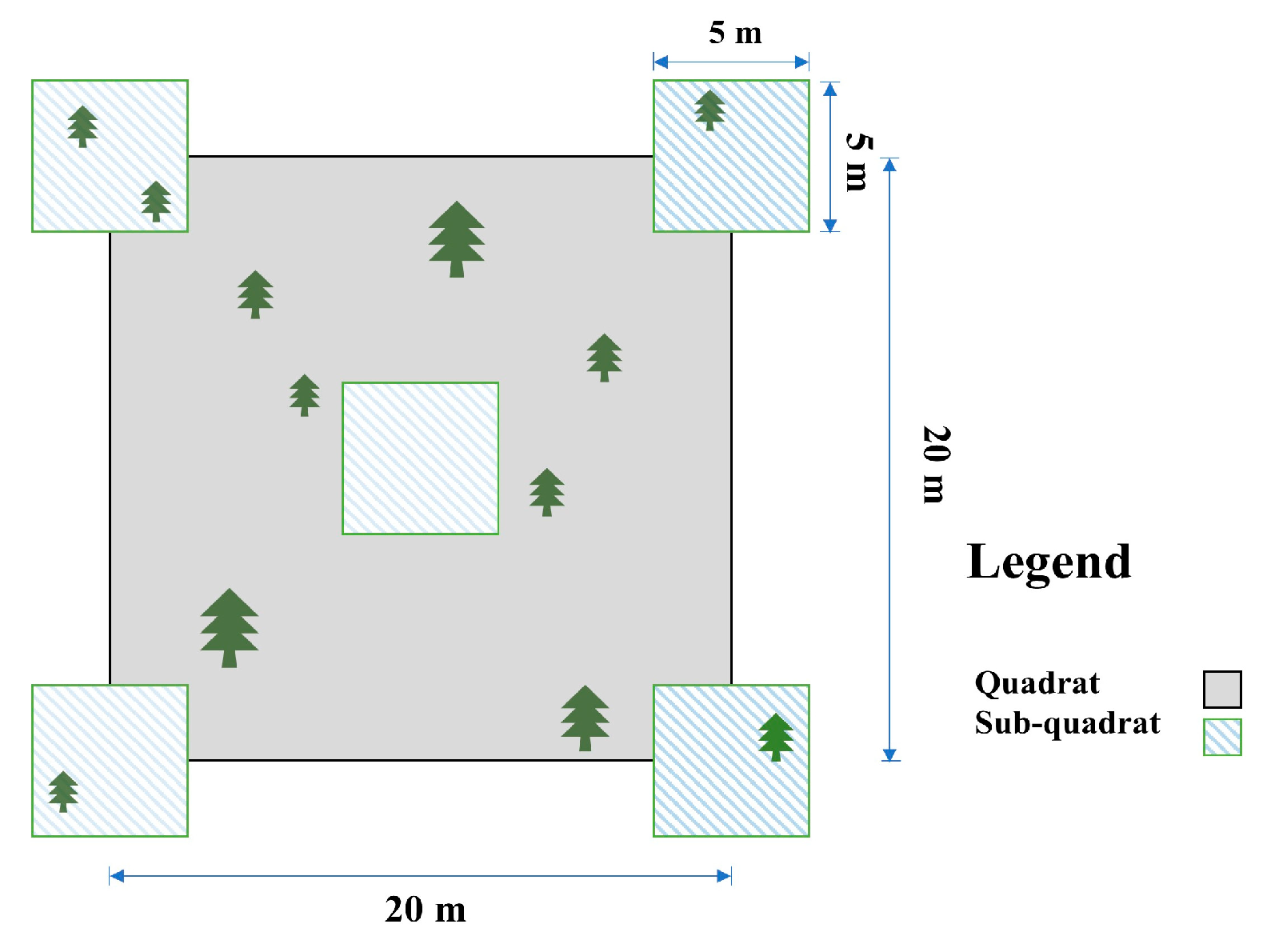


| Mixed Stand Type | Species Mixing Ratio | Average Diameter at Breast Height (cm) | Average Tree Height (m) |
|---|---|---|---|
| T1 | CL:LF = 1:3 | 19.18 ± 2.75 | 14.20 ± 2.55 |
| T2 | CL:PB = 1:3 | 12.54 ± 0.43 | 8.77 ± 1.30 |
| T3 | CL:PB:LF = 1:2:1 | 12.47 ± 0.73 | 9.66 ± 0.52 |
| T4 | CL:PB:SS = 1:2:1 | 13.11 ± 0.44 | 9.13 ± 0.76 |
| T5 | CL:PB:CF = 1:2:1 | 16.79 ± 0.86 | 14.49 ± 2.95 |
| T6 | CL:PB:PE = 1:2:1 | 12.23 ± 1.27 | 8.04 ± 1.17 |
| Layer | Num. | Scientific Name | Family Name | Mixed Pattern | |||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | ||||
| Shrub Layer | 1 | Litsea cubeba (Lour.) Pers. | Lauraceae | 0 | 0 | 0 | 0 | 0 | 10.19 |
| 2 | E. japonica | Theaceae | 16.93 | 13.93 | 12.14 | 5.52 | 11.74 | 14.32 | |
| 3 | Rubus corchorifolius L. f. | Rosaceae | 19.05 | 4.77 | 3.9 | 11.85 | 8.31 | 8.65 | |
| 4 | Morinda officinalis How | Rubiaceae | 15.43 | 8.75 | 5 | 0 | 8.55 | 11.61 | |
| 5 | L. chinense | Hamamelidaceae | 10.34 | 5.72 | 13.78 | 10.91 | 8.69 | 10.15 | |
| 6 | S. paniculata | Symplocaceae | 9.07 | 11.99 | 9.29 | 7.87 | 7.76 | 11.06 | |
| 7 | Raphiolepis indica (L.) Lindl. | Rosaceae | 0 | 5.57 | 0 | 2.78 | 4.32 | 13.18 | |
| 8 | Smilax china L. | Smilacaceae | 0 | 0 | 0 | 0 | 8.92 | 4.75 | |
| 9 | Toxicodendron vernicifluum(Stokes) F. A. Barkley | Anacardiaceae | 9.15 | 11.77 | 6.63 | 11.12 | 7.16 | 4.75 | |
| 10 | L. formosana | Hamamelidaceae | 7.48 | 0 | 9.85 | 0 | 0 | 0 | |
| 11 | Mussaenda pubescens W. T. Aiton | Rubiaceae | 26.52 | 0 | 0 | 0 | 16.04 | 0 | |
| 12 | Clerodendrum cytrophyllum Turcz. | Lamiaceae | 0 | 0 | 0 | 0 | 10.32 | 0 | |
| 13 | Dalbergia hupeana Hance | Fabaceae | 0 | 8.17 | 9.97 | 3.75 | 4.32 | 0 | |
| 14 | P. bournei | Lauraceae | 0 | 15.02 | 15.13 | 9.68 | 3.61 | 0 | |
| 15 | Camellia japonica L. | Theaceae | 14.09 | 3.05 | 7.28 | 3.06 | 4.32 | 0 | |
| 16 | Ardisia japonica (Thunb.) Blume | Primulaceae | 0 | 14.06 | 7.24 | 9.44 | 0 | 0 | |
| 17 | Ficus pandurate Hance | Moraceae | 0 | 0 | 0 | 21.61 | 0 | 0 | |
| Herb Layer | 1 | D. dichotoma | Gleicheniaceae | 46.91 | 53.9 | 29.24 | 32.33 | 63.82 | 40.24 |
| 2 | W. japonica | Blechnaceae | 34.41 | 27.7 | 27.22 | 16.01 | 33.95 | 31.83 | |
| 3 | Cornopteris decurrentialata (Hook.) Nakai | Athyriaceae | 0 | 0 | 0 | 0 | 0 | 22.45 | |
| 4 | Smilax glabra Roxb. | Smilacaceae | 7.56 | 0 | 13.1 | 8.12 | 0 | 4.55 | |
| 5 | Lophatherum gracile Brongn. | Poaceae | 13.25 | 7.85 | 16.91 | 8.66 | 0 | 11.16 | |
| 6 | Dryopteris chinensis (Baker) Koidz. | Dryopteridaceae | 17.62 | 0 | 0 | 7.1 | 0 | 27.46 | |
| 7 | Melastoma dodecandrum Lour. | Melastomataceae | 0 | 0 | 0 | 0 | 10.76 | 10 | |
| 8 | Ficus tikoua Bur. | Moraceae | 0 | 0 | 0 | 0 | 6.68 | 0 | |
| 9 | Adiantum flabellulatum L. | Pteridaceae | 0 | 0 | 14.37 | 21.93 | 6.68 | 0 | |
| 10 | Ophiopogon bodinieri H. Lév. | Asparagaceae | 0 | 0 | 8.71 | 11.31 | 0 | 0 | |
| 11 | Miscanthus floridulus (Labill.) Warb. | Poaceae | 0 | 10.76 | 15.02 | 11.31 | 0 | 0 | |
| 12 | S. china | Smilacaceae | 0 | 9.32 | 0 | 0 | 0 | 0 | |
| 13 | Hedyotis auricularia L. | Rubiaceae | 0 | 0 | 0 | 13.1 | 0 | 0 | |
| Mixed Pattern | Aboveground Biomass/(g·m−2) | Proportion of the Total Biomass/% | |||
|---|---|---|---|---|---|
| Shrub Layer | Herb Layer | Total Biomass | Shrub Layer | Herb Layer | |
| T1 | 76.52 ± 1.63 a | 102.63 ± 0.82 b | 179.15 ± 2.12 b | 42.71 | 57.29 |
| T2 | 141.24 ± 4.92 c | 122.71 ± 1.70 c | 263.95 ± 4.13 c | 53.51 | 46.49 |
| T3 | 198.01 ± 1.71 e | 142.36 ± 5.82 d | 340.36 ± 4.35 e | 58.18 | 41.82 |
| T4 | 240.26 ± 3.27 f | 183.09 ± 3.38 e | 423.35 ± 2.13 f | 56.75 | 43.25 |
| T5 | 165.99 ± 1.66 d | 142.69 ± 7.58 d | 308.67 ± 6.23 d | 53.77 | 46.23 |
| T6 | 85.08 ± 2.12 b | 75.55 ± 1.79 a | 160.63 ± 1.94 a | 52.97 | 47.03 |
| Biomass Indexes | Bs | Bh | Bt | |
|---|---|---|---|---|
| Plant diversity indexes in shrub layer | D | 0.337 | 0.173 | 0.281 |
| H | 0.541 * | 0.368 | 0.485 * | |
| E | −0.317 | −0.336 | −0.329 | |
| S | 0.656 ** | 0.488 * | 0.603 ** | |
| Plant diversity indexes in herb layer | D | 0.429 | 0.274 | 0.378 |
| H | 0.593 ** | 0.466 | 0.554 * | |
| E | 0.349 | 0.31 | 0.34 | |
| S | 0.567 * | 0.417 | 0.519 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Guo, H.; Jiang, J. Understory Plant Diversity in Cunninghamia lanceolata (Lamb.) Hook. Plantations Under Different Mixed Planting Patterns. Forests 2025, 16, 1290. https://doi.org/10.3390/f16081290
Wang M, Guo H, Jiang J. Understory Plant Diversity in Cunninghamia lanceolata (Lamb.) Hook. Plantations Under Different Mixed Planting Patterns. Forests. 2025; 16(8):1290. https://doi.org/10.3390/f16081290
Chicago/Turabian StyleWang, Minsi, Hongting Guo, and Jiang Jiang. 2025. "Understory Plant Diversity in Cunninghamia lanceolata (Lamb.) Hook. Plantations Under Different Mixed Planting Patterns" Forests 16, no. 8: 1290. https://doi.org/10.3390/f16081290
APA StyleWang, M., Guo, H., & Jiang, J. (2025). Understory Plant Diversity in Cunninghamia lanceolata (Lamb.) Hook. Plantations Under Different Mixed Planting Patterns. Forests, 16(8), 1290. https://doi.org/10.3390/f16081290





