Multiplication of Axillary Shoots of Adult Quercus robur L. Trees in RITA® Bioreactors
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Micropropagation in Semisolid Medium
2.2. Experiments in Bioreactors
2.2.1. Explant Type and Support
2.2.2. Frequency of Immersion
2.2.3. Media Composition
2.3. Rooting
2.4. Data Recording and Statistical Analysis
3. Results
3.1. Effect of the Support
3.2. Effect of Immersion Frequency
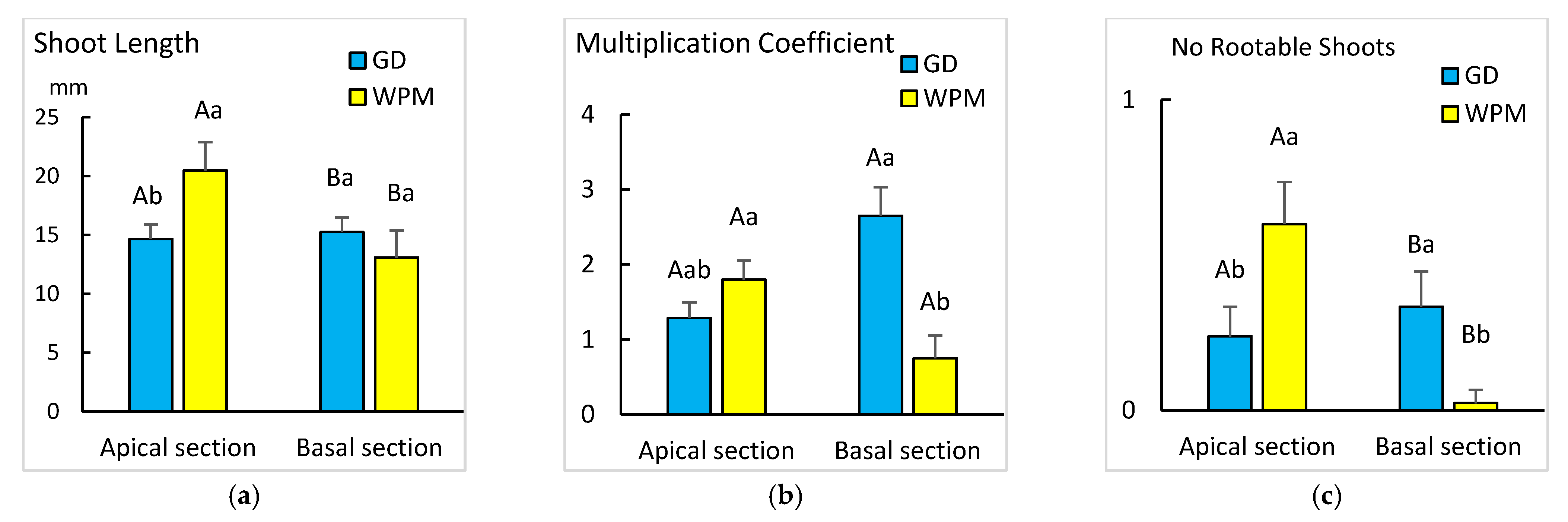
3.3. Effect of the Culture Media
3.4. Effect of Silver Nitrate and Activated Charcoal
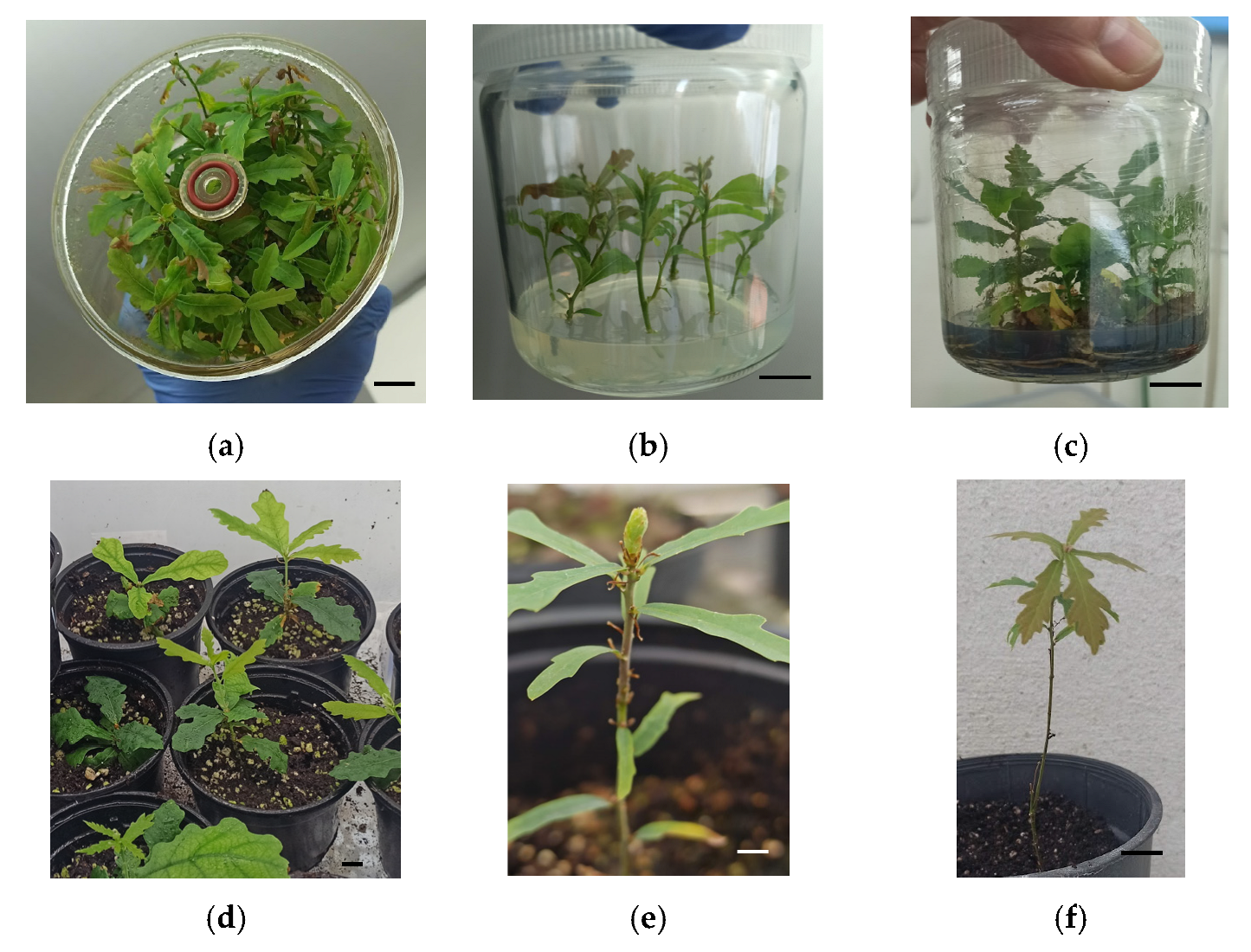
3.5. Rooting and Acclimation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jenkins, M.; Schaap, B. Forest Ecosystem Services. In Background Analytical Study 1; Global Forest Goals; United Nations Forum on Forests: New York, NY, USA, 2018; p. 41. Available online: https://www.un.org/esa/forests/wp-content/uploads/2018/05/UNFF13_BkgdStudy_ForestsEcoServices.pdf (accessed on 30 May 2025).
- FAO. The State of the World’s Forests 2024—Forest-Sector Innovations Towards a More Sustainable Future. 2024. Available online: https://openknowledge.fao.org/items/ec487897-97b5-43ec-bc2e-5ddfc76c8e85 (accessed on 29 May 2025).
- Scion. Forest Ecosystem Services. 2021. Available online: https://www.scionresearch.com/__data/assets/pdf_file/0009/78678/Forest_ecosystem_services_infosheet.pdf (accessed on 29 May 2025).
- Goded, S.; Ekroos, J.; Domínguez, J.; Azcarate, J.G.; Guitián, J.A.; Smith, H.G. Effects of eucalyptus plantations on avian and herb species richness and composition in North-West Spain. Glob. Ecol. Conserv. 2019, 19, e00690. [Google Scholar] [CrossRef]
- García-Fernández, F.; Vidal, M.; Regos, A.; Domínguez, J. Eucalyptus cover as the primary driver of native forest bird reductions: Evidence from a stand-scale analysis in NW Iberia. Forest Ecol. Manag. 2025, 586, 122714. [Google Scholar] [CrossRef]
- Eaton, E.; Caudullo, G.; Oliveira, S.; de Rigo, D. Quercus robur and Quercus petraea in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; pp. 160–163. [Google Scholar]
- Zamora, F. Barrel aging; types of wood. In Red Wine Technology; Morata, A., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 125–147. [Google Scholar] [CrossRef]
- Krutovsky, K.V.; Popova, A.A.; Yakovlev, I.A.; Yanbaev, Y.A.; Matveev, S.M. Response of pedunculate oak (Quercus robur L.) to adverse environmental conditions in genetic and dendrochronological studies. Plants 2025, 14, 109. [Google Scholar] [CrossRef]
- Savill, P.S.; Kanowski, P.J. Tree improvement programs for European oaks: Goals and strategies. Ann. Sci. For. 1993, 50 (Suppl. S1), 368–383. [Google Scholar] [CrossRef][Green Version]
- Szuba, A.; Kalemba, E.M.; Wawrzyniak, M.K.; Suszka, J.; Chmielarz, P. Deterioration in the quality of recalcitrant Quercus robur seeds during six months of storage at subzero temperatures: Ineffective activation of prosurvival mechanisms and evidence of freezing stress from an untargeted metabolomic study. Metabolites 2022, 12, 756. [Google Scholar] [CrossRef] [PubMed]
- Chmielarz, P.; Kotlarski, S.; Kalemba, E.M.; Martins, J.P.R.; Michalak, M. Successful in vitro shoot multiplication of Quercus robur L. trees aged up to 800 years. Plants 2023, 12, 2230. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.P.R.; Wawrzyniak, M.K.; Kalemba, E.M.; Ley-López, J.M.; Mendes, M.M.; Naskręt-Barciszewska, M.Z.; Barciszewski, J.; Chmielarz, P. Differential morphophysiological and epigenetic responses during in vitro multiplication of Quercus robur depending on donor age and plant growth regulators. Plant Cell Tiss. Organ Cult. 2024, 159, 62. [Google Scholar] [CrossRef]
- Favre, J.M.; Juncker, B. In vitro growth of buds taken from seedlings and adult plant material in Quercus robur L. Plant Cell Tiss. Organ Cult. 1987, 8, 49–60. [Google Scholar] [CrossRef]
- Chalupa, V. Vegetative propagation of oak (Quercus robur and Q petraea) by cutting and tissue culture. Ann. Sci. For. 1993, 50 (Suppl. S1), 295s–307s. [Google Scholar] [CrossRef]
- Meier-Dinkel, A.; Becker, B.; Duckstein, D. Micropropagation and ex vitro rooting of several clones of late-flushing Quercus robur L. Ann. Sci. For. 1993, 50 (Suppl. S1), 319s–322s. [Google Scholar]
- Gebhardt, K.; Frühwacht-Wilms, U.; Weisgerber, H. Micropropagation and restricted-growth storage of adult oak genotypes. Ann. Sci. For. 1993, 50 (Suppl. S1), 323s–329s. [Google Scholar] [CrossRef]
- Juncker, B.; Favre, J.M. Long-term effects of culture establishment from shoot-tip explants in micropropagating oak (Quercus robur L). Ann. Sci. For. 1994, 51, 581–588. [Google Scholar] [CrossRef]
- Vieitez, A.M.; Sánchez, M.C.; Amo-Marco, J.B.; Ballester, A. Forced flushing of branch segments as a method for obtaining reactive explants of mature Quercus robur trees for micropropagation. Plant Cell Tissue Organ Cult. 1994, 37, 287–295. [Google Scholar] [CrossRef]
- Sanchez, M.C.; San-Jose, M.C.; Ballester, A.; Vieitez, A.M. Requirements for in vitro rooting of Quercus robur and Q. rubra shoots derived from mature trees. Tree Physiol. 1993, 16, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Bonga, J.M. Clonal propagation of mature trees: Problems and possible solutions. In Cell and Tissue Culture in Forestry: General Principles and Biotechnology; Bonga, J.M., Durzan, D.J., Eds.; Martinus Nijhoff Publishers: Dordrecht, The Netherlands, 1987; Volume 1, pp. 249–271. Available online: https://link.springer.com/chapter/10.1007/978-94-017-0994-1_15 (accessed on 25 April 2025).
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tiss. Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Watt, M.P. The status of temporary immersion system (TIS) technology for plant micropropagation. Afr. J. Biotechn. 2012, 11, 14025–14035. [Google Scholar] [CrossRef]
- Georgiev, V.; Schumann, A.; Pavlov, A.; Bley, T. Temporary immersion systems in plant biotechnology. Eng. Life Sci. 2014, 14, 607–621. [Google Scholar] [CrossRef]
- Vidal, N.; Sánchez, C. Use of bioreactor systems in the propagation of forest trees. Eng. Life Sci. 2019, 19, 896–915. [Google Scholar] [CrossRef] [PubMed]
- Mallón, R.; Covelo, P.; Vieitez, A.M. Improving secondary embryogenesis in Quercus robur: Application of temporary immersion for mass propagation. Trees 2012, 26, 731–741. [Google Scholar] [CrossRef]
- Mallón, R.; Vieitez, A.M.; Vidal, N. High-efficiency Agrobacterium-mediated transformation in Quercus robur: Selection by use of a temporary immersion system and assessment by quantitative PCR. Plant Cell Tiss. Organ Cult. 2013, 114, 171–185. [Google Scholar] [CrossRef]
- Pérez, M.; Bueno, M.A.; Escalona, M.; Toorop, P.; Rodríguez, R.; Cañal, M.J. Temporary immersion systems (RITA®) for the improvement of cork oak somatic embryogenic culture proliferation and somatic embryo production. Trees 2013, 27, 1277–1284. [Google Scholar] [CrossRef]
- Kong, L.; Holtz, C.T.; Nairn, C.J.; Houke, H.; Powell, W.A.; Baier, K.; Merkle, S.A. Application of airlift bioreactors to accelerate genetic transformation in American chestnut. Plant Cell Tiss. Organ Cult. 2014, 117, 39–50. [Google Scholar] [CrossRef]
- McGuigan, L.; Fernandes, P.; Oakes, A.; Stewart, K.; Powell, W. Transformation of American chestnut (Castanea dentata (Marsh.) Borkh) using RITA® temporary immersion bioreactors and We Vitro containers. Forests 2020, 11, 1196. [Google Scholar] [CrossRef]
- Vidal, N.; Blanco, B.; Cuenca, B. A temporary immersion system for micropropagation of axillary shoots of hybrid chestnut. Plant Cell Tissue Organ Cult. 2015, 123, 229–243. [Google Scholar] [CrossRef]
- Liu, Z.; Bi, W.L.; Shukla, M.R.; Saxena, P.K. In vitro technologies for American chestnut (Castanea dentata (Marshall) Borkh) conservation. Plants 2022, 11, 464. [Google Scholar] [CrossRef]
- Thiesen, F.; Bubner, B. Unlocking the secrets of stable European Beech in vitro propagation. In Book of Abstracts of the Second Conference of the COST Action CA21157 COPYTREE, In Vitro Culture of Woody Crops: Problem Solving by New Approaches. The Second Conference of the COST Action CA21157 COPYTREE, Bulduri, Latvia, 22–24 April 2024; p. 92.
- Gatti, E.; Sgarbi, E.; Ozudogru, E.A.; Lambardi, M. The effect of Plantform™ bioreactor on micropropagation of Quercus robur in comparison to a conventional in vitro culture system on gelled medium, and assessment of the microenvironment influence on leaf structure. Plant Biosyst. 2017, 151, 1129–1136. [Google Scholar] [CrossRef]
- Saher, S.; Piqueras, A.; Hellin, E.; Olmos, E. Prevention of hyperhydricity in micropropagated carnation shoots by bottom cooling: Implications of oxidative stress. Plant Cell Tiss. Org. Cult. 2005, 81, 149–158. [Google Scholar] [CrossRef]
- Polivanova, O.B.; Bedarev, V.A. Hyperhydricity in plant tissue culture. Plants 2022, 11, 3313. [Google Scholar] [CrossRef]
- Gresshoff, P.M.; Doy, C.H. Development and differentiation of haploid Lycopersicon esculentum (tomato). Planta 1972, 107, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Proc. Inter. Plant Propagators Soc. 1980, 30, 421–427. [Google Scholar]
- San-José, M.C.; Ballester, A.; Vieitez, A.M. Factors affecting in vitro propagation of Quercus robur L. Tree Physiol. 1988, 4, 281–290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Nezami-Alanagh, E.; Garoosi, G.A.; Landín, M.; Gallego, P.P. Computer-based tools provide new insight into the key factors that cause physiological disorders of pistachio rootstocks cultured in vitro. Sci. Rep. 2019, 9, 9740. [Google Scholar] [CrossRef]
- San José, M.C.; Blázquez, N.; Cernadas, M.J.; Janeiro, L.V.; Cuenca, B.; Sánchez, C.; Vidal, N. Temporary immersion systems to improve alder micropropagation. Plant Cell Tissue Organ Cult. 2020, 143, 265–275. [Google Scholar] [CrossRef]
- Regueira, M.; Rial, E.; Blanco, B.; Bogo, B.; Aldrey, A.; Correa, B.; Varas, E.; Sánchez, C.; Vidal, N. Micropropagation of axillary shoots of Salix viminalis using a temporary immersion system. Trees 2018, 32, 61–71. [Google Scholar] [CrossRef]
- Gago, D.; Sánchez, C.; Aldrey, A.; Christie, C.B.; Bernal, M.Á.; Vidal, N. Micropropagation of plum (Prunus domestica L.) in bioreactors using photomixotrophic and photoautotrophic conditions. Horticulturae 2022, 8, 286. [Google Scholar] [CrossRef]
- Caboni, E.; Frattarelli, A.; Giorgioni, M.; Meneghini, M.; Damiano, C. Improving micropropagation of hazelnut Italian cultivars through temporary immersion system. Acta Hortic. 2009, 845, 255–260. [Google Scholar] [CrossRef]
- Latawa, Y.; Shukla, M.R.; Saxena, P.K. An efficient temporary immersion system for micropropagation of hybrid hazelnut. Botany 2016, 94, 1–8. [Google Scholar] [CrossRef]
- Vieitez, A.M.; Corredoira, E.; Martínez, M.T.; San-José, M.C.; Sánchez, C.; Valladares, S.; Vidal, N.; Ballester, A. Application of biotechnological tools to Quercus improvement. Eur. J. Forest Res. 2012, 131, 519–539. [Google Scholar] [CrossRef]
- Grira, M.; Prinsen, E.; Werbrouck, S.P.O. The effect of topophysis on the in vitro development of Handroanthus guayacan and on its metabolism of meta-Topolin Riboside. Plants 2023, 12, 3577. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, T.P.; Steinmacher, D. Plant growth regulation in cell and tissue culture in vitro. Plants 2024, 13, 327. [Google Scholar] [CrossRef]
- Ree, J.F.; Powell, C.; Folgado, R.; Pence, V.C.; Walters, C.; Maschinski, J. Development of a micropropagation protocol for the ex situ conservation of Nuttall’s Scrub Oak (Quercus dumosa). Plants 2024, 13, 1148. [Google Scholar] [CrossRef]
- Ree, J.F.; Powell, C.; Folgado, R.; Pence, V.; Walters, C.; Maschinski, J. Establishing tissue culture lines from mature Nuttall’s scrub oak (Quercus dumosa Nutt.) for ex situ conservation. In Vitro Cell Dev. Biol.-Plant 2025, 61, 102–116. [Google Scholar] [CrossRef]
- Vieitez, A.M.; San-José, M.C.; Vieitez, E. In vitro plantlet regeneration from juvenile and mature Quercus robur L. J. Hort. Sci. 1985, 60, 99–106. [Google Scholar] [CrossRef]
- Gao, H.; Xia, X.; An, L.; Xin, X.; Liang, Y. Reversion of hyperhydricity in pink (Dianthus chinensis L.) plantlets by AgNO3 and its associated mechanism during in vitro culture. Plant Sci. 2017, 254, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Naing, A.H.; Park, K.I.; Kim, C.K. Silver nitrate reduces hyperhydricity in shoots regenerated from the hypocotyl of snapdragon cv. Maryland Apple Blossom. Sci. Hortic. 2023, 308, 111593. [Google Scholar] [CrossRef]
- Sreelekshmi, R.; Siril, E.A. Effective reversal of hyperhydricity leading to efficient micropropagation of Dianthus chinensis L. 3Biotech 2021, 11, 95. [Google Scholar] [CrossRef]
- Drisya Ravi, R.S.; Siril, E.A.; Nair, B.R. The effect of silver nitrate on micropropagation of Moringa oleifera Lam. an important vegetable crop of tropics with substantial nutritional value. Physiol. Mol. Biol. Plants 2019, 25, 1311–1322. [Google Scholar] [CrossRef]
- Hassanein, A.M.; Salem, J.M.; Faheed, F.A.; El-Nagish, A. Effect of anti-ethylene compounds on isoenzyme patterns and genome stability during long term culture of Moringa oleifera. Plant Cell Tissue Organ Cult. 2018, 132, 201–212. [Google Scholar] [CrossRef]
- Pan, M.J.; van Staden, J. The use of charcoal in in vitro culture—A review. Plant Growth Reg. 1998, 26, 155–163. [Google Scholar] [CrossRef]
- Elmaataoui, S.; Mazri, M.A.; Meziani, R.; Bouchiha, F. Effects of culture medium strength and antioxidants on adventitious bud multiplication, hyperhydricity and tissue browning of date palm cv. Aziza Bouzid. World J. Adv. Res. Rev. 2020, 6, 103–109. [Google Scholar] [CrossRef]





| Tree Characteristics | Lines Established In Vitro | ||
|---|---|---|---|
| Name | Age (Estimated) | Origin | Abbreviation |
| Chaián 1 | 60 y | Basal shoots | Ch1BS |
| Chaián 2 | 60 y | Basal shoots | Ch2BS |
| Carballo das Mentiras | 200 y | Epicormic branches | CM |
| San Lourenzo | 300 y | Basal shoots | SLo |
| RUS | 800 y | Epicormic branches | RUS |
| Experiments | ||||
|---|---|---|---|---|
| Factor | Levels | Genotype | Experiment No | Other Factors in the Same Exp. |
| Support | Rockwool cubes | Ch1BS, Ch2BS, CM, RUS | Exp. 1 | Explant type |
| No support | RUS | Exp. 6 | Explant type; SN, AC | |
| Immersions | 3/6 per day | CM, RUS | Exp. 2 | Explant type |
| Explant type | Apical sections Basal sections with callus | Ch1BS, Ch2BS, CM, RUS | Exp. 1 | Support |
| CM, RUS | Exp. 2 | Immersions | ||
| SLo | Exp. 3 | Culture medium | ||
| Ch1BS, Ch2BS, CM | Exp. 4 | Culture medium | ||
| RUS | Exp. 5 | Culture medium | ||
| RUS | Exp. 6 | Support; SN, AC | ||
| Culture medium | GD, MS ½ N | SLo | Exp. 3 | Explant type |
| GD, MS ½ N, WPM | Ch1BS, Ch2BS, CM | Exp. 4 | Explant type | |
| GD, WPM | RUS | Exp. 5 | Explant type | |
| Silver Nitrate (SN) | Present, Absent, | RUS | Exp. 6 | Explant type; Support |
| Act. Charcoal (AC) | Combined | |||
| Genotype | |||||
|---|---|---|---|---|---|
| Explant Type | Support | Ch1BS | Ch2BS | CM | RUS |
| Apical sections | No support | 80 ± 13.3 a 1,2 | 85 ± 7.4 a | 83 ± 17.3 a | 87 ± 19.2 a |
| Cubes | 5 ± 3.5 c | 10 ± 7.3 c | 7 ± 2.8 c | 17 ± 9.6 c | |
| Basal sections | No support | 26 ± 4.8 b | 27 ± 14.1 b | 21 ± 7.7 b | 42 ± 12.1 b |
| Cubes | 17 ± 5.4 b | 10 ± 1.9 c | 7 ± 6.0 b | 13 ± 7.4 c | |
| Apical Sections | Basal Sections | ||||
|---|---|---|---|---|---|
| Genotype | Variable | 3 imm | 6 imm | 3 imm | 6 imm |
| Carballo das Mentiras | |||||
| Normal Shoot No. | 0.8 ± 0.25 Ba 1,2 | 1.2 ± 0.19 Ba | 1.8 ± 0.21 Aa | 1.6 ± 0.27 Aa | |
| Hyperhydric Shoot No. | 0.38 ± 0.18 Aa | 0.13 ± 0.07 Ab | 0.03 ± 0.01 Ba | 0.20 ± 0.14 Ba | |
| Shoot Length (mm) | 17.2 ± 3.79 Aa | 17.9 ± 1.59 Aa | 24.6 ± 1.74 Aa | 19.0 ± 2.21 Aa | |
| Multiplication Coefficient | 0.9 ± 0.30 Bb | 1.6 ± 0.20 Ba | 2.5 ± 0.32 Aa | 2.1 ± 0.32 Aa | |
| Rootable Shoot No. | 0.4 ± 0.18 Ab | 1.0 ± 0.12 Aa | 1.1 ± 0.11 Aa | 0.7 ± 0.45 Aa | |
| RUS | |||||
| Normal Shoot No. | 1.7 ± 0.26 Aa | 1.7 ± 0.25 Aa | 2.3 ± 0.36 Aa | 2.3 ± 0.35 Aa | |
| Hyperhydric Shoot No. | 0.23 ± 0.11 Aa | 0.14 ± 0.07 Aa | 0.33 ± 0.13 Aa | 0.13 ± 0.09 Aa | |
| Shoot Length (mm) | 16.7 ± 1.25 Aa | 19.4 ± 1.17 Aa | 14.7 ± 0.93 Ba | 15.5 ± 1.22 Ba | |
| Multiplication Coefficient | 1.9 ± 0.28 Ba | 1.7 ± 0.24 Ba | 2.5 ± 0.35 Aa | 2.4 ± 0.36 Aa | |
| Rootable Shoot No. | 0.6 ± 0.17 Aa | 0.6 ± 0.11 Aa | 0.7 ± 0.16 Aa | 0.8 ± 0.16 Aa | |
| Variable | ||||
|---|---|---|---|---|
| Medium | Explant | SL (mm) | MC | NRS |
| GD | Apical + Cubes | 31.3 ± 5.06 Bb 1,2 | 1.3 ± 0.34 Bb | 0.3 ± 0.12 Bb |
| Basal − Cubes | 41.6 ± 2.80 Ba | 7.3 ± 1.06 Aa | 2.3 ± 0.45 Aa | |
| MS ½ N | Apical + Cubes | 37.7 ± 2.53 Ab | 2.2 ± 0.32 Ab | 0.9 ± 0.13 Bab |
| Basal − Cubes | 50.6 ± 3.20 Aa | 6.3 ± 0.53 Aa | 1.5 ± 0.19 Ba | |
| Factors | Variables | |||||
|---|---|---|---|---|---|---|
| Genotype | Medium | Explant | Subculture | SL (mm) | MC | NRS |
| Ch1BS | GD | Apical | Rep1 | 17.3 ± 1.89 Ba 1,2 | 1.3 ± 0.19 Ba | 0.3 ± 0.12 Ba |
| Rep2 | 16.7 ± 0.96 Ba | 1.8 ± 0.14 Ba | 0.5 ± 0.10 Ba | |||
| Basal | Rep1 | 20.8 ± 2.75 Aa | 3.4 ± 0.60 Aa | 0.9 ± 0.30 Aa | ||
| Rep2 | 22.0 ± 1.25 Aa | 4.4 ± 0.47 Aa | 0.9 ± 0.16 Aa | |||
| MS ½ N | Apical | 1st Subc | 25.6 ± 1.56 Aa | 2.1 ± 0.24 Aa | 0.9 ± 0.07 Aa | |
| 2nd Subc | 24.9 ± 2.35 Aa | 1.6 ± 0.20 Ab | 0.8 ± 0.11 Aa | |||
| Basal | 1st Subc | 26.3 ± 3.30 Aa | 2.4 ± 0.43 Aa | 0.8 ± 0.23 Ba | ||
| 2nd Subc | 11.1 ± 1.74 Ab | 0.8 ± 0.21 Ab | 0.1 ± 0.09 Bb | |||
| Ch2BS | GD | Apical | Rep1 | 16.7 ± 1.93 Ba | 1.7 ± 0.25 Ba | 0.3 ± 0.12 Ba |
| Rep2 | 13.8 ± 1.14 Ba | 2.2 ± 0.30 Ba | 0.3 ± 0.10 Ba | |||
| Basal | Rep1 | 26.0 ± 2.13 Aa | 3.4 ± 0.42 Aa | 1.4 ± 0.46 Aa | ||
| Rep2 | 22.4 ± 1.22 Ab | 4.2 ± 0.46 Aa | 0.9 ± 0.15 Aa | |||
| MS ½ N | Apical | 1st Subc | 28.4 ± 1.79 Ba | 2.0 ± 0.19 Aa | 0.9 ± 0.13 Aa | |
| 2nd Subc | 25.9 ± 1.64 Ba | 1.4 ± 0.18 Ab | 0.7 ± 0.12 Aa | |||
| Basal | 1st Subc | 33.9 ± 2.34 Aa | 3.1 ± 0.43 Aa | 1.1 ± 0.20 Aa | ||
| 2nd Subc | 9.8 ± 1.69 Ab | 1.1 ± 0.24 Ab | 0.3 ± 0.17 Ab | |||
| CM | GD | Apical | Rep1 | 25.7 ± 1.75 Aa | 2.0 ± 0.31 Aa | 0.7 ± 0.10 Aa |
| Rep2 | 17.1 ± 1.09 Ab | 1.5 ± 0.13 Aa | 0.8 ± 0.28 Aa | |||
| Basal | Rep1 | 25.3 ± 1.88 Aa | 2.3 ± 0.31 Aa | 0.6 ± 0.11 Aa | ||
| Rep2 | 21.2 ± 1.58 Ab | 1.9 ± 0.22 Aa | 0.6 ± 0.12 Aa | |||
| MS ½ N | Apical | 1st Subc | 30.3 ± 2.96 Aa | 2.1 ± 0.28 Aa | 0.7 ± 0.11 Aa | |
| 2nd Subc | 26.6 ± 2.24 Ab | 1.6 ± 0.22 Ab | 0.6 ± 0.09 Aa | |||
| Basal | 1st Subc | 24.8 ± 2.16 Ba | 1.7 ± 0.35 Ba | 0.5 ± 0.12 Ba | ||
| 2nd Subc | 15.4 ± 3.53 Bb | 0.6 ± 0.14 Bb | 0.2 ± 0.09 Ba | |||
| WPM | Apical | 1st Subc | 25.0 ± 1.57 Aa | 1.9 ± 0.19 Aa | 0.7 ± 0.09 Aa | |
| 2nd Subc | 21.4 ± 1.16 Ab | 1.5 ± 0.12 Ab | 0.8 ± 0.09 Aa | |||
| Basal | 1st Subc | 24.7 ± 2.12 Aa | 2.7 ± 0.40 Aa | 0.8 ± 0.16 Aa | ||
| 2nd Subc | 15.4 ± 2.64 Ab | 1.4 ± 0.33 Ab | 0.3 ± 0.10 Ab | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmielarz, P.; Sánchez, C.; Martins, J.P.R.; Ley-López, J.M.; Covelo, P.; Cernadas, M.J.; Aldrey, A.; Rico, S.; Vielba, J.M.; Christie, B.; et al. Multiplication of Axillary Shoots of Adult Quercus robur L. Trees in RITA® Bioreactors. Forests 2025, 16, 1285. https://doi.org/10.3390/f16081285
Chmielarz P, Sánchez C, Martins JPR, Ley-López JM, Covelo P, Cernadas MJ, Aldrey A, Rico S, Vielba JM, Christie B, et al. Multiplication of Axillary Shoots of Adult Quercus robur L. Trees in RITA® Bioreactors. Forests. 2025; 16(8):1285. https://doi.org/10.3390/f16081285
Chicago/Turabian StyleChmielarz, Paweł, Conchi Sánchez, João Paulo Rodrigues Martins, Juan Manuel Ley-López, Purificación Covelo, María José Cernadas, Anxela Aldrey, Saleta Rico, Jesús María Vielba, Bruce Christie, and et al. 2025. "Multiplication of Axillary Shoots of Adult Quercus robur L. Trees in RITA® Bioreactors" Forests 16, no. 8: 1285. https://doi.org/10.3390/f16081285
APA StyleChmielarz, P., Sánchez, C., Martins, J. P. R., Ley-López, J. M., Covelo, P., Cernadas, M. J., Aldrey, A., Rico, S., Vielba, J. M., Christie, B., & Vidal, N. (2025). Multiplication of Axillary Shoots of Adult Quercus robur L. Trees in RITA® Bioreactors. Forests, 16(8), 1285. https://doi.org/10.3390/f16081285








