Dendrometer-Based Analysis of Intra-Annual Growth and Water Status in Two Pine Species in a Mediterranean Forest Stand Under a Semi-Arid Climate
Abstract
1. Introduction
2. Material and Methods
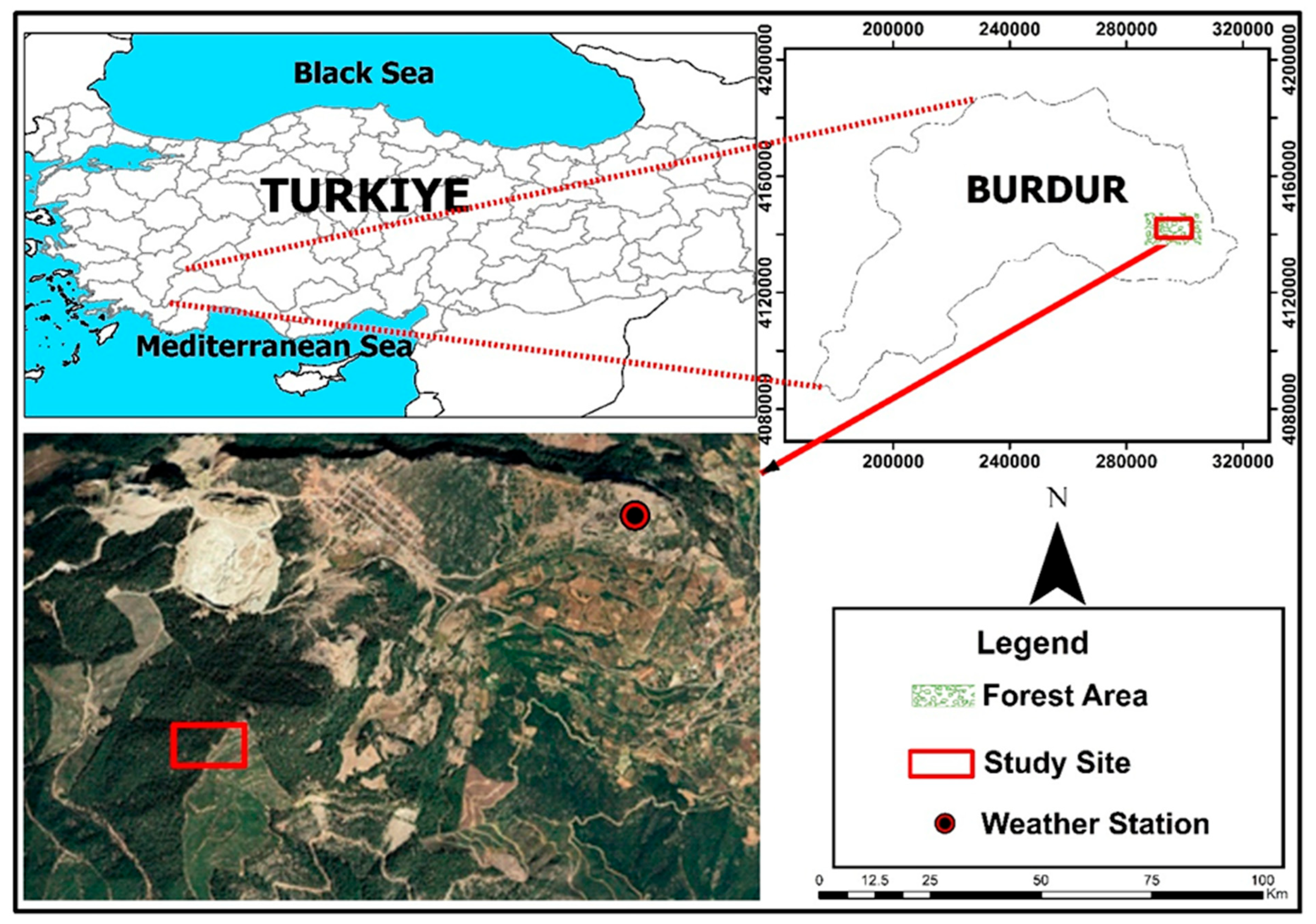
2.1. Location of the Study Site and Stand Characteristics
2.2. Meteorological Observations
2.3. Extraction of Stem Radial Increment and Tree Water Status from Dendrometer Data
2.4. Seasonal Growth Pattern Assessment
2.5. Statistical Analyses and Modeling
Species) + β6·(VPD × Species) + β7·(SWP × VPD × Species) + a + ε
3. Results
3.1. Environmental Conditions During the Study Period
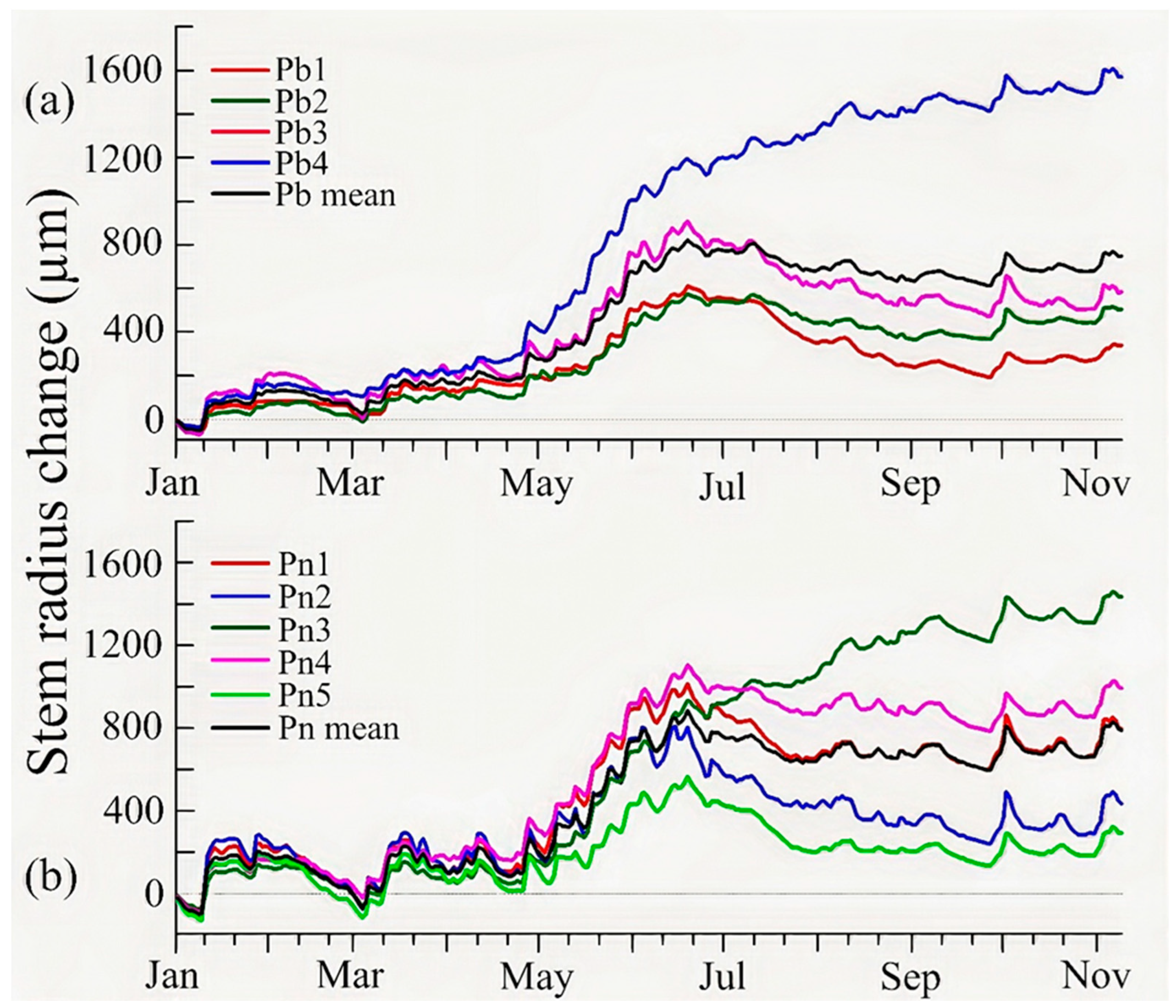
3.2. Stem Growth and Tree Water Status Dynamics
3.3. Impact of Environmental Factors on Stem Radius Changes
4. Discussion
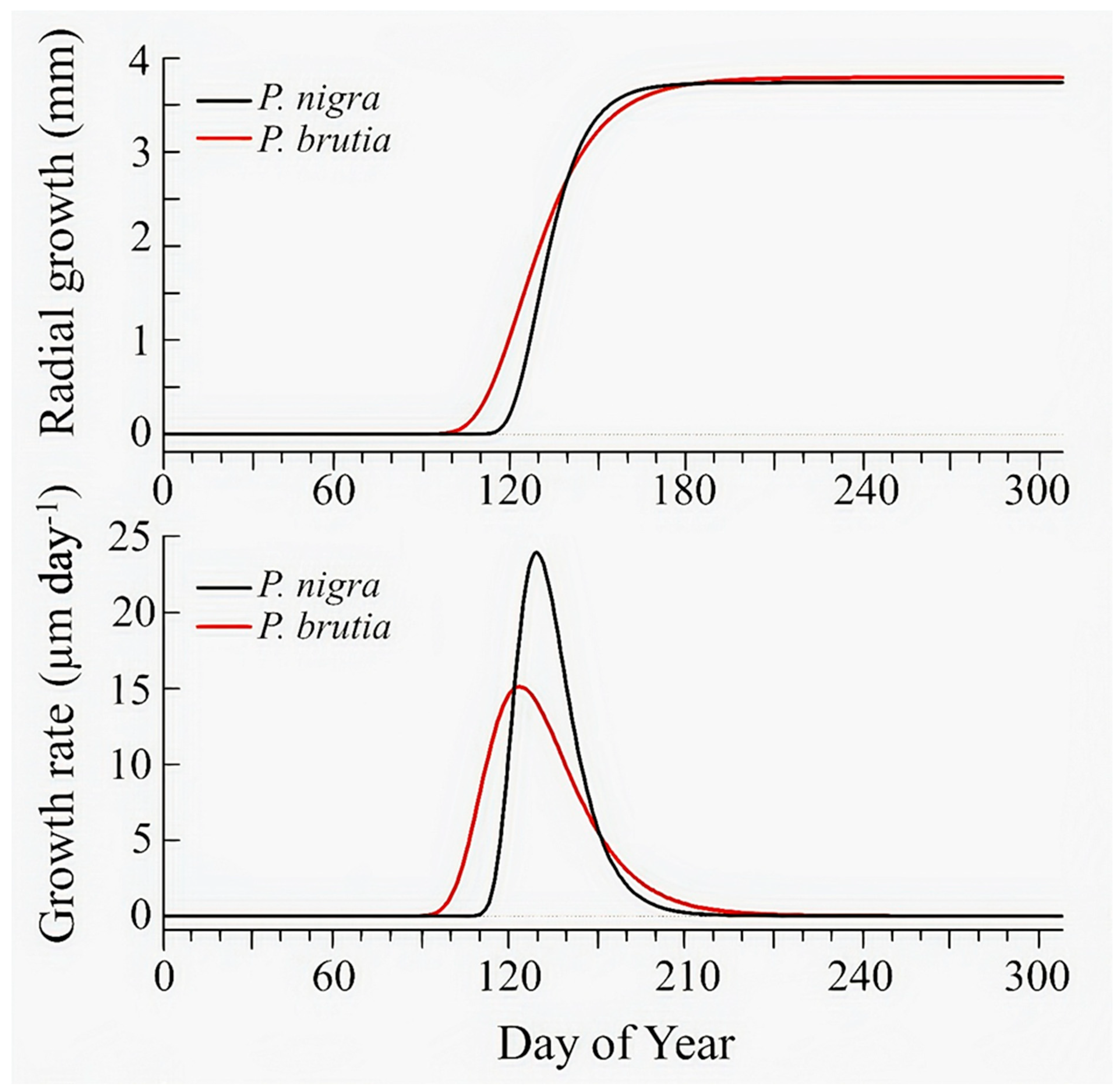
4.1. Species-Specific Growth Patterns
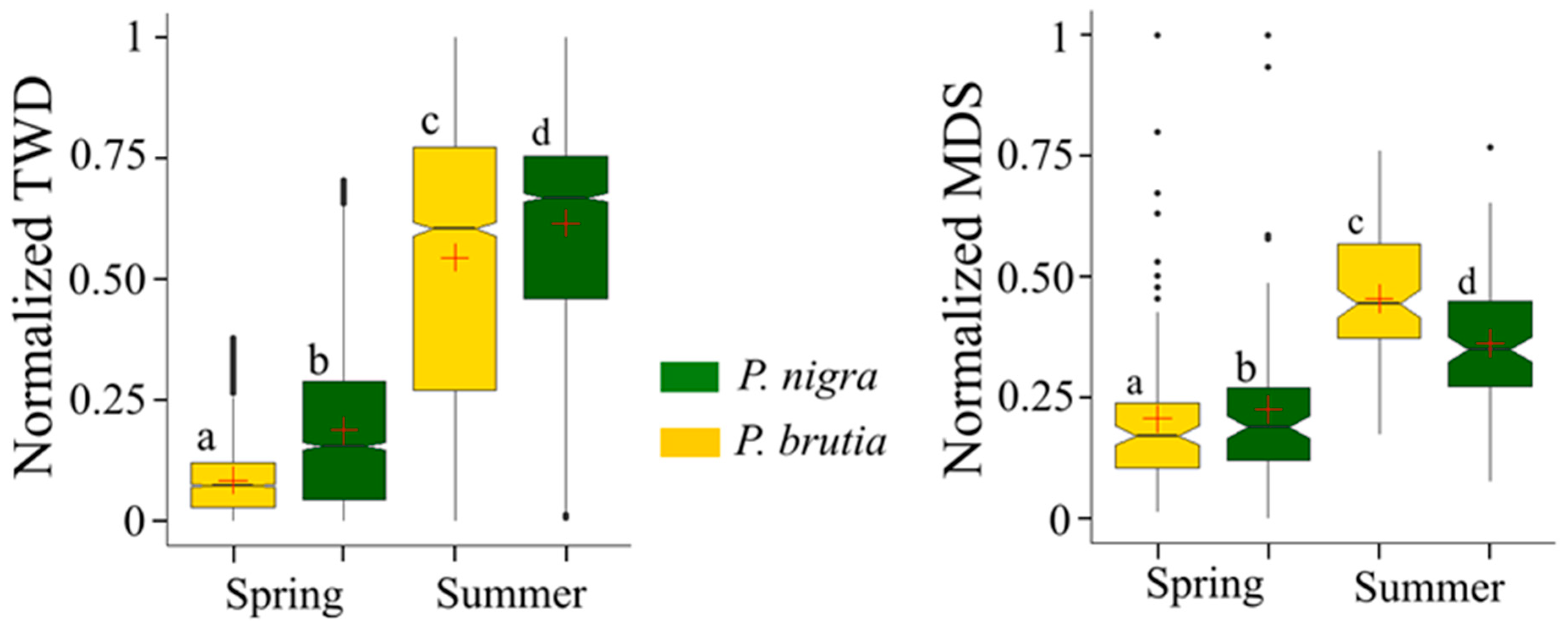
4.2. Species-Specific Tree Water Status Dynamics
4.3. Environmental Determinants of Tree Growth and Tree Water Status Dynamics
4.4. Limitations of the Study
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forzieri, G.; Dakos, V.; McDowell, N.G.; Ramdane, A.; Cescatti, A. Emerging signals of declining forest resilience under climate change. Nature 2022, 608, 534–539. [Google Scholar] [CrossRef]
- Lionello, P.; Scarascia, L. The relation between climate change in the Mediterranean region and global warming. Reg. Environ. Change 2018, 18, 1481–1493. [Google Scholar] [CrossRef]
- Noto, L.V.; Cipolla, G.; Francipane, A.; Pumo, D. Climate change in the Mediterranean Basin (Part I): Induced alterations on climate forcings and hydrological processes. Water Resour. Manag. 2023, 37, 2287–2305. [Google Scholar] [CrossRef]
- Cramer, W.; Guiot, J.; Fader, M.; Garrabou, J.; Gattuso, J.-P.; Iglesias, A.; Lange, M.A.; Lionello, P.; Llasat, M.C.; Paz, S.; et al. Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Clim. Change 2008, 8, 972–980. [Google Scholar] [CrossRef]
- Forner, A.; Valladares, F.; Bonal, D.; Granier, A.; Grossiord, C.; Aranda, I. Extreme droughts affecting Mediterranean tree species’ growth and water-use efficiency: The importance of timing. Tree Physiol. 2018, 38, 1127–1137. [Google Scholar] [CrossRef]
- Vatandaşlar, C.; Türkeş, M.; Semerci, A.; Karahan, A. Analyzing climate-induced mortality of Taurus fir based on temporal forest management plans and climatic variations and droughts in the Central Mediterranean sub-region of Turkey. Eur. J. For. Res. 2023, 142, 61–89. [Google Scholar] [CrossRef]
- GDF General Directorate of Forestry (Türkiye). Official Statistics. 2022. Available online: https://www.ogm.gov.tr/tr/e-kutuphane/resmi-istatistikler (accessed on 27 September 2024). (In Turkish)
- Boydak, M. Silvicultural characteristics and natural regeneration of Pinus brutia Ten.—A review. J. Plant Ecol. 2004, 171, 153–163. [Google Scholar] [CrossRef]
- Mauri, A.; Di Leo, M.; de Rigo, D.; Caudullo, G. Pinus halepensis and Pinus brutia in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; p. e0166b8+. [Google Scholar]
- Akkemik, Ü. Pinus nigra. In Türkiye’nin Doğal ve Egzotik Ağaç ve Çalıları–1, Gymnospermler–Angiospermler; Akkemik, Ü., Ed.; General Directorate of Forestry Publications: Ankara, Türkiye, 2018; pp. 182–184. (In Turkish) [Google Scholar]
- Atalay, İ.; Sezer, İ.; Çukur, H. Kızılçam (Pinus brutia Ten.) ormanlarının ekolojik özellikleri ve tohum nakli açısından bölgelere ayrılması. In Orman Ağaçları ve Tohumları Islah Araştırma Müdürlüğü Yayın No. 6; Ege Üniversitesi Basım Evi: İzmir, Türkiye, 1998. (In Turkish) [Google Scholar]
- Güney, C.O.; Güney, A.; Sarı, A.; Kavgacı, A.; Ryan, K.C.; Hood, S.M. Modeling post-fire mortality of Turkish pine (Pinus brutia Ten.). For. Ecol. Manag. 2024, 572, 122265. [Google Scholar] [CrossRef]
- Ganatsas, P.; Spanos, I. Root system assymetry of Mediterranean pines. Plant Soil. 2005, 278, 75–83. [Google Scholar] [CrossRef]
- Enescu, C.M.; de Rigo, D.; Caudullo, G.; Mauri, A.; Houston Durrant, T. Pinus nigra in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; p. e015138+. [Google Scholar]
- Atalay, İ.; Efe, R. Ecological attributes and distribution of Anatolian black pine [Pinus nigra Arnold. subsp. pallasiana Lamb. Holmboe] in Turkey. J. Environ. Biol. 2012, 33, 509–519. [Google Scholar]
- Sevgi, O.; Yılmaz, O.Y. Karaçam ormanlarının yayılışı. In Karaçam; Sevgi, O., Tecimen, B., Okan, T., Eds.; Türkiye Ormancılar Derneği Yayın No: E/22/62; Türkiye Ormancılar Derneği: Ankara, Türkiye, 2022; ISBN 978-605-71791-2-8. (In Turkish) [Google Scholar]
- Genç, M. Silvikültürün Temel Esasları; Süleyman Demirel Üniversitesi Yayınları, Yayın No. 44; Süleyman Demirel Üniversitesi: Isparta, Türkiye, 2004. (In Turkish) [Google Scholar]
- Valor, T.; Hood, S.M.; Pique, M.; Larranaga, A.; Casals, P. Resin ducts and bark thickness influence pine resistance to bark beetles after prescribed fire. For. Ecol. Manag. 2021, 494, 119322. [Google Scholar] [CrossRef]
- Sarris, D.; Mazza, G. Mediterranean pine root systems under drought. In Pines and Their Mixed Forest Ecosystems in the Mediterranean Basin; Ne’eman, G., Osem, Y., Eds.; Managing Forest Ecosystems; Springer: Cham, Switzerland, 2021; Volume 38, pp. 129–141. [Google Scholar]
- Zweifel, R.; Haeni, M.; Buchmann, N.; Eugster, W. Are trees able to grow in periods of stem shrinkage? New Phytol. 2016, 211, 839–849. [Google Scholar] [CrossRef]
- Leštianska, A.; Fleischer, P., Jr.; Merganičová, K.; Fleischer, P., Sr.; Střelcová, K. Intra-annual dynamics of stem circumference variation and water status of four coniferous tree species (Pinus sylvestris, Picea abies, Larix decidua and Abies alba) under warmer and water-limited conditions. Biologia 2025, 80, 1125–1142. [Google Scholar] [CrossRef]
- Zweifel, R.; Zimmermann, L.; Newbery, D.M. Modeling tree water deficit from microclimate: An approach to quantifying drought stress. Tree Physiol. 2005, 25, 147–156. [Google Scholar] [CrossRef]
- Güney, A.; Gülsoy, S.; Şentürk, Ö.; Niessner, A.; Küppers, M. Environmental control of daily stem radius increment in the montane conifer Cedrus libani. J. For. Res. 2020, 31, 1159–1171. [Google Scholar] [CrossRef]
- Köse, N.; Akkemik, Ü.; Dalfes, H.N.; Özeren, M.S.; Tolunay, D. Tree-ring growth of Pinus nigra Arn. subsp. pallasiana under different climate conditions throughout western Anatolia. Dendrochronologia 2012, 30, 295–301. [Google Scholar] [CrossRef]
- Deligöz, A.; Cankara, F.G. Differences in physiological and biochemical responses to summer drought of Pinus nigra subsp. pallasiana and Pinus brutia in a natural mixed stand. J. For. Res. 2020, 31, 1479–1487. [Google Scholar] [CrossRef]
- Bayar, E.; Deligöz, A. Ecophysiological Behavior of Mediterranean Woody Species Under Summer Drought. Bosque 2021, 42, 311–321. Available online: https://revistabosque.org/index.php/bosque/article/view/197 (accessed on 1 July 2025).
- ISRIC World Soil Information. 2024. Available online: https://soilgrids.org/ (accessed on 28 October 2024).
- Raffelsbauer, V.; Spannl, S.; Peña, K.; Pucha-Cofrep, D.; Steppe, K.; Bräuning, A. Tree circumference changes and species-specific growth recovery after extreme dry events in a montane rainforest in Southern Ecuador. Front. Plant Sci. 2019, 10, 342. [Google Scholar] [CrossRef]
- Aryal, S.; Häusser, M.; Grießinger, J.; Fan, Z.; Bräuning, A. “dendRoAnalyst”: A tool for processing and analysing dendrometer data. Dendrochronologia 2020, 64, 125772. [Google Scholar] [CrossRef]
- Duchesne, L.; Houle, D.; D’Orangeville, L. Influence of climate on seasonal patterns of stem increment of balsam fir in a boreal forest of Québec, Canada. Agric. For. Meteorol. 2012, 162, 108–114. [Google Scholar] [CrossRef]
- Li, W.; Yue, F.; Wang, C.; Liao, J.; Zhang, X. Climatic influences on intra-annual stem variation of Larix principis-rupprechtii in a semi-arid region. Front. For. Global Change 2022, 5, 948022. [Google Scholar] [CrossRef]
- Makowski, D.; Ben-Shachar, M.S.; Patil, I.; Lüdecke, D. Methods and algorithms for correlation analysis in R. J. Open Source Softw. 2020, 5, 2306. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation, R Package Version 1.0.10. 2022. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 1 July 2025).
- Mazza, G.; Markou, L.; Sarris, D. Species-specific growth dynamics and vulnerability to drought at the single tree level in a Mediterranean reforestation. Trees 2021, 35, 1697–1710. [Google Scholar] [CrossRef]
- Çatal, Y.; Güzel, B.; Genç, M. Determination of free-to-grow stages for natural mixed Brutian pine–Anatolian black pine stands. Turk. J. For. Sci. 2017, 1, 145–154. [Google Scholar] [CrossRef]
- Chambel, M.R.; Climent, J.; Pichot, C.; Ducci, F. Mediterranean pines (Pinus halepensis Mill. and Pinus brutia Ten.). In Forest Tree Breeding in Europe; Pâques, L., Ed.; Managing Forest Ecosystems; Springer: Dordrecht, The Netherlands, 2013; Volume 25, pp. 179–213. [Google Scholar] [CrossRef]
- Fontaine, M.; Aerts, R.; Özkan, K.; Mert, A.; Gülsoy, A.; Süel, H.; Waelkens, M.; Muys, B. Elevation and exposition rather than soil types determine communities and site suitability in Mediterranean mountain forests of southern Anatolia, Turkey. For. Ecol. Manag. 2007, 247, 18–25. [Google Scholar] [CrossRef]
- Güner, Ş.T.; Çömez, A.; Özkan, K.; Karataş, R.; Çelik, N. Modeling the productivity of Anatolian black pine plantations in Turkey. J. Fac. For. Istanb. Univ. 2016, 66, 159–172. (In Turkish) [Google Scholar] [CrossRef]
- Şahin, A. Yield Research in Calabrian Pine (Pinus brutia Ten.) Stands of Mersin Region. Ph.D. Thesis, Department of Forest Engineering, Artvin Çoruh University, Artvin, Türkiye, 2015; p. 337. (In Turkish). [Google Scholar]
- Kahriman, A.; Şahin, A.; Sönmez, T.; Yavuz, M. Growth models for natural stands of Calabrian pine in the central Mediterranean region of Türkiye. Šum. List 2023, 147, 107–119. [Google Scholar] [CrossRef]
- Carus, S.; Sevgi, O.; Yılmaz, O.Y.; Tecimen, H.B.; Akburak, S. Doğal Karaçam (Pinus nigra Arnold) meşcerelerinde tek ağaç çap artım modeli: Alaçam Dağları örneği. In Karaçam; Sevgi, O., Tecimen, B., Okan, T., Eds.; Türkiye Ormancılar Derneği Yayın No: E/22/62; Türkiye Ormancılar Derneği: Ankara, Türkiye, 2022; pp. 400–416. ISBN 978-605-71791-2-8. (In Turkish) [Google Scholar]
- Forner, A.; Aranda, I.; Granier, A.; Valladares, F. Differential impact of the most extreme drought event over the last half century on growth and sap flow in two coexisting Mediterranean trees. Plant Ecol. 2014, 215, 703–719. [Google Scholar] [CrossRef]
- Houminer, N.; Riov, J.; Moshelion, M.; Osem, Y.; David-Schwartz, R. Comparison of morphological and physiological traits between Pinus brutia, Pinus halepensis, and their vigorous F1 hybrids. Forests 2022, 13, 1477. [Google Scholar] [CrossRef]
- Sabater, A.M.; Vicente, E.; Morcillo, L.; del Campo, A.; Larsen, E.K.; Moutahir, H.; Pastor, F.; Palau, J.L.; Bellot, J.; Vilagrosa, A. Water-based forest management of Mediterranean pine forests. In Pines and Their Mixed Forest Ecosystems in the Mediterranean Basin; Ne’eman, G., Osem, Y., Eds.; Managing Forest Ecosystems; Springer: Cham, Switzerland, 2021; Volume 38, pp. 615–642. [Google Scholar] [CrossRef]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Tordoni, E.; Petruzzellis, F.; Di Bonaventura, A.; Pavanetto, N.; Tomasella, M.; Nardini, A.; Boscutti, F.; Martini, F.; Bacaro, G. Projections of leaf turgor loss point shifts under future climate change scenarios. Glob. Change Biol. 2022, 28, 6640–6652. [Google Scholar] [CrossRef]
- Schumann, K.; Schuldt, B.; Fischer, M.; Ammer, C.; Leuschner, C. Xylem safety in relation to the stringency of plant water potential regulation of European beech, Norway spruce, and Douglas-fir trees during severe drought. Trees 2024, 38, 607–623. [Google Scholar] [CrossRef]
- Andivia, E.; Zuccarini, P.; Grau, B.; de Herralde, F.; Villar-Salvador, P.; Savé, R. Rooting big and deep rapidly: The ecological roots of pine species distribution in southern Europe. Trees 2018, 33, 293–303. [Google Scholar] [CrossRef]
- Tapias, R.; Climent, J.; Pardos, J.A.; Gil, L. Life histories of Mediterranean pines. Plant Ecol. 2004, 171, 53–68. [Google Scholar] [CrossRef]
- Ulusuan, M.D.; Eler, Ü. Variation of bark thickness on single stem of crimean pine (Pinus nigra Arnold subsp pallasiana (Lamb) Holmboe) In Natural Stands: Acase study of Isparta region. Turk. J. For. 2023, 24, 188–196. (In Turkish) [Google Scholar] [CrossRef]
- Fernandes, P.M.; Vega, J.A.; Jimenez, E.; Rigolot, E. Fire resistance of European pines. For. Ecol. Manag. 2008, 256, 246–255. [Google Scholar] [CrossRef]
- Camarero, J.J.; Olano, J.M.; Parras, A. Plastic bimodal xylogenesis in conifers from continental Mediterranean climates. New Phytol. 2010, 185, 471–480. [Google Scholar] [CrossRef]
- Altieri, S.; Niccoli, F.; Kabala, J.P.; Liyaqat, I.; Battipaglia, G. Influence of drought and minimum temperature on tree growth and water use efficiency of Mediterranean species. Dendrochronologia 2024, 83, 126162. [Google Scholar] [CrossRef]
- Bachofen, C.; Tumber-Dávila, S.J.; Mackay, D.S.; McDowell, N.G.; Carminati, A.; Klein, T.; Stocker, B.D.; Mencuccini, M.; Grossiord, C. Tree water uptake patterns across the globe. New Phytol. 2024, 242, 1891–1910. [Google Scholar] [CrossRef]
- Dong, M.; Wang, B.; Jiang, Y.; Ding, X. Environmental controls of diurnal and seasonal variations in the stem radius of Platycladus orientalis in northern China. Forests 2019, 10, 784. [Google Scholar] [CrossRef]
- Urban, J.; Ingwers, M.; McGuire, M.A.; Teskey, R.O. Stomatal conductance increases with rising temperature. Plant Signal. Behav. 2017, 12, e1356534. [Google Scholar] [CrossRef]


| Species/Tree ID | Height (m) | DBH (cm) | Age |
|---|---|---|---|
| Pinus nigra Arn. subsp. pallasiana (Lamb.) Holmboe | |||
| Pn1 | 15.5 | 38.5 | 88 |
| Pn2 | 15 | 45 | 89 |
| Pn3 | 15 | 42 | 89 |
| Pn4 | 18 | 52 | 89 |
| Pn5 | 16 | 46 | 80 |
| Species’ mean | 15.9 ± 1.1 | 46.2 ± 3.6 | 87 ± 3.5 |
| Pinus brutia Ten. | |||
| Pb1 | 15 | 45 | 85 |
| Pb2 | 19 | 59 | 89 |
| Pb3 | 17 | 54 | 70 |
| Pb4 | 17 | 52 | 70 |
| Species’ mean | 17 ± 1.4 | 52.5 ± 1.7 | 78.5 ± 8.6 |
| Variable | 2023 | Long Term Average x | |
|---|---|---|---|
| Mean ± SD * | Range | ||
| Air temperature (°C) | 13.3 ± 8.2 | −7.2–33.4 | 13.6 |
| Global radiation (W m−2) ** | 349 ± 208 | 3.3–959.5 | – |
| Relative humidity (%) | 63.4 ± 24.8 | 6.9–100 | 57.4 |
| Daily precipitation (mm) *** | 1.3 ± 0.4 | 0–15.7 | 1.03 |
| Soil water potential (bar) | −5.2 ± 6.5 | −0.16–−14.5 | – |
| Variable | GR | Tmean | Tmax | Tmin | RH | SWP | Pp | VPD |
|---|---|---|---|---|---|---|---|---|
| MDS Pb | 0.47 *** | 0.63 *** | 0.60 *** | 0.67 *** | −0.40 *** | −0.51 *** | −0.17 *** | 0.55 *** |
| MDS Pn | 0.28 *** | 0.41 *** | 0.37 *** | 0.44 *** | −0.16 *** | −0.21 *** | 0.01 | 0.29 *** |
| TWD Pb | 0.301 ** | 0.69 *** | 0.69 *** | 0.68 *** | −0.62 *** | −0.78 *** | −0.39 *** | 0.72 *** |
| TWD Pn | −0.26 *** | 0.62 ** | 0.62 * | 0.61 *** | −0.60 *** | −0.77 *** | −0.33 *** | 0.67 *** |
| GRO Pb | 0.40 ** | 0.77 ** | 0.77 ** | 0.76 ** | −0.41 ** | 0.61 ** | −0.17 | 00.62 ** |
| GRO Pn | 0.40 ** | 0.77 ** | 0.77 ** | 0.76 ** | −0.41 ** | 0.61 * | −0.14 | 0.62 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özçelik, M.S. Dendrometer-Based Analysis of Intra-Annual Growth and Water Status in Two Pine Species in a Mediterranean Forest Stand Under a Semi-Arid Climate. Forests 2025, 16, 1229. https://doi.org/10.3390/f16081229
Özçelik MS. Dendrometer-Based Analysis of Intra-Annual Growth and Water Status in Two Pine Species in a Mediterranean Forest Stand Under a Semi-Arid Climate. Forests. 2025; 16(8):1229. https://doi.org/10.3390/f16081229
Chicago/Turabian StyleÖzçelik, Mehmet S. 2025. "Dendrometer-Based Analysis of Intra-Annual Growth and Water Status in Two Pine Species in a Mediterranean Forest Stand Under a Semi-Arid Climate" Forests 16, no. 8: 1229. https://doi.org/10.3390/f16081229
APA StyleÖzçelik, M. S. (2025). Dendrometer-Based Analysis of Intra-Annual Growth and Water Status in Two Pine Species in a Mediterranean Forest Stand Under a Semi-Arid Climate. Forests, 16(8), 1229. https://doi.org/10.3390/f16081229





