Climate-Driven Habitat Shifts and Conservation Implications for the Submediterranean Oak Quercus pyrenaica Willd.
Abstract
1. Introduction
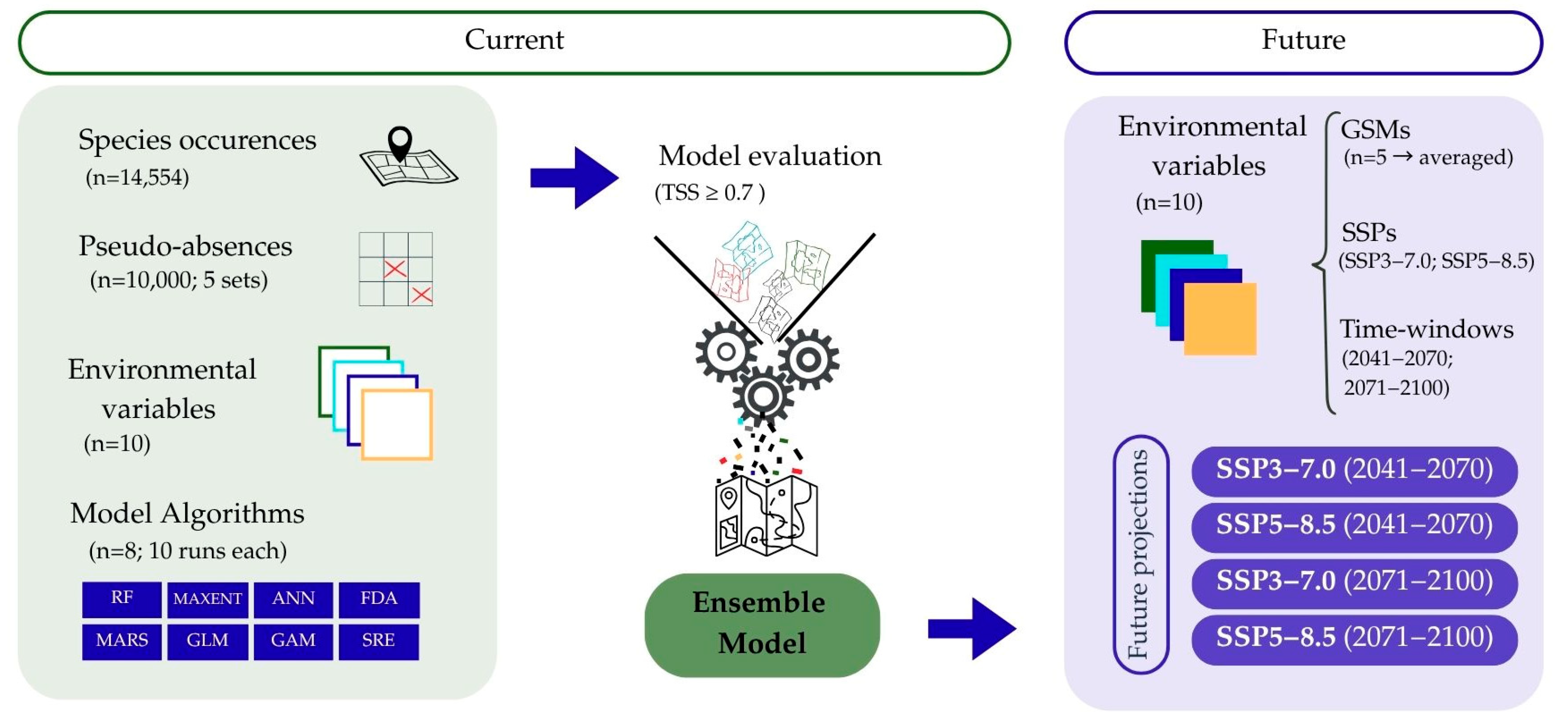
2. Materials and Methods
2.1. Study Area and Species Occurrence Data
2.2. Environmental Variables
2.3. Modeling Approach: Calibration, Fitting, and Evaluation
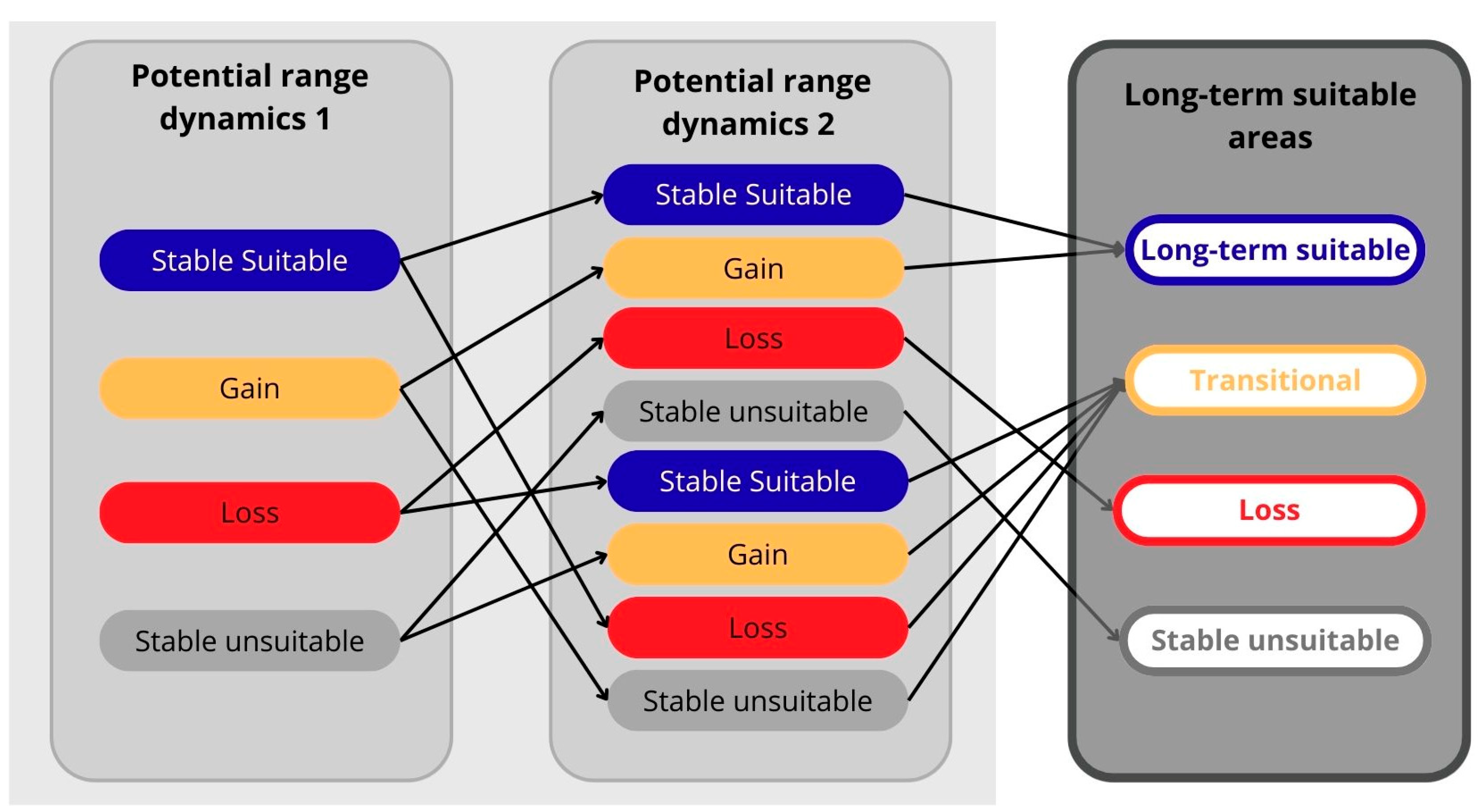
2.4. Present-to-Future Range Shifts
3. Results
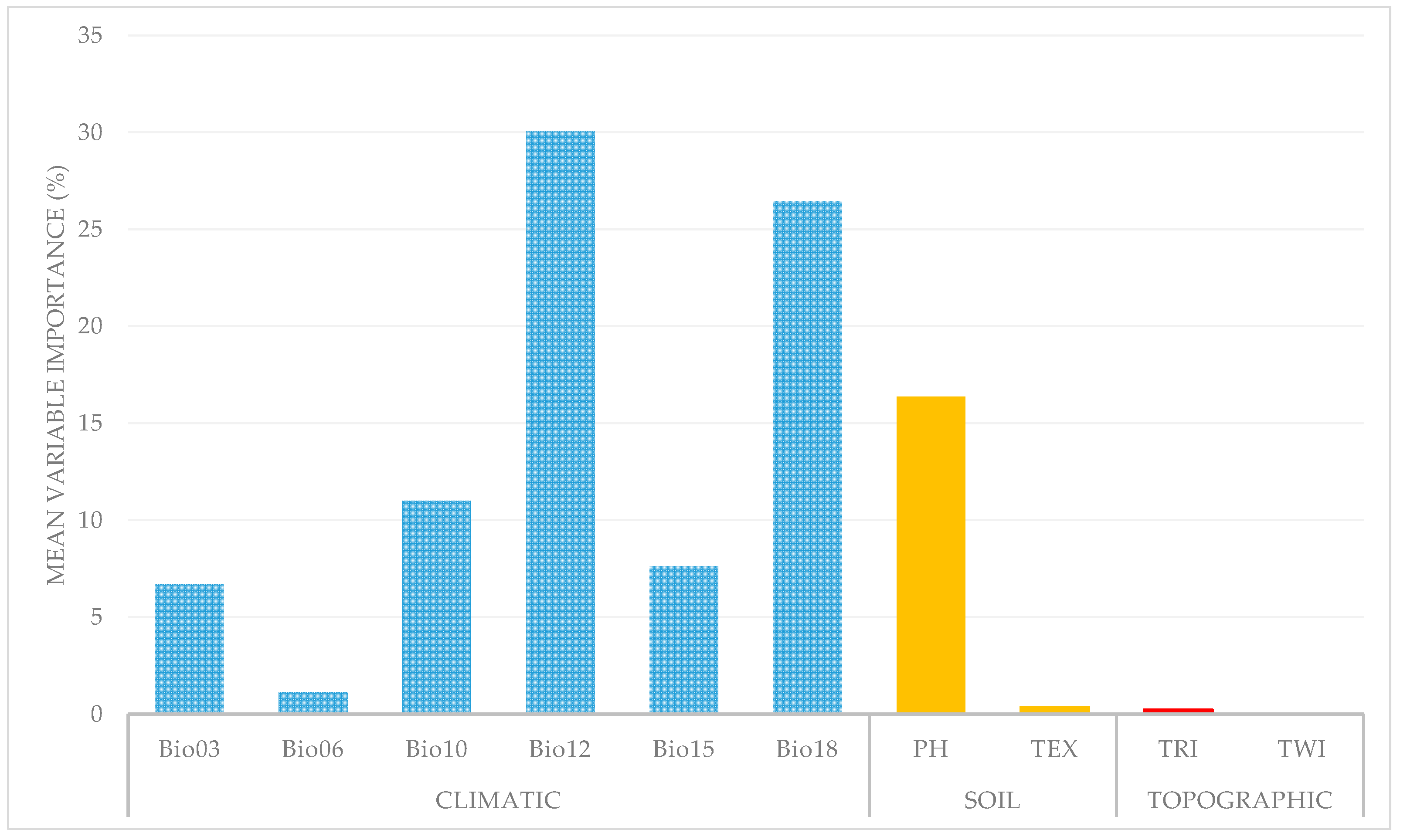
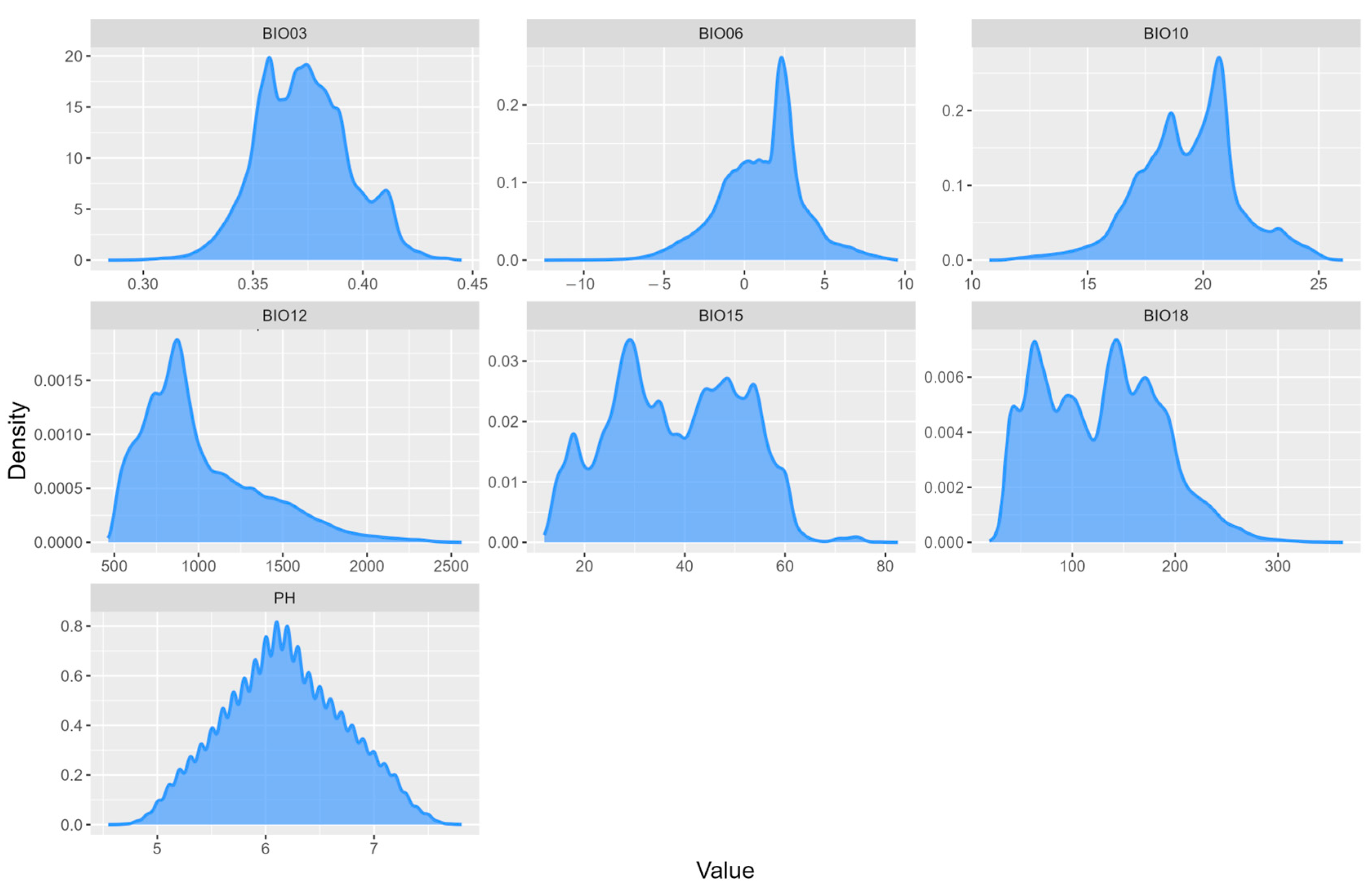
3.1. Model Evaluation and Environmental Variable Contribution
3.2. Potential Current Distribution and Future Range Shifts
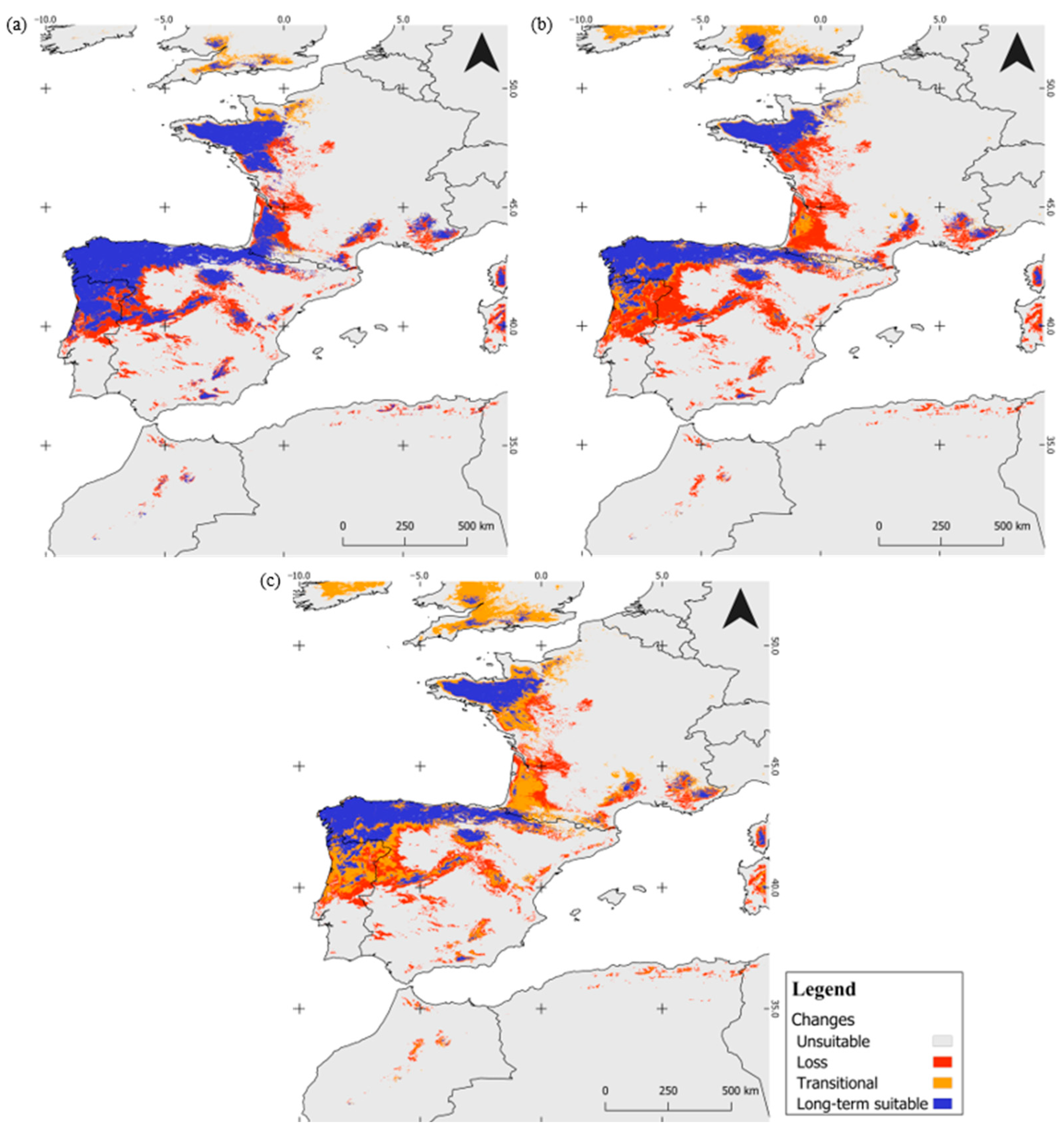
3.3. Long-Term-Suitable Areas and Implications for Species Conservation
4. Discussion
4.1. Current and Future Scenarios
4.2. What Can We Do in the Future to Conserve and Restore?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SDM | Species Distribution Models |
| IP | Iberian Peninsula |
| ES | Ecosystem Services |
References
- Castro, E.; González, M.; Tenorio, M.; Bombín, R.; Antón, M.; Fuster, M.; Manzaneque, A.; Manzaneque, F.; Saiz, J.; Juaristi, C.; et al. Los Bosques Ibéricos, 4th ed.; Tenorio, M., Juaristi, C., Ollero, H., Eds.; Editorial Planeta: Barcelona, Spain, 2005. [Google Scholar]
- de la Serna, B.V. Comprehensive Study of Quercus Pyrenaica Willd. Forests at Iberian Peninsula: Indicator Species, Bioclimatic, and Syntaxonomical Characteristics. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2014. [Google Scholar]
- de la Serna, B.V.; Sánchez-Mata, D.; Gavilán, R.G. Marcescent Quercus Pyrenaica Forest on the Iberian Peninsula. In Geobotany Studies; Springer: Cham, Switzerland, 2016; pp. 257–283. [Google Scholar]
- Amigo, J.; Romero, M.I. Vegetación Atlántica Bajo Clima Mediterráneo: Un Caso En El Noroeste Ibérico. Phytocoenologia 1994, 22, 583–603. [Google Scholar] [CrossRef]
- Amigo, J.; Izco, J.; Guitián, J.; Romero, M.I. Reinterpretación Del Robledal Termófilo Galaico-Portugués: Rusco Aculeati-Quercetum Roboris. Lazaroa 1998, 19, 85–98. [Google Scholar]
- Monteiro-Henriques, T. Landscape and Phytosociology of the Paiva River’s Hidrographical Basin. Ph.D. Thesis, Technical University of Lisbon, Higher Institute of Agronomy, Lisbon, Portugal, 2010. [Google Scholar]
- Marín, S.d.T.; Rodríguez-Calcerrada, J.; Arenas-Castro, S.; Prieto, I.; González, G.; Gil, L.; de la Riva, E.G. Fagus Sylvatica and Quercus Pyrenaica: Two Neighbors with Few Things in Common. For. Ecosyst. 2023, 10, 1–14. [Google Scholar] [CrossRef]
- Vila-Viçosa, C.M.; Capelo, J.H.; Alves, P.; Almeida, R.S.; Vázquez, F.M. New Annotated Checklist of the Portuguese Oaks (Quercus L., Fagaceae). Mediterr. Bot. 2023, 44, e79286. [Google Scholar] [CrossRef]
- Carvalho, J.P.; Santos, J.; Reimão, D.; Alves, P.; Grosso-Silva, J.; Santos, T.; Pinto, M.A.; Marques, G.; Martins, L.; Carvalheira, M.; et al. O Carvalho Negral; Carvalho, J., Ed.; Universidade de Trás-os-Montes e Alto Douro-CEGE: Vila Real, Portugal, 2005. [Google Scholar]
- Pinto-Gomes, C.; Paiva-Ferreira, R.; Meireles, C. New Proposals on Portuguese Vegetation. Lazaroa 2007, 28, 67–77. [Google Scholar] [CrossRef]
- Pérez-Luque, A.J.; Benito, B.M.; Bonet-García, F.J.; Zamora, R. Ecological Diversity within Rear-Edge: A Case Study from Mediterranean Quercus Pyrenaica Willd. Forests 2021, 12, 10. [Google Scholar] [CrossRef]
- Vila-Viçosa, C.M. Os Carvalhais Marcescentes do Centro e Sul de Portugal—Estudo e Conservação. Master’s Thesis, Universidade de Évora e Instituto Superior de Agronomia, Évora, Portugal, 2012. [Google Scholar]
- Vila-Viçosa, C.; Arenas-Castro, S.; Marcos, B.; Honrado, J.; García, C.; Vázquez, F.M.; Almeida, R.; Gonçalves, J. Combining Satellite Remote Sensing and Climate Data in Species Distribution Models to Improve the Conservation of Iberian White Oaks (Quercus l.). ISPRS Int. J. Geo-Inf. 2020, 9, 735. [Google Scholar] [CrossRef]
- Salvatore, P.; de Rigo, D.; Caudullo, G. Quercus Pubescens in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Durrant, T.H., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; pp. 156–157. ISBN 978-92-76-17290-1. [Google Scholar]
- Rivas-Martínez, S.; Sáenz, S.R.; Penas, A. Worldwide Bioclimatic Classification System. Glob. Geobot. 2011, 1, 1–634. [Google Scholar]
- Rivas-Martínez, S.; Penas, Á.; del Río, S.; Díaz González, T.E.; Rivas-Sáenz, S. Bioclimatology of the Iberian Peninsula and the Balearic Islands. In The Vegetation of the Iberian Peninsula. Plant and Vegetation; Loidi, J., Ed.; Springer: Cham, Switzerland, 2017; Volume 12, pp. 29–80. [Google Scholar]
- Vila-Viçosa, C.; Gonçalves, J.; Honrado, J.; Lomba, Â.; Almeida, R.S.; Vázquez, F.M.; Garcia, C. Late Quaternary Range Shifts of Marcescent Oaks Unveil the Dynamics of a Major Biogeographic Transition in Southern Europe. Sci. Rep. 2020, 10, 21598. [Google Scholar] [CrossRef] [PubMed]
- García-Mijangos, I.; Campos, J.A.; Biurrun, I.; Herrera, M.; Loidi, J. Marcescent Forests of the Iberian Peninsula: Floristic and Climatic Characterization. In Geobotany Studies; Springer: Cham, Switzerland, 2015; pp. 119–138. [Google Scholar]
- de Dios, R.S.; Benito-Garzón, M.; Sainz-Ollero, H. Present and Future Extension of the Iberian Submediterranean Territories as Determined from the Distribution of Marcescent Oaks. Plant Ecol. 2009, 204, 189–205. [Google Scholar] [CrossRef]
- Passos, I.; Vila-Viçosa, C.; Gonçalves, J.; Ribeiro, M.M.; Figueiredo, A. Tracking Submediterranean Ecotone Shifts Under Climate Change Scenarios Using Marcescent Oaks as Indicators. Sci. Rep. 2025. accepted. [Google Scholar]
- Vogel, J.; Paton, E.; Aich, V. Seasonal Ecosystem Vulnerability to Climatic Anomalies in the Mediterranean. Biogeosciences 2021, 18, 5903–5927. [Google Scholar] [CrossRef]
- Barredo, J.I.; Caudullo, G.; Dosio, A. Mediterranean Habitat Loss under Future Climate Conditions: Assessing Impacts on the Natura 2000 Protected Area Network. Appl. Geogr. 2016, 75, 83–92. [Google Scholar] [CrossRef]
- Lionello, P.; Scarascia, L. The Relation between Climate Change in the Mediterranean Region and Global Warming. Reg. Environ. Change 2018, 18, 1481–1493. [Google Scholar] [CrossRef]
- Smith, A.J.; Goetz, E.M. Climate Change Drives Increased Directional Movement of Landscape Ecotones. Landsc. Ecol. 2021, 36, 3105–3116. [Google Scholar] [CrossRef]
- Walter, J.A.; Atkins, J.W.; Hulshof, C.M. Climate and Topography Control Variation in the Tropical Dry Forest–Rainforest Ecotone. Ecology 2024, 105, e4442. [Google Scholar] [CrossRef] [PubMed]
- Wasson, K.; Woolfolk, A.; Fresquez, C. Ecotones as Indicators of Changing Environmental Conditions: Rapid Migration of Salt Marsh—Upland Boundaries. Estuaries Coasts 2013, 36, 654–664. [Google Scholar] [CrossRef]
- Swanston, C.W.; Janowiak, M.K.; Brandt, L.A.; Butler, P.R.; Handler, S.D.; Shannon, P.D.; Derby Lewis, A.; Hall, K.; Fahey, R.T.; Scott, L.; et al. Forest Adaptation Resources: Climate Change Tools and Approaches for Land Managers, 2nd ed.; U.S. Department of Agriculture, Forest Service, Northeastern Research Station: Newtown Square, PA, USA, 2016.
- Sousa-Silva, R.; Verbist, B.; Lomba, Â.; Valent, P.; Suškevičs, M.; Picard, O.; Hoogstra-Klein, M.A.; Cosofret, V.-C.; Bouriaud, L.; Ponette, Q.; et al. Adapting Forest Management to Climate Change in Europe: Linking Perceptions to Adaptive Responses. For. Policy Econ. 2018, 90, 22–30. [Google Scholar] [CrossRef]
- Palik, B.J.; Clark, P.W.; D′Amato, A.W.; Swanston, C.; Nagel, L. Operationalizing Forest-Assisted Migration in the Context of Climate Change Adaptation: Examples from the Eastern USA. Ecosphere 2022, 13, e4260. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Guillera-Arroita, G. Modelling of Species Distributions, Range Dynamics and Communities under Imperfect Detection: Advances, Challenges and Opportunities. Ecography 2017, 40, 281–295. [Google Scholar] [CrossRef]
- Sillero, N.; Arenas-Castro, S.; Enriquez-Urzelai, U.; Vale, C.G.; Sousa-Guedes, D.; Martínez-Freiría, F.; Real, R.; Barbosa, A.M. Want to Model a Species Niche? A Step-by-Step Guideline on Correlative Ecological Niche Modelling. Ecol. Model. 2021, 456, 109671. [Google Scholar] [CrossRef]
- Pecchi, M.; Marchi, M.; Burton, V.; Giannetti, F.; Moriondo, M.; Bernetti, I.; Bindi, M.; Chirici, G. Species Distribution Modelling to Support Forest Management. A Literature Review. Ecol. Model. 2019, 411, 108817. [Google Scholar] [CrossRef]
- Zurell, D.; Franklin, J.; König, C.; Bouchet, P.J.; Dormann, C.F.; Elith, J.; Fandos, G.; Feng, X.; Guillera-Arroita, G.; Guisan, A.; et al. A Standard Protocol for Reporting Species Distribution Models. Ecography 2020, 43, 1261–1277. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J. A Review of Evidence about Use and Performance of Species Distribution Modelling Ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- Araújo, M.B.; Anderson, R.P.; Barbosa, A.M.; Beale, C.M.; Dormann, C.F.; Early, R.; Garcia, R.A.; Guisan, A.; Maiorano, L.; Naimi, B.; et al. Standards for Distribution Models in Biodiversity Assessments. Sci. Adv. 2019, 5, aat4858. [Google Scholar] [CrossRef] [PubMed]
- Domisch, S.; Friedrichs, M.; Hein, T.; Borgwardt, F.; Wetzig, A.; Jähnig, S.C.; Langhans, S.D. Spatially Explicit Species Distribution Models: A Missed Opportunity in Conservation Planning? Divers. Distrib. 2019, 25, 758–769. [Google Scholar] [CrossRef]
- Gaytán, Á.; Ricarte, A.; González-Bornay, G. Hoverfly Diversity (Diptera: Syrphidae) of Pyrenean Oak Woodlands in Central-Western Spain: A Preliminary Study with Conservation Outcomes. J. Insect Conserv. 2020, 24, 163–173. [Google Scholar] [CrossRef]
- Rodríguez-de la Cruz, D.; Perfecto-Arribas, S.; Delgado-Sánchez, L. Diversity Analysis of Macrofungi and Lichenised Fungi in Pyrenean Oak (Quercus pyrenaica Willd.) and Chestnut (Castanea sativa L.) Forests: Implications for the Conservation of Forest Habitats in Castilla y León (Central-Northwest Spain). Forests 2025, 16, 9. [Google Scholar] [CrossRef]
- Diez-Hermano, S.; Poveda, J.; Benito, Á.; Peix, Á.; Martín-Pinto, P.; Diez, J.J. Soil Mycobiome and Forest Endophytic Fungi: Is There a Relationship between Them? For. Ecol. Manag. 2024, 562, 121924. [Google Scholar] [CrossRef]
- Aldea, J.; del Río, M.; Cattaneo, N.; Riofrío, J.; Ordóñez, C.; Uzquiano, S.; Bravo, F. Short-Term Effect of Thinning on Inter- and Intra-Annual Radial Increment in Mediterranean Scots Pine-Oak Mixed Forests. For. Ecol. Manag. 2023, 549, 121462. [Google Scholar] [CrossRef]
- Stavi, I.; Thevs, N.; Welp, M.; Zdruli, P. Provisioning Ecosystem Services Related with Oak (Quercus) Systems: A Review of Challenges and Opportunities. Agrofor. Syst. 2022, 96, 293–313. [Google Scholar] [CrossRef]
- Bartolomé, J.; Amat, A.C.; Rubines, J.; Sesma, J.; López-Garrido, O.; Ibáñez, M.; Hernández-Castellano, C.; Lavín, S.; Gort-Esteve, A.; Hernández-Rodríguez, A.; et al. Neutral Impact of Cattle Grazing in Pyrenean Oak Forests Integrity. Sustainability 2024, 16, 10939. [Google Scholar] [CrossRef]
- Díaz-Maroto, I.J.; Vila-Lameiro, P. Deciduous and Semi-Deciduous Oak Forests (Quercus Robur, Q. Petraea and Q. Pyrenaica) Floristic Composition in the Northwest Iberian Peninsula. Biologia 2007, 62, 163–172. [Google Scholar] [CrossRef]
- Proença, V.A.M. Galicio-Portuguese Oak Forest of Quercus Robur and Quercus Pyrenaica: Biodiversity Patterns and Forest Response to Fire. Ph.D. Thesis, Universidade de Lisboa, Lisboa, Portugal, 2009. [Google Scholar]
- Piñar Fuentes, J.C.; Cano-Ortiz, A.; Musarella, C.M.; Quinto Canas, R.; Pinto Gomes, C.J.; Spampinato, G.; del Río, S.; Cano, E. Bioclimatology, Structure, and Conservation Perspectives of Quercus Pyrenaica, Acer Opalus Subsp. Granatensis, and Corylus Avellana Deciduous Forests on Mediterranean Bioclimate in the South-Central Part of the Iberian Peninsula. Sustainability 2019, 11, 6500. [Google Scholar] [CrossRef]
- del Río Gonźalez, S.; Herrero Cembranos, L.; Penas Merino, Á. Bioclimatic Analysis of the Quercus Pyrenaica Forests in Spain. Phytocoenologia 2007, 37, 541–560. [Google Scholar] [CrossRef]
- Gavilán, R.G.; Mata, D.S.; Vilches, B.; Entrocassi, G. Modeling Current Distribution of Spanish Quercus Pyrenaica Forests Using Climatic Parameters. Phytocoenologia 2007, 37, 561–581. [Google Scholar] [CrossRef]
- Díaz, J.B. El Cambio Climático y La Composición Florística de Los Robledales de Quercus Pyrenaica de La Sierra de Guadarrama. Conserv. óN Veg. 2018, 22, 14–15. [Google Scholar] [CrossRef]
- Chevalier, M.; Broennimann, O.; Cornuault, J.; Guisan, A. Data Integration Methods to Account for Spatial Niche Truncation Effects in Regional Projections of Species Distribution. Ecol. Appl. 2021, 31, e02427. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, M.; Zarzo-Arias, A.; Guélat, J.; Mateo, R.G.; Guisan, A. Accounting for Niche Truncation to Improve Spatial and Temporal Predictions of Species Distributions. Front. Ecol. Evol. 2022, 10, 1–14. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at High Resolution for the Earth′s Land Surface Areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef] [PubMed]
- Reif, A.; Xystrakis, F.; Gärtner, S.; Sayer, U. Floristic Change at the Drought Limit of European Beech (Fagus sylvatica L.) to Downy Oak (Quercus Pubescens) Forest in the Temperate Climate of Central Europe. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 646–654. [Google Scholar] [CrossRef]
- Hengl, T.; De Jesus, J.M.; Heuvelink, G.B.M.; Gonzalez, M.R.; Kilibarda, M.; Blagotić, A.; Shangguan, W.; Wright, M.N.; Geng, X.; Bauer-Marschallinger, B.; et al. SoilGrids250m: Global Gridded Soil Information Based on Machine Learning. PLoS ONE 2017, 12, e0169748. [Google Scholar] [CrossRef] [PubMed]
- Riley, S.J.; De Gloria, S.D.; Elliot, R. A Terrain Ruggedness Index That Quantifies Topographic Heterogeneity. Intermt. J. Sci. 1999, 5, 23–27. [Google Scholar]
- Beven, K.J.; Kirkby, M.J. A Physically Based, Variable Contributing Area Model of Basin Hydrology. Hydrol. Sci. Bull. 1979, 24, 43–69. [Google Scholar] [CrossRef]
- Dormann, C.F.; Schymanski, S.J.; Cabral, J.; Chuine, I.; Graham, C.; Hartig, F.; Kearney, M.; Morin, X.; Römermann, C.; Schröder, B.; et al. Correlation and Process in Species Distribution Models: Bridging a Dichotomy. J. Biogeogr. 2012, 39, 2119–2131. [Google Scholar] [CrossRef]
- McSweeney, C.F.; Jones, R.G.; Lee, R.W.; Rowell, D.P. Selecting CMIP5 GCMs for Downscaling over Multiple Regions. Clim. Dyn. 2015, 44, 3237–3260. [Google Scholar] [CrossRef]
- Scafetta, N. Impacts and Risks of “Realistic” Global Warming Projections for the 21st Century. Geosci. Front. 2024, 15, 101774. [Google Scholar] [CrossRef]
- Huard, D.; Fyke, J.; Capellán-Pérez, I.; Matthews, H.D.; Partanen, A.-I. Estimating the Likelihood of GHG Concentration Scenarios from Probabilistic Integrated Assessment Model Simulations. Earth′s Future 2022, 10, e2022EF002715. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A Platform for Ensemble Forecasting of Species Distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Thuiller, W.; Georges, D.; Gueguen, M.; Engler, R.; Breiner, F.; Lafourcade, B.; Patin, R.; Blancheteau, H. Biomod2: Ensemble Platform for Species Distribution Modeling Version 4.2-6.2. Available online: https://cran.r-project.org/web/packages/biomod2/index.html (accessed on 24 April 2025).
- Swets, J.A. Measuring the Accuracy of Diagnostic Systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models; Cambridge University Press: Cambridge, UK, 2017. ISBN 9781139028271.
- Freeman, E.A.; Moisen, G.G. A Comparison of the Performance of Threshold Criteria for Binary Classification in Terms of Predicted Prevalence and Kappa. Ecol. Model. 2008, 217, 48–58. [Google Scholar] [CrossRef]
- Stewart, S.B.; Fedrigo, M.; Kasel, S.; Roxburgh, S.H.; Choden, K.; Tenzin, K.; Allen, K.; Nitschke, C.R. Predicting Plant Species Distributions Using Climate-Based Model Ensembles with Corresponding Measures of Congruence and Uncertainty. Divers. Distrib. 2022, 28, 1105–1122. [Google Scholar] [CrossRef]
- Zittis, G.; Bruggeman, A.; Lelieveld, J. Revisiting Future Extreme Precipitation Trends in the Mediterranean. Weather Clim. Extrem. 2021, 34, 100380. [Google Scholar] [CrossRef] [PubMed]
- Todaro, V.; D’Oria, M.; Secci, D.; Zanini, A.; Tanda, M.G. Climate Change over the Mediterranean Region: Local Temperature and Precipitation Variations at Five Pilot Sites. Water 2022, 14, 2499. [Google Scholar] [CrossRef]
- Vessella, F.; López-Tirado, J.; Simeone, M.C.; Schirone, B.; Hidalgo, P.J. A Tree Species Range in the Face of Climate Change: Cork Oak as a Study Case for the Mediterranean Biome. Eur. J. For. Res. 2017, 136, 555–569. [Google Scholar] [CrossRef]
- Almeida, A.M.; Martins, M.J.; Campagnolo, M.L.; Fernandez, P.; Albuquerque, T.; Gerassis, S.; Gonçalves, J.C.; Ribeiro, M.M. Prediction Scenarios of Past, Present, and Future Environmental Suitability for the Mediterranean Species Arbutus unedo L. Sci. Rep. 2022, 12, 84. [Google Scholar] [CrossRef]
- Fyllas, N.M.; Koufaki, T.; Sazeides, C.I.; Spyroglou, G.; Theodorou, K. Potential Impacts of Climate Change on the Habitat Suitability of the Dominant Tree Species in Greece. Plants 2022, 11, 1616. [Google Scholar] [CrossRef] [PubMed]
- Mauri, A.; Girardello, M.; Forzieri, G.; Manca, F.; Beck, P.S.A.; Cescatti, A.; Strona, G. Assisted Tree Migration Can Reduce but Not Avert the Decline of Forest Ecosystem Services in Europe. Glob. Environ. Change 2023, 80, 102676. [Google Scholar] [CrossRef]
- Puig-Gironès, R.; Muriana, M.; Real, J.; Sabaté, S. Unravelling the Influence of Annual Weather Conditions and Mediterranean Habitat Types on Acorn Production, Availability and Predation. For. Ecol. Manag. 2023, 543, 121149. [Google Scholar] [CrossRef]
- Pérez-Ramos, I.M.; Marañón, T. Factors Affecting Post-Dispersal Seed Predation in Two Coexisting Oak Species: Microhabitat, Burial and Exclusion of Large Herbivores. For. Ecol. Manag. 2008, 255, 3506–3514. [Google Scholar] [CrossRef]
- Gómez, J.M.; Puerta-Piñero, C.; Schupp, E.W. Effectiveness of Rodents as Local Seed Dispersers of Holm Oaks. Oecologia 2008, 155, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Luo, Y.; Wang, R.; Du, F.K. The Distinct Fruit Size and Physical Defense Promote Divergent Secondary Seed Dispersal Strategies of Three Oak Species. For. Ecol. Manag. 2023, 529, 120642. [Google Scholar] [CrossRef]
- Grivet, D.; Smouse, P.E.; Sork, V.L. A Novel Approach to an Old Problem: Tracking Dispersed Seeds. Mol. Ecol. 2005, 14, 3585–3595. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Labourdette, D.; Nogués-Bravo, D.; Ollero, H.S.; Schmitz, M.F.; Pineda, F.D. Forest Composition in Mediterranean Mountains Is Projected to Shift along the Entire Elevational Gradient under Climate Change. J. Biogeogr. 2012, 39, 162–176. [Google Scholar] [CrossRef]
- Granda, E.; Alla, A.Q.; Laskurain, N.A.; Loidi, J.; Sánchez-Lorenzo, A.; Camarero, J.J. Coexisting Oak Species, Including Rear-Edge Populations, Buffer Climate Stress through Xylem Adjustments. Tree Physiol. 2018, 38, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Souza, T.; Chase, J.M.; Haddad, N.M.; Vancine, M.H.; Didham, R.K.; Melo, F.L.P.; Aizen, M.A.; Bernard, E.; Chiarello, A.G.; Faria, D.; et al. Species Turnover Does Not Rescue Biodiversity in Fragmented Landscapes. Nature 2025, 640, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Hampe, A.; Jump, A.S. Climatic Relicts: Past, Present, Future. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 313–333. [Google Scholar] [CrossRef]
- Gómez, A.; Lunt, D.H. Refugia within Refugia: Patterns of Phylogeographic Concordance in the Iberian Peninsula. In Phylogeography of Southern European Refugia: Evolutionary Perspectives on the Origins and Conservation of European Biodiversity; Weiss, S., Ferrand, N., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 155–158. [Google Scholar]
- Bagnoli, F.; Della Rocca, G.; Spanu, I.; Fineschi, S.; Vendramin, G.G. The Origin of the Afro-Mediterranean Cypresses: Evidence from Genetic Analysis. Perspect. Plant Ecol. Evol. Syst. 2020, 46, 125564. [Google Scholar] [CrossRef]
- Cortés-Molino, Á.; Linares, J.C.; Viñegla, B.; Lechuga, V.; Salvo-Tierra, A.E.; Flores-Moya, A.; Fernández-Luque, I.; Carreira, J.A. Unexpected Resilience in Relict Abies Pinsapo Boiss Forests to Dieback and Mortality Induced by Climate Change. Front. Plant Sci. 2022, 13, 991720. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.A.; da Silva, C.R.B.; Moore, M.P.; Diamond, S.E. When Will a Changing Climate Outpace Adaptive Evolution? WIREs Clim. Change 2023, 14, e852. [Google Scholar] [CrossRef]
- Hernández, L.; Sánchez de Dios, R.; Montes, F.; Sainz-Ollero, H.; Cañellas, I. Exploring Range Shifts of Contrasting Tree Species across a Bioclimatic Transition Zone. Eur. J. For. Res. 2017, 136, 481–492. [Google Scholar] [CrossRef]
- Kopsierker, L.; Costa Domingo, G.; Underwood, E. Climate Mitigation Potential of Large-Scale Nature Restoration in Europe. Analysis of the Climate Mitigation Potential of Restoring Habitats Listed in Annex I of the Habitats Directive; Institute for European Environmental Policy: London, UK, 2022. [Google Scholar]
- Costa, J.C.; Monteiro-Henriques, T.; Bingre, P.; Espírito-Santo, D. Warm-Temperate Forests of Central Portugal: A Mosaic of Syntaxa. In Geobotany Studies; Springer: Cham, Switzerland, 2015; pp. 97–117. [Google Scholar]
- Gustafson, E.J.; Kern, C.C.; Kabrick, J.M. Can Assisted Tree Migration Today Sustain Forest Ecosystem Goods and Services for the Future? For. Ecol. Manag. 2023, 529, 120723. [Google Scholar] [CrossRef]
- Weiss, G.; Emery, M.R.; Corradini, G.; Živojinović, I. New Values of Non-Wood Forest Products. Forests 2020, 11, 165. [Google Scholar] [CrossRef]
- Thirgood, J.V. Man and the Mediterranean Forest. A History of Resource Depletion; Academic Press: Toronto, ON, Canada, 1981. [Google Scholar]
- Aguiar, C.; Vila-Viçosa, C. Trás-Os-Montes and Beira Alta. In The Vegetation of the Iberian Peninsula; Loidi, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1, pp. 367–394. [Google Scholar]
- Silva, V.; Catry, F.X.; Fernandes, P.M.; Rego, F.C.; Bugalho, M.N. Trade-offs between Fire Hazard Reduction and Conservation in a Natura 2000 Shrub–Grassland Mosaic. Appl. Veg. Sci. 2020, 23, 39–52. [Google Scholar] [CrossRef]
- Valbuena-Carabaña, M.; González-Martínez, S.C.; Gil, L. Coppice Forests and Genetic Diversity: A Case Study in Quercus Pyrenaica Willd. from Central Spain. For. Ecol. Manag. 2008, 254, 225–232. [Google Scholar] [CrossRef]
- Millar, C.I.; Stephenson, N.L.; Stephens, S.L. Climate Change and Forests of the Future: Managing in the Face of Uncertainty. Ecol. Appl. 2007, 17, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; She, Y.; She, J.; Zhang, J.; Zhang, X.; Luo, C. Assessing Habitat Suitability and Selecting Optimal Habitats for Relict Tree Cathaya Argyrophylla in Hunan, China: Integrating Pollen Size, Environmental Factors, and Niche Modeling for Conservation. Ecol. Indic. 2022, 145, 109669. [Google Scholar] [CrossRef]
- Aykurt, C.; Özkan, K.; Gülben, M.; Şentürk, Ö.; Berberoğlu, E.; Türkan, S.; Öz, Z.; Göktürk, R.S.; Akgül, H.; Günaydın, S.; et al. Patterns of Functional Diversity, Species Diversity, and Endemicity Driven by Elevation and Topographic Complexity in a Mediterranean Mountain Refuge. Ecol. Evol. 2025, 15, e71354. [Google Scholar] [CrossRef] [PubMed]
- Sanz, R.; Pulido, F.; Camarero, J.J. Boreal Trees in the Mediterranean: Recruitment of Downy Birch (Betula Alba) at Its Southern Range Limit. Ann. For. Sci. 2011, 68, 793–802. [Google Scholar] [CrossRef]
- Pérez-Luque, A.J.; Bonet-García, F.J.; Zamora, R. Colonization Pattern of Abandoned Croplands by Quercus Pyrenaica in a Mediterranean Mountain Region. Forests 2021, 12, 1584. [Google Scholar] [CrossRef]
- Frietsch, M.; Loos, J.; Löhr, K.; Sieber, S.; Fischer, J. Future-Proofing Ecosystem Restoration through Enhancing Adaptive Capacity. Commun. Biol. 2023, 6, 377. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pineda, E.; Blanco-García, A.; Lindig-Cisneros, R.; O’Neill, G.A.; Lopez-Toledo, L.; Sáenz-Romero, C. Pinus Pseudostrobus Assisted Migration Trial with Rain Exclusion: Maintaining Monarch Butterfly Biosphere Reserve Forest Cover in an Environment Affected by Climate Change. New For. 2021, 52, 995–1010. [Google Scholar] [CrossRef]
- Etterson, J.R.; Cornett, M.W.; White, M.A.; Kavajecz, L.C. Assisted Migration across Fixed Seed Zones Detects Adaptation Lags in Two Major North American Tree Species. Ecol. Appl. 2020, 30, e02092. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Tu, Z.; Zhang, Y.; Zhong, W.; Xia, H.; Hao, Z.; Zhang, C.; Li, H. Predicting the Impact of Climate Change on the Distribution of Two Relict Liriodendron Species by Coupling the MaxEnt Model and Actual Physiological Indicators in Relation to Stress Tolerance. J. Environ. Manag. 2022, 322, 116024. [Google Scholar] [CrossRef] [PubMed]
- Van de Peer, T.; Verheyen, K.; Baeten, L.; Ponette, Q.; Muys, B. Biodiversity as Insurance for Sapling Survival in Experimental Tree Plantations. J. Appl. Ecol. 2016, 53, 1777–1786. [Google Scholar] [CrossRef]
- Kaviriri, D.K.; Liu, T.; Yang, L. Potential Distribution and Ecological Niche of Quercus Mongolica under Different Climate Scenarios. Plant Ecol. 2025, 226, 703–720. [Google Scholar] [CrossRef]
- Van de Peer, T.; Mereu, S.; Verheyen, K.; Saura, J.M.C.; Morillas, L.; Roales, J.; Cascio, M.L.; Spano, D.; Paquette, A.; Muys, B. Tree Seedling Vitality Improves with Functional Diversity in a Mediterranean Common Garden Experiment. For. Ecol. Manag. 2018, 409, 614–633. [Google Scholar] [CrossRef]
- Hartl, D.; Clark, A. Principles of Population Genetics; Sinauer and Associates: Sunderland, MA, USA, 2007. [Google Scholar]
- Ribeiro, M.M.; LeProvost, G.; Gerber, S.; Vendramin, G.G.; Anzidei, M.; Decroocq, S.; Marpeau, A.; Mariette, S.; Plomion, C. Origin Identification of Maritime Pine Stands in France Using Chloroplast Simple-Sequence Repeats. Ann. For. Sci. 2002, 59, 53–62. [Google Scholar] [CrossRef]
- Yousefzadeh, H.; Amirchakhmaghi, N.; Naseri, B.; Shafizadeh, F.; Kozlowski, G.; Walas, Ł. The Impact of Climate Change on the Future Geographical Distribution Range of the Endemic Relict Tree Gleditsia Caspica (Fabaceae) in Hyrcanian Forests. Ecol. Inform. 2022, 71, 101773. [Google Scholar] [CrossRef]
- Ruiz de la Torre, J. Flora Mayor; Icona (Organismo Autonomo Parques Nacionales): Madrid, Spain, 2006. [Google Scholar]


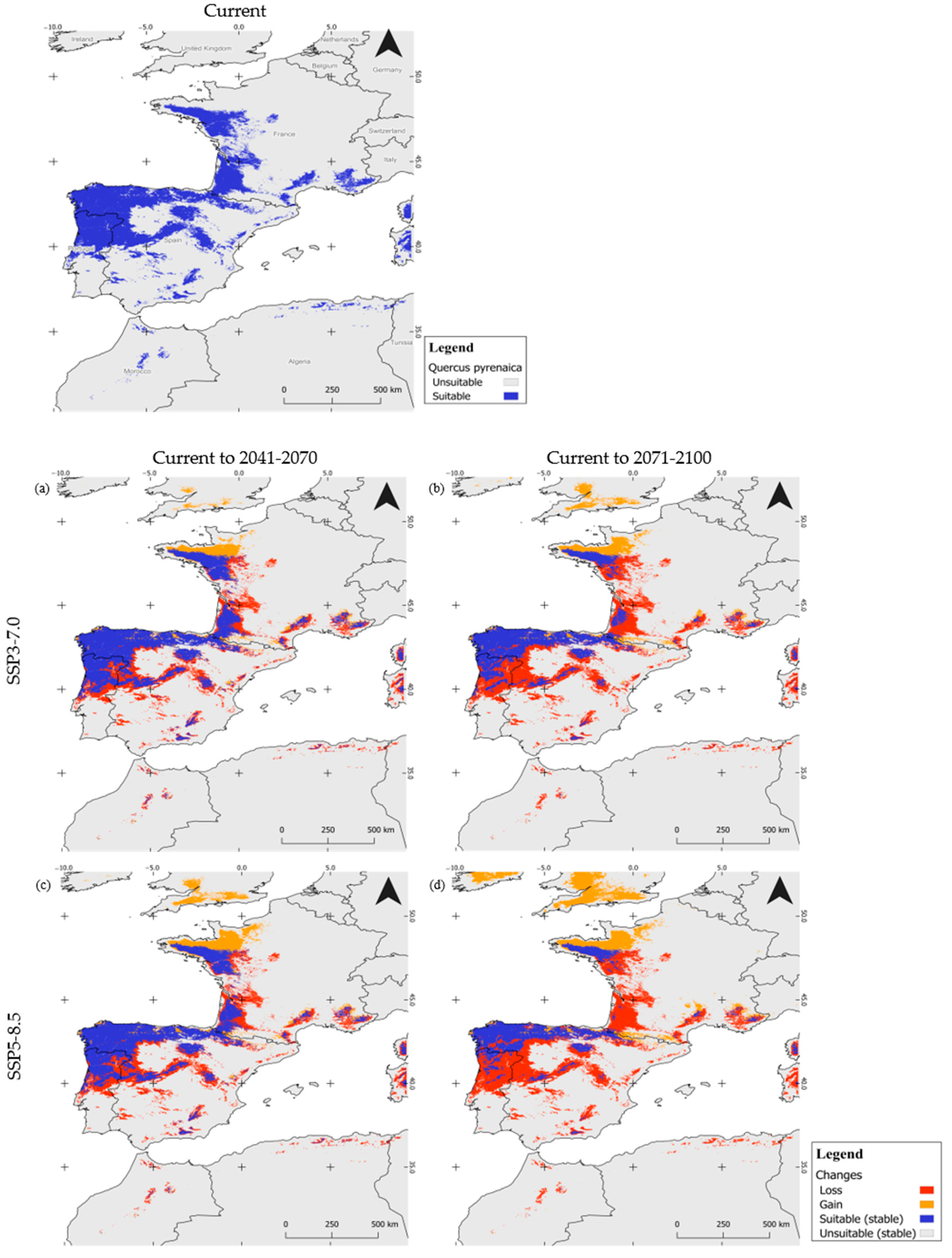
| SSP Scenarios | Time Frames | Gain | Stable | Loss | Total Suitable Area | |||
|---|---|---|---|---|---|---|---|---|
| Km2 | % | Km2 | % | Km2 | % | Km2 | ||
| SSP3-7.0 | 2041–2070 | 56,540 | 10.42 | 303,008 | 55.86 | 182,879 | 33.71 | 359,548 |
| 2071–2100 | 77,272 | 13.72 | 209,214 | 37.15 | 276,673 | 49.13 | 286,486 | |
| SSP5-8.5 | 2041–2070 | 89,047 | 15.49 | 282,048 | 49.06 | 203,839 | 35.45 | 371,095 |
| 2071–2100 | 150,728 | 23.68 | 161,301 | 25.34 | 324,586 | 50.99 | 312,029 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passos, I.; Vila-Viçosa, C.; Gonçalves, J.; Figueiredo, A.; Ribeiro, M.M. Climate-Driven Habitat Shifts and Conservation Implications for the Submediterranean Oak Quercus pyrenaica Willd. Forests 2025, 16, 1226. https://doi.org/10.3390/f16081226
Passos I, Vila-Viçosa C, Gonçalves J, Figueiredo A, Ribeiro MM. Climate-Driven Habitat Shifts and Conservation Implications for the Submediterranean Oak Quercus pyrenaica Willd. Forests. 2025; 16(8):1226. https://doi.org/10.3390/f16081226
Chicago/Turabian StylePassos, Isabel, Carlos Vila-Viçosa, João Gonçalves, Albano Figueiredo, and Maria Margarida Ribeiro. 2025. "Climate-Driven Habitat Shifts and Conservation Implications for the Submediterranean Oak Quercus pyrenaica Willd." Forests 16, no. 8: 1226. https://doi.org/10.3390/f16081226
APA StylePassos, I., Vila-Viçosa, C., Gonçalves, J., Figueiredo, A., & Ribeiro, M. M. (2025). Climate-Driven Habitat Shifts and Conservation Implications for the Submediterranean Oak Quercus pyrenaica Willd. Forests, 16(8), 1226. https://doi.org/10.3390/f16081226









