Growth and Water-Use Efficiency of European Beech and Turkey Oak at Low-Elevation Site
Abstract
1. Introduction
2. Materials and Methods
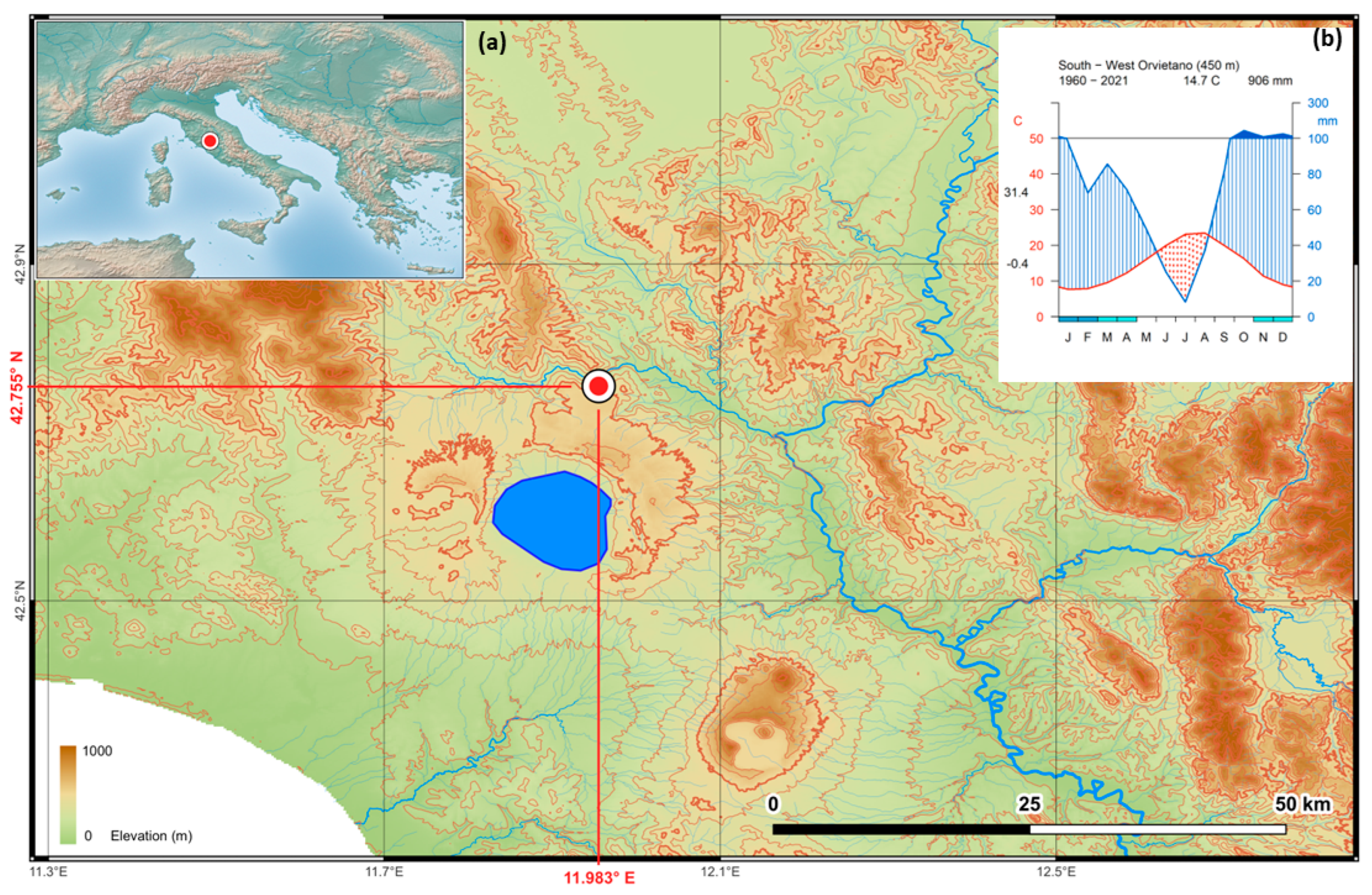
2.1. Study Area
2.2. Meteorological Data
2.3. Tree Sampling and Dendrochronological Analysis
2.4. Sample Preparation, δ13C, Δ13C and iWUE Calculation
2.5. Statistical Analysis
3. Results
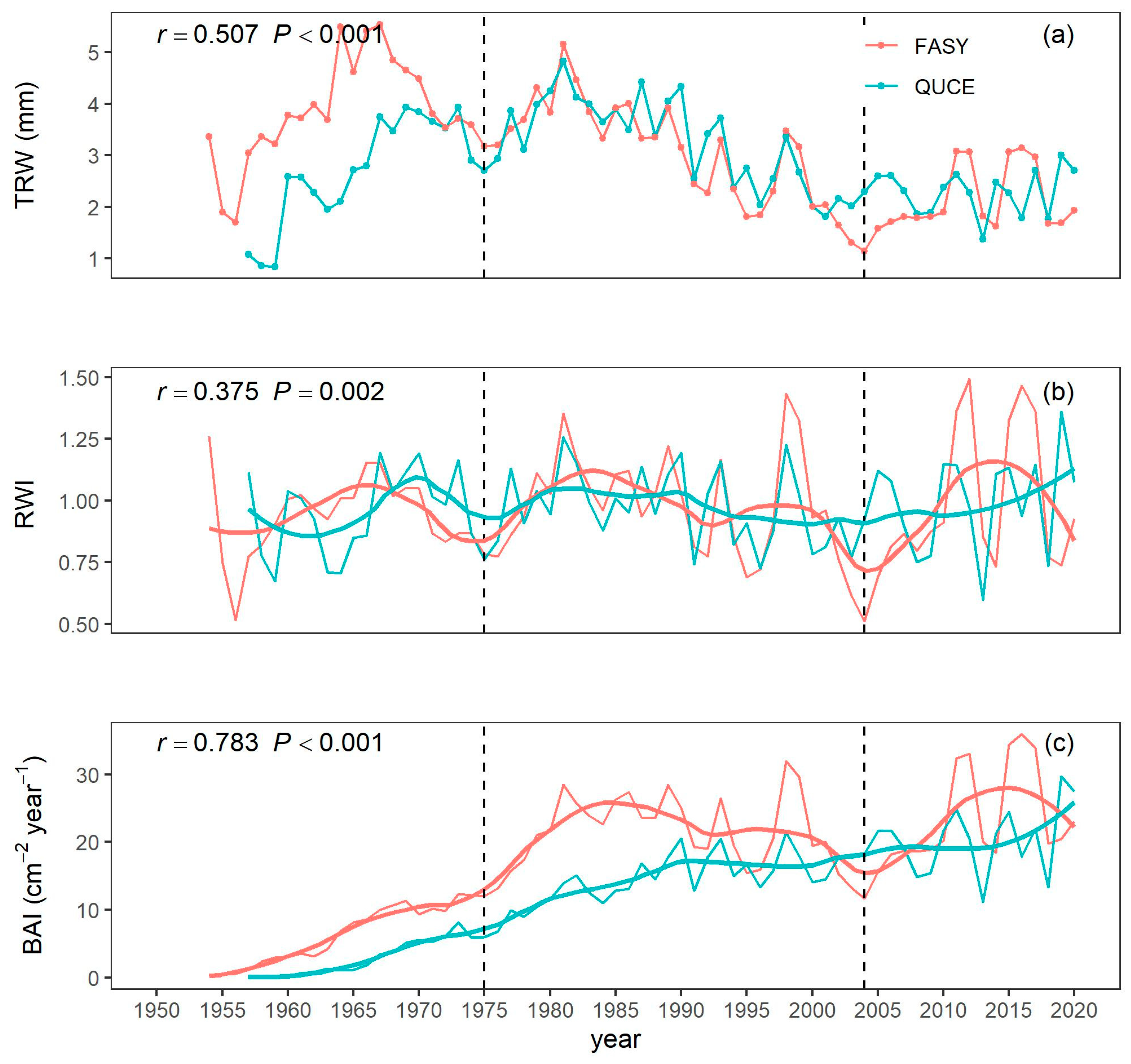
3.1. Growth Patterns and Trends
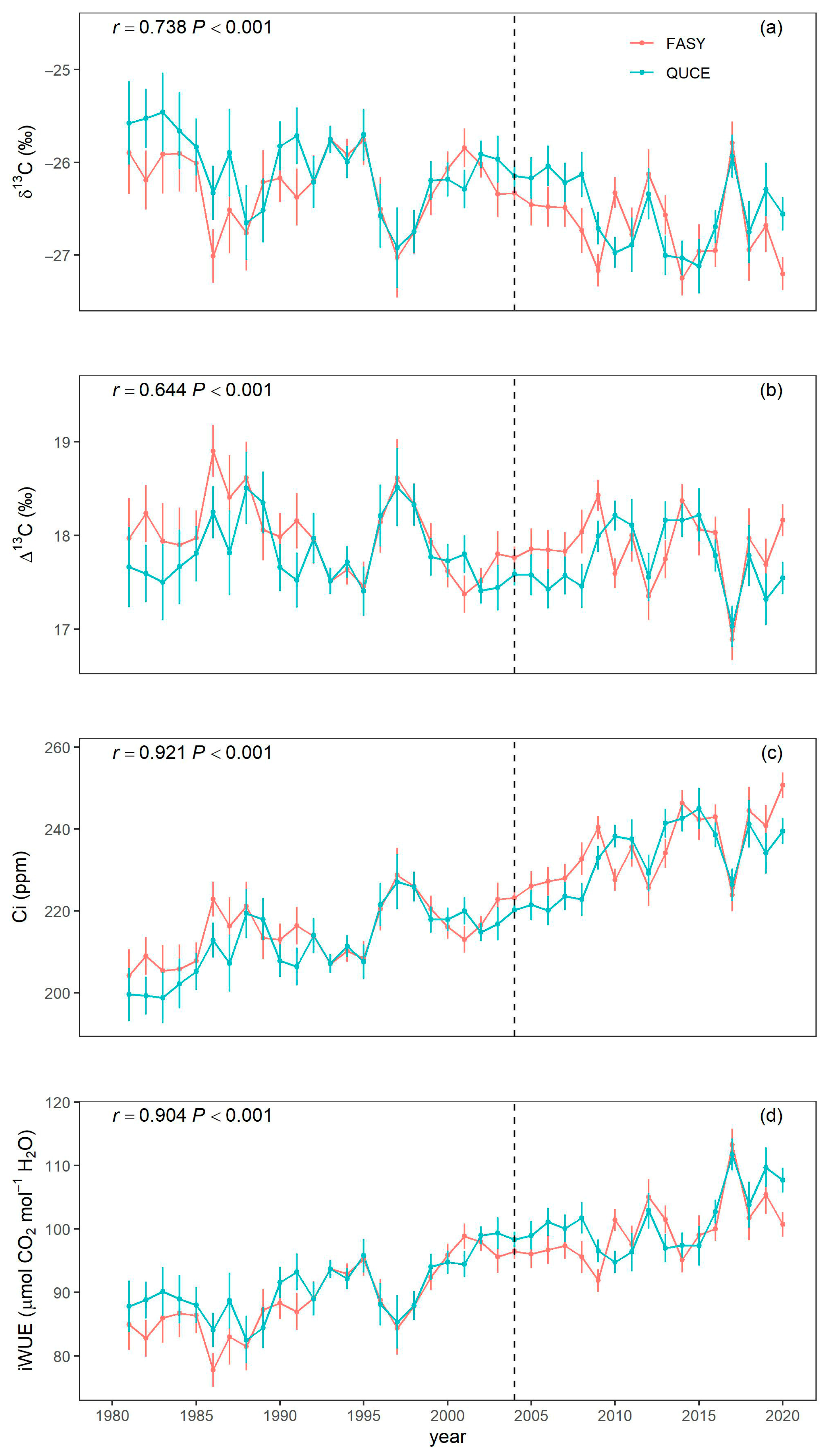
3.2. Isotope-Derived Parameter Temporal Dynamics and Relation to CO2 Increment
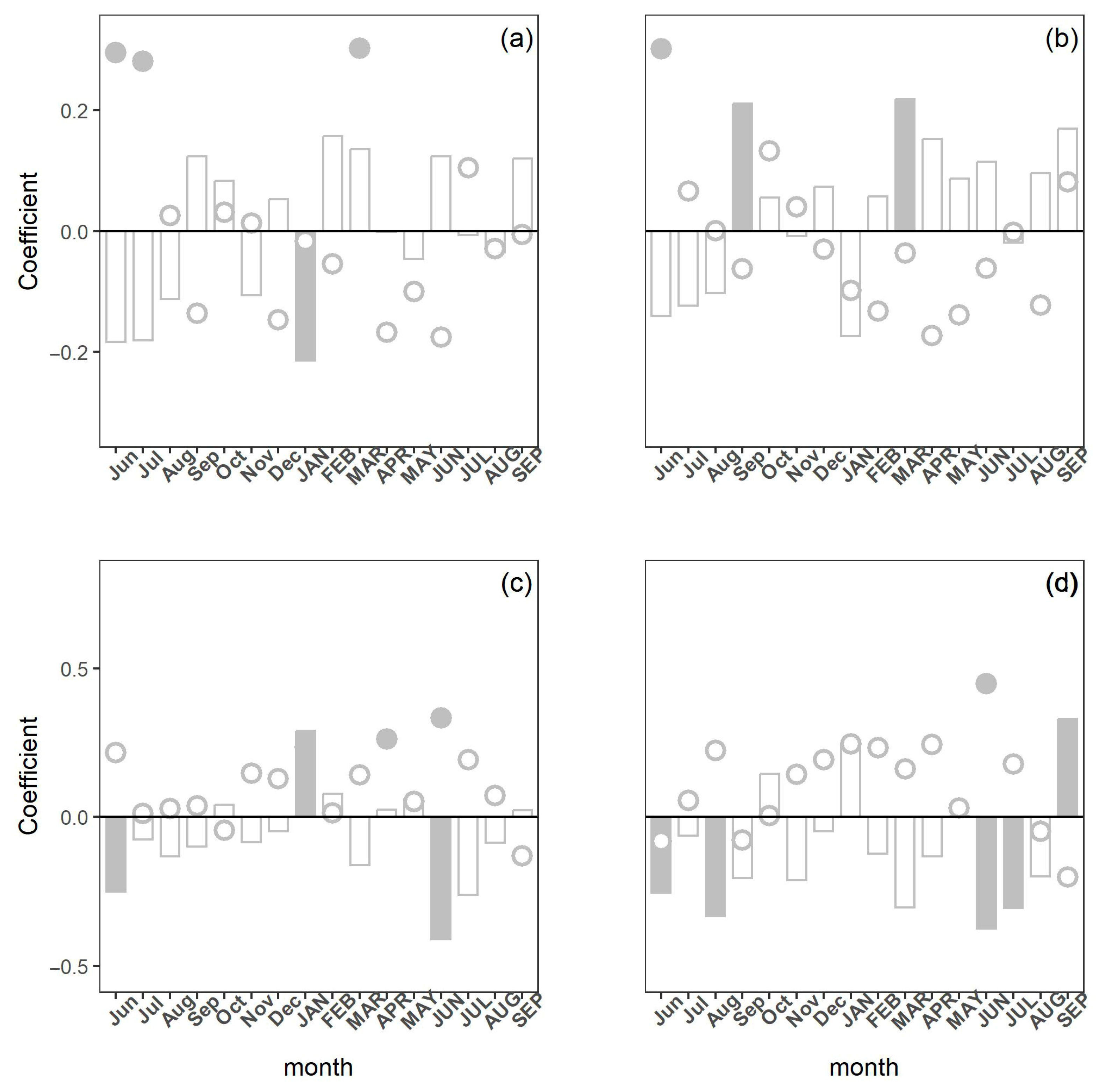
3.3. Climatic Influence on Tree Growth and Carbon Isotope Discrimination
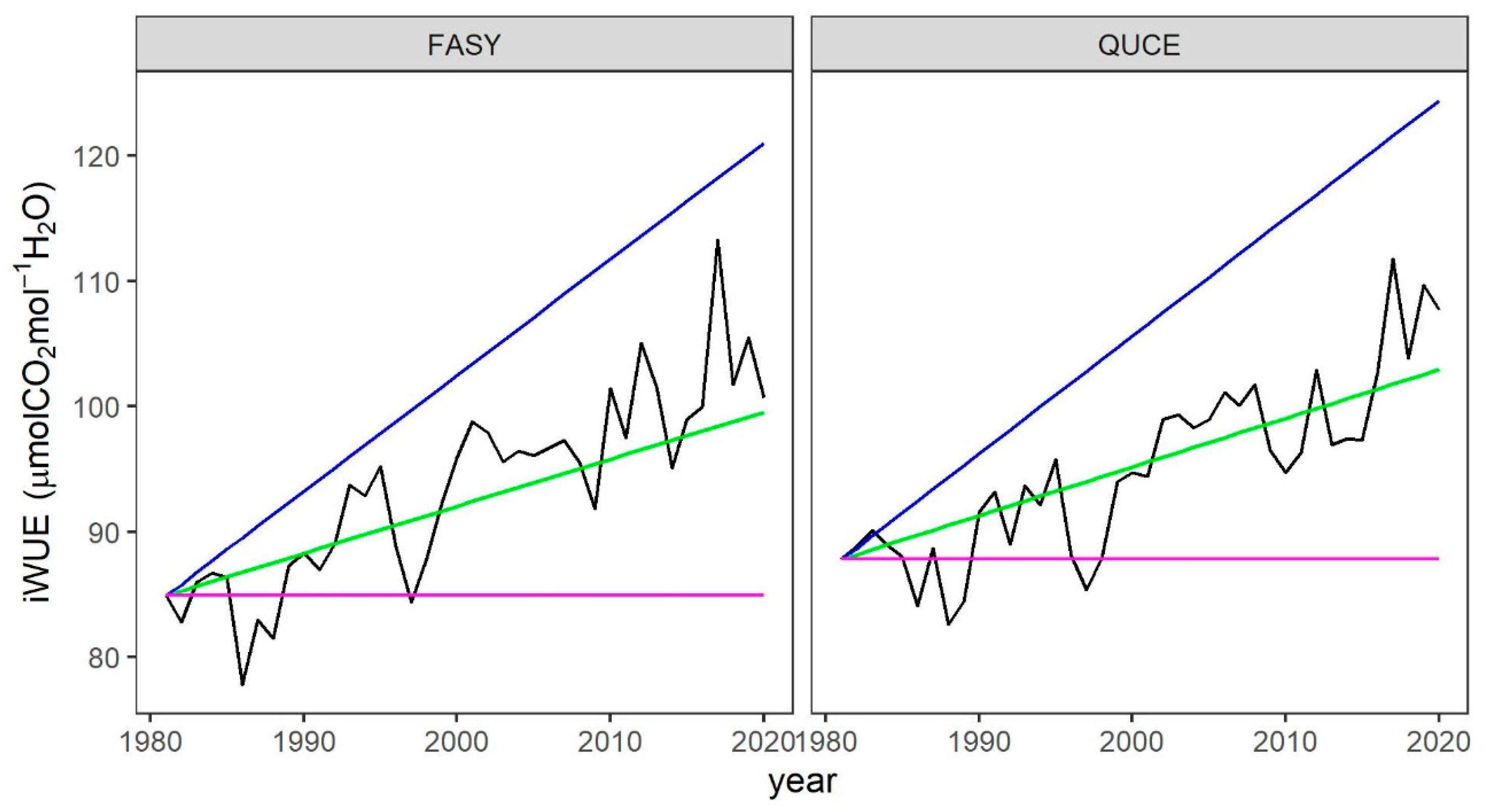
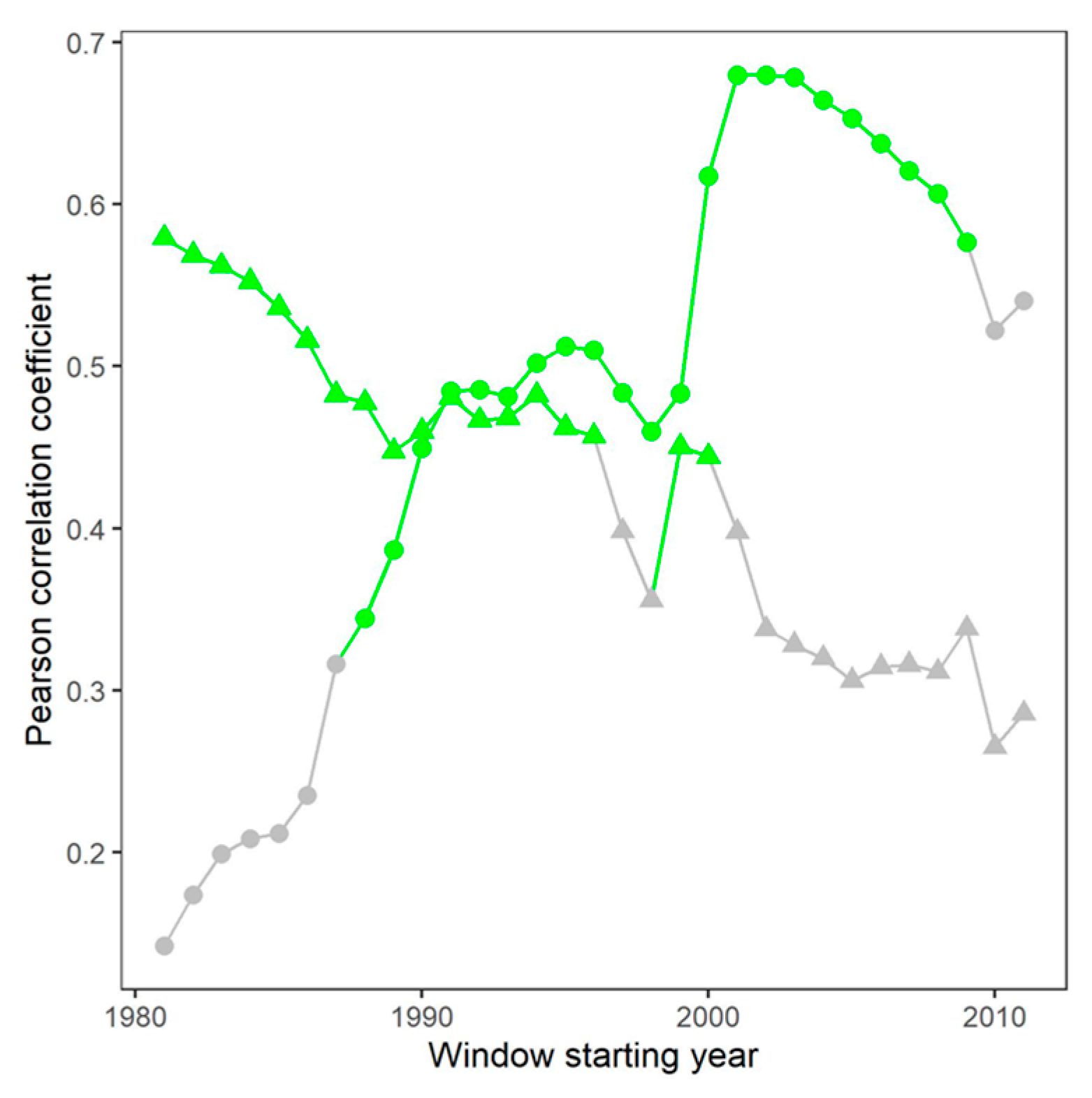
3.4. Interaction Between iWUE and Radial Growth
4. Discussion
4.1. Tree Growth and the Influence of Climatic Factors
4.2. Carbon Isotope Composition, Discrimination, Ci and iWUE
4.3. iWUE and Radial Growth
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Laurentis, D.; Papitto, G.; Cindolo, C.; Cocciufa, C.; Clementel, F.; Morelli, S.; Rizzo, M.; Zanotelli, S. Inventario Forestale Nazionale INFC2015; Carabinieri Command of Forestry, Environmental and Agri-Food Units Studies and Projects Office of the Carabinieri Command for the Protection of Biodiversity and Parks: Rome, Italy, 2015. [Google Scholar]
- Lados, B.B.; Benke, A.; Borovics, A.; Köbölkuti, Z.A.; Molnár, C.; Nagy, L.; Gy Tóth, E.; Cseke, K. What we know about Turkey oak (Quercus cerris L.)—from evolutionary history to species ecology. Forestry 2024, 97, 497–511. [Google Scholar] [CrossRef]
- Jump, A.S.; Hunt, J.M.; Penuelas, J. Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob. Change Biol. 2006, 12, 2163–2174. [Google Scholar] [CrossRef]
- Piovesan, G.; Biondi, F.; Di Filippo, A.; Alessandrini, A.; Maugeri, M. Drought-driven growth reduction in old beech (Fagus sylvatica L.) forests of the central Apennines, Italy. Glob. Change Biol. 2008, 14, 1265–1281. [Google Scholar] [CrossRef]
- Lindner, M.; Fitzgerald, J.; Zimmermann, N.; Reyer, C.; Delzon, S.; van der Maaten, E.; Schelhaas, M.; Lasch, P.; Eggers, J.; van der Maaten-Theunissen, M.; et al. Climate change and European forests: What do we know, what are the uncertainties, and what are the implications for forest management? J. Environ. Manag. 2014, 146, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Peuke, A.D.; Schraml, C.; Hartung, W.; Rennenberg, H. Identification of drought-sensitive beech ecotypes by physiological parameters. New Phytol. 2002, 154, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Fotelli, M.N.; Rennenberg, H.; Geßler, A. Effects of drought on the competitive interference of an early successional species (Rubus fruticosus) on Fagus sylvatica L. seedlings: 15N uptake and partitioning, responses of amino acids and other N compounds. Plant Biol. 2002, 4, 311–320. [Google Scholar] [CrossRef]
- Kostić, S.; Levanič, T.; Orlović, S.; Matović, B.; Stojanović, D.B. Turkey oak (Quercus cerris L.) is more drought tolerant and better reflects climate variations compared to pedunculate oak (Quercus robur L.) in lowland mixed forests in northwestern Serbia: A stable carbon isotope ratio (δ13C) and radial growth approach. Ecol. Indic. 2022, 142, 109242. [Google Scholar] [CrossRef]
- Móricz, N.; Illés, G.; Mészáros, I.; Garamszegi, B.; Berki, I.; Bakacsi, Z.; Kámpel, J.; Szabó, O.; Rasztovits, E.; Cseke, K.; et al. Different drought sensitivity traits of young sessile oak (Quercus petraea (Matt.) Liebl.) and Turkey oak (Quercus cerris L.) stands along a precipitation gradient in Hungary. For. Ecol. Manag. 2021, 492, 119165. [Google Scholar] [CrossRef]
- Gómez-Guerrero, A.; Doane, T. Chapter Seven—The Response of Forest Ecosystems to Climate Change. In Climate Change Impacts on Soil Processes and Ecosystem Properties; Horwath, W.R., Kuzyakov, Y., Eds.; Developments in Soil Science; Elsevier: Amsterdam, The Netherlands, 2018; Volume 35, pp. 185–206. [Google Scholar]
- Kasper, J.; Weigel, R.; Walentowski, H.; Gröning, A.; Petritan, A.M.; Leuschner, C. Climate warming-induced replacement of mesic beech by thermophilic oak forests will reduce the carbon storage potential in aboveground biomass and soil. Ann. For. Sci. 2021, 78, 89. [Google Scholar] [CrossRef]
- Jump, A.S.; Cavin, L.; Hunter, P.D. Monitoring and managing responses to climate change at the retreating range edge of forest trees. J. Environ. Monit. 2010, 12, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, A.; Biondi, F.; Čufar, K.; de Luis, M.; Grabner, M.; Maugeri, M.; Presutti Saba, E.; Schirone, B.; Piovesan, G. Bioclimatology of beech (Fagus sylvatica L.) in the Eastern Alps: Spatial and altitudinal climatic signals identified through a tree-ring network. J. Biogeogr. 2007, 34, 1873–1892. [Google Scholar] [CrossRef]
- Hartl-Meier, C.; Zang, C.; Büntgen, U.; Esper, J.; Rothe, A.; Göttlein, A.; Dirnböck, T.; Treydte, K. Uniform climate sensitivity in tree-ring stable isotopes across species and sites in a mid-latitude temperate forest. Tree Physiol. 2014, 35, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Dittmar, C.; Zech, W.; Elling, W. Growth variations of Common beech (Fagus sylvatica L.) under different climatic and environmental conditions in EuropeÐa dendroecological study. For. Ecol. Manag. 2003, 173, 63–78. [Google Scholar] [CrossRef]
- Cavin, L.; Jump, A.S. Highest drought sensitivity and lowest resistance to growth suppression are found in the range core of the tree Fagus sylvatica L. not the equatorial range edge. Glob. Change Biol. 2017, 23, 362–379. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, S. Structure and management of beech (Fagus sylvatica L.) forests in Italy. IForest 2009, 2, 105–113. [Google Scholar] [CrossRef]
- Piovesan, G.; Biondi, F.; Bernabei, M.; Di Filippo, A.; Schirone, B. Spatial and altitudinal bioclimatic zones of the Italian peninsula identified from a beech (Fagus sylvatica L.) tree-ring network. Acta Oecologica 2005, 27, 197–210. [Google Scholar] [CrossRef]
- Cavin, L.; Mountford, E.P.; Peterken, G.F.; Jump, A.S. Extreme drought alters competitive dominance within and between tree species in a mixed forest stand. Funct. Ecol. 2013, 27, 1424–1435. [Google Scholar] [CrossRef]
- Höhn, M.; Major, E.; Avdagic, A.; Bielak, K.; Bosela, M.; Coll, L.; Dinca, L.; Giammarchi, F.; Ibrahimspahic, A.; Mataruga, M.; et al. Local characteristics of the standing genetic diversity of european beech with high within-region differentiation at the eastern part of the range. Can. J. For. Res. 2021, 51, 1791–1798. [Google Scholar] [CrossRef]
- Rose, L.; Leuschner, C.; Köckemann, B.; Buschmann, H. Are marginal beech (Fagus sylvatica L.) provenances a source for drought tolerant ecotypes? Eur. J. For. Res. 2009, 128, 335–343. [Google Scholar] [CrossRef]
- Saurer, M.; Siegwolf, R.T.W.; Schweingruber, F.H. Carbon isotope discrimination indicates improving water-use efficiency of trees in northern Eurasia over the last 100 years. Glob. Change Biol. 2004, 10, 2109–2120. [Google Scholar] [CrossRef]
- Cherubini, P.; Battipaglia, G.; Innes, J.L. Tree Vitality and Forest Health: Can Tree-Ring Stable Isotopes Be Used as Indicators? Curr. For. Rep. 2021, 7, 69–80. [Google Scholar] [CrossRef]
- Geßler, A.; Ferrio, J.P.; Hommel, R.; Treydte, K.; Werner, R.A.; Monson, R.K. Stable isotopes in tree rings: Towards a mechanistic understanding of isotope fractionation and mixing processes from the leaves to the wood. Tree Physiol. 2014, 34, 796–818. [Google Scholar] [CrossRef] [PubMed]
- Mathias, J.M.; Thomas, R.B. Global tree intrinsic water use efficiency is enhanced by increased atmospheric CO2 and modulated by climate and plant functional types. Proc. Natl. Acad. Sci. USA 2021, 118, e2014286118. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, G.; De Angelis, P.; Brugnoli, E.; Cherubini, P.; Scarascia-Mugnozza, G. Tree-ring Δ13C reveals the impact of past forest management on water-use efficiency in a Mediterranean oak coppice in Tuscany (Italy). Ann. For. Sci. 2010, 67, 510. [Google Scholar] [CrossRef]
- McCarroll, D.; Gagen, M.H.; Loader, N.J.; Robertson, I.; Anchukaitis, K.J.; Los, S.; Young, G.H.F.; Jalkanen, R.; Kirchhefer, A.; Waterhouse, J.S. Correction of tree ring stable carbon isotope chronologies for changes in the carbon dioxide content of the atmosphere. Geochim. Cosmochim. Acta 2009, 73, 1539–1547. [Google Scholar] [CrossRef]
- Heilman, K.A.; Trouet, V.M.; Belmecheri, S.; Pederson, N.; Berke, M.A.; McLachlan, J.S. Increased water use efficiency leads to decreased precipitation sensitivity of tree growth, but is offset by high temperatures. Oecologia 2021, 197, 1095–1110. [Google Scholar] [CrossRef] [PubMed]
- Olano, J.M.; Sangüesa-Barreda, G.; García-López, M.A.; García-Hidalgo, M.; Rozas, V.; García-Cervigón, A.I.; Delgado-Huertas, A.; Hernández-Alonso, H. Water use efficiency and climate legacies dominate beech growth at its rear edge. J. Ecol. 2023, 111, 2160–2171. [Google Scholar] [CrossRef]
- Rezaie, N.; D’Andrea, E.; Bräuning, A.; Matteucci, G.; Bombi, P.; Lauteri, M. Do atmospheric CO2 concentration increase, climate and forest management affect iWUE of common beech? Evidences from carbon isotope analyses in tree rings. Tree Physiol. 2018, 38, 1110–1126. [Google Scholar] [CrossRef] [PubMed]
- Puchi, P.F.; Dalmonech, D.; Vangi, E.; Battipaglia, G.; Tognetti, R.; Collalti, A. Contrasting patterns of water use efficiency and annual radial growth among European beech forests along the Italian peninsula. Sci. Rep. 2024, 14, 6526. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Canadell, J.G.; Ogaya, R. Increased water-use efficiency during the 20th century did not translate into enhanced tree growth. Glob. Ecol. Biogeogr. 2011, 20, 597–608. [Google Scholar] [CrossRef]
- Levanič, T.; Čater, M.; McDowell, N.G. Associations between growth, wood anatomy, carbon isotope discrimination and mortality in a Quercus robur forest. Tree Physiol. 2011, 31, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Linares, J.C.; Camarero, J.J. From pattern to process: Linking intrinsic water-use efficiency to drought-induced forest decline. Glob. Change Biol. 2012, 18, 1000–1015. [Google Scholar] [CrossRef]
- Canham, C.D.; Papaik, M.J.; Uriarte, M.; McWilliams, W.H.; Jenkins, J.C.; Twery, M.J. Neighborhood analyses of canopy tree competition along environmental gradients in New England forests. Ecol. Appl. 2006, 16, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, L.; Xu, H.; Creed, I.F.; Blanco, J.A.; Wei, X.; Sun, G.; Asbjornsen, H.; Bishop, K. Forest water-use efficiency: Effects of climate change and management on the coupling of carbon and water processes. For. Ecol. Manag. 2023, 534, 120853. [Google Scholar] [CrossRef]
- Verdonck, S.; Geussens, A.; Zweifel, R.; Thomaes, A.; Van Meerbeek, K.; Muys, B. Mitigating drought stress in European beech and pedunculate oak: The role of competition reduction. For. Ecosyst. 2025, 13, 100303. [Google Scholar] [CrossRef]
- Urrutia-jalabert, R.; Malhi, Y.; Barichivich, J.; Lara, A.; Delgado-huertas, A.; Rodríguez, C.G.; Cuq, E. Increased water use efficiency but contrasting tree growth patterns in Fitzroya cupressoides forests of southern Chile during recent decades. J. Geophys. Res. Biogeosci. 2015, 120, 2505–2524. [Google Scholar] [CrossRef]
- Voelker, S.L.; Meinzer, F.C.; Lachenbruch, B.; Brooks, J.R.; Guyette, R.P. Drivers of radial growth and carbon isotope discrimination of bur oak (Quercus macrocarpa Michx.) across continental gradients in precipitation, vapour pressure deficit and irradiance. Plant. Cell Environ. 2014, 37, 766–779. [Google Scholar] [CrossRef] [PubMed]
- Marchi, M.; Bucci, G.; Iovieno, P.; Ray, D. ClimateDT: A Global Scale-Free Dynamic Downscaling Portal for Historic and Future Climate Data. Environ. 2024, 11, 82. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Phipps, R.L.; Whiton, J.C. Decline in long-term growth trends of white oak. Can. J. For. Res. 1988, 18, 24–32. [Google Scholar] [CrossRef]
- Buras, A.; Wilmking, M. Correcting the calculation of Gleichläufigkeit. Dendrochronologia 2015, 34, 29–30. [Google Scholar] [CrossRef]
- Eckstein, D.; Bauch, J. Beitrag zur Rationalisierung eines dendrochronologischen Verfahrens und zur Analyse seiner Aussagesicherheit. Forstwiss. Cent. 1969, 88, 230–250. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Xu, G.; Zeng, X.; Wu, G.; Zhang, X.; Qin, D. Tree growth and intrinsic water-use efficiency of inland riparian forests in northwestern China: Evaluation via δ13C and δ18O analysis of tree rings. Tree Physiol. 2014, 34, 966–980. [Google Scholar] [CrossRef] [PubMed]
- Portarena, S.; Farinelli, D.; Famiani, F.; Cinosi, N.; Traini, C.; Rezaei, N.; Brugnoli, E. Differential tolerance to summer stress conditions in two olive cultivars using the dendro-isotopic approach. Dendrochronologia 2024, 84, 126182. [Google Scholar] [CrossRef]
- Farquhar, G.D.; O’Leary, M.H.; Berry, J.A. On the Relationship between Carbon Isotope Discrimination and Intercellular Carbon Dioxide Concentration in Leaves. Aust. J. Plant Physiol. 1982, 9, 121–137. [Google Scholar] [CrossRef]
- Seibt, U.; Rajabi, A.; Griffiths, H.; Berry, J.A. Carbon isotopes and water use efficiency: Sense and sensitivity. Oecologia 2008, 155, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.C.; Poulter, B.; Saurer, M.; Esper, J.; Huntingford, C.; Helle, G.; Treydte, K.; Zimmermann, N.E.; Schleser, G.H.; Ahlström, A.; et al. Water-use efficiency and transpiration across European forests during the Anthropocene. Nat. Clim. Chang. 2015, 5, 579–583. [Google Scholar] [CrossRef]
- Zang, C.; Biondi, F. treeclim: An R package for the numerical calibration of proxy-climate relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Mazza, G.; Monteverdi, M.C.; Altieri, S.; Battipaglia, G. Climate-driven growth dynamics and trend reversal of Fagus sylvatica L. and Quercus cerris L. in a low-elevation beech forest in Central Italy. Sci. Total Environ. 2024, 908, 168250. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, A.; Biondi, F.; Maugeri, M.; Schirone, B.; Piovesan, G. Bioclimate and growth history affect beech lifespan in the Italian Alps and Apennines. Glob. Change Biol. 2012, 18, 960–972. [Google Scholar] [CrossRef]
- Martinez del Castillo, E.; Zang, C.S.; Buras, A.; Hacket-Pain, A.; Esper, J.; Serrano-Notivoli, R.; Hartl, C.; Weigel, R.; Klesse, S.; Resco de Dios, V.; et al. Climate-change-driven growth decline of European beech forests. Commun. Biol. 2022, 5, 163. [Google Scholar] [CrossRef] [PubMed]
- Buonincontri, M.P.; Bosso, L.; Smeraldo, S.; Chiusano, M.L.; Pasta, S.; Di Pasquale, G. Shedding light on the effects of climate and anthropogenic pressures on the disappearance of Fagus sylvatica in the Italian lowlands: Evidence from archaeo-anthracology and spatial analyses. Sci. Total Environ. 2023, 877, 162893. [Google Scholar] [CrossRef] [PubMed]
- Magri, D. Patterns of post-glacial spread and the extent of glacial refugia of European beech (Fagus sylvatica). J. Biogeogr. 2008, 35, 450–463. [Google Scholar] [CrossRef]
- Magri, D.; Vendramin, G.G.; Comps, B.; Dupanloup, I.; Geburek, T.; Gömöry, D.; Latałowa, M.; Litt, T.; Paule, L.; Roure, J.M.; et al. A new scenario for the Quaternary history of European beech populations: Palaeobotanical evidence and genetic consequences, A new scenario for the Quaternary history of European beech populations: Palaeobotanical evidence and genetic consequences. New Phytol. 2006, 171, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Čufar, K.; Prislan, P.; De Luis, M.; Gričar, J. Tree-ring variation, wood formation and phenology of beech (Fagus sylvatica) from a representative site in Slovenia, SE Central Europe. Trees-Struct. Funct. 2008, 22, 749–758. [Google Scholar] [CrossRef]
- D’Andrea, E.; Rezaie, N.; Prislan, P.; Gričar, J.; Collalti, A.; Muhr, J.; Matteucci, G. Frost and drought: Effects of extreme weather events on stem carbon dynamics in a Mediterranean beech forest. Plant Cell Environ. 2020, 43, 2365–2379. [Google Scholar] [CrossRef] [PubMed]
- Scartazza, A.; Sbrana, C.; D’Andrea, E.; Matteucci, G.; Rezaie, N.; Lauteri, M. Above-and belowground interplay: Canopy CO2 uptake, carbon and nitrogen allocation and isotope fractionation along the plant-ectomycorrhiza continuum. Plant Cell Environ. 2023, 46, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Zwieniecki, M.A.; Tixier, A.; Sperling, O. Temperature-assisted redistribution of carbohydrates in trees. Am. J. Bot. 2015, 102, 1216–1218. [Google Scholar] [CrossRef] [PubMed]
- Móricz, N.; Mészáros, I.; Illés, G.Z.; Garamszegi, B.; Eötvös, C.B.; Kern, A.; Hirka, A.; Berki, I.; Borovics, A.; Hollós, R.; et al. Radial growth projections reveal site-specific futures of different oak species with contrasting water availability in SW Hungary. Front. For. Glob. Chang. 2025, 8, 1581222. [Google Scholar] [CrossRef]
- Zweifel, R.; Zimmermann, L.; Zeugin, F.; Newbery, D.M. Intra-annual radial growth and water relations of trees: Implications towards a growth mechanism. J. Exp. Bot. 2006, 57, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Sass-Klaassen, U.; Sabajo, C.R.; den Ouden, J. Vessel formation in relation to leaf phenology in pedunculate oak and European ash. Dendrochronologia 2011, 29, 171–175. [Google Scholar] [CrossRef]
- Michelot, A.; Simard, S.; Rathgeber, C.; Dufrêne, E.; Damesin, C. Comparing the intra-annual wood formation of three European species (Fagus sylvatica, Quercus petraea and Pinus sylvestris) as related to leaf phenology and non-structural carbohydrate dynamics. Tree Physiol. 2012, 32, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Walde, M.G.; Lehmann, M.M.; Gessler, A.; Vitasse, Y.; Diao, H. Stable Isotope Labelling Reveals Water and Carbon Fluxes in Temperate Tree Saplings Before Budbreak. Plant Cell Environ. 2024, 48, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Trumbore, S.; Czimczik, C.I.; Sierra, C.A.; Muhr, J.; Xu, X. Non-structural carbon dynamics and allocation relate to growth rate and leaf habit in California oaks. Tree Physiol. 2015, 35, 1206–1222. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, E.; Rezaie, N.; Battistelli, A.; Gavrichkova, O.; Kuhlmann, I.; Matteucci, G.; Moscatello, S.; Proietti, S.; Scartazza, A.; Trumbore, S.; et al. Winter’s bite: Beech trees survive complete defoliation due to spring late-frost damage by mobilizing old C reserves. New Phytol. 2019, 224, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.K.; Scherrer, D.; Camarero, J.J.; Ziche, D.; Babst, F.; Bigler, C.; Bolte, A.; Dorado-Liñán, I.; Etzold, S.; Fonti, P.; et al. Climate sensitivity and drought seasonality determine post-drought growth recovery of Quercus petraea and Quercus robur in Europe. Sci. Total Environ. 2021, 784, 147222. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, R.; Deslauriers, A.; Prislan, P.; Rademacher, T.; Rezaie, N.; Richardson, A.D.; Vitasse, Y.; Rossi, S. From Roots to Leaves: Tree Growth Phenology in Forest Ecosystems. Curr. For. Rep. 2025, 11, 12. [Google Scholar] [CrossRef]
- Belmecheri, S.; Lavergne, A. Compiled records of atmospheric CO2 concentrations and stable carbon isotopes to reconstruct climate and derive plant ecophysiological indices from tree rings. Dendrochronologia 2020, 63, 125748. [Google Scholar] [CrossRef]
- Duquesnay, A.; Bréda, N.; Stievenard, M.; Dupouey, J.L. Changes of tree-ring δ13C and water-use efficiency of beech (Fagus sylvatica L.) in north-eastern France during the past century. Plant Cell Environ. 1998, 21, 565–572. [Google Scholar] [CrossRef]
- Raffalli-Delerce, G.; Masson-Delmotte, V.; Dupouey, J.L.; Stievenard, M.; Breda, N.; Moisselin, J.M. Reconstruction of summer droughts using tree-ring cellulose isotopes: A calibration study with living oaks from Brittany (western France). Tellus B Chem. Phys. Meteorol. 2004, 56, 160–174. [Google Scholar] [CrossRef]
- Treydte, K.S.; Frank, D.C.; Saurer, M.; Helle, G.; Schleser, G.H.; Esper, J. Impact of climate and CO2 on a millennium-long tree-ring carbon isotope record. Geochim. Cosmochim. Acta 2009, 73, 4635–4647. [Google Scholar] [CrossRef]
- Saurer, M.; Spahni, R.; Frank, D.C.; Joos, F.; Leuenberger, M.; Loader, N.J.; Mccarroll, D.; Gagen, M.; Poulter, B.E.N.; Siegwolf, R.T.W.; et al. Spatial variability and temporal trends in water-use efficiency of European forests. Glob. Change Biol. 2014, 20, 3700–3712. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; Buckley, T.N.; Turnbull, T.L. Diminishing CO2-driven gains in water-use efficiency of global forests. Nat. Clim. Chang. 2020, 10, 466–471. [Google Scholar] [CrossRef]
- Giammarchi, F.; Cherubini, P.; Pretzsch, H.; Tonon, G. The increase of atmospheric CO2 affects growth potential and intrinsic water-use efficiency of Norway spruce forests: Insights from a multi-stable isotope analysis in tree rings of two Alpine chronosequences. Trees-Struct. Funct. 2017, 31, 503–515. [Google Scholar] [CrossRef]
- Tognetti, R.; Lombardi, F.; Lasserre, B.; Cherubini, P.; Marchetti, M. Tree-Ring Stable Isotopes Reveal Twentieth-Century Increases in Water-Use Efficiency of Fagus sylvatica and Nothofagus spp. in Italian and Chilean Mountains. PLoS ONE 2014, 9, e113136. [Google Scholar] [CrossRef] [PubMed]
- Soh, W.K.; Yiotis, C.; Murray, M.; Parnell, A.; Wright, I.J.; Spicer, R.A.; Lawson, T.; Caballero, R.; McElwain, J.C. Rising CO2 drives divergence in water use efficiency of evergreen and deciduous plants. Sci. Adv. 2019, 5, eaax7906. [Google Scholar] [CrossRef] [PubMed]
- Dorado-Liñán, I.; Valbuena-Carabaña, M.; Cañellas, I.; Gil, L.; Gea-Izquierdo, G. Climate Change Synchronizes Growth and iWUE Across Species in a Temperate-Submediterranean Mixed Oak Forest. Front. Plant Sci. 2020, 11, 706. [Google Scholar] [CrossRef] [PubMed]
- Shestakova, T.A.; Voltas, J.; Saurer, M.; Berninger, F.; Esper, J.; Andreu-Hayles, L.; Daux, V.; Helle, G.; Leuenberger, M.; Loader, N.J.; et al. Spatio-temporal patterns of tree growth as related to carbon isotope fractionation in European forests under changing climate. Glob. Ecol. Biogeogr. 2019, 28, 1295–1309. [Google Scholar] [CrossRef]
- Santruckova, H.; Šantrůček, J.; Šetlík, J.; Svoboda, M.; Kopáček, J. Carbon isotopes in tree rings of Norway spruce exposed to atmospheric pollution. Environ. Sci. Technol. 2007, 41, 5778–5782. [Google Scholar] [CrossRef] [PubMed]
- Michelot-Antalik, A.; Granda, E.; Fresneau, C.; Damesin, C. Evidence of a seasonal trade-off between growth and starch storage in declining beeches: Assessment through stem radial increment, non-structural carbohydrates and intra-ring δ13C. Tree Physiol. 2019, 39, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, B.; Alberti, G.; Maltoni, A.; Tani, A.; Piussi, P. Beech coppice conversion to high forest: Results from a 31-year experiment in Eastern Pre-Alps. Ann. For. Sci. 2017, 74, 44. [Google Scholar] [CrossRef]
- Notarangelo, M.; La Marca, O. Growth analysis of an aged Turkey oak coppice under conversion into high forest. Ann. Silvic. Res. 2021, 46, 84–92. [Google Scholar] [CrossRef]
- Merlin, M.; Perot, T.; Perret, S.; Korboulewsky, N.; Vallet, P. Effects of stand composition and tree size on resistance and resilience to drought in sessile oak and Scots pine. For. Ecol. Manag. 2015, 339, 22–33. [Google Scholar] [CrossRef]
- Bayer, D.; Pretzsch, H. Reactions to gap emergence: Norway spruce increases growth while european beech features horizontal space occupation–evidence by repeated 3D TLS measurements. Silva Fenn. 2017, 51, 7748. [Google Scholar] [CrossRef]
- Scartazza, A.; Di Baccio, D.; Bertolotto, P.; Gavrichkova, O.; Matteucci, G. Investigating the European beech (Fagus sylvatica L.) leaf characteristics along the vertical canopy profile: Leaf structure, photosynthetic capacity, light energy dissipation and photoprotection mechanisms. Tree Physiol. 2016, 36, 1060. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, F.; Pelleri, F.; Manetti, M.C.; Sansone, D.; Battipaglia, G. Effects of thinning intensity on productivity and water use efficiency of Quercus robur L. For. Ecol. Manag. 2020, 473, 118282. [Google Scholar] [CrossRef]
| Stands | Basal Area (m2 ha−1) | Tree Density (n ha−1) | Mean Tree Diameter (cm) |
|---|---|---|---|
| Quercus cerris | 46 | 480 | 34.9 |
| Fagus sylvatica | 47 | 428 | 36.9 |
| Species | N | DBH (cm) | Tree Age (Year) | Mean TRW (mm) | EPS | GLK Sel | DBH Iso (cm) |
|---|---|---|---|---|---|---|---|
| Quercus cerris | 32 | 42 ± 3 | 61 ± 3 | 3.26 ± 0.32 | 0.891 | 66–84 | 39.5 ± 4.1 |
| Fagus sylvatica | 13 | 45 ± 2 | 60 ± 2 | 3.33 ± 0.24 | 0.866 | 63–82 | 50.1 ± 3.1 |
| Quercus cerris | Fagus sylvatica | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Mean | SD | Min | Max | Mean | SD | Min | Max |
| δ13C (‰) | −26.2 | 0.5 | −27.1 | −25.5 | −26.4 | 0.8 | −27.3 | −25.7 |
| Δ13C (‰) | 17.8 | 0.4 | 17.0 | 18.5 | 17.9 | 0.8 | 16.8 | 18.9 |
| Ci (ppm) | 220.9 | 13.2 | 198.8 | 245.0 | 223.3 | 12.7 | 204.7 | 250.7 |
| iWUE (mol mol−1H2O) | 95.0 | 7.0 | 82.6 | 111.7 | 93.5 | 7.5 | 77.8 | 113.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezaie, N.; D’Andrea, E.; Ciolfi, M.; Brugnoli, E.; Portarena, S. Growth and Water-Use Efficiency of European Beech and Turkey Oak at Low-Elevation Site. Forests 2025, 16, 1210. https://doi.org/10.3390/f16081210
Rezaie N, D’Andrea E, Ciolfi M, Brugnoli E, Portarena S. Growth and Water-Use Efficiency of European Beech and Turkey Oak at Low-Elevation Site. Forests. 2025; 16(8):1210. https://doi.org/10.3390/f16081210
Chicago/Turabian StyleRezaie, Negar, Ettore D’Andrea, Marco Ciolfi, Enrico Brugnoli, and Silvia Portarena. 2025. "Growth and Water-Use Efficiency of European Beech and Turkey Oak at Low-Elevation Site" Forests 16, no. 8: 1210. https://doi.org/10.3390/f16081210
APA StyleRezaie, N., D’Andrea, E., Ciolfi, M., Brugnoli, E., & Portarena, S. (2025). Growth and Water-Use Efficiency of European Beech and Turkey Oak at Low-Elevation Site. Forests, 16(8), 1210. https://doi.org/10.3390/f16081210






