Leaf Chemistry Patterns in Populations of a Key Lithophyte Tree Species in Brazil’s Atlantic Forest Inselbergs
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Characteristics of the Soil and the Species Studied
2.3. Tree Selection and Leaf Collection
2.4. Chemical and Isotopic Analyses of the Leaves
2.5. Nutritional Relationships and Nutrient Retranslocation
2.6. Statistical Analysis
3. Results
3.1. SLA, Soil, and Leaf Chemistry Relations
3.2. Carbon and Nutrients in Leaves
3.3. Stoichiometric Relationships
3.4. Natural Abundance of δ13C and δ15N
3.5. Nutrient Retranslocation Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | Green Leaves | Senescent Leaves | ||||||
|---|---|---|---|---|---|---|---|---|
| TI | PA | SA | All | TI | PA | SA | All | |
| C | 0.52 | 0.60 | 0.13 | 0.06 | −0.15 | 0.10 | 0.17 | 0.02 |
| N | 0.20 | −0.42 | 0.57 | 0.16 | −0.12 | 0.08 | −0.02 | 0.20 |
| P | 0.68 * | −0.40 | 0.82 * | 0.33 | 0.00 | 0.13 | −0.20 | 0.07 |
| K | 0.05 | 0.05 | 0.32 | 0.52 * | −0.02 | −0.42 | −0.43 | 0.22 |
| Ca | −0.30 | −0.22 | −0.22 | −0.20 | −0.13 | −0.67 | 0.50 | 0.03 |
| Mg | −0.27 | −0.17 | −0.27 | 0.26 | −0.50 | −0.38 | −0.27 | 0.12 |
| Mn | −0.05 | −0.02 | −0.48 | −0.08 | 0.05 | −0.23 | 0.50 | 0.13 |
| δ13C | −0.45 | 0.27 | −0.10 | 0.10 | 0.20 | 0.02 | −0.32 | 0.26 |
| δ15N | 0.25 | −0.30 | −0.40 | −0.05 | 0.00 | −0.15 | 0.02 | −0.01 |
References
- Porembski, S.; Barthlott, W. Granitic and gneissic outcrops (inselbergs) as centers of diversity for desiccation-tolerant vascular plants. Plant Ecol. 2000, 151, 19–28. [Google Scholar] [CrossRef]
- de Paula, L.F.; Forzza, R.C.; Neri, A.V.; Bueno, M.L.; Porembski, S. Sugar Loaf Land in southeastern Brazil: A centre of diversity for mat-forming bromeliads on inselbergs. Bot. J. Linn. Soc. 2016, 181, 459–476. [Google Scholar] [CrossRef]
- Couto, D.R.; Porembski, S.; Barthlott, W.; de Paula, L.F. Hyperepilithics—An overlooked life form of vascular plants on tropical vertical rock walls. Austral Ecol. 2023, 48, 1074–1082. [Google Scholar] [CrossRef]
- Couto, D.R.; Francisco, T.M.; De Paula, L.F.; Paula, R.R.; Nascimento, M.T. Woody vegetation on tropical inselbergs: Floristic-structural characterization and aboveground carbon storage. J. Mt. Sci. 2025, 22, 1517–1534. [Google Scholar] [CrossRef]
- Francisco, T.M.; Couto, D.R.; Moreira, M.M.; Fontana, A.P.; De Fraga, C.N. Inselbergs from Brazilian Atlantic Forest: High biodiversity refuges of vascular epiphytes from Espirito Santo. Biodivers. Conserv. 2023, 32, 2561–2584. [Google Scholar] [CrossRef]
- Porembski, S. Tropical inselbergs: Habitat types, adaptive strategies and diversity patterns. Braz. J. Bot. 2007, 30, 579–586. [Google Scholar] [CrossRef]
- Scarano, F.R. Plant communities at the periphery of the Atlantic rain forest: Rare-species bias and its risks for conservation. Biol. Conserv. 2009, 142, 1201–1208. [Google Scholar] [CrossRef]
- Francisco, T.M.; Couto, D.R.; Evans, D.M.; Garbin, M.L.; Ruiz-Miranda, C.R. Structure and robustness of an epiphyte–phorophyte commensalistic network in a neotropical inselberg. Austral Ecol. 2018, 43, 903–914. [Google Scholar] [CrossRef]
- Couto, D.R.; Francisco, T.M.; Nascimento, M.T. Commensalistic epiphyte–phorophyte networks in woody vegetation of tropical inselbergs: Patterns of organization and structure. Austral Ecol. 2022, 47, 911–927. [Google Scholar] [CrossRef]
- Couto, D.R.; Dias, H.M.; Pereira, M.C.A.; Fraga, C.N.D.; Pezzopane, J.E.M. Vascular epiphytes on Pseudobombax (Malvaceae) in rocky outcrops (inselbergs) in Brazilian Atlantic Rainforest: Basis for conservation of a threatened ecosystem. Rodriguésia 2016, 67, 583–601. [Google Scholar] [CrossRef]
- Couto, D.R.; Francisco, T.M.; Garbin, M.L.; Dias, H.M.; Pereira, M.C.A.; Neto, L.M.; Pezzopane, J.E.M. Surface roots as a new ecological zone for occurrence of vascular epiphytes: A case study on Pseudobombax trees on inselbergs. Plant Ecol. 2019, 220, 1071–1084. [Google Scholar] [CrossRef]
- Benites, V.M.; Schaefer, C.E.G.; Simas, F.N.; Santos, H.G. Soils associated with rock outcrops in the Brazilian mountain ranges Mantiqueira and Espinhaço. Braz. J. Bot. 2007, 30, 569–577. [Google Scholar] [CrossRef]
- Larson, D.W.; Matthes, U.; Kelly, P.E. Cliff Ecology: Pattern and Process in Cliff Ecosystems, 1st ed.; Cambridge University Press: New York, NY, USA, 2000; 340p. [Google Scholar]
- Scarano, F.R. Structure, function and floristic relationships of plant communities in stressful habitats marginal to the Brazilian Atlantic rainforest. Ann. Bot. 2002, 90, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Monge, M.; Fontana, A.P.; de Fraga, C.N.; Kollmann, L.J.C.; Nakajima, J.N. Cololobus ruschianus (Vernonieae, Asteraceae), a threatened narrow endemic species from Atlantic Forest inselbergs, Espírito Santo, Brazil. Syst. Bot. 2021, 46, 1114–1120. [Google Scholar] [CrossRef]
- Vaughn, K.J.; Porensky, L.M.; Wilkerson, M.L.; Balachowski, J.; Peffer, E.; Riginos, C.; Young, T.P. Restoration Ecology. Nat. Educ. Knowl. 2010, 3, 66. [Google Scholar]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere, 1st ed.; Princeton University Press: Princeton, NJ, USA, 2017; 439p. [Google Scholar]
- Reich, P.B. The world-wide ‘fast–slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Carvalho-Sobrinho, J.G.; Yoshikawa, V.N. Pseudobombax in Flora e Funga do Brasil, Jardim Botânico do Rio de Janeiro. 2025. Available online: https://floradobrasil.jbrj.gov.br/FB9193 (accessed on 25 May 2025).
- Zanne, A.E.; Lopez-Gonzalez, G.; Coomes, D.A.; Ilic, J.; Jansen, S.; Lewis, S.L.; Miller, R.B.; Swenson, N.G.; Wiemann, M.C.; Chave, J. Global Wood Density Database. Dryad. 2025. Available online: https://datadryad.org/dataset/doi:10.5061/dryad.234 (accessed on 20 March 2025).
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- Paula, R.R.; Guillemot, J.; Delarmelina, W.M.; de Souza, P.H.; de Moraes, C.R.; Campanharo, I.F.; Mendes, L.J.; Trivelin, P.C.O.; Klippel, V.H.; Trazzi, P.A.; et al. Exploring the forestry potential of two legume species with contrasting ecological strategies in a seasonally dry tropical region. Trees 2022, 36, 1413–1424. [Google Scholar] [CrossRef]
- He, M.; Yan, Z.; Cui, X.; Gong, Y.; Li, K.; Han, W. Scaling the leaf nutrient resorption efficiency: Nitrogen vs phosphorus in global plants. Sci. Total Environ. 2020, 729, 138920. [Google Scholar] [CrossRef]
- Querejeta, J.I.; Prieto, I.; Armas, C.; Casanoves, F.; Diémé, J.S.; Diouf, M.; Yossi, H.; Kaya, B.; Pugnaire, F.I.; Rusch, G.M. Higher leaf nitrogen content is linked to tighter stomatal regulation of transpiration and more efficient water use across dryland trees. New Phytol. 2022, 235, 1351–1364. [Google Scholar] [CrossRef]
- Malavolta, E. Manual de Nutrição Mineral de Plantas, 1st ed.; Editora Agronômica Ceres: Piracicaba, SP, Brazil, 2006; 638p. [Google Scholar]
- Handley, L.L.; Raven, J.A. The use of natural abundance of nitrogen isotopes in plant physiology and ecology. Plant Cell Environ. 1992, 15, 965–985. [Google Scholar] [CrossRef]
- Craine, J.M.; Elmore, A.J.; Aidar, M.P.; Bustamante, M.; Dawson, T.E.; Hobbie, E.A.; Kahmen, A.; Mack, M.C.; McLauchlan, K.K.; Michelsen, A.; et al. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol. 2009, 183, 980–992. [Google Scholar] [CrossRef] [PubMed]
- Hobbie, E.A.; Högberg, P. Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New Phytol. 2012, 196, 367–382. [Google Scholar] [CrossRef]
- Espindola, J.A.A.; Guerra, J.G.M.; Almeida, D.L.D.; Teixeira, M.G.; Urquiaga, S. Decomposição e liberação de nutrientes acumulados em leguminosas herbáceas perenes consorciadas com bananeira. Rev. Bras. Ciênc. Solo 2006, 30, 321–328. [Google Scholar] [CrossRef]
- Bachega, L.R.; Bouillet, J.P.; de Cássia Piccolo, M.; Saint-André, L.; Bouvet, J.M.; Nouvellon, Y.; Gonçalves, J.L.M.; Robin, A.; Laclau, J.P. Decomposition of Eucalyptus grandis and Acacia mangium leaves and fine roots in tropical conditions did not meet the Home Field Advantage hypothesis. For. Ecol. Manag. 2016, 359, 33–43. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Seibt, U.; Rajabi, A.; Griffiths, H.; Berry, J.A. Carbon isotopes and water use efficiency: Sense and sensitivity. Oecologia 2008, 155, 441–454. [Google Scholar] [CrossRef]
- Wang, W.; Pataki, D.E. Spatial patterns of plant isotope tracers in the Los Angeles urban region. Landsc. Ecol. 2010, 25, 35–52. [Google Scholar] [CrossRef]
- Brugnoli, E.; Farquhar, G.D. Photosynthetic Fractionation of Carbon Isotopes. In Photosynthesis. Advances in Photosynthesis and Respiration, 1st ed.; Springer: Dordrecht, The Netherlands, 2000; pp. 399–434. [Google Scholar] [CrossRef]
- Ma, W.T.; Yu, Y.Z.; Wang, X.; Gong, X.Y. Estimation of intrinsic water-use efficiency from δ13C signature of C3 leaves: Assumptions and uncertainty. Front. Plant Sci. 2023, 13, 1037972. [Google Scholar] [CrossRef]
- Oliveira-Filho, A.T.; Tameirão-Neto, E.; Carvalho, W.A.; Werneck, M.; Brina, A.E.; Vidal, C.V.; Rezende, S.C.; Pereira, J.A.A. Análise florística do compartimento arbóreo de áreas de Floresta Atlântica sensu lato na região das Bacias do Leste (Bahia, Minas Gerais, Espírito Santo e Rio de Janeiro). Rodriguésia 2005, 56, 185–235. [Google Scholar] [CrossRef][Green Version]
- Covre, J.M.C.; Couto, D.R.; Dias, H.M.; Zorzanelli, J.P.F. Vascular plants on inselberg landscapes in Espírito Santo state: Bases for the creation of a protected area in southeastern Brazil. Acta Sci. Biol. Sci. 2021, 43, e54760. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.D.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- EMBRAPA; Centro Nacional de Pesquisa de Solos (Rio de Janeiro, RJ). Manual de Métodos de Análise de Solo, 2nd ed.; EMBRAPA: Brasília, Brazil; Centro Nacional de Pesquisa de Solos: Rio de Janeiro, Brazil, 1997; 212p. [Google Scholar]
- Freitas, C.A.A.D.; Souza, R.C.D.; Pereira, M.G.; Caldeira, M.V.W.; Couto, D.R.; Kunz, S.H.; Moreau, J.S.; Dias, H.M.; Momolli, D.R. Contribution of Pseudobombax aff. petropolitanum to Nutrient Cycling in Woody Vegetation from a Neotropical Inselberg. Floresta Ambiente 2022, 29, e20220034. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, M.; Zhang, H.; Ren, T.; Liu, C.; He, N. Divergent response and adaptation of specific leaf area to environmental change at different spatio-temporal scales jointly improve plant survival. Glob. Change Biol. 2023, 29, 1144–1159. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Ellison, A.M. A Primer of Ecological Statistics, 1st ed.; Sinauer Associates: Sunderland, UK, 2004; pp. 1–640. [Google Scholar]
- Allen, S.E.; Grimshaw, H.M.; Parkinson, J.A.; Allen, E. Chemical Analysis of Ecological Materials, 2nd ed.; Blackwell Scientific: Oxford, UK, 1989; pp. 81–159. [Google Scholar]
- Standard Reference Material 1515; Apple Leaves. National Institute of Standards and Technology, U.S. Department of Commerce: Gaithersburg, MD, USA, 2017.
- Van Heerwaarden, L.M.; Toet, S.; Aerts, R. Nitrogen and phosphorus resorption efficiency and proficiency in six sub-arctic bog species after 4 years of nitrogen fertilization. J. Ecol. 2003, 91, 1060–1070. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Xiong, K.; Yu, Y.; Min, X. Changes of leaf functional traits in karst rocky desertification ecological environment and the driving factors. Glob. Ecol. Conserv. 2020, 24, e01381. [Google Scholar] [CrossRef]
- Pires, F.R.; Esposti, M.; Caten, A.; Guarçoni, A. Levantamento da fertilidade nas principais unidades de mapeamento do Espírito Santo. Rev. Ciênc. Agron. 2003, 34, 115–123. [Google Scholar]
- Aerts, R.; Chapin, F.S., III. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res. 1999, 30, 1–67. [Google Scholar] [CrossRef]
- Cunha, F.V.; Leles, P.S.S.; Pereira, M.G.; Bellumath, V.G.H.; Alonso, J.M. Acúmulo e decomposição da sera-pilheira em quatro formações florestais. Ciência Florest. 2013, 23, 379–387. [Google Scholar] [CrossRef]
- Riggs, C.E.; Hobbie, S.E.; Cavender-Bares, J.; Savage, J.A.; Wei, X. Contrasting effects of plant species traits and moisture on the decomposition of multiple senescent fractions. Oecologia 2015, 179, 573–584. [Google Scholar] [CrossRef]
- Han, M.; Chen, Y.; Sun, L.; Yu, M.; Li, R.; Li, S.; Su, J.; Zhu, B. Linking rhizosphere soil microbial activity and plant resource acquisition strategy. J. Ecol. 2023, 111, 875–888. [Google Scholar] [CrossRef]
- Porembski, S.; Martinelli, G.; Ohlemüller, R.; Barthlott, W. Diversity and ecology of saxicolous vegetation mats on inselbergs in the Brazilian Atlantic rainforest. Divers. Distrib. 1998, 4, 107–119. [Google Scholar] [CrossRef]
- Zhang, K.; Su, Y.; Yang, R. Biomass and nutrient allocation strategies in a desert ecosystem in the Hexi Corridor, northwest China. J. Plant Res. 2017, 130, 699–708. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. The role of plants in the effects of global change on nutrient availability and stoichiometry in the plant-soil system. Plant Physiol. 2012, 160, 1741–1761. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed Editora: Porto Alegre, Brazil, 2017; pp. 1–731. [Google Scholar]
- Kong, D.X.; Li, Y.Q.; Wang, M.L.; Bai, M.; Zou, R.; Tang, H.; Wu, H. Effects of light intensity on leaf photosynthetic characteristics, chloroplast structure, and alkaloid content of Mahonia bodinieri (Gagnep.) Laferr. Acta Physiol. Plant 2016, 38, 120. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, S.; Chen, Y.; Ding, D.; Han, W. The unimodal latitudinal pattern of K, Ca and Mg concentration and its potential drivers in forest foliage in eastern China. For. Ecosyst. 2024, 11, 100193. [Google Scholar] [CrossRef]
- Smith, J.L.; Paul, E.A. The significance of soil microbial biomass estimations. In Soil Biochemistry, 1st ed.; Routledge: New York, NY, USA, 1991; pp. 357–398. [Google Scholar]
- Honvault, N.; Faucon, M.P.; McLaren, T.; Houben, D.; Frossard, E.; Oberson, A. Influence of cover crop residue traits on phosphorus availability and subsequent uptake by plants. Nutr. Cycl. Agroecosyst. 2024, 128, 131–148. [Google Scholar] [CrossRef]
- Stevenson, F.J.; Cole, M.A. Cycles of Soil: Carbon, Nitrogen, Phosphorus, Sulfur, Micronutrients, 1st ed.; Wiley: Hoboken, NJ, USA, 1986; pp. 1–448. [Google Scholar]
- Barbosa, V.; Barreto-Garcia, P.; Gama-Rodrigues, E.; Paula, A.D. Biomassa, carbono e nitrogênio na serapilheira acumulada de florestas plantadas e nativa. Floresta Ambiente 2017, 24, e20150243. [Google Scholar] [CrossRef]
- Maluf, H.J.G.M.; Soares, E.M.B.; Silva, I.R.D.; Neves, J.C.L.; Silva, L.D.O.G. Decomposição de resíduos de culturas e mineralização de nutrientes em solo com diferentes texturas. Rev. Bras. Ciênc. Solo 2015, 39, 1681–1689. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S., III; Pons, T.L. Plant Physiological Ecology, 2nd ed.; Springer Science & Business Media: New York, NY, USA, 2008; pp. 1–591. [Google Scholar]
- Chapin, F.S., III; Matson, P.A.; Vitousek, P. Principles of Terrestrial Ecosystem Ecology, 2nd ed.; Springer Science & Business Media: New York, NY, USA, 2011; pp. 1–511. [Google Scholar]
- Chapin, F.S., III. Adaptations and physiological responses of wild plants to nutrient stress. In Genetic Aspects of Plant Mineral Nutrition: Proceedings of the Second International Symposium on Genetic Aspects of Plant Mineral Nutrition, Organized by the University of Wisconsin, Madison, 16–20 June 1985; Springer: Dordrecht, The Netherlands, 1987; pp. 15–25. [Google Scholar] [CrossRef]
- Escudero, A.; Del Arco, J.M.; Sanz, I.C.; Ayala, J. Effects of leaf longevity and retranslocation efficiency on the retention time of nutrients in the leaf biomass of different woody species. Oecologia 1992, 90, 80–87. [Google Scholar] [CrossRef]
- Wan, F.; Ross-Davis, A.L.; Davis, A.S.; Song, X.; Chang, X.; Zhang, J.; Liu, Y. Nutrient retranslocation in Larix principis-rupprechtii Mayr relative to fertilization and irrigation. New For. 2021, 52, 69–88. [Google Scholar] [CrossRef]
- Güsewell, S. N: P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.A.; Howarth, R.W. Nutrients in synergy. Nature 2007, 449, 1000–1001. [Google Scholar] [CrossRef]
- Du, E.; Terrer, C.; Pellegrini, A.F.; Ahlström, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2000, 13, 221–226. [Google Scholar] [CrossRef]
- Falkengren-Grerup, U.; Michelsen, A.; Olsson, M.O.; Quarmby, C.; Sleep, D. Plant nitrate use in deciduous woodland: The relationship between leaf N, 15N natural abundance of forbs and soil N mineralisation. Soil Biol. Biochem. 2004, 36, 1885–1891. [Google Scholar] [CrossRef]
- Silva, A.C.; Santos, A.D.; Paiva, A.D. Translocação de nutrientes em folhas de Hevea brasiliensis (clone) e em acículas de Pinus oocarpa. Rev. Univ. Alfenas 1998, 4, 11–18. [Google Scholar]
- Zhang, J.; Yu, W.; Wang, Y.; Huang, Z.; Liu, G. Effects of climate, soil, and leaf traits on nutrient resorption efficiency and proficiency of different plant functional types across arid and semiarid regions of northwest China. BMC Plant Biol. 2024, 24, 1093. [Google Scholar] [CrossRef]
- Larcher, W. Utilização de carbono e produção de matéria seca. In Ecologia Vegetal; Larcher, W., Ed.; EPUE: São Paulo, Brazil, 1986; 319p. [Google Scholar]
- Estiarte, M.; Campioli, M.; Mayol, M.; Penuelas, J. Variability and limits of nitrogen and phosphorus resorption during foliar senescence. Plant Commun. 2023, 4, 100503. [Google Scholar] [CrossRef]
- Vergutz, L.; Manzoni, S.; Porporato, A.; Novais, R.F.; Jackson, R.B. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol. Monogr. 2012, 82, 205–220. [Google Scholar] [CrossRef]
- Das, C.; Mondal, N.K. A case study of nutrient retranslocation in four deciduous tree species of West Bengal tropical forest, India. Trop. Ecol. 2024, 65, 580–591. [Google Scholar] [CrossRef]
- González, I.; Sixto, H.; Rodríguez-Soalleiro, R.; Cañellas, I.; Fuertes, A.; Oliveira, N. How can leaf-senescent from different species growing in short rotation coppice contribute to the soil nutrient pool? For. Ecol. Manag. 2022, 520, 120405. [Google Scholar] [CrossRef]
- Santiago, L.S. Extending the leaf economics spectrum to decomposition: Evidence from a tropical forest. Ecology 2007, 88, 1126–1131. [Google Scholar] [CrossRef]
- Zirbel, C.R.; Bassett, T.; Grman, E.; Brudvig, L.A. Plant functional traits and environmental conditions shape community assembly and ecosystem functioning during restoration. J. Appl. Ecol. 2017, 54, 1070–1079. [Google Scholar] [CrossRef]
- Vitt, P.; Finch, J.; Barak, R.S.; Braum, A.; Frischie, S.; Redlinski, I. Seed sourcing strategies for ecological restoration under climate change: A review of the current literature. Front. Conserv. Sci. 2022, 3, 938110. [Google Scholar] [CrossRef] [PubMed]
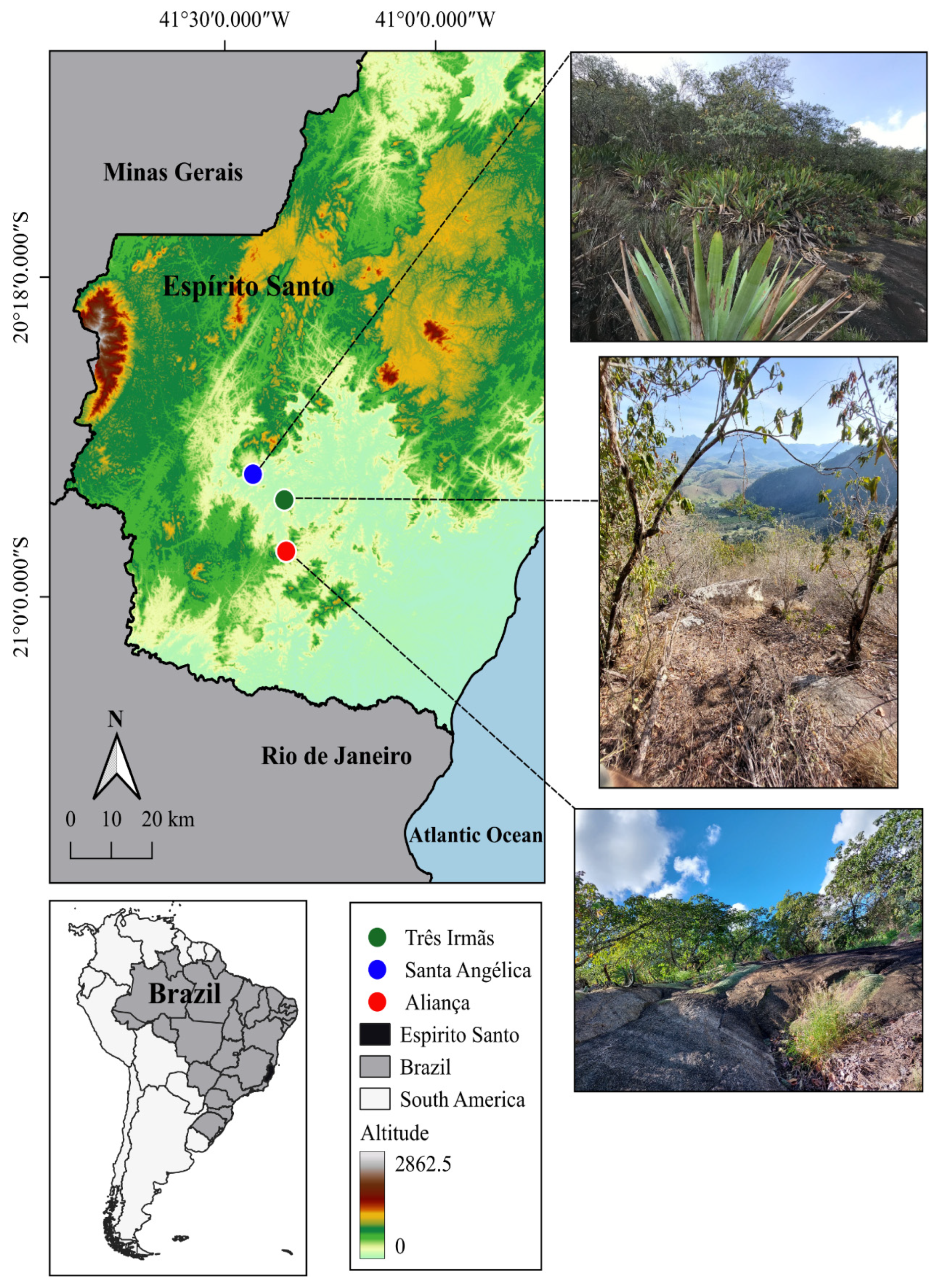
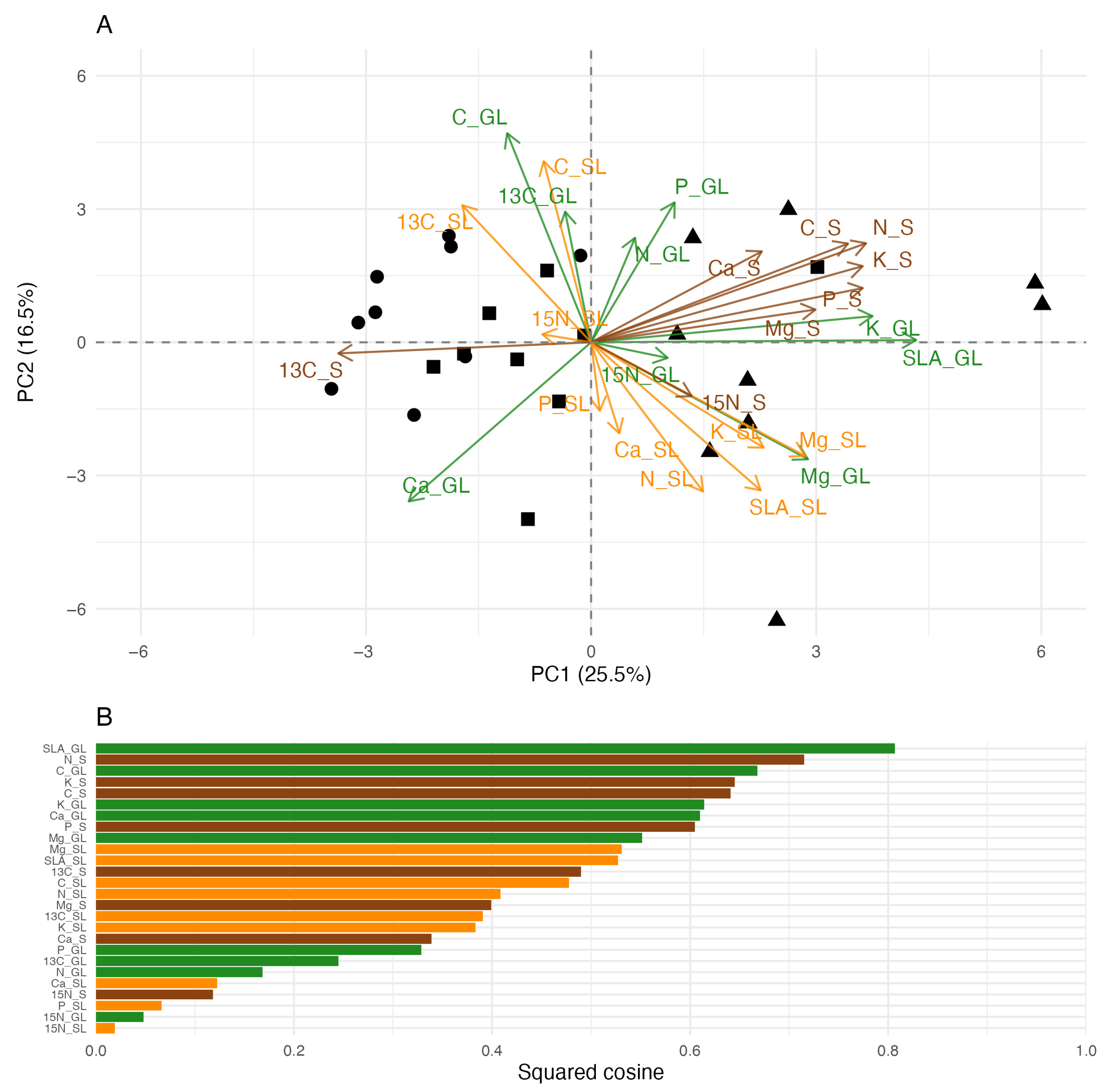
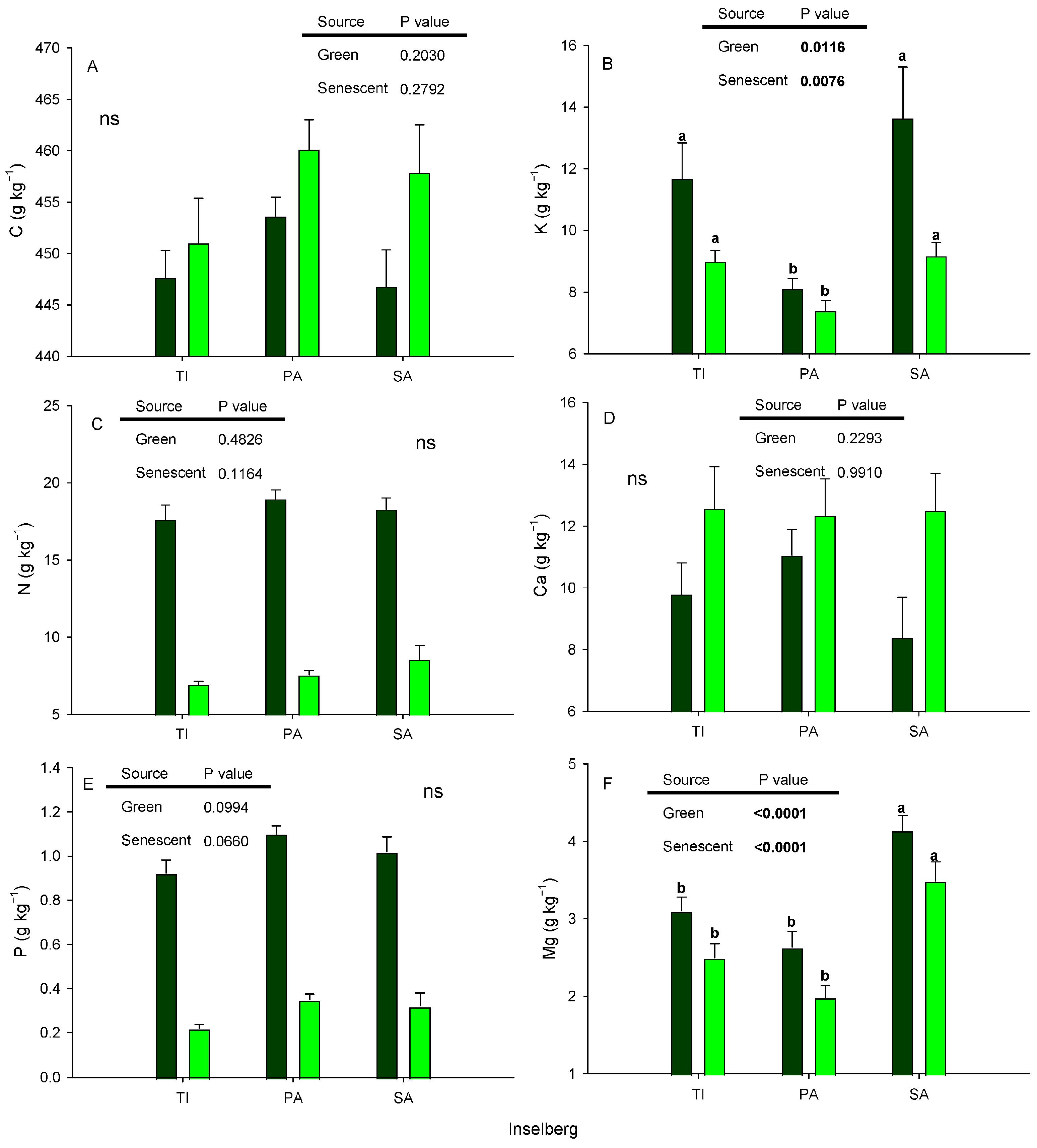
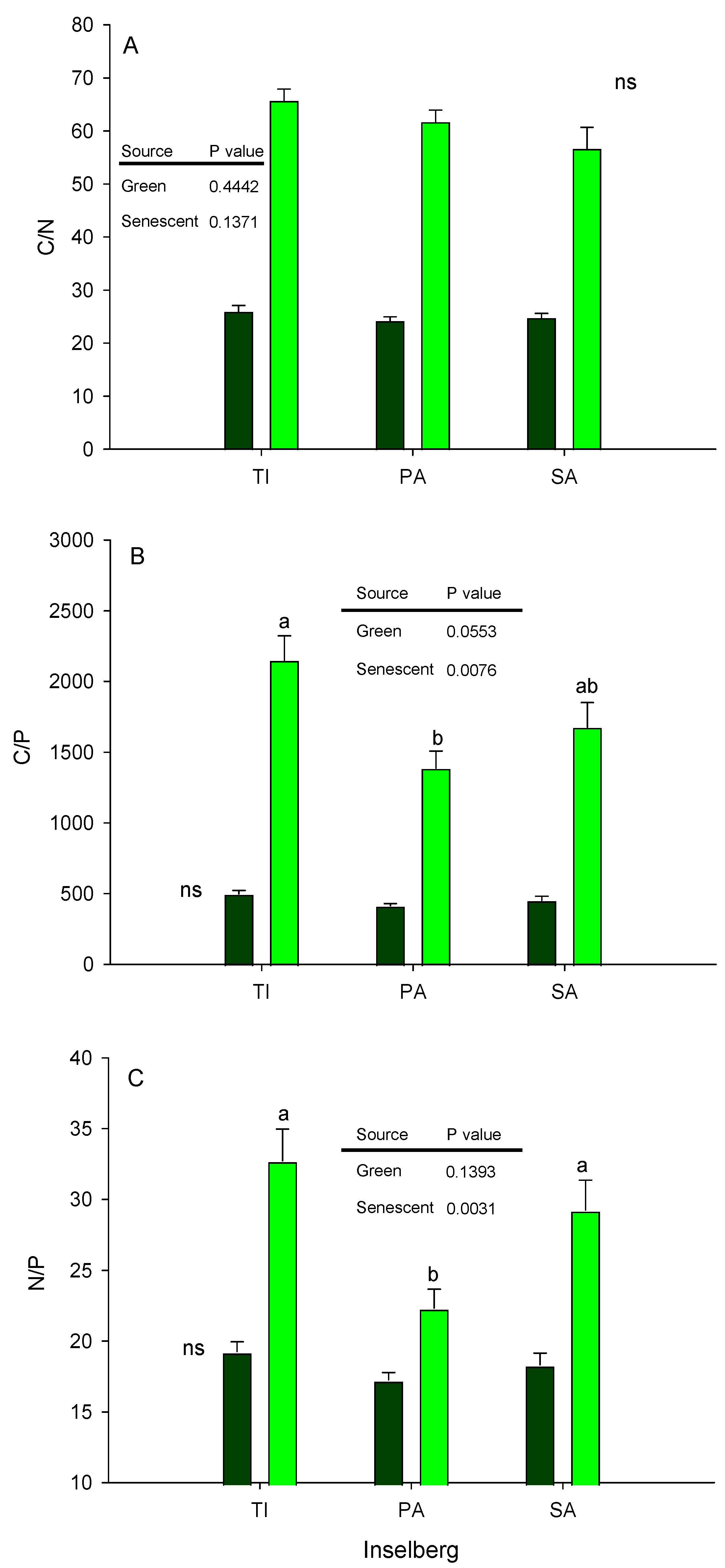


| Variables | p Value | TI | PA | SA |
|---|---|---|---|---|
| pH | 0.0240 | 4.86 ± 0.083 a | 4.56 ± 0.089 ab | 4.53 ± 0.074 b |
| C (kg ha−1) | 0.0004 | 62.65 ± 3.44 b | 56.47 ± 5.26 b | 82.69 ± 5.91 a |
| N (kg ha−1) | <0.0001 | 4.78 ± 0.25 b | 4.81 ± 0.40 b | 6.94 ± 0.43 a |
| P (kg ha−1) | <0.0001 | 1.07 ± 0.11 b | 1.61 ± 0.28 b | 3.13 ± 0.12 a |
| K (kg ha−1) | 0.0001 | 106.62 ± 9.28 a | 59.99 ± 11.44 b | 106.11 ± 6.06 a |
| Ca (kg ha−1) | 0.9804 | 975.90 ± 157.13 a | 923.08 ± 211.36 a | 943.87 ± 186.49 a |
| Mg (kg ha−1) | 0.0800 | 111.13 ± 17.64 a | 58.14 ± 60.34 a | 178.49 ± 11.64 a |
| δ13C (‰) | <0.0001 | −27.04 ± 0.12 b | −25.33 ± 0.24 a | −27.63 ± 0.18 b |
| δ15N (‰) | <0.0001 | 5.04 ± 0.16 b | 6.02 ± 0.21 a | 6.59 ± 0.22 a |
| C/N ratio | 0.0066 | 15.27 ± 0.23 a | 13.77 ± 0.36 b | 13.85 ± 0.42 b |
| C/P ratio | <0.0001 | 65.11 ± 6.64 a | 38.74 ± 2.34 b | 28.18 ± 4.63 b |
| N/P ratio | 0.0002 | 5.02 ± 0.54 a | 3.35 ± 0.18 b | 2.35 ± 0.41 b |
| Inselberg | Leaf Area (cm2) | Leaf Mass (g) | SLA (cm2 g−1) | |||
|---|---|---|---|---|---|---|
| Green | Senescent | Green | Senescent | Green | Senescent | |
| TI | 686.9 ± 39.3 | 531.5 ± 48.6 | 8.6 ± 0.6 | 7.2 ± 0.5 | 81.2 ± 4.8 b | 73.8 ± 3.4 ab |
| PA | 683.6 ± 33.5 | 544.6 ± 49.8 | 9.0 ± 0.5 | 7.6 ± 0.8 | 76.4 ± 2.5 b | 71.7 ± 1.4 b |
| SA | 760.6 ± 48.1 | 504.9 ± 46.1 | 7.5 ± 0.7 | 6.1 ± 0.8 | 104.3 ± 4.8 a | 82.8 ± 5.0 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerônimo Júnior, R.A.d.C.; Paula, R.R.; Francisco, T.M.; Couto, D.R.; Covre, J.M.C.; Villela, D.M. Leaf Chemistry Patterns in Populations of a Key Lithophyte Tree Species in Brazil’s Atlantic Forest Inselbergs. Forests 2025, 16, 1186. https://doi.org/10.3390/f16071186
Jerônimo Júnior RAdC, Paula RR, Francisco TM, Couto DR, Covre JMC, Villela DM. Leaf Chemistry Patterns in Populations of a Key Lithophyte Tree Species in Brazil’s Atlantic Forest Inselbergs. Forests. 2025; 16(7):1186. https://doi.org/10.3390/f16071186
Chicago/Turabian StyleJerônimo Júnior, Roberto Antônio da Costa, Ranieri Ribeiro Paula, Talitha Mayumi Francisco, Dayvid Rodrigues Couto, João Mário Comper Covre, and Dora Maria Villela. 2025. "Leaf Chemistry Patterns in Populations of a Key Lithophyte Tree Species in Brazil’s Atlantic Forest Inselbergs" Forests 16, no. 7: 1186. https://doi.org/10.3390/f16071186
APA StyleJerônimo Júnior, R. A. d. C., Paula, R. R., Francisco, T. M., Couto, D. R., Covre, J. M. C., & Villela, D. M. (2025). Leaf Chemistry Patterns in Populations of a Key Lithophyte Tree Species in Brazil’s Atlantic Forest Inselbergs. Forests, 16(7), 1186. https://doi.org/10.3390/f16071186








