Canopy Cover Drives Odonata Diversity and Conservation Prioritization in the Protected Wetland Complex of Thermaikos Gulf (Greece)
Abstract
1. Introduction
2. Materials and Methods
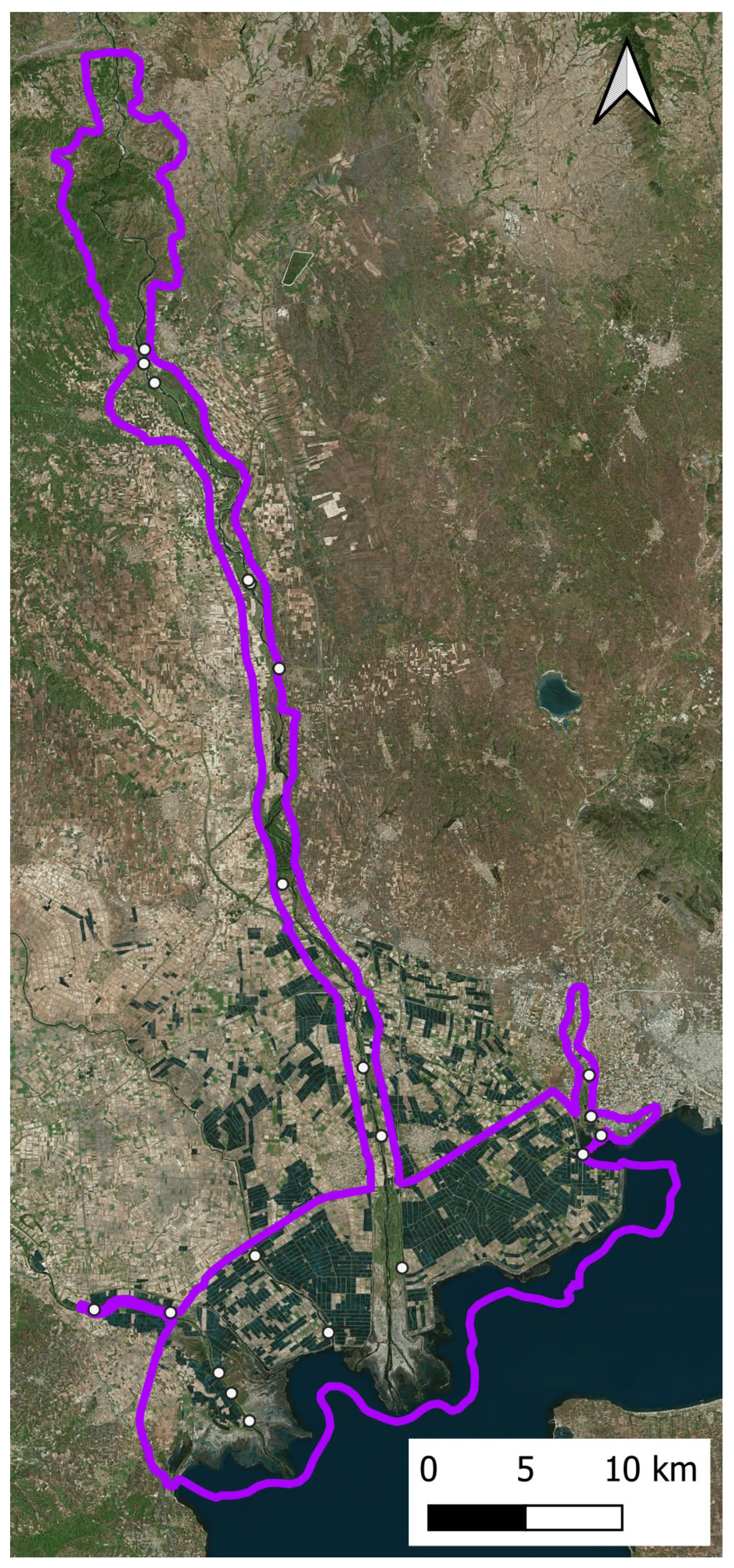
2.1. Study Area
2.2. Sampling and Taxonomic Identification
2.3. Data Analyses
2.3.1. Hypothesis 1
2.3.2. Hypotheses 2,3
2.3.3. Hypothesis 4
3. Results
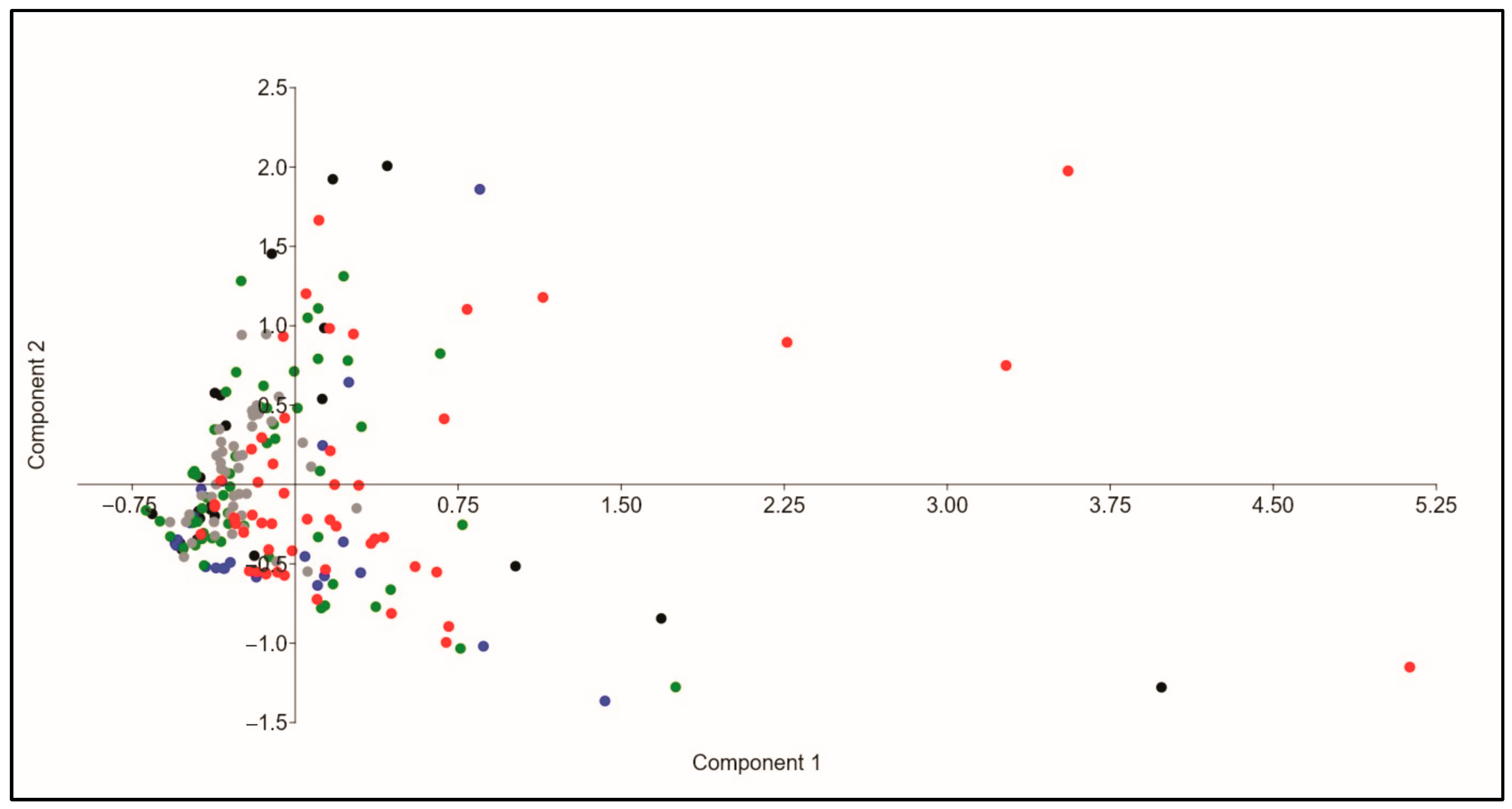
3.1. Composition of Odonate Assemblages
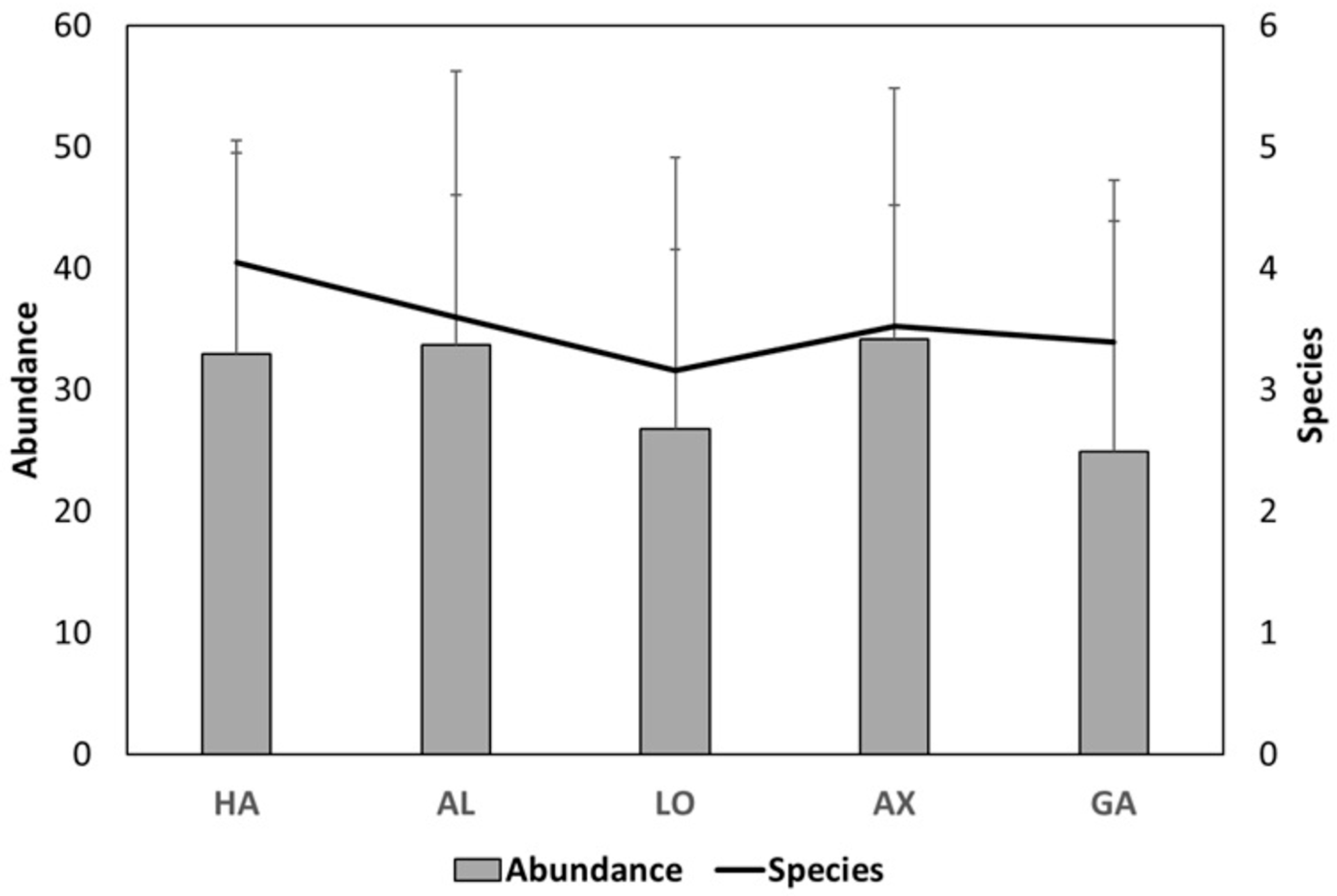
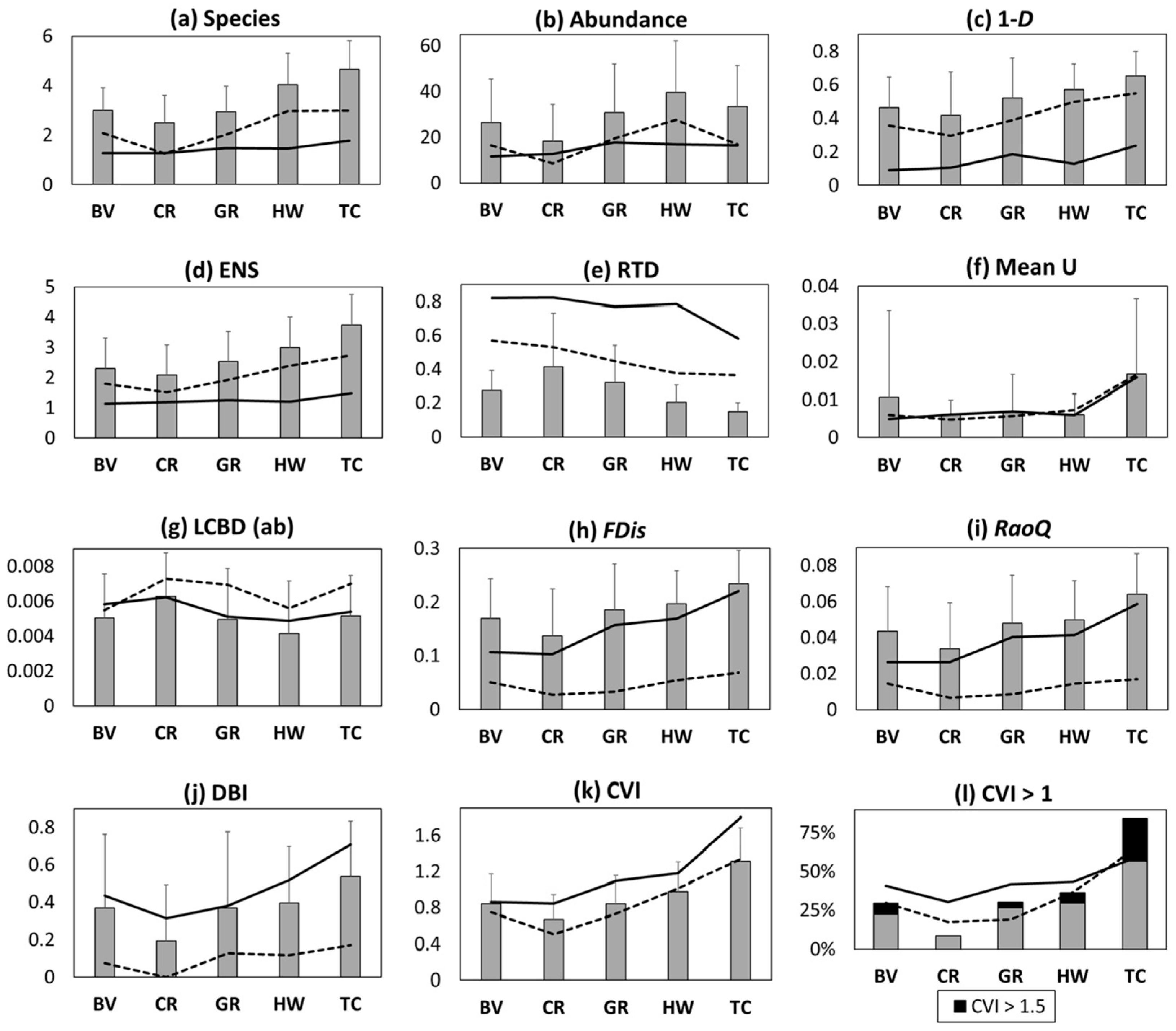
3.2. Richness and Diversity Patterns
4. Discussion
4.1. Land Use Drives Differential Assemblage Composition
4.2. Ecophysiological Restrictions Influence Diversity Patterns
4.3. Canopy Cover Enhances Odonate Diversity and Site Conservation Value
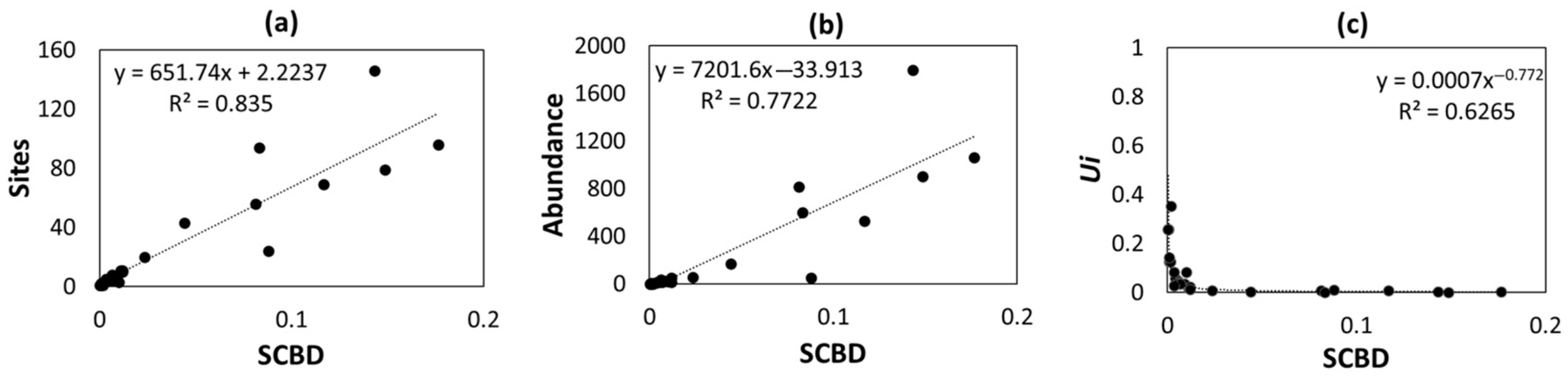
4.4. Agriculture Influences β-Diversity Patterns
4.5. Conclusions and Implications for Conservation
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudgeon, D. Multiple threats imperil freshwater biodiversity in the Anthropocene. Curr. Biol. 2019, 29, R960–R967. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zarfl, C.; Bremerich, V.; David, J.N.W.; Hogan, Z.; Kalinkat, G.; Tockner, K.; Jähnig, S.C. The global decline of freshwater megafauna. Glob. Change Biol. 2019, 25, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Sullivan, C.A. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Strayer, D.L.; Dudgeon, D. Freshwater biodiversity conservation: Recent progress and future challenges. J. N. Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Reidy Liermann, C.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Couceiro, S.R.M.; Hamada, N.; Forsberg, B.R.; Pimentel, T.P.; Luz, S.L.B. A macroinvertebrate multimetric index to evaluate the biological condition of streams in the Central Amazon region of Brazil. Ecol. Indic. 2012, 18, 118–125. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S., III; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Leal, C.G.; Lennox, G.D.; Ferraz, S.F.B.; Ferreira, J.; Gardner, T.A.; Thomson, J.R.; Berenguer, E.; Lees, A.C.; Hughes, R.M.; MacNally, R.; et al. Integrated terrestrial–freshwater planning doubles conservation of tropical aquatic species. Science 2020, 370, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Boix, D.; García-Berthou, E.; Gascón, S.; Benejam, L.L.; Tornés, E.; Sala, J.; Benito, J.; Munné, A.; Solà, C.; Sabater, S. Response of community structure to sustained drought in Mediterranean rivers. J. Hydrol. 2010, 383, 135–146. [Google Scholar] [CrossRef]
- Shanafield, M.; Bourke, S.A.; Zimmer, M.A.; Costigan, K.H. An overview of the hydrology of non–perennial rivers and streams. WIREs Water 2021, 8, e1504. [Google Scholar] [CrossRef]
- Commission of the European Communities. Wise Use and Conservation of Wetlands. Communication from the Commission to the Council and the European Parliament. COM(95) 189 Final; Office for Official Publications of the European Communities: Luxembourg; Brussels, Belgium, 1995. [Google Scholar]
- Zotos, A.; Kokkoris, I.P.; Charalampopoulos, I.; Bekri, E.S.; Dimopoulos, P. Wetlands in crisis: The silent desertification threat on the Greek wetlands. Land 2024, 13, 1567. [Google Scholar] [CrossRef]
- Rahel, F.J. Homogenization of freshwater faunas. Annu. Rev. Ecol. Evol. Syst. 2002, 33, 291–315. [Google Scholar] [CrossRef]
- Sargac, J.; Johnson, R.K.; Burdon, F.J.; Truchy, A.; Rîşnoveanu, G.; Goethals, P.; McKie, B.G. Forested riparian buffers change the taxonomic and functional composition of stream invertebrate communities in agricultural catchments. Water 2021, 13, 1028. [Google Scholar] [CrossRef]
- Vilenica, M.; Rebrina, F.; Matoničkin Kepčija, R.; Šegota, V.; Rumišek, M.; Ružanović, L.; Brigić, A. Aquatic macrophyte vegetation promotes taxonomic and functional diversity of Odonata assemblages in intermittent karst rivers in the Mediterranean. Diversity 2022, 14, 31. [Google Scholar] [CrossRef]
- Beaujour, P.M.; Loranger-Merciris, G.; Cézilly, F. Sites and species contribution to the β-diversity of Odonata assemblages in Haiti: Implications for conservation. Glob. Ecol. Conserv. 2024, 50, e02816. [Google Scholar] [CrossRef]
- Allan, J.D.; Castillo, M.M. Stream Ecology: Structure and Function of Running Waters; Chapman and Hall: New York, NY, USA, 2007. [Google Scholar]
- Ball-Damerow, J.E.; M’Gonigle, L.K.; Resh, V.H. Changes in occurrence, richness, and biological traits of dragonflies and damselflies (Odonata) in California and Nevada over the past century. Biodivers. Conserv. 2014, 23, 2107–2126. [Google Scholar] [CrossRef]
- Gimenez, B.C.; Higuti, J. Land use effects on the functional structure of aquatic insect communities in Neotropical streams. Inland Waters 2017, 7, 305–313. [Google Scholar] [CrossRef]
- Corbet, P.S. Dragonflies: Behaviour and Ecology of Odonata; Harley Books: Colchester, UK, 1999. [Google Scholar]
- Cordoba-Aguilar, A.; Beatty, C.D.; Bried, J.T. Dragonflies & Damselflies: Model Organisms for Ecological and Evolutionary Research, 2nd ed.; Oxford University Press: Oxford, UK, 2022. [Google Scholar]
- Kalkman, V.J.; Clausnitzer, V.; Dijkstra, K.D.B.; Orr, A.G.; Paulson, D.; van Tol, J. Global diversity of dragonflies (Odonata) in freshwater. Hydrobiologia 2008, 595, 351–363. [Google Scholar] [CrossRef]
- Rodrigues, M.E.; de Oliveira Roque, F.; Quintero, J.M.O.; de Castro Pena, J.C.; de Souza, D.C.; De Marco, P. Nonlinear responses in damselfly community along a gradient of habitat loss in a savanna landscape. Biol. Conserv. 2016, 194, 113–120. [Google Scholar] [CrossRef]
- Dolný, A.; Harabiš, F.; Bárta, D.; Lhota, S.; Drozd, P. Aquatic insects indicate terrestrial habitat degradation: Changes in taxonomical structure and functional diversity of dragonflies in tropical rainforest of East Kalimantan. Trop. Zool. 2012, 25, 141–157. [Google Scholar] [CrossRef]
- Oliveira-Junior, J.M.B.; De Marco, P.; Dias-Silva, K.; Leitão, R.P.; Leal, C.G.; Pompeu, P.S.; Gardner, T.A.; Hughes, R.M.; Juen, L. Effects off human disturbance and riparian conditions on Odonata (Insecta) assemblages in eastern Amazon basin streams. Limnologica 2017, 66, 31–39. [Google Scholar] [CrossRef]
- Monteiro-Júnior, C.S.; Juen, L.; Hamada, N. Analysis of urban impacts of aquatic habitats in the central Amazon basin: Adult odonates as bioindicators of environmental quality. Ecol. Indic. 2015, 48, 303–311. [Google Scholar] [CrossRef]
- Clausnitzer, V.; Kalkman, V.J.; Ram, M.; Collen, B.; Baillie, J.E.; Bedjanič, M.; Darwall, W.R.T.; Dijkstra, K.-D.B.; Dowf, R.; Hawking, J.; et al. Odonata enter the biodiversity crisis debate: The first global assessment of an insect group. Biol. Conserv. 2009, 142, 1864–1869. [Google Scholar] [CrossRef]
- De Knijf, G.; Billqvist, M.; van Grunsven, R.H.A.; Prunier, F.; Vinko, D.; Trottet, A.; Bellotto, V.; Clay, J.; Allen, D.J. Measuring the Pulse of European Biodiversity. European Red List of Dragonflies & Damselflies (Odonata); European Commission: Brussels, Belgium, 2024. [Google Scholar]
- The Greek Red List. Available online: https://redlist.necca.gov.gr (accessed on 27 May 2025).
- Smith, J.; Samways, M.J.; Taylor, S. Assessing riparian quality using two complementary sets of bioindicators. Biodivers. Conserv. 2007, 16, 2695–2713. [Google Scholar] [CrossRef]
- Silva, D.P.; De Marco, P., Jr.; Resende, D.C. Adult Odonate abundance and community assemblage measures as indicators of stream ecological integrity: A case study. Ecol. Indic. 2010, 10, 744–752. [Google Scholar] [CrossRef]
- Simaika, J.P.; Samways, M.J. Comparative assessment of indices of freshwater habitat conditions using different invertebrate taxon sets. Ecol. Indic. 2011, 11, 370–378. [Google Scholar] [CrossRef]
- De Marco Júnior, P.; Batista, J.D.; Cabette, H.S.R. Community assembly of adult odonates in tropical streams: An ecophysiological hypothesis. PLoS ONE 2015, 10, e0123023. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Juen, L.; Rodrigues, M.E. The Zygoptera/Anisoptera ratio as a tool to assess anthropogenic changes in Atlantic Forest streams. Biodivers. Conserv. 2021, 30, 1315–1329. [Google Scholar] [CrossRef]
- Ribeiro, C.; Rodrigues, M.E.; Sahlén, G.; de Oliveira Roque, F. Dragonflies within and outside a protected area: A comparison revealing the role of well–preserved atlantic forests in the preservation of critically endangered, phytotelmatous species. J. Insect Conserv. 2022, 26, 271–282. [Google Scholar] [CrossRef]
- Flenner, I.; Sahlén, G. Dragonfly community re–organization in boreal forestlakes: Rapid species turnover driven by climate change? Insect Conserv. Divers. 2008, 1, 169–179. [Google Scholar] [CrossRef]
- Harabiš, F.; Dolný, A. Human altered ecosystems: Suitable habitats as well as ecological traps for dragonflies (Odonata): The matter of scale. J. Insect Conserv. 2012, 16, 121–130. [Google Scholar] [CrossRef]
- Golfieri, B.; Hardersen, S.; Maiolini, B.; Surian, N. Odonates as indicators of the ecological integrity of the river corridor: Development and application of the Odonate River Index (ORI) in northern Italy. Ecol. Indic. 2016, 61, 234–247. [Google Scholar] [CrossRef]
- Rodrigues, M.E.; Roque, F.O.; Ferreira, R.G.N.; Saito, V.S.; Samways, M.J. Egg–laying traits reflect shifts in dragonfly assemblages in response to different amount of tropical forest cover. Insect Conserv. Divers. 2018, 11, 231–240. [Google Scholar] [CrossRef]
- de Bello, F.; Carmona, C.P.; Mason, N.W.H.; Sebastià, M.-T.; Lepš, J. Which trait dissimilarity for functional diversity: Trait means or trait overlap? J. Veg. Sci. 2013, 24, 807–819. [Google Scholar] [CrossRef]
- Rocha-Ortega, M.; Rodríguez, P.; Córdoba-Aguilar, A. Spatial and temporal effects of land use change as potential drivers of odonate community composition but not species richness. Biodivers. Conserv. 2019, 28, 451–466. [Google Scholar] [CrossRef]
- Mendes, T.P.; de Assis Montag, L.F.; Alvarado, S.T.; Juen, L. Assessing habitat quality on alpha and beta diversity of Odonata larvae (Insect) in logging areas in Amazon forest. Hydrobiologia 2021, 848, 1147–1161. [Google Scholar] [CrossRef]
- Legendre, P.; De Cáceres, M. Beta diversity as the variance of community data: Dissimilarity coefficients and partitioning. Ecol. Lett. 2013, 16, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Olsen, K.; Svenning, J.C.; Balslev, H. Niche breadth predicts geographical range size and northern range shift in European dragonfly species (Odonata). Diversity 2022, 14, 719. [Google Scholar] [CrossRef]
- Wildermuth, H. Dragonflies recognize the water of rendezvous and oviposition sites by horizontally polarized light: A behavioural field test. Naturwissenschaften 1998, 85, 297–302. [Google Scholar] [CrossRef]
- Bossard, M.; Feranec, J.; Otahel, J. CORINE Land Cover Technical Guide: Addendum 2000; European Environment Agency: Copenhagen, Denmark, 2000. [Google Scholar]
- Dimopoulos, P.; Bergmeier, E.; Fischer, P. Natura 2000 habitat types of Greece evaluated in the light of distribution, threat and responsibility. Biol. Environ. 2006, 106, 175–187. [Google Scholar] [CrossRef]
- Zanaga, D.; Van De Kerchove, R.; Daems, D.; De Keersmaecker, W.; Brockmann, C.; Kirches, G.; Wevers, J.; Cartus, O.; Santoro, M.; Fritz, S.; et al. ESA WorldCover 10 m 2021 v200. 2022. Available online: https://zenodo.org/records/7254221 (accessed on 27 May 2025).
- Schorr, M.; Paulson, D.; Deliry, C. World Odonata List. 2025. Available online: https://www.pugetsound.edu/puget-sound-museum-natural-history/biodiversity-resources/insects/dragonflies/world-odonata-list (accessed on 27 May 2025).
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. The ‘vegan’ Package. 2007. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 10 July 2025).
- Anderson, M.J. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible assymetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- McCune, B.; Mefford, M.J. PC-ORD, version 6.21; Multivariate Analysis of Ecological Data; MjM Software: Gleneden Beach, OR, USA, 2011. [Google Scholar]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Ellingsen, K.E.; Clarke, K.R.; Somerfield, P.J.; Warwick, R.M. Taxonomic distinctness as a measure of diversity applied over a large scale: The benthos of the Norwegian continental shelf. J. Anim. Ecol. 2005, 74, 1069–1079. [Google Scholar] [CrossRef]
- Freitag, S.; Jaarsveld, A.V. Relative occupancy, endemism, taxonomic distinctiveness and vulnerability: Prioritizing regional conservation actions. Biodivers. Conserv. 1997, 6, 211–232. [Google Scholar] [CrossRef]
- Laliberte, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Botta-Dukat, Z. Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. J. Veg. Sci. 2005, 16, 533–540. [Google Scholar] [CrossRef]
- Dijkstra, K.-D.B.; Lewington, R. Field Guide to the Dragonflies of Britain and Europe; British Wildlife Publishing: Gillingham, Dorset, UK, 2006. [Google Scholar]
- Cancellario, T.; Miranda, R.; Baquero, E.; Fontaneto, D.; Martínez, A.; Mammola, S. Climate change will redefine taxonomic, functional, and phylogenetic diversity of Odonata in space and time. npj Biodivers. 2022, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Boudot, J.-P.; Kalkman, V.J. Atlas of the European Dragonflies and Damselflies; KNNV Publishing: Zeist, The Netherlands, 2015. [Google Scholar]
- Harabiš, F.; Hronková, J. European database of the life-history, morphological and habitat characteristics of dragonflies (Odonata). Eur. J. Entomol. 2020, 117, 302–308. [Google Scholar] [CrossRef]
- Jödicke, R. Die Binsenjungfern und Winterlibellen Europas: Lestidae; Die Neue Brehm-Bucherei: Westarp, Germany, 1997. [Google Scholar]
- Sternberg, K. Die postglaziale Besiedlung Mitteleuropas durch Libellen, mit besonderer Berücksichtigung Südwestdeutschlands (Insecta, Odonata). The postglacial colonization of Central Europe by dragonflies, with special reference to southwestern Germany (Insecta, Odonata). J. Biogeogr. 1998, 25, 319–337. [Google Scholar] [CrossRef]
- Viza, A.; Garcia-Raventós, A.; Ll Riera, J.; Maynou, X.; Martín, R.; Prunier, F.; El Haissoufi, M.; Múrria, C. Species-specific functional traits rather than phylogenetic relatedness better predict future range-shift responses of odonates. Insect Conserv. Diver. 2023, 16, 574–587. [Google Scholar] [CrossRef]
- Schneider, W. Eine Massenwanderung von Selysiothemis nigra (Vander Linden, 1825) (Odonata: Macrodiplactidae) und Lindenia tetraphylla (Vander Linden, 1825) (Odonata: Gomphidae) in Südjordanien. Entomol. Z. 1981, 91, 97–102. [Google Scholar]
- Martens, A. Die Federlibellen Europas (Platycnemididae); Westarp & Spektrum: Magdeburg, Germany, 1996. [Google Scholar]
- Sternberg, K.; Buchwald, R. Die Libellen Baden Band 2: Grosslibellen (Anisoptera); Ulmer Verlang: Stuttgart, Germany, 2000. [Google Scholar]
- Rüppell, G.; Hilfert-Rüppell, D.; Rehfeldt, G.; Schütte, C. Die Prachtlibellen Europas; Westarp Wissenschaften: Hohenwarsleben, Germany, 2005. [Google Scholar]
- Harabiš, F.; Dolný, A. The effect of ecological determinants on the dispersal abilities of Central European dragonflies (Odonata). Odonatologica 2011, 41, 17–26. [Google Scholar]
- Wildermuth, H. Perching behaviour in Lindenia tetraphylla - a distinctive feature among European Clubtails (Odonata: Gomphidae). Not. Odonatol. 2014, 8, 55–66. [Google Scholar]
- Hilfert-Rüppell, D. To stay or not to stay: Decision-making during territorial behaviour of Calopteryx haemorrhoidalis and Calopteryx splendens splendens (Zygoptera: Calopterygidae). Int. J. Odonatol. 1999, 2, 167–175. [Google Scholar] [CrossRef]
- Wildermuth, H.; Martens, A. Taschenlexikon der Libellen Europas: Alle Arten von den Azoren bis zum Ural im Porträt; Quelle & Meyer: Wiebelsheim, Germany, 2014. [Google Scholar]
- Suhling, F.; Müller, O. Die Flußjungfern Europas (Gomphidae); Westarp: Magdeburg, Germany; 237p.
- Sternberg, K.; Buchwald, R. Die Libellen Baden Band 1: Kleinlibellen (Zygoptera); Ulmer Verlang: Stuttgart, Germany, 1999. [Google Scholar]
- Samways, M.J.; Simaika, J.P. Manual of Freshwater Assessment for South Africa: Dragonfly Biotic Index; South African National Biodiversity Institute (SANBI): Pretoria, South Africa, 2016. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Baselga, A.; Orme, C.D.L. betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Dray, S.; Bauman, D.; Blanchet, G.; Borcard, D.; Clappe, S.; Guénard, G.; Jombart, T.; Larocque, G.; Legendre, P.; Madi, N.; et al. Package ‘adespatial’. 2018. Available online: https://cran.r-project.org/package=adespatial (accessed on 27 May 2025).
- Worthen, W.B.; Guevara-Mora, M. The Effects of Light Environment on Adult Odonate Communities in Disturbed and Intact Forest: The Importance of Small-Scale Effects. Diversity 2024, 16, 557. [Google Scholar] [CrossRef]
- Carvalho, F.G.; Duarte, L.; Nakamura, G.; dos Santos-Seger, G.D.; Juen, L. Changes of phylogenetic and taxonomic diversity of Odonata (Insecta) in response to land use in Amazonia. Forests 2021, 12, 1061. [Google Scholar] [CrossRef]
- Pereira-Moura, L.; Veras, D.S.; de Carvalho, F.G.; Juen, L.; Couceiro, S.R.M. Habitat specificity and morphology-main filters for the distribution of Odonata in the Cerrado Maranhense, Brazil. Aquat. Ecol. 2023, 57, 443–458. [Google Scholar] [CrossRef]
- Renner, S.; Périco, E.; Dalzochio, M.S.; Sahlén, G. Water body type and land cover shape the dragonfly communities (Odonata) in the Pampa biome, Rio Grande do Sul, Brazil. J. Insect Conserv. 2018, 22, 113–125. [Google Scholar] [CrossRef]
- Seidu, I.; Danquah, E.; Ayine Nsor, C.; Amaning Kwarteng, D.; Lancaster, L.T. Odonata community structure and patterns of land use in the Atewa Range Forest Reserve, Eastern Region (Ghana). Int. J. Odonatol. 2017, 20, 173–189. [Google Scholar] [CrossRef]
- Kietzka, G.J.; Pryke, J.S.; Samways, M.J. Comparative effects of urban and agricultural land transformation on Odonata assemblages in a biodiversity hotspot. Basic Appl. Ecol. 2018, 33, 89–98. [Google Scholar] [CrossRef]
- Dolný, A.; Ožana, S.; Burda, M.; Harabiš, F. Effects of landscape patterns and their changes to species richness, species composition, and the conservation value of Odonates (Insecta). Insects 2021, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- Pires, M.M.; Sahlén, G.; Périco, E. Agricultural land use affects the heterogeneity of Odonata communities in the Brazilian Pampa. J. Insect Conserv. 2022, 26, 503–514. [Google Scholar] [CrossRef]
- Calvão, L.B.; Brito, J.D.S.; Ferreira, D.; Cunha, E.J.; Oliveira-Junior, J.M.B.; Juen, L. Effects of the loss of forest cover on odonate communities in eastern Amazonia. J. Insect Conserv. 2023, 27, 205–218. [Google Scholar] [CrossRef]
- Juen, L.; De Marco, P., Jr. Odonate biodiversity in terra-firme forest streamlets in Central Amazonia: On the relative effects of neutral and niche drivers at small geographical extents. Insect Conserv. Divers. 2011, 4, 265–274. [Google Scholar] [CrossRef]
- Corbet, P.S.; May, M.L. Fliers and perchers among Odonata: Dichotomy or multidimensional continuum? A provisional reappraisal. Int. J. Odonatol. 2008, 11, 155–171. [Google Scholar] [CrossRef]
- Monteiro Júnior, C.S.; Couceiro, S.R.M.; Hamada, N.; Juen, L. Effect of vegetation removal for road building on richness and composition of Odonata communities in Amazonia, Brazil. Int. J. Odonatol. 2013, 16, 135–144. [Google Scholar] [CrossRef]
- Koparde, P.; Mhaske, P.; Patwardhan, A. Habitat correlates of Odonata species diversity in the northern Western Ghats, India. Odonatologica 2015, 44, 21–43. [Google Scholar]
- Calvão, L.B.; Nogueira, D.S.; de Assis Montag, L.F.; Lopes, M.A.; Juen, L. Are Odonata communities impacted by conventional or reduced impact logging? For. Ecol. Manag. 2016, 382, 143–150. [Google Scholar] [CrossRef]
- Hofhansl, F.P.; Schneeweihs, S. Banderillas: Effects of deforestation on dragonflies (Insecta, Odonata) in the Pacific lowland of Costa Rica. Zugleich Kat. Der Oberösterr. Landesmuseen N.S. 2008, 80, 237–247. [Google Scholar]
- French, S.K.; McCauley, S.J. Canopy cover affects habitat selection by adult dragonflies. Hydrobiologia 2018, 818, 129–143. [Google Scholar] [CrossRef]
- Miguel, T.B.; Oliveira-Junior, J.M.B.; Ligeiro, R.; Juen, L. Odonata (Insecta) as a tool for the biomonitoring of environmental quality. Ecol. Indic. 2017, 81, 555–566. [Google Scholar] [CrossRef]
- Hendriks, J.A.; Mariaty; Maimunah, S.; Anirudh, N.B.; Holly, B.A.; Erkens, R.H.; Harrison, M.E. Odonata (Insecta) communities in a lowland mixed mosaic forest in Central Kalimantan, Indonesia. Ecologies 2023, 4, 55–73. [Google Scholar] [CrossRef]
- Resende, D.C. Activity patterns and thermoregulation in a tropical dragonfly assemblage. Odonatologica 2002, 31, 129–138. [Google Scholar]
- Heiser, M.; Schmitt, T. Do different dispersal capacities influence the biogeography of the western Palearctic dragonflies (Odonata)? Biol. J. Linn. Soc. 2010, 99, 177–195. [Google Scholar] [CrossRef]
- Dutra, S.; De Marco, P. Bionomic differences in odonates and their influence on the efficiency of indicator species of environmental quality. Ecol. Indic. 2015, 49, 132–142. [Google Scholar] [CrossRef]
- Carvalho, F.G.; Pinto, N.S.; Oliveira-Junior, J.M.B.; Juen, L. Effects of marginal vegetation removal on Odonata communities. Acta Limnol. Bras. 2013, 25, 10–18. [Google Scholar] [CrossRef]
- Corbet, P.S.; Brooks, S.J. Dragonflies; Collins New Naturalist Library; HarperCollins Publishers: London, UK, 2008. [Google Scholar]
- Kietzka, G.J.; Pryke, J.S.; Samways, M.J.; Leather, S.R.; Sahlen, G. Landscape ecological networks are successful in supporting a diverse dragonfly assemblage. Insect Conserv. Divers. 2015, 8, 229–237. [Google Scholar] [CrossRef]
- Cunningham-Minnick, M.J.; Meyer, T.B.; Crist, T.O. Shifts in dragonfly community structure across aquatic ecotones. Int. J. Odonatol. 2019, 22, 121–133. [Google Scholar] [CrossRef]
- Remsburg, A.J.; Olson, A.C.; Samways, M.J. Shade alone reduces adult dragonfly (Odonata: Libellulidae) abundance. J. Insect Behav. 2008, 21, 460–468. [Google Scholar] [CrossRef]
- Horváth, G. Reflection-polarization patterns at flat water surfaces and their relevance for insect polarization vision. J. Theor. Biol. 1995, 175, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Holuša, O. Expansion of Erythromma lindenii (Selys, 1840) (Odonata: Coenagrionidae) is still ongoing: Settlement of Central Europe by various migratory routes. Libellula 2023, 42, 49–61. [Google Scholar]
- Tatarkiewicz, D. Sites of the emergence of the Scarce Chaser Libellula fulva (O.F. Müller, 1764) (Odonata: Libellulidae) in the forest of Puszcza Notecka. Odonatrix 2010, 6, 21–29. [Google Scholar]
- Hacet, N.; Aktaç, N. Two new records of Odonata (Gomphidae) for Turkey, Gomphus flavipes (Charpentier, 1825) and Ophiogomphus cecilia (Geoffroy in Fourcroy, 1785), with distributional notes on G. flavipes and G. ubadschii Schmidt, 1953. Entomol. News 2008, 119, 81–89. [Google Scholar] [CrossRef]
- Hardersen, S. The dragonflies: Species, phenology, larval habitats (Odonata). In Invertebrati di una foresta della Pianura Padana, Bosco della Fontana, Secondo contributo; Ceretti, P.H., Mason, S., Nardi, F., Tisato, G., Zapparoli, M., Eds.; Conservatione Habitat Invertebrati: Verona, Italy, 2004; pp. 29–50. [Google Scholar]
- Borkenstein, A.; Jödicke, R. Thermoregulatory behaviour of Sympetrum striolatum at low temperatures with special reference to the role of direct sunlight (Odonata: Libellulidae). Odonatologica 2022, 51, 83–109. [Google Scholar] [CrossRef]
- Basel, A.M.; Simaika, J.P.; Samways, M.J.; Midgley, G.F.; Latombe, G.; MacFadyen, S.; Hui, C. Drivers of compositional turnover in narrow-ranged and widespread dragonflies and damselflies in Africa. Insect Conserv. Divers. 2024, 17, 501–511. [Google Scholar] [CrossRef]
- Devictor, V.; Julliard, R.; Jiguet, F. Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos 2008, 117, 507–514. [Google Scholar] [CrossRef]
- De, K.; Dey, D.; Shruti, M.; Uniyal, V.P.; Adhikari, B.S.; Johnson, J.A.; Hussain, S.A. β-diversity of odonate community of the Ganga River: Partitioning and insights from local and species contribution. Wetl. Ecol. Manag. 2023, 31, 899–912. [Google Scholar] [CrossRef]
- Nascimento, A.C.L.; Martins, A.R.; Dias-Silva, K.; Juen, L.; Mortati, A.F.; Oliveira-Junior, J.M.B. Species and Site Contributions to Odonata adult assemblage uniqueness in Brazilian Amazon streams. J. Insect Conserv. 2025, 29, 13. [Google Scholar] [CrossRef]
- Kokkoris, I.; Dimopoulos, P.; Xystrakis, F.; Tsiripidis, I. National scale ecosystem condition assessment with emphasis on forest types in Greece. One Ecosyst. 2018, 3, e25434. [Google Scholar] [CrossRef]

| Suborder | Species | Haliacmon | Almyravlakas | Loudias | Axios | Gallikos |
|---|---|---|---|---|---|---|
| Zygoptera | Calopteryx splendens | 253 | 556 | |||
| Zygoptera | Erythromma lindenii | 5 | ||||
| Zygoptera | Erythromma viridulum | 2 | 16 | 17 | ||
| Zygoptera | Ischnura elegans | 101 | 130 | 73 | 580 | 188 |
| Zygoptera | Chalcolestes parvidens | 1 | ||||
| Zygoptera | Lestes barbarus | 4 | 2 | |||
| Zygoptera | Lestes macrostigma | 17 | 1 | |||
| Zygoptera | Platycnemis pennipes | 206 | 5 | 1 | 231 | 87 |
| Anisoptera | Aeshna affinis | 16 | ||||
| Anisoptera | Aeshna mixta | 9 | 5 | 2 | ||
| Anisoptera | Anax imperator | 13 | 15 | |||
| Anisoptera | Anax parthenope | 2 | 5 | 12 | 2 | 29 |
| Anisoptera | Isoaeshna isoceles | 4 | 1 | |||
| Anisoptera | Gomphus vulgatissimus | 2 | ||||
| Anisoptera | Lindenia tetraphylla | 3 | 3 | 18 | ||
| Anisoptera | Onychogomphus forcipatus | 2 | 9 | 4 | ||
| Anisoptera | Ophiogomphus cecilia | 1 | ||||
| Anisoptera | Stylurus flavipes | 1 | ||||
| Anisoptera | Crocothemis erythraea | 306 | 168 | 175 | 888 | 257 |
| Anisoptera | Libellula fulva | 21 | 3 | |||
| Anisoptera | Orthetrum albistylum | 112 | 38 | 49 | 271 | 130 |
| Anisoptera | Orthetrum brunneum | 1 | 41 | 7 | ||
| Anisoptera | Orthetrum cancellatum | 57 | 15 | 7 | 79 | 10 |
| Anisoptera | Orthetrum coerulescens | 9 | 21 | 24 | ||
| Anisoptera | Sympetrum fonscolombii | 206 | 65 | 84 | 323 | 227 |
| Anisoptera | Sympetrum meridionale | 7 | 6 | |||
| Anisoptera | Sympetrum sanguineum | 1 | 9 | |||
| Anisoptera | Sympetrum striolatum | 1 |
| Analysis | Factor (Classes) | Total | Zygoptera | Anisoptera | |||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| PERMDIST | River | 1.296 | 0.24 | 0.544 | 0.69 | 2.751 | 0.031 |
| Habitat | 4.337 | 0.001 | 2.578 | 0.011 | 3.176 | 0.002 | |
| CLC | 5.024 | 0.001 | 1.827 | 0.061 | 3.63 | 0.001 | |
| ESA WorldCover | 1.159 | 0.352 | 0.58 | 0.673 | 0.378 | 0.846 | |
| PERMANOVA | River | 1.09 | 0.332 | 0.948 | 0.477 | 1.733 | 0.016 |
| Habitat | 1.224 | 0.127 | 1.199 | 0.235 | 1.033 | 0.413 | |
| CLC | 0.675 | 0.649 | 2.354 | 0.08 | 0.015 | 0.998 | |
| ESA WorldCover | 2.536 | 0.001 | 1.843 | 0.057 | 2.433 | 0.003 | |
| (CR-HW) | 3.743 | 0.003 | 2.774 | 0.016 | |||
| (BV-CR) | 3.834 | 0.005 | 2.761 | 0.01 | |||
| (CR-GR) | 3.307 | 0.008 | |||||
| (BV-GR) | 2.759 | 0.015 | 3.937 | 0.003 | |||
| (BV-TC) | 2.372 | 0.026 | |||||
| (CR-TC) | 2.391 | 0.04 | 2.628 | 0.017 | |||
| (GR-TC) | 2.391 | 0.04 | 2.492 | 0.029 | |||
| (GR-HW) | 2.917 | 0.012 | |||||
| Factor | Variable | H | p | Dunn’s Post Hoc Test |
|---|---|---|---|---|
| River | RTD (A) | 11.53 | 0.0212 | GA-HA * |
| Mean U (A,Z) | 12.66 | 0.0130 | HA-LO * | |
| Habitat | Mean U (A) | 32.84 | 0.0006 | 92A0-92D0 **, 92A0-1410 * |
| LCBD (ab) | 42.69 | 1.23 × 10−5 | 92A0-92D0 *, 92A0-1410 * | |
| WorldCover | S (A,Z) | 68.77 | 6.51 × 10−15 | BV-TC ***, CR-TC ***, GR-TC ***, BV-HW **, CR-HW **, GR-HW ** |
| Abundance (Z) | 19.32 | 6.79 × 10−4 | CR-HW **, CR-TC ** | |
| 1-D (Z) | 31.83 | 2.07 × 10−6 | BV-TC ***, CR-TC ***, GR-TC ** | |
| ENS (A,Z) | 56.22 | 1.80 × 10−11 | BV-TC ***, CR-TC ***, GR-TC ***, CR-HW **, BV-HW * | |
| RTD (A,Z) | 60.86 | 1.92 × 10−12 | BV-TC ***, CR-TC ***, GR-TC ***, CR-HW **, GR-HW ** | |
| Mean U (A,Z) | 35.67 | 3.39 × 10−7 | GR-TC ***, HW-TC ***, BV-TC **, CR-TC * | |
| LCBD (ab) (Z) | 18.35 | 1.10 × 10−3 | CR-HW * | |
| FDis (A) | 29.86 | 5.22 × 10−6 | CR-TC ***, BV-TC *, GR-TC *, HW-TC * | |
| RaoQ (A) | 26.73 | 2.26 × 10−5 | CR-TC ***, GR-TC **, BV-TC *, HW-TC * | |
| DBI (A) | 29.06 | 7.61 × 10−6 | CR-TC ***, GR-TC **, BV-TC *, CR-HW * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaltsas, D.; Alvanou, L.; Ekklisiarchos, I.; Raptis, D.I.; Avtzis, D.N. Canopy Cover Drives Odonata Diversity and Conservation Prioritization in the Protected Wetland Complex of Thermaikos Gulf (Greece). Forests 2025, 16, 1181. https://doi.org/10.3390/f16071181
Kaltsas D, Alvanou L, Ekklisiarchos I, Raptis DI, Avtzis DN. Canopy Cover Drives Odonata Diversity and Conservation Prioritization in the Protected Wetland Complex of Thermaikos Gulf (Greece). Forests. 2025; 16(7):1181. https://doi.org/10.3390/f16071181
Chicago/Turabian StyleKaltsas, Dimitris, Lydia Alvanou, Ioannis Ekklisiarchos, Dimitrios I. Raptis, and Dimitrios N. Avtzis. 2025. "Canopy Cover Drives Odonata Diversity and Conservation Prioritization in the Protected Wetland Complex of Thermaikos Gulf (Greece)" Forests 16, no. 7: 1181. https://doi.org/10.3390/f16071181
APA StyleKaltsas, D., Alvanou, L., Ekklisiarchos, I., Raptis, D. I., & Avtzis, D. N. (2025). Canopy Cover Drives Odonata Diversity and Conservation Prioritization in the Protected Wetland Complex of Thermaikos Gulf (Greece). Forests, 16(7), 1181. https://doi.org/10.3390/f16071181






