Endophytic Bacterial Consortia Isolated from Disease-Resistant Pinus pinea L. Increase Germination and Plant Quality in Susceptible Pine Species (Pinus radiata D. Don)
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Characterization of Endophytic Bacteria from Pinus pinea L. Roots
2.1.1. Sampling and Isolation
2.1.2. Molecular Typing and Identification
2.1.3. Screening of Endophytic Bacteria for Plant Growth-Promoting Traits
2.2. Bacterial Formulations and PGPB Inoculation in Pinus radiata D. Don Seeds
2.2.1. Bacterial Formulations
2.2.2. Pinus radiata Seed Inoculation and Climatic Chamber Experiment
2.2.3. Seedling Emergence
2.2.4. Seedling Height, Root Size, and Biomass
2.3. Plant Biochemical Profile
2.3.1. Photosynthetic Pigments
2.3.2. Total Soluble Sugars and Starch
2.3.3. Total Phenolic Compounds
2.3.4. Total Flavonoid Content
2.3.5. Free Amino Acids
2.3.6. Malondialdehyde (MDA) Content
2.4. Statistical Analysis
2.5. Data Visualization
3. Results
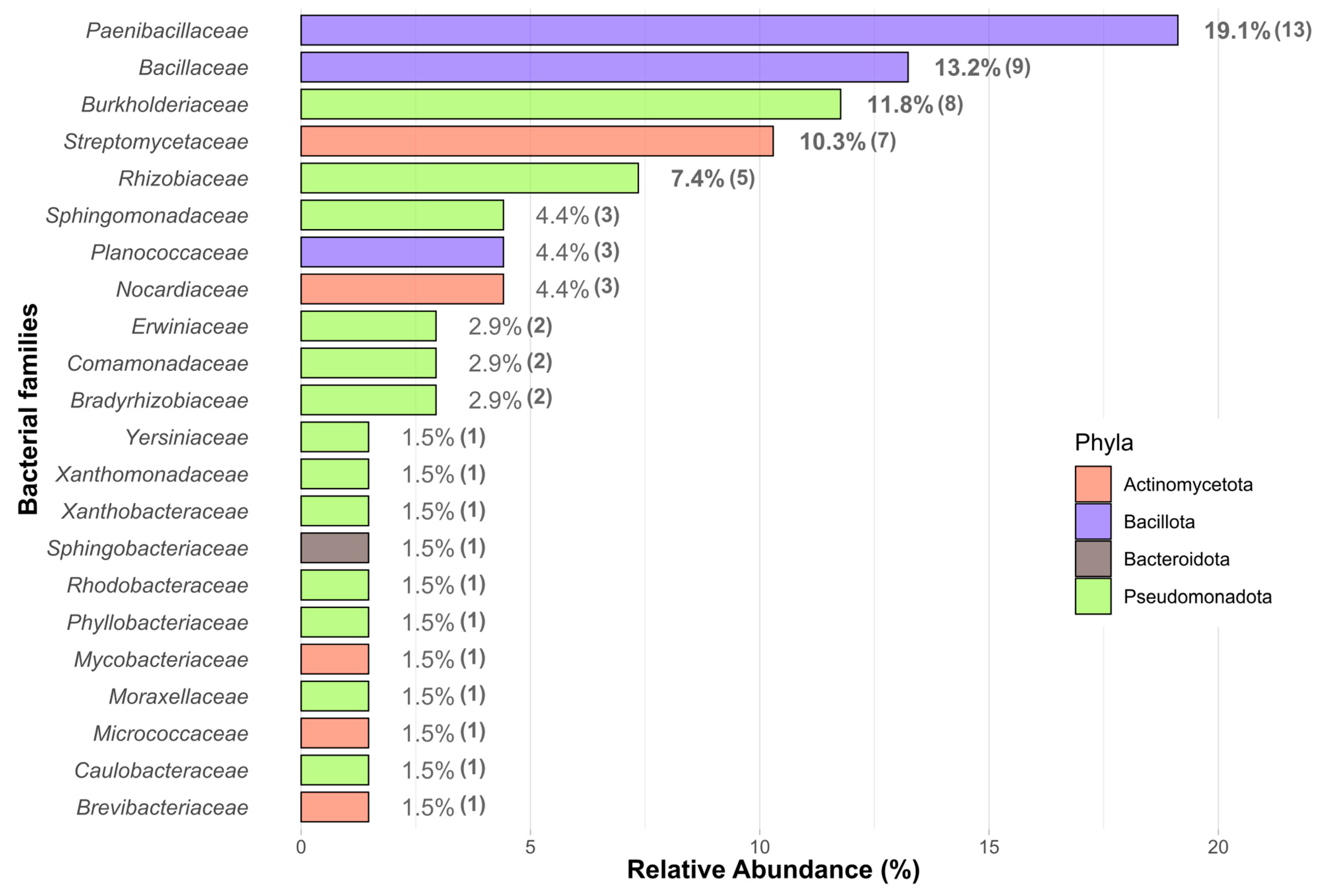
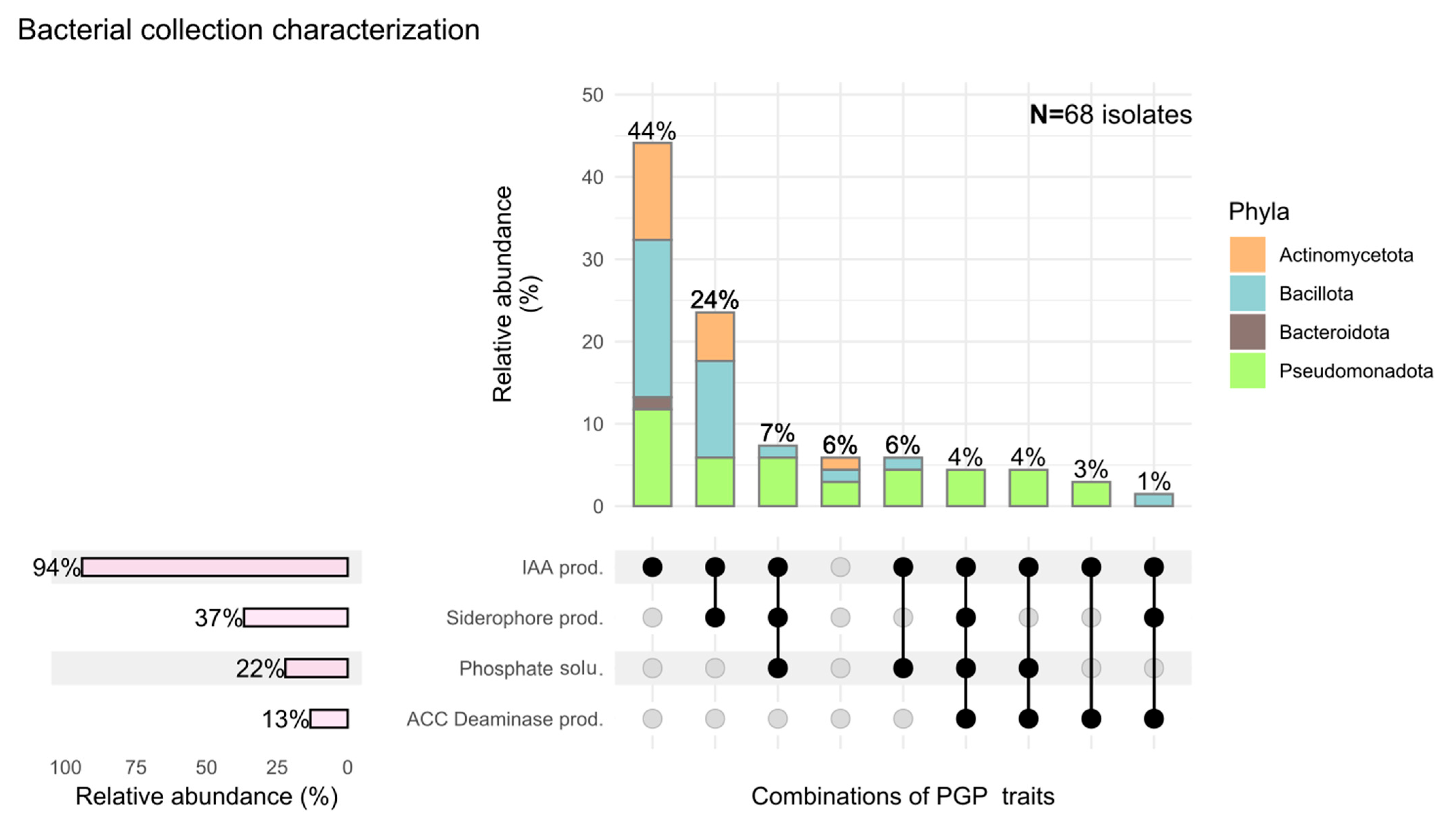
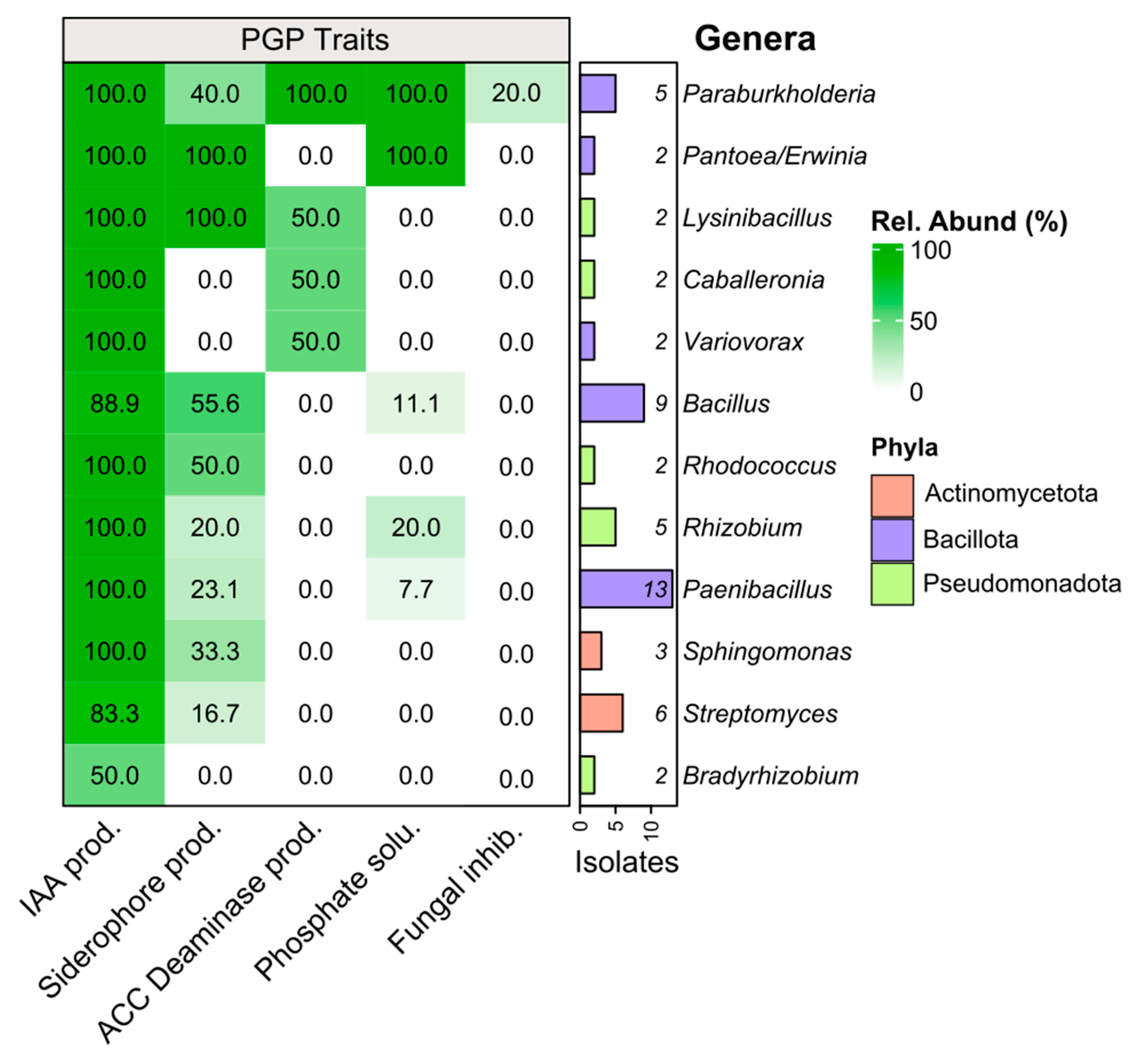
3.1. Diversity and PGP-Related Functions of Pinus pinea L. Endophytes
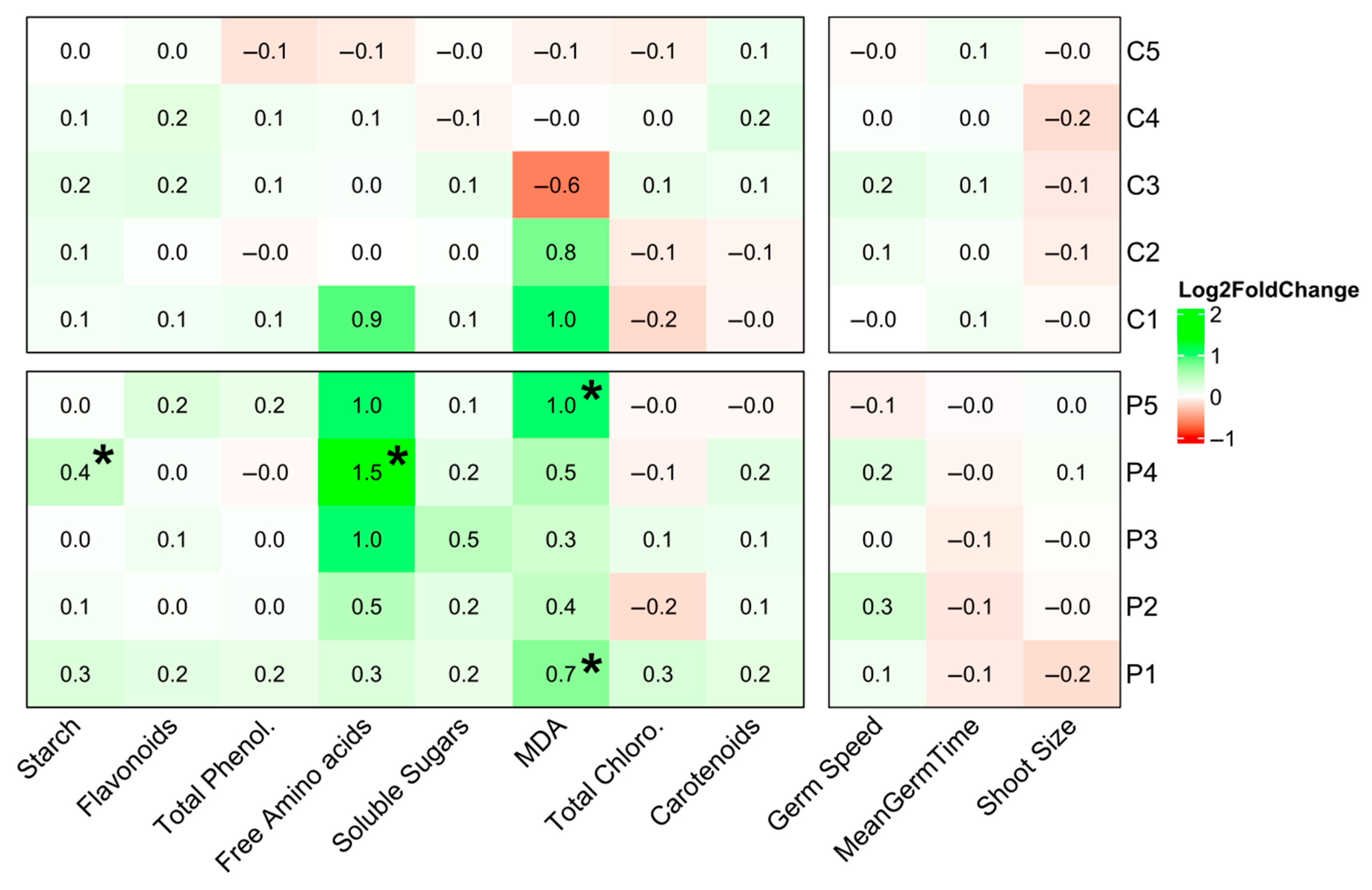
3.2. Pinus radiata D. Don Seeds Germination, Morphology, and Plant Quality After PGPB Inoculation
4. Discussion
4.1. Endophyte Diversity and Plant Growth-Promoting Traits
4.2. Bacterial Formulations Impact on Germination and Seedling Quality Profiles
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PGP | Plant growth-promoting |
| PGPB | Plant growth-promoting bacteria |
| IAA | Indole-3-acetic acid |
| ACC | 1-aminocyclopropane-1-carboxylate |
| TSB | Tryptic soy broth |
| TSA | Tryptic soy agar |
| R2A | Reasoner’s 2A agar |
| PDA | Potato dextrose agar |
| CAS | Chrome Azurol S |
| NBRIP | National Botanical Research Institute’s phosphate |
| OD | Optical density |
| MDA | Malonaldehyde |
References
- Mead, D.J. Sustainable Management of Pinus Radiata Plantations.; FAO Forestry Paper No. 170; FAO: Rome, Italy, 2013. [Google Scholar]
- Ayrilmis, N.; Buyuksari, U.; Avci, E.; Koc, E. Utilization of Pine (Pinus pinea L.) Cone in Manufacture of Wood Based Composite. For. Ecol. Manage. 2009, 259, 65–70. [Google Scholar] [CrossRef]
- Pignatti, G.; Facciotto, G.; Incollu, G.; Maltoni, S.; Marongiu, M.; Sperandio, G.; Verani, S.; Puxeddu, M. Sustainable Forest Management in Radiata Pine Plantations: A Case Study in Sardinia (Italy). Environ. Sci. Proc. 2020, 3, 51. [Google Scholar] [CrossRef]
- Mosquera-Losada, M.R.; Rigueiro-Rodríguez, A.; Ferreiro-Domínguez, N. Effect of Liming and Organic and Inorganic Fertilization on Soil Carbon Sequestered in Macro-and Microaggregates in a 17-Year Old Pinus Radiata Silvopastoral System. J. Environ. Manage. 2015, 150, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Balla, A.; Silini, A.; Cherif-Silini, H.; Bouket, A.C.; Moser, W.K.; Nowakowska, J.A.; Oszako, T.; Benia, F.; Belbahri, L. The Threat of Pests and Pathogens and the Potential for Biological Control in Forest Ecosystems. Forests 2021, 12, 1579. [Google Scholar] [CrossRef]
- Amaral, J.; Correia, B.; António, C.; Rodrigues, A.M.; Gómez-Cadenas, A.; Valledor, L.; Hancock, R.D.; Alves, A.; Pinto, G. Pinus Susceptibility to Pitch Canker Triggers Specific Physiological Responses in Symptomatic Plants: An Integrated Approach. Front. Plant Sci. 2019, 10, 509. [Google Scholar] [CrossRef]
- Monteiro, P.; Valledor, L.; Osorio, S.; Camisón, Á.; Vallarino, J.G.; Gómez-Cadenas, A.; Díez, J.J.; Pinto, G. Physiological, Metabolic and Hormonal Responses of Two Pinus Spp. with Contrasting Susceptibility to Brown-Spot Needle Blight Disease. Tree Physiol. 2024, 44, tpae003. [Google Scholar] [CrossRef]
- Pimentel, C.S.; Gonçalves, E.V.; Firmino, P.N.; Calvão, T.; Fonseca, L.; Abrantes, I.; Correia, O.; Máguas, C. Differences in Constitutive and Inducible Defences in Pine Species Determining Susceptibility to Pinewood Nematode. Plant Pathol. 2017, 66, 131–139. [Google Scholar] [CrossRef]
- Zamora-Ballesteros, C.; Pinto, G.; Amaral, J.; Valledor, L.; Alves, A.; Diez, J.J.; Martín-García, J. Dual Rna-Sequencing Analysis of Resistant (Pinus pinea) and Susceptible (Pinus radiata) Hosts during Fusarium Circinatum Challenge. Int. J. Mol. Sci. 2021, 22, 5231. [Google Scholar] [CrossRef]
- Feng, Z.; Liang, Q.; Yao, Q.; Bai, Y.; Zhu, H. The Role of the Rhizobiome Recruited by Root Exudates in Plant Disease Resistance: Current Status and Future Directions. Environ. Microbiome 2024, 19, 91. [Google Scholar] [CrossRef]
- Leitão, F.; Pinto, G.; Amaral, J.; Monteiro, P.; Henriques, I. New Insights into the Role of Constitutive Bacterial Rhizobiome and Phenolic Compounds in Two Pinus Spp. with Contrasting Susceptibility to Pine Pitch Canker. Tree Physiol. 2021, 42, 600–615. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant Growth-Promoting Bacterial Endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Pavlo, A.; Leonid, O.; Iryna, Z.; Natalia, K.; Maria, P.A. Endophytic Bacteria Enhancing Growth and Disease Resistance of Potato (Solanum tuberosum L.). Biol. Control 2011, 56, 43–49. [Google Scholar] [CrossRef]
- Glandorf, D.C.M.; Verheggen, P.; Jansen, T.; Jorritsma, J.-W.; Smit, E.; Leeflang, P.; Wernars, K.; Thomashow, L.S.; Laureijs, E.; Thomas-Oates, J.E.; et al. Effect of Genetically Modified Pseudomonas Putida WCS358r on the Fungal Rhizosphere Microflora of Field-Grown Wheat. Appl. Environ. Microbiol. 2001, 67, 3371–3378. [Google Scholar] [CrossRef] [PubMed]
- Abro, M.A.; Sun, X.; Li, X.; Jatoi, G.H.; Guo, L.-D. Biocontrol Potential of Fungal Endophytes against Fusarium oxysporum f. Sp. Cucumerinum Causing Wilt in Cucumber. Plant Pathol. J. 2019, 35, 598. [Google Scholar] [CrossRef]
- Sarma, B.K.; Yadav, S.K.; Singh, S.; Singh, H.B. Microbial Consortium-Mediated Plant Defense against Phytopathogens: Readdressing for Enhancing Efficacy. Soil Biol. Biochem. 2015, 87, 25–33. [Google Scholar] [CrossRef]
- Kondo, Y.R.; da Cruz, S.P.; Chanway, C.; Kaschuk, G. Inoculation with Azospirillum brasilense or Bacillus Spp. Improves Root Growth and Nutritional Quality of Araucaria (Araucaria angustifolia) Seedlings. For. Ecol. Manage. 2024, 568, 122092. [Google Scholar] [CrossRef]
- Bahramov, R.; Mamatyusupov, A.; Tokhtaboeva, F.; Khomidov, J.; Yuldashev, H. A Comprehensive Application of Fertilizers for Growing Plantations in Forest Nurseries: A Brief Review. IOP Conf. Ser. Earth Environ. Sci. 2020, 614, 12117. [Google Scholar] [CrossRef]
- Jung, T.; Orlikowski, L.; Henricot, B.; Abad-Campos, P.; Aday, A.G.; Aguín Casal, O.; Bakonyi, J.; Cacciola, S.O.; Cech, T.; Chavarriaga, D.; et al. Widespread Phytophthora Infestations in European Nurseries Put Forest, Semi-Natural and Horticultural Ecosystems at High Risk of Phytophthora Diseases. For. Pathol. 2016, 46, 134–163. [Google Scholar] [CrossRef]
- Boix-Fayos, C.; de Vente, J. Challenges and Potential Pathways towards Sustainable Agriculture within the European Green Deal. Agric. Syst. 2023, 207, 103634. [Google Scholar] [CrossRef]
- Hallmann, J.; Quadt-Hallmann, A.; Mahaffee, W.F.; Kloepper, J.W. Bacterial Endophytes in Agricultural Crops. Can. J. Microbiol. 1997, 43, 895–914. [Google Scholar] [CrossRef]
- Ali, S.; Charles, T.C.; Glick, B.R. Delay of Flower Senescence by Bacterial Endophytes Expressing 1-Aminocyclopropane-1-Carboxylate Deaminase. J. Appl. Microbiol. 2012, 113, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Gao, J.; Chen, X.; Zhang, M.; Yang, F.; Du, Y.; Munir, I.; Xue, J.; Zhang, X. Isolation and Characterization of Plant Growth-Promoting Endophytic Bacteria Paenibacillus polymyxa SK1 from Lilium lancifolium. Biomed Res. Int. 2020, 2020, 8650957. [Google Scholar] [CrossRef] [PubMed]
- Proença, D.N.; Francisco, R.; Kublik, S.; Schöler, A.; Vestergaard, G.; Schloter, M.; Morais, P. V The Microbiome of Endophytic, Wood Colonizing Bacteria from Pine Trees as Affected by Pine Wilt Disease. Sci. Rep. 2017, 7, 4205. [Google Scholar] [CrossRef]
- Mesanza, N.; Iturritxa, E.; Patten, C.L. Native Rhizobacteria as Biocontrol Agents of Heterobasidion annosum Ss and Armillaria mellea Infection of Pinus Radiata. Biol. Control 2016, 101, 8–16. [Google Scholar] [CrossRef]
- Iturritxa, E.; Trask, T.; Mesanza, N.; Raposo, R.; Elvira-Recuenco, M.; Patten, C.L. Biocontrol of Fusarium circinatum Infection of Young Pinus radiata Trees. Forests 2017, 8, 32. [Google Scholar] [CrossRef]
- Shen, S.Y.; Fulthorpe, R. Seasonal Variation of Bacterial Endophytes in Urban Trees. Front. Microbiol. 2015, 6, 427. [Google Scholar] [CrossRef]
- Versalovic, J. Genomic Fingerprinting of Bacteria Using Repetitive Sequence-Based Polymerase Chain Reaction. Methods Mol. Cell Biol. 1994, 5, 25–40. [Google Scholar] [CrossRef]
- Lane, D.J. 16S/23S RRNA Sequencing. In Nucleic acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley and sons: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A New Generation of Protein Database Search Programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Kim, O.; Cho, Y.; Lee, K.; Yoon, S.; Kim, M.; Na, H.; Park, S.; Jeon, Y.S.; Lee, J.; Yi, H.; et al. Introducing EzTaxon-e: A Prokaryotic 16S RRNA Gene Sequence Database with Phylotypes That Represent Uncultured Species. Int. J. Syst. Evol. Microbiol. 2012, 62, 716–721. [Google Scholar] [CrossRef]
- Fidalgo, C.; Henriques, I.; Rocha, J.; Tacão, M.; Alves, A. Culturable Endophytic Bacteria from the Salt Marsh Plant Halimione portulacoides: Phylogenetic Diversity, Functional Characterization, and Influence of Metal (Loid) Contamination. Environ. Sci. Pollut. Res. 2016, 23, 10200–10214. [Google Scholar] [CrossRef]
- Dworkin, M.; Foster, J. Experiments with Some Microorganisms Which Utilize Ethane and Hydrogen. J. Bacteriol. 1958, 75, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.A.; Weber, R.P. Colorimetric Estimation of Indolacetic Acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, C.S. An Efficient Microbiological Growth Medium for Screening Phosphate Solubilizing Microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Miranda, S.; Cabirol, N.; George-Téllez, R.; Zamudio-Rivera, L.S.; Fernández, F.J. O-CAS, a Fast and Universal Method for Siderophore Detection. J. Microbiol. Methods 2007, 70, 127–131. [Google Scholar] [CrossRef]
- Anith, K.N.; Nysanth, N.S.; Natarajan, C. Novel and Rapid Agar Plate Methods for In Vitro Assessment of Bacterial Biocontrol Isolates’ Antagonism Against Multiple Fungal Phytopathogens. Lett. Appl. Microbiol. 2021, 73, 229–236. [Google Scholar] [CrossRef]
- Martínez-Álvarez, P.; Alves-Santos, F.M.; Diez, J.J. In Vitro and In Vivo Interactions Between Trichoderma viride and Fusarium circinatum. Silva Fenn. 2012, 46, 303–316. [Google Scholar] [CrossRef]
- Silva-Castro, I.; Diez, J.J.; Martín-Ramos, P.; Pinto, G.; Alves, A.; Martín-Gil, J.; Martín-García, J. Application of Bioactive Coatings Based on Chitosan and Propolis for Pinus Spp. Protection against Fusarium circinatum. Forests 2018, 9, 685. [Google Scholar] [CrossRef]
- López-Hidalgo, C.; Meijón, M.; Lamelas, L.; Valledor, L. The Rainbow Protocol: A Sequential Method for Quantifying Pigments, Sugars, Free Amino Acids, Phenolics, Flavonoids and MDA from a Small Amount of Sample. Plant Cell Environ. 2021, 44, 1977–1986. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts: I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant Growth Promoting Bacteria in Agriculture: Two Sides of a Coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- Reed, L.; Glick, B.R. The Recent Use of Plant-Growth-Promoting Bacteria to Promote the Growth of Agricultural Food Crops. Agriculture 2023, 13, 1089. [Google Scholar] [CrossRef]
- Stewart, E.J. Growing Unculturable Bacteria. J. Bacteriol. 2012, 194, 4151–4160. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Anderson, I.C.; Killham, K.; Moore, E.R.B. Diversity of Predominant Endophytic Bacteria in European Deciduous and Coniferous Trees. Can. J. Microbiol. 2008, 54, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Bal, A.; Anand, R.; Berge, O.; Chanway, C.P. Isolation and Identification of Diazotrophic Bacteria from Internal Tissues of Pinus contorta and Thuja plicata. Can. J. For. Res. 2012, 42, 807–813. [Google Scholar] [CrossRef]
- Harirchi, S.; Sar, T.; Ramezani, M.; Aliyu, H.; Etemadifar, Z.; Nojoumi, S.A.; Yazdian, F.; Awasthi, M.K.; Taherzadeh, M.J. Bacillales: From Taxonomy to Biotechnological and Industrial Perspectives. Microorganisms 2022, 10, 2355. [Google Scholar] [CrossRef]
- Liu, Y.; Ponpandian, L.N.; Kim, H.; Jeon, J.; Hwang, B.S.; Lee, S.K.; Park, S.-C.; Bae, H. Distribution and Diversity of Bacterial Endophytes from Four Pinus Species and Their Efficacy as Biocontrol Agents for Devastating Pine Wood Nematodes. Sci. Rep. 2019, 9, 12461. [Google Scholar] [CrossRef]
- Carper, D.L.; Carrell, A.A.; Kueppers, L.M.; Frank, A.C. Bacterial Endophyte Communities in Pinus Flexilis Are Structured by Host Age, Tissue Type, and Environmental Factors. Plant Soil 2018, 428, 335–352. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Li, Y.; Liu, Z.; Li, D.; Wen, X.; Feng, Y.; Zhang, X. Pinewood Nematode Alters the Endophytic and Rhizospheric Microbial Communities of Pinus massoniana. Microb. Ecol. 2021, 81, 807–817. [Google Scholar] [CrossRef]
- Khalid, A.; Tahir, S.; Arshad, M.; Zahir, Z.A. Relative Efficiency of Rhizobacteria for Auxin Biosynthesis in Rhizosphere and Non-Rhizosphere Soils. Soil Res. 2004, 42, 921–926. [Google Scholar] [CrossRef]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V.; Jeffery, L.D. Structure, Variation, and Assembly of the Root-Associated Microbiomes of Rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef]
- Ali, B.; Sabri, A.N.; Ljung, K.; Hasnain, S. Auxin Production by Plant Associated Bacteria: Impact on Endogenous IAA Content and Growth of Triticum aestivum L. Lett. Appl. Microbiol. 2009, 48, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Bal, H.B.; Das, S.; Dangar, T.K.; Adhya, T.K. ACC Deaminase and IAA Producing Growth Promoting Bacteria from the Rhizosphere Soil of Tropical Rice Plants. J. Basic Microbiol. 2013, 53, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Mohite, B. Isolation and Characterization of Indole Acetic Acid (IAA) Producing Bacteria from Rhizospheric Soil and Its Effect on Plant Growth. J. Soil Sci. Plant Nutr. 2013, 13, 638–649. [Google Scholar] [CrossRef]
- Lebrazi, S.; Fadil, M.; Chraibi, M.; Fikri-Benbrahim, K. Screening and Optimization of Indole-3-Acetic Acid Production by Rhizobium Sp. Strain Using Response Surface Methodology. J. Genet. Eng. Biotechnol. 2020, 18, 21. [Google Scholar] [CrossRef]
- Liu, W.H.; Chen, F.F.; Wang, C.E.; Fu, H.H.; Fang, X.Q.; Ye, J.R.; Shi, J.Y. Indole-3-Acetic Acid in Burkholderia pyrrocinia JK-SH007: Enzymatic Identification of the Indole-3-Acetamide Synthesis Pathway. Front. Microbiol. 2019, 10, 484770. [Google Scholar] [CrossRef]
- Ait Bessai, S.; Bensidhoum, L.; Nabti, E. hafid Optimization of IAA Production by Telluric Bacteria Isolated from Northern Algeria. Biocatal. Agric. Biotechnol. 2022, 41, 102319. [Google Scholar] [CrossRef]
- Leveau, J.H.J.; Gerards, S. Discovery of a Bacterial Gene Cluster for Catabolism of the Plant Hormone Indole 3-Acetic Acid. FEMS Microbiol. Ecol. 2008, 65, 238–250. [Google Scholar] [CrossRef]
- Zarkan, A.; Liu, J.; Matuszewska, M.; Gaimster, H.; Summers, D.K. Local and Universal Action: The Paradoxes of Indole Signalling in Bacteria. Trends Microbiol. 2020, 28, 566–577. [Google Scholar] [CrossRef]
- Walpola, B.C.; Hettiarachchi, R.H.A.N. Comparison of Qualitative and Quantitative Methods for Isolation of Phosphate Solubilizing Microorganisms. Vidyodaya J. Sci. 2020, 23, 14–22. [Google Scholar]
- Sarwar, S.; Khaliq, A.; Yousra, M.; Sultan, T.; Ahmad, N.; Khan, M. Screening of Siderophore-Producing PGPRs Isolated from Groundnut (Arachis hypogaea L.) Rhizosphere and Their Influence on Iron Release in Soil. Commun. Soil Sci. Plant Anal. 2020, 51, 1680–1692. [Google Scholar] [CrossRef]
- Khan, A.; Doshi, H.V.; Thakur, M.C. Bacillus Spp.: A Prolific Siderophore Producer. In Bacilli and Agrobiotechnology; Islam, M.T., Rahman, M., Pandey, P., Jha, C.K., Aeron, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 309–323. ISBN 978-3-319-44409-3. [Google Scholar]
- Tiwari, S.; Prasad, V.; Lata, C. Chapter 3—Bacillus: Plant Growth Promoting Bacteria for Sustainable Agriculture and Environment. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, J.S., Singh, D.P., Eds.; Elsevier: New York, NY, USA, 2019; pp. 43–55. ISBN 978-0-444-64191-5. [Google Scholar]
- Abeysinghe, S. Effect of Combined Use of Bacillus subtilis CA32 and Trichoderma harzianum RU01 on Biological Control of Rhizoctonia Solani on Solanum melongena and Capsicum annuum. Plant Pathol. J. 2009, 8, 9–16. [Google Scholar] [CrossRef]
- Madhaiyan, M.; Selvakumar, G.; Alex, T.H.; Cai, L.; Ji, L. Plant Growth Promoting Abilities of Novel Burkholderia-Related Genera and Their Interactions With Some Economically Important Tree Species. Front. Sustain. Food Syst. 2021, 5, 618305. [Google Scholar] [CrossRef]
- Ghosh, R.; Barman, S.; Mukherjee, R.; Mandal, N.C. Role of Phosphate Solubilizing Burkholderia Spp. for Successful Colonization and Growth Promotion of Lycopodium cernuum L.(Lycopodiaceae) in Lateritic Belt of Birbhum District of West Bengal, India. Microbiol. Res. 2016, 183, 80–91. [Google Scholar] [CrossRef]
- Donoso, R.; Leiva-Novoa, P.; Zúñiga, A.; Timmermann, T.; Recabarren-Gajardo, G.; González, B. Biochemical and Genetic Bases of Indole-3-Acetic Acid (Auxin Phytohormone) Degradation by the Plant-Growth-Promoting Rhizobacterium Paraburkholderia phytofirmans PsJN. Appl. Environ. Microbiol. 2017, 83, e01991-16. [Google Scholar] [CrossRef]
- Barrera-Galicia, G.C.; Peniche-Pavía, H.A.; Peña-Cabriales, J.J.; Covarrubias, S.A.; Vera-Núñez, J.A.; Délano-Frier, J.P. Metabolic Footprints of Burkholderia sensu Lato Rhizosphere Bacteria Active against Maize Fusarium Pathogens. Microorganisms 2021, 9, 2061. [Google Scholar] [CrossRef]
- Carrión, V.J.; Cordovez, V.; Tyc, O.; Etalo, D.W.; de Bruijn, I.; de Jager, V.C.L.; Medema, M.H.; Eberl, L.; Raaijmakers, J.M. Involvement of Burkholderiaceae and Sulfurous Volatiles in Disease-Suppressive Soils. ISME J. 2018, 12, 2307–2321. [Google Scholar] [CrossRef]
- Puri, A.; Padda, K.P.; Chanway, C.P. Evidence of Endophytic Diazotrophic Bacteria in Lodgepole Pine and Hybrid White Spruce Trees Growing in Soils with Different Nutrient Statuses in the West Chilcotin Region of British Columbia, Canada. For. Ecol. Manage. 2018, 430, 558–565. [Google Scholar] [CrossRef]
- Pahari, A.; Mishra, B.B. Characterization of Siderophore Producing Rhizobacteria and Its Effect on Growth Performance of Different Vegetables. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1398–1405. [Google Scholar] [CrossRef]
- Kumar, P.; Thakur, S.; Dhingra, G.K.; Singh, A.; Pal, M.K.; Harshvardhan, K.; Dubey, R.C.; Maheshwari, D.K. Inoculation of Siderophore Producing Rhizobacteria and Their Consortium for Growth Enhancement of Wheat Plant. Biocatal. Agric. Biotechnol. 2018, 15, 264–269. [Google Scholar] [CrossRef]
- Zou, X.; Binkley, D.; Doxtader, K.G. A New Method for Estimating Gross Phosphorus Mineralization and Immobilization Rates in Soils. Plant Soil 1992, 147, 243–250. [Google Scholar] [CrossRef]
- Davey, M.W.; Stals, E.; Panis, B.; Keulemans, J.; Swennen, R.L. High-Throughput Determination of Malondialdehyde in Plant Tissues. Anal. Biochem. 2005, 347, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhong, S.; Grierson, D. Recent Advances in Ethylene Research. J. Exp. Bot. 2009, 60, 3311–3336. [Google Scholar] [CrossRef] [PubMed]
- Belimov, A.A.; Safronova, V.I.; Sergeyeva, T.A.; Egorova, T.N.; Matveyeva, V.A.; Tsyganov, V.E.; Borisov, A.Y.; Tikhonovich, I.A.; Kluge, C.; Preisfeld, A.; et al. Characterization of Plant Growth Promoting Rhizobacteria Isolated from Polluted Soils and Containing 1-Aminocyclopropane-1-Carboxylate Deaminase. Can. J. Microbiol. 2001, 47, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Silveira, V.; Balbuena, T.S.; Santa-Catarina, C.; Floh, E.I.S.; Guerra, M.P.; Handro, W. Biochemical Changes during Seed Development in Pinus taeda L. Plant Growth Regul. Plant Growth Regul. 2004, 44, 147–156. [Google Scholar] [CrossRef]
- Pullman, G.S.; Buchanan, M. Identification and Quantitative Analysis of Stage-Specific Carbohydrates in Loblolly Pine (Pinus taeda) Zygotic Embryo and Female Gametophyte Tissues. Tree Physiol. 2008, 28, 985–996. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Plant Growth-Promoting Bacteria of Soil: Designing of Consortia Beneficial for Crop Production. Microorganisms 2023, 11, 2864. [Google Scholar] [CrossRef]
- Pascale, A.; Proietti, S.; Pantelides, I.S.; Stringlis, I.A. Modulation of the Root Microbiome by Plant Molecules: The Basis for Targeted Disease Suppression and Plant Growth Promotion. Front. Plant Sci. 2020, 10, 1741. [Google Scholar] [CrossRef]
| Plant Growth-Promoting Traits | ||||||
|---|---|---|---|---|---|---|
| Strains | Genera | Bacterial Formulation | Phosphate Solu. | IAA (µg/mL) | Siderophores | Fungal Inhib. |
| Paenibacillus T.M5R4 | Paenibacillus | P1; P2; P3; P4; P5 | - | 21.7 | - | - |
| Bacillus R.M2R7 | Bacillus | P2; P3; P4; P5 | - | 6.46 | Positive | - |
| Acinetobacter T.M2R22 | Acinetobacter | P3; P4; P5 | Positive | 13.5 | Positive | - |
| Paraburkholderia R.M1R3 | Paraburkholderia | P4; P5 | Positive | 12.65 | Positive | - |
| Rahnella T.M2R17 | Rahnella | P5 | Positive | 67.01 | - | Positive |
| Caballeronia R.M3R3 | Caballeronia | C1; C2; C3; C4; C5 | - | 16.44 | - | - |
| Rhodococcus T.M4R4 | Rhodococcus | C2; C3; C4; C5 | - | 35.5 | Positive | - |
| Mesorhizobium R.M1R2 | Mesorhizobium | C3; C4; C5 | Positive | 33.15 | Positive | - |
| Burkholderia R.M1R13 | Burkholderia | C4; C5 | Positive | 13.01 | Positive | - |
| Paraburkholderia R.M2R9 | Paraburkholderia | C5 | Positive | 11.35 | - | Positive |
| Cont. | C5 | C4 | C3 | C2 | C1 | P5 | P4 | P3 | P2 | P1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Germ. Percentage | 70.8% | 75% | 72.9% | 89.5% | 79.2% | 77.1% | 66.7% | 81.3% | 68.8% | 83.3% | 70.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitão, F.; Alves, M.; Henriques, I.; Pinto, G. Endophytic Bacterial Consortia Isolated from Disease-Resistant Pinus pinea L. Increase Germination and Plant Quality in Susceptible Pine Species (Pinus radiata D. Don). Forests 2025, 16, 1161. https://doi.org/10.3390/f16071161
Leitão F, Alves M, Henriques I, Pinto G. Endophytic Bacterial Consortia Isolated from Disease-Resistant Pinus pinea L. Increase Germination and Plant Quality in Susceptible Pine Species (Pinus radiata D. Don). Forests. 2025; 16(7):1161. https://doi.org/10.3390/f16071161
Chicago/Turabian StyleLeitão, Frederico, Marta Alves, Isabel Henriques, and Glória Pinto. 2025. "Endophytic Bacterial Consortia Isolated from Disease-Resistant Pinus pinea L. Increase Germination and Plant Quality in Susceptible Pine Species (Pinus radiata D. Don)" Forests 16, no. 7: 1161. https://doi.org/10.3390/f16071161
APA StyleLeitão, F., Alves, M., Henriques, I., & Pinto, G. (2025). Endophytic Bacterial Consortia Isolated from Disease-Resistant Pinus pinea L. Increase Germination and Plant Quality in Susceptible Pine Species (Pinus radiata D. Don). Forests, 16(7), 1161. https://doi.org/10.3390/f16071161







