The Effects of Tree Species on Soil Organic Carbon Mineralization in Reservoir Water-Level Drawdown Zones
Abstract
1. Introduction
2. Materials and Methods
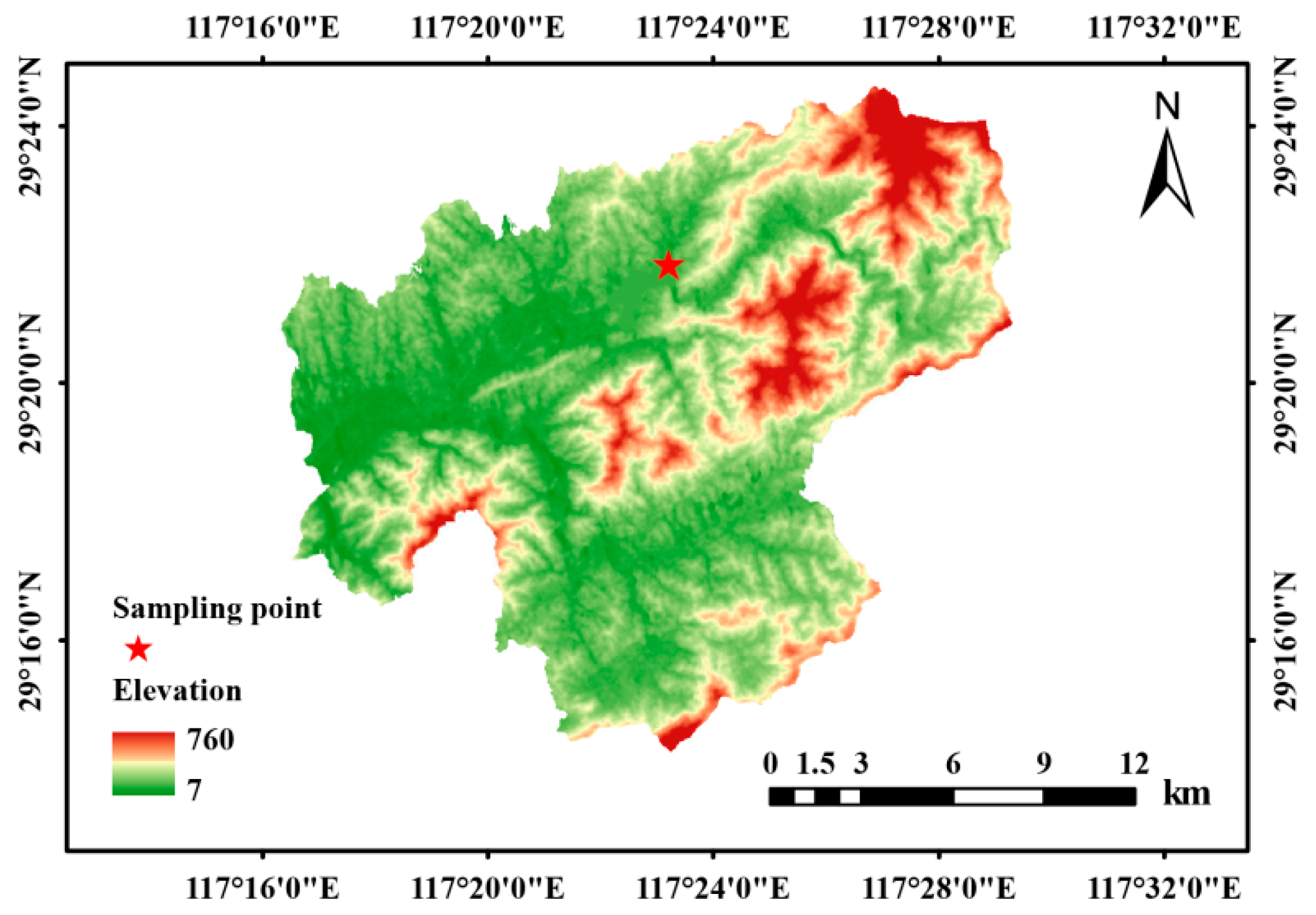
2.1. Study Site
2.2. Experimental Design
2.3. Sampling
2.4. Methods
2.5. Statistical Analyses
3. Results
3.1. Soil Biochemical Properties of Different Tree Species
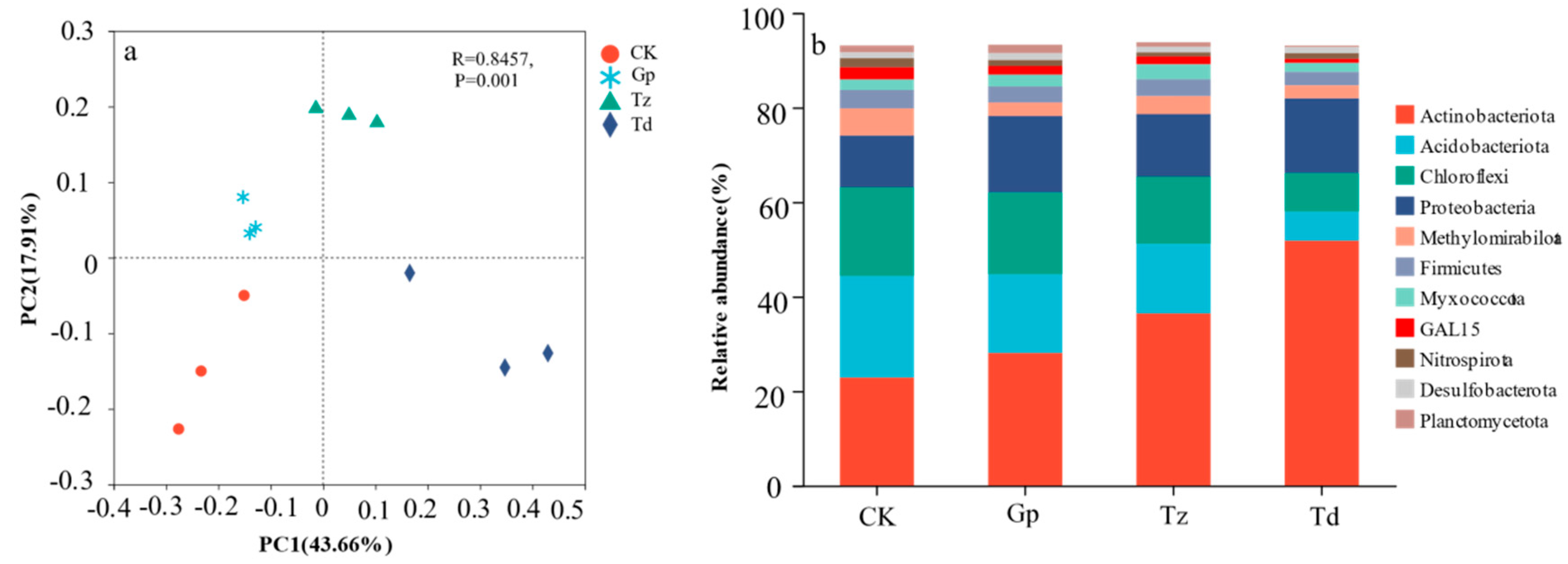
3.2. Characteristics of Soil Bacterial Community in Different Tree Species
3.3. Characteristics of SOC Mineralization of Different Tree Species
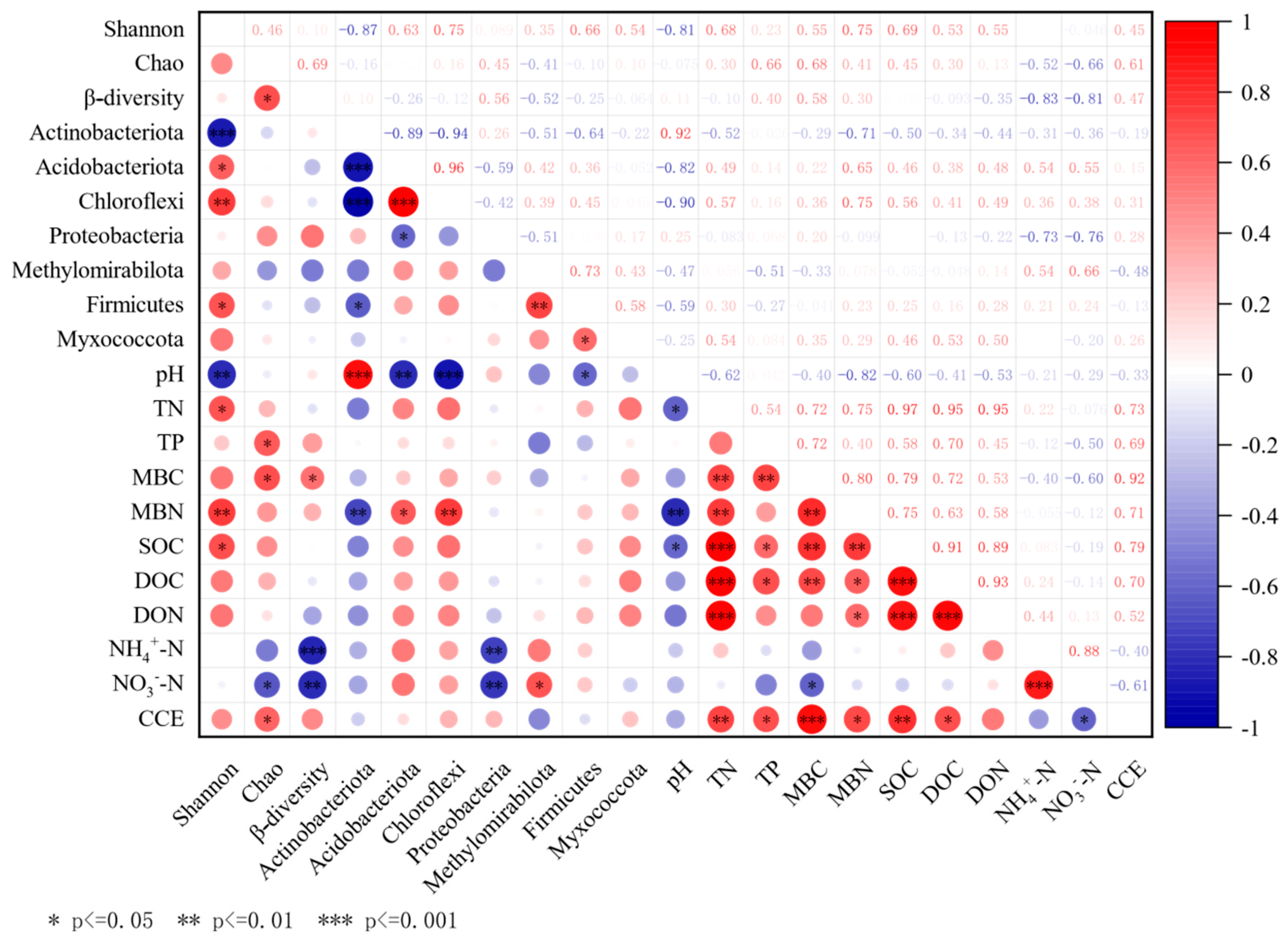
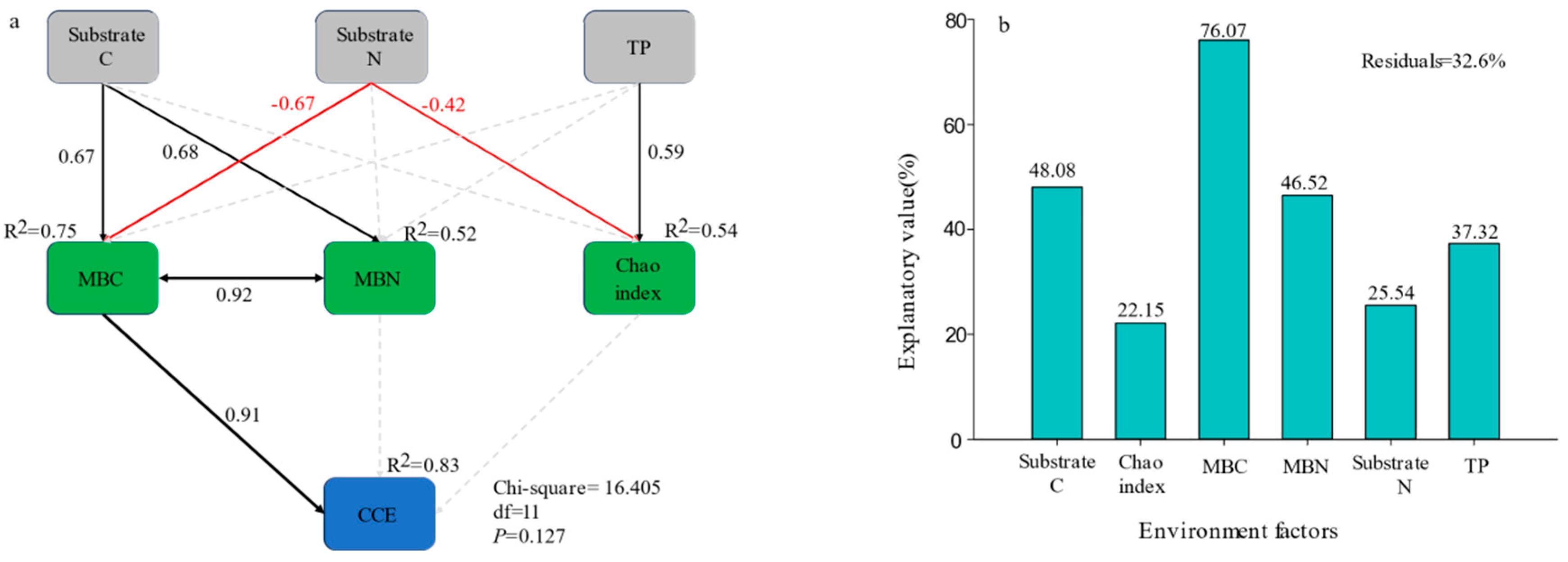
3.4. Factors Affecting SOC Mineralization in Different Tree Species
4. Discussion
4.1. The Effects of Different Tree Species on Soil Biochemical Properties and Microorganisms
4.2. Factors Affecting SOC Mineralization in Different Tree Species
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, H.W.; Cui, H.L.; Fu, C.X.; Li, R.; Qi, F.Y.; Liu, Z.L.; Yang, G.; Xiao, K.Q.; Qiao, M. Unveiling the crucial role of soil microorganisms in carbon cycling: A review. Sci. Total Environ. 2024, 909, 168627. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Morrissey, E.M.; Mau, R.L.; Hayer, M.; Piñeiro, J.; Mack, M.; Marks, J.; Bell, S.; Miller, S.; Schwartz, E.; et al. The temperature sensitivity of soil: Microbial biodiversity, growth, and carbon mineralization. ISME J. 2021, 15, 2738–2747. [Google Scholar] [CrossRef] [PubMed]
- Morrien, E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; de Hollander, M. Soil networks become more connected and take up more carbon as nature restoration progresses. Nat. Commun. 2017, 8, 14349. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liu, S.G.; Kim, D.G.; Peng, C.; Sweeney, S.; Shangguan, Z. Past and future carbon sequestration benefits of China’s grain for green program. Glob. Environ. Change 2017, 47, 13–20. [Google Scholar] [CrossRef]
- Wu, J.G.; Zhang, X.Q.; Xu, D.Y. The mineralization of soil organic carbon under different land uses in the liupan mountain forest zone. Acta Phytoecol. Sin. 2004, 28, 530–538. [Google Scholar]
- Ladegaard-Pedersen, P.; Elberling, B.; Vesterdal, L. Soil carbon stocks, mineralization rates, and CO2 effluxes under 10 tree species on contrasting soil types. Can. J. For. Res. 2005, 35, 1277–1284. [Google Scholar] [CrossRef]
- Fang, T.; Li, Y.C.; Yao, Z.X.; Yu, Y.F. Effects of planting broadleaf trees and Moso bamboo on soil carbon mineralization and microbial community structure. Chin. J. Appl. Ecol. 2021, 32, 82–92. [Google Scholar]
- Thoms, C.; Gattinger, A.; Jacob, M.; Thomas, F.M.; Gleixner, G. Direct and indirect effects of tree diversity drive soil microbial diversity in temperate deciduous forest. Soil Biol. Biochem. 2010, 42, 1558–1565. [Google Scholar] [CrossRef]
- Segura, J.H.; Nilsson, M.B.; Sparrman, T.; Serk, H.; Öquist, M.G. Boreal tree species affect soil organic matter composition and saprotrophic mineralization rates. Plant Soil 2019, 441, 173–190. [Google Scholar] [CrossRef]
- Drake, J.E.; Darby, B.A.; Giasson, M.A.; Kramer, M.A.; Finzi, A.C. Stoichiometry constrains microbial response to root exudation- insights from a model and a field experiment in a temperate forest. Biogeosciences 2013, 10, 821–838. [Google Scholar] [CrossRef]
- Wang, Q.K.; Zhong, M.C. Composition and mineralization of soil organic carbon pools in four single-tree species forest soils. J. For. Res. 2016, 27, 1277–1285. [Google Scholar] [CrossRef]
- Deemer, B.R.; Harrison, J.A.; Li, S.Y.; Beaulieu, J.J.; Delsontro, T.; Barros, N.; José, F.B.N.; Powers, S.M.; dos Santos, M.A.; Vonk, J.A. Greenhouse gas emissions from reservoir water surfaces: A new global synthesis. Bioscience 2016, 66, 949–964. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, R.; Muller, R.A.; Clow, D.; Verpoorter, C.; Sobek, S. Organic carbon burial in global lakes and reservoirs. Nat. Commun. 2017, 8, 1694. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Yoon, T.K.; Lee, S.H.; Kang, H.; Park, J.H. Enhanced greenhouse gas emission from exposed sediments along a hydroelectric reservoir during an extreme drought event. Environ. Res. Lett. 2016, 11, 124003. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Zhang, Y.J.; Yan, Y.; Liu, Y.H.; Xu, Y.; Liu, Y.Q.; Guo, X.M.; Lou, Y.L.; Lu, S.B. Response to different temperatures under forest recovery types on soil organic carbon mineralization in subtropics. Acta Ecol. Sin. 2018, 38, 5056–5066. [Google Scholar]
- Xu, X.C.; Zhang, Q.Q.; Teng, Q.M.; Zhao, M.S.; Li, Y.C. Rhizosphere effects of moso bamboo and dominant tree species of secondary broadleaved forest on soil organic carbon mineralization. Chin. J. Appl. Ecol. 2023, 34, 2374–2382. [Google Scholar]
- Lu, R.K. Soil Agrochemical Analysis Methods; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Chen, C.R.; Xu, Z.H.; Keay, P.; Zhang, S.L. Total soluble nitrogen in forest soils as determined by persulfate oxidation and by high temperature catalytic oxidation. Aust. J. Soil Res. 2005, 43, 515–523. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen:the effects of fumigation time and temperature. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Chen, C.R.; Xu, Z.H.; Mathers, N.J. Soil carbon pools in adjacent natural and plantation forests of subtropical Australia. Soil Sci. Soc. Am. J. 2004, 68, 282–291. [Google Scholar] [CrossRef]
- Ran, K.; Wang, Z.J.; Li, P. Response difference of bacterial and fungal structure and diversity in rhizosphere soil of typical urban tree along urban-rural environmental gradient. Acta Ecol. Sin. 2023, 43, 9758–9769. [Google Scholar]
- Smith, J.L.; Schobel, R.R.; Mcneal, B.L.; Campbell, G.S. Potential errors in the first-order model for estimating soil nitrogen mineralization potentials. Soil Sci. Soc. Am. J. 1980, 44, 996–1000. [Google Scholar] [CrossRef]
- Lü, D.; Yang, Y.H.; Zhao, W.H.; Lei, S.Y.; Zhang, X.P. Fine root biomass distribution and coupling to soil physicochemical properties under different restored vegetation types. Acta Ecol. Sin. 2018, 38, 3979–3987. [Google Scholar]
- Li, J.W.; Li, M.Y.; Dong, L.B.; Wang, K.B.; Liu, Y.L.; Hai, X.Y.; Pan, Y.Y.; Lv, W.W.; Wang, X.Z.; Shangguan, Z.P.; et al. Plant productivity and microbial composition drive soil carbon and nitrogen sequestrations following cropland abandonment. Sci. Total Environ. 2020, 744, 140802. [Google Scholar] [CrossRef]
- Hong, S.B.; Piao, S.L.; Chen, A.P.; Liu, Y.W.; Peng, L.L. Afforestation neutralizes soil pH. Nat. Commun. 2018, 9, 520. [Google Scholar] [CrossRef]
- Mei, Y.; Huang, P.; Wang, P.; Zhu, K. Effects of water level fluctuations and vegetation restoration on soil prokaryotic microbial community structure in the riparian zone of the three Gorges reservoir. Environ. Sci. 2024, 45, 2715–2726. [Google Scholar]
- Hong, S.B.; Yin, G.D.; Piao, S.L.; Dybzinski, R.; Cong, N.; Li, X.Y.; Wang, K.; Peñuelas, J.; Zeng, H.; Chen, A.P. Divergent responses of soil organic carbon to afforestation. Nat. Sustain. 2020, 3, 694–700. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Hang, Z.Q.; Wang, G.X.; Liu, J.E.; Wang, G.; Wang, H. Characterization of soil organic carbon fractions at Spartina alterniflora saltmarsh in North Jiangsu. Acta Ecol. Sin. 2014, 34, 4175–4182. [Google Scholar]
- Li, Y.; Li, Z.; Cui, S.; Liang, G.P.; Zhang, Q.P. Microbial-derived carbon components are critical for enhancing soil organic carbon in no-tillage croplands: A global perspective. Soil Tillage Res. 2021, 205, 104758. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Bai, S.B. Effects of nitrogen forms on nutrient uptake and growth of trees. Chin. J. Appl. Ecol. 2003, 14, 2044–2048. [Google Scholar]
- Li, Z.L.; Zeng, Z.Q.; Tian, D.H.; Wang, J.S.; Wang, B.X.; Han, Y.H.; Chen, Q.Q.; Chen, W.N.; Yang, J.L.; Meng, C.; et al. Global variations and controlling factors of soil nitrogen turnover rate. Earth-Sci. Rev. 2020, 207, 103250. [Google Scholar] [CrossRef]
- Han, X.; Yuan, C.Y.; Li, J.H.; Hong, Z.W.; Liu, X.; Du, T.; Li, H.; You, C.M.; Tan, B.; Zhu, P.; et al. Effects of tree species and soil layers on soil extractable nitrogen content. Ecol. Environ. Sci. 2022, 31, 2143–2151. [Google Scholar]
- Zhu, S.-G.; Tao, H.-Y.; Li, W.-B.; Zhou, R.; Gui, Y.-W.; Zhu, L.; Zhang, X.-L.; Wang, W.; Wang, B.-Z.; Mei, F.-J.; et al. Phosphorus availability mediates plant–plant interaction and field productivity in maize-grass pea intercropping system: Field experiment and its global validation. Agric. Syst. 2023, 205, 103584. [Google Scholar] [CrossRef]
- Chen, X.L.; Chen, H.Y.H.; Chang, S.X. Meta-analysis shows that plant mixtures increase soil phosphorus availability and plant productivity in diverse ecosystems. Nat. Ecol. Evol. 2022, 6, 1112–1121. [Google Scholar] [CrossRef]
- Fang, X.H.; Zhang, J.C.; Meng, M.J.; Guo, X.P.; Wu, Y.W.; Liu, X.; Zhao, K.L.; Ding, L.Z.; Shao, Y.F.; Fu, W.J. Forest-type shift and subsequent intensive management affected soil organic carbon and microbial community in southeastern China. Eur. J. For. Res. 2017, 136, 689–697. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef]
- Jiao, S.; Chen, W.M.; Wang, J.L.; Du, N.N.; Li, Q.P.; Wei, G. Soil microbiomes with distinct assemblies through vertical soil profiles drive the cycling of multiple nutrients in reforested ecosystems. Microbiome 2018, 6, 146. [Google Scholar] [CrossRef]
- Liu, X.S.; Siemann, E.; Cui, C.; Liu, Y.Q.; Guo, X.M.; Zhang, L. Moso bamboo (Phyllostachys edulis) invasion effects on litter, soil and microbial PLFA characteristics depend on sites and invaded forests. Plant Soil 2019, 79, 439–449. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Yang, Y.K.; Zou, Y.; Zou, Y.; DIlida, Y.; Zhai, C.; Sun, D.; Li, M.; Li, Y.; Liu, P. cultivable symbiotic actinomycetes of dictyostelid cellular slime molds in different regions. J. Fungal Res. 2023, 21, 131–142. [Google Scholar]
- Yang, Y.; Dou, Y.X.; Wang, B.R.; Wang, Y.Q.; Liang, C.; An, S.S.; Soromotin, A.; Kuzyakov, Y. Increasing contribution of microbial residues to soil organic carbon in grassland restoration chronosequence. Soil Biol. Biochem. 2022, 170, 108688. [Google Scholar] [CrossRef]
- Yin, S.; Bai, J.H.; Wang, W.; Zhang, G.L.; Jia, J.; Cui, B.S.; Liu, X.H. Effects of soil moisture on carbon mineralization in floodplain wetlands with different flooding frequencies. J. Hydrol. 2019, 574, 1074–1084. [Google Scholar] [CrossRef]
- Wei, Z.Q.; Wu, S.H.; Zhou, S.L.; Lin, C. Installation of impervious surface in urban areas affects microbial biomass, activity (potential C mineralisation), and functional diversity of the fine earth. Soil Res. 2013, 51, 59–67. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, T.T.; Shi, L.L.; Kurganova, I.; Lopes de Gerenyu, V.; Kalinina, O.; Giani, L.; Kuzyakov, Y. Organic carbon accumulation and microbial activities in arable soils after abandonment: A chronosequence study. Geoderma 2023, 435, 116496. [Google Scholar] [CrossRef]
- Shu, X.Y.; Hu, Y.F.; Liu, W.J.; Xia, L.; Zhang, Y.; Zhou, W.; Liu, W.; Zhang, Y. Linking between soil properties, bacterial communities, enzyme activities, and soil organic carbon mineralization under ecological restoration in an alpine degraded grassland. Front. Microbiol. 2023, 14, 1131836. [Google Scholar] [CrossRef]
- Yang, H.; Cao, J.H.; Zhang, L.K.; Hou, Y.L.; Mao, L.F. Pool sizes and turnover of soil organic carbon of farmland soil in Karst area of Guilin. J. Northeast Agric. Univ. (Engl. Ed.) 2011, 18, 39–45. [Google Scholar] [CrossRef]
- Patoine, G.L.; Eisenhauer, N.; Cesarz, S.; Phillips, H.R.P.; Guerra, C.A.; Xu, X.F.; Zhang, L.H. Drivers and trends of global soil microbial carbon over two decades. Nat. Commun. 2022, 13, 4195. [Google Scholar] [CrossRef]
- Guo, Z.; Han, J.C.; Li, J.; Xu, Y.; Wang, X.L.; Li, Y. Effects of long-term fertilization on soil organic carbon mineralization and microbial community structure. PLoS ONE 2019, 14, e0211163. [Google Scholar] [CrossRef]
- Kemmitt, S.J.; Lanyon, C.V.; Waite, I.S.; Wen, Q.; Addiscott, T.M.; Bird, N.R.A.; O’Donnell, A.G.; Brookes, P.C. Mineralization of native soil organic matter is not regulated by the size, activity or composition of the soil microbial biomass-a new perspective. Soil Biol. Biochem. 2008, 40, 61–73. [Google Scholar] [CrossRef]

| Item | CK | G. pensilis | T. Zhongshanshan | T. distichum |
|---|---|---|---|---|
| pH | 4.35 ± 0.02 c | 4.55 ± 0.05 c | 4.89 ± 0.07 b | 5.63 ± 0.08 a |
| TP (g·kg−1) | 0.34 ± 0.02 c | 0.42 ± 0.02 ab | 0.43 ± 0.02 a | 0.37 ± 0.01 bc |
| TN (g·kg−1) | 1.08 ± 0.069 ab | 1.20 ± 0.08 a | 1.21 ± 0.22 a | 0.72 ± 0.01 b |
| SOC (g·kg−1) | 6.507 ± 0.66 ab | 7.99 ± 0.15 a | 7.64 ± 1.17 ab | 5.36 ± 0.20 b |
| MBC (mg·kg−1) | 149.40 ± 15.62 bc | 207.45 ± 5.14 a | 173.36 ± 14.78 ab | 131.42 ± 5.06 c |
| MBN (mg·kg−1) | 11.24 ± 1.75 ab | 13.16 ± 1.35 a | 11.18 ± 0.67 ab | 7.16 ± 1.09 b |
| DOC (mg·kg−1) | 57.171 ± 0.98 c | 72.25 ± 2.52 b | 96.35 ± 5.32 a | 39.40 ± 1.58 d |
| DON (mg·kg−1) | 8.49 ± 0.42 b | 7.85 ± 0.28 b | 12.41 ± 1.11 a | 3.98 ± 0.01 c |
| NH4+-N (mg·kg−1) | 120.151 ± 9.52 a | 85.36 ± 4.11 c | 115.90 ± 4.28 ab | 98.12 ± 2.03 bc |
| NO3−-N (mg·kg−1) | 2.86 ± 0.32 a | 0.92 ± 0.11 c | 1.73 ± 0.12 b | 1.56 ± 0.14 bc |
| Species | OTU Richness | Shannon Index | Chao Richness | Coverage/% |
|---|---|---|---|---|
| CK | 2630 ± 53 b | 6.03 ± 0.06 a | 3337 ± 75 b | 98.03 ± 0.00 a |
| G. pensilis | 3159 ± 112 a | 6.31 ± 0.09 a | 3974 ± 149 a | 97.65 ± 0.00 b |
| T. Zhongshanshan | 2843 ± 117 ab | 5.95 ± 0.08 a | 3607 ± 190 ab | 97.87 ± 0.00 ab |
| T. distichum | 2718 ± 146 b | 4.90 ± 0.51 b | 3530 ± 184 ab | 97.80 ± 0.00 ab |
| Species | Potentially Carbon Mineralizable (C0) (mg·kg−1) | Constant (K) | Relation Coefficient (R2) |
|---|---|---|---|
| CK | 281.468 ± 41.174 b | 0.023 ± 0.002 b | 0.997 ± 0.001 ab |
| G. pensilis | 614.831 ± 89.932 a | 0.018 ± 0.002 b | 0.999 ± 0.000 a |
| T. Zhongshanshan | 513.859 ± 59.407 a | 0.017 ± 0.003 b | 0.999 ± 0.001 a |
| T. distichum | 227.177 ± 24.311 b | 0.031 ± 0.003 a | 0.994 ± 0.002 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, F.; Yang, J.; Zhang, Y.; Qiu, L.; Chen, Z.; Wang, X.; Zhang, T.; Li, S.; Tong, J.; et al. The Effects of Tree Species on Soil Organic Carbon Mineralization in Reservoir Water-Level Drawdown Zones. Forests 2025, 16, 1145. https://doi.org/10.3390/f16071145
Zhang J, Wang F, Yang J, Zhang Y, Qiu L, Chen Z, Wang X, Zhang T, Li S, Tong J, et al. The Effects of Tree Species on Soil Organic Carbon Mineralization in Reservoir Water-Level Drawdown Zones. Forests. 2025; 16(7):1145. https://doi.org/10.3390/f16071145
Chicago/Turabian StyleZhang, Jiayi, Fang Wang, Jia Yang, Yanting Zhang, Li Qiu, Ziting Chen, Xi Wang, Tianya Zhang, Songzhe Li, Jiacheng Tong, and et al. 2025. "The Effects of Tree Species on Soil Organic Carbon Mineralization in Reservoir Water-Level Drawdown Zones" Forests 16, no. 7: 1145. https://doi.org/10.3390/f16071145
APA StyleZhang, J., Wang, F., Yang, J., Zhang, Y., Qiu, L., Chen, Z., Wang, X., Zhang, T., Li, S., Tong, J., Lu, S., & Zhang, Y. (2025). The Effects of Tree Species on Soil Organic Carbon Mineralization in Reservoir Water-Level Drawdown Zones. Forests, 16(7), 1145. https://doi.org/10.3390/f16071145





