1. Introduction
The accumulation and stabilization of soil organic carbon (SOC) are critical components of global carbon (C) cycling and climate change mitigation [
1]. Globally, forest ecosystems store approximately 861 Pg C, with over 40% sequestered in soils, often exceeding the C stored in aboveground and belowground biomass [
2,
3,
4,
5,
6]. SOC represents a long-term carbon sink whose dynamics are modulated by various biotic and abiotic factors. These include stand age, vegetation type, soil characteristics, climate, and forest management [
7,
8]. Due to the growing interest in carbon neutrality and sustainable forest development, accurately quantifying and modeling of SOC is essential for national greenhouse gas inventories and implementing sustainable forest management practices. Therefore, accurate estimates and models of carbon stocks both in aboveground biomass (the most studied component) and in belowground components (which can represent over 60% of a stand’s C stocks depending on the soil type) [
4,
5,
6] are essential.
In Chile,
Pinus radiata D. Don is the most widely planted tree species, covering over 1.3 million hectares and constituting around 56% of the total area under plantation [
9]. Due to its rapid growth, adaptability to various environmental limitations, and high productivity under intensive silvicultural practices, it dominates the Chilean forestry sector alongside
Eucalyptus sp. plantations [
9,
10,
11,
12,
13]. Numerous studies have investigated the productivity and aboveground carbon stocks of radiata pine stands, particularly with respect to biomass. However, research on forest floor and belowground carbon, particularly SOC, has received comparatively less attention, especially in mature stands [
4,
5,
6,
14]. SOC comprises a significant portion of the total ecosystem carbon, particularly in the deep mineral horizons which function as long-term carbon reservoirs [
15,
16]. Therefore, improving our understanding of SOC in intensively managed radiata pine plantations is crucial for optimizing national carbon budgets and developing site-specific management strategies.
The diversity of soil types in south-central Chile, ranging from volcanic ash in the Andes, sandy soils in the central valley, and granitic and/or metamorphic substrates in the Coastal Range and coastal zone, adds complexity to SOC assessments [
4,
17]. Volcanic ash soils, particularly Andisols, have been shown to exhibit high SOC retention. This phenomenon is attributed to the presence of amorphous minerals such as allophane, imogolite, and iron oxides. These minerals stabilize soil organic matter (SOM), the organic material embedded in mineral soil [
18], leading to low bulk density and high carbon concentrations [
19,
20,
21,
22]. Conversely, sandy soils have low water holding capacity and limited nutrient availability. This characteristic accelerates SOM decomposition and reduces SOC storage [
4,
5]. Granitic soils, characterized by their coarse texture and limited nutrient-holding capacity [
22], exhibit the potential to enhance long-term SOC accumulation through intensive forestry practices, such as fertilization. These practices promote vegetation growth and the accumulation of SOM [
4,
6]. Metamorphic soils, characterized by their complex mineral compositions, have been shown to exert a regulatory effect on the rate of SOM decomposition, thereby contributing to stabilizing the SOC [
6]. Recent studies in southern and central Chile suggest that the carbon stocks in adult radiata pine plantations can vary by more than 40% depending on soil type [
4,
5,
6]. This highlights the necessity of incorporating soil properties, such as soil texture, soil water holding capacity, and fertility, into the estimation of SOC to ensure precision and accuracy.
The variability of SOM and SOC is influenced by a combination of soil properties and climatic factors, as these elements affect microbial activity and the turnover rate of organic matter [
18,
23]. Climatic variables, particularly precipitation, temperature, and potential evapotranspiration, play a key role in forest growth, productivity, and biomass accumulation of forests [
10,
12]. Furthermore, they have been shown to influence the following: organic matter inputs to the soil, soil respiration, and the decomposition rates of SOM, affecting both the carbon dynamics in the forest floor and mineral soil [
24]. Many studies have identified a negative correlation between mean temperature and C stock, while a positive correlation has been observed with annual precipitation [
25,
26,
27]. For example, higher SOC stocks are typically observed in humid temperate regions than in warm zones due to slower decomposition of SOM and higher litter accumulation [
4,
8,
15,
28].
Although mineral soil is the main long-term carbon pool, the forest floor plays a crucial role in short-term carbon dynamics and nutrient cycling. It contributes organic matter to mineral soil from aboveground biomass. The forest floor is composed of accumulated leaf litter, decomposing woody material, and humus [
29]. The structure and biomass of the forest floor are highly sensitive to environmental conditions, stand age, species, and soil fertility [
4,
18,
30,
31]. Volcanic soils often support thick, slowly decomposing organic layers, while sandy soils tend to have thinner, rapidly cycling litter layers [
4]. However, not all studies reflect this trend, suggesting that soil types may interact with climatic factors in forest soil carbon stocks [
5,
32]. Integrating forest floor carbon evaluations with SOC assessments provides a more comprehensive understanding of total ecosystem carbon stocks and temporal dynamics.
In the current climate change context, where temperature increases and precipitation decreases are expected [
33], it is especially important to understand how climate, soil, and site characteristics affect carbon stocks in forest plantations. In our study, we developed empirical models to estimate carbon stocks in the total biomass, the mineral soil (SOC), and the forest floor in sites with adult
Pinus radiata plantations. We used environmental variables that can be easily acquired, as well as soil variables that were measured and sampled in situ, such as soil bulk density, texture, nitrogen content, and soil water availability. Field data were collected from plots in central Chile across soils of different parent materials (granitic, sandy, metamorphic, and recent ash), enabling us to calibrate predictive linear models for SOC at depths of up to 1 m. Our fitted models require data that can be easily collected in the field. Unlike other models, such as process-based models or spatially explicit models [
34,
35,
36], ours do not require large amounts of input data. Our objectives were therefore to assess the aboveground and belowground carbon stocks in radiata pine plantations across four different soil types, and to model these carbon stocks in relation to climate and soil properties.
2. Materials and Methods
2.1. Study Area and Experimental Design
This study includes the sites presented and detailed by Bozo et al. [
5] and Asmussen et al. [
6]. In brief, twenty sites were selected to represent a productivity gradient of adult radiata pine plantations under intensive management at harvesting age (18–25 years old, the age at which radiata pine plantations are typically harvested in Chile) (
Table 1 and
Figure 1). These sites cover a significant portion of the productive area of radiata pine plantations in Chile [
9]. The sites were located in central and south-central Chile, spanning from the Maule (35°25′ S) and Araucanía (38°54′ S) administrative regions, and from 72°58′ W to 71°43′ W from west to east. The altitudes ranged from 106 to 849 m above sea level. The soils were classified as coarse volcanic sand, granitic, metamorphic, and recent volcanic ash [
17,
37]. The selected stands at each soil site were planted between 1998 and 2004, with an initial planting density ranging from 1000 to 1250 trees ha
−1.
Table 1.
Site characteristics of selected locations that consider a range of productivity sites for contrasting sandy, granitic, metamorphic, and recent ash soils.
Table 1.
Site characteristics of selected locations that consider a range of productivity sites for contrasting sandy, granitic, metamorphic, and recent ash soils.
| Soil | Soil Order a | DBH | Height | VHA | Age |
|---|
| (cm) | (m) | (m3 ha−1) | (Years) |
|---|
| Sandy | Entisol | 29.2 | 28.3 | 333.8 | 22 |
| Sandy | Entisol | 34.3 | 27.4 | 431.4 | 21 |
| Sandy | Entisol | 35.6 | 30.2 | 470.8 | 22 |
| Sandy | Entisol | 34.4 | 30.0 | 517.5 | 23 |
| Sandy | Entisol | 36.9 | 36.3 | 625.4 | 23 |
| Granitic | Inceptisol | 29.1 | 27.3 | 363.0 | 19 |
| Granitic | Inceptisol | 30.4 | 30.6 | 397.9 | 20 |
| Granitic | Ultisol | 34.2 | 25.8 | 437.4 | 22 |
| Granitic | Ultisol | 36.6 | 30.6 | 574.6 | 21 |
| Granitic | Inceptisol | 36.0 | 34.2 | 669.8 | 25 |
| Recent Ash | Andisol | 35.5 | 31.3 | 387.6 | 21 |
| Recent Ash | Andisol | 33.8 | 32.0 | 432.2 | 21 |
| Recent Ash | Andisol | 34.8 | 37.1 | 512.6 | 20 |
| Recent Ash | Andisol | 41.1 | 30.5 | 549.1 | 22 |
| Recent Ash | Andisol | 38.2 | 37.0 | 646.3 | 23 |
| Metamorphic | Entisol | 27.7 | 22.7 | 252.7 | 20 |
| Metamorphic | Inceptisol | 29.7 | 27.1 | 381.5 | 21 |
| Metamorphic | Alfisol | 32.0 | 30.0 | 402.8 | 20 |
| Metamorphic | Alfisol | 35.8 | 30.2 | 530.1 | 21 |
| Metamorphic | Ultisol | 40.6 | 33.5 | 690.4 | 25 |
Figure 1.
Location of
Pinus radiata D. Don stands evaluated by soil type and climate characteristics and soil water holding capacity. (
a) Study area in central-south Chile (black frame); (
b) sites and mean summer (January to March) temperature (°C, Sum. temp.); (
c) sites and annual precipitation (mm yr
−1, MAP); (
d) sites and soil water holding capacity up to 1 m deep (mm, SWHC) [
39].
Figure 1.
Location of
Pinus radiata D. Don stands evaluated by soil type and climate characteristics and soil water holding capacity. (
a) Study area in central-south Chile (black frame); (
b) sites and mean summer (January to March) temperature (°C, Sum. temp.); (
c) sites and annual precipitation (mm yr
−1, MAP); (
d) sites and soil water holding capacity up to 1 m deep (mm, SWHC) [
39].
Soil preparation for all sites included subsoiling to a depth of 60 cm and disking. Traditional operational fertilization was applied at a rate of 100 to 150 g per plant of an NPK mix (10 to 15 g of nitrogen, 10.9 to 13.2 g of phosphorus, and 8.3 to 12.5 g of potassium), plus 2 to 3 g per plant of boron after planting. Weed control operations considered the pre-planting total area and included two years of banded weed control after planting in most conditions. All selected stands were pruned to a minimum height of 5.5 m and thinned using one or two commercial thinning practices according to the silvicultural program applied to each site’s stand. Final stand stocking at harvest reached 400 to 500 trees ha−1.
Field measurements were conducted at sandy and volcanic ash soil sites between March and May 2022, and at granitic and metamorphic soil sites between May and November 2023. At each site, three 1000 m2 inventory plots were established to measure the diameter at breast height (DBH, in cm at a height of 1.3 m) and the total height (H, in m) of all trees. The forest floor and mineral soil were also sampled within each inventory plot. A total of 60 plots (15 plots per soil type) were measured across all sites.
2.2. Volume and Carbon Stocks of Above- and Belowground Biomass Estimations
We used individual tree measurements from each plot (considering the number of trees, DBH, and H) to estimate tree over bark volume (V, m
3 tree
−1). The calculations considered a local volume equation of individual trees developed by CMPC Forestal Mininco Company [
40] (Equation (1)).
where V is the individual tree over bark volume (m
3 tree
−1), D is the DBH (cm), and H is the total height (m).
We estimated the aboveground biomass of each tree component (stem, bark, branches, and needles) using the allometric equations for radiata pine published by Sandoval et al. [
41]. Then, we calculated the total aboveground biomass (AGB, kg tree
−1) as the sum of each tree component’s biomass. We calculated the belowground biomass (BGB, kg tree
−1) using the allometric equation for the radiata pine root component published by Zerihun and Montagu [
42]. We estimated the carbon stock of the aboveground and belowground tree biomass by multiplying the AGB and the BGB by a carbon factor (CF), corresponding to the carbon fraction of the biomass. We used 0.48, as recommended by the IPCC [
43].
Finally, individual plot stocking (NHA, trees ha−1), stand volume (VHA, m3 ha−1), aboveground biomass carbon stock (AGBC, Mg ha−1), and belowground biomass carbon stock (BGBC, Mg ha−1) were estimated by adding and scaling to a hectare level the numbers of trees per plot, individual tree volume, aboveground biomass carbon, and belowground biomass carbon. The sum of the last two estimates provided the total biomass carbon stocks (TBC, Mg ha−1).
2.3. Forest Floor Carbon Sampling and Calculations
Samples of the forest floor from the organic horizons (Oi, Oe, and Oa layers) were collected using a 25 cm diameter circular cutting frame (490.9 cm2) at ten systematically selected sampling points within each plot. At each point, litter (pine needles in all stages of decomposition) and coarse woody debris were collected separately. A total of 1200 forest floor samples were collected from all evaluated plots (20 sites × 3 plots × 10 sampling points × 2 forest floor sampling types). Each sample was stored in a labeled paper bag, and taken to the laboratory, where it was dried at 65 °C until it reached a constant weight. The dry weight of the litter and woody debris was recorded for each sample using a balance with a precision of 0.01 g. After weighing, the samples of each forest floor type (litter and woody debris) were composited and homogenized separately for each plot. Each pair of composite samples per plot was ground separately using a 250 μm sieve blade mill. Five-gram subsample aliquots were obtained for analyzing the carbon concentration in the litter and woody debris components of each plot.
The carbon stock of litter and coarse woody debris was determined by multiplying the dry biomass weight at each sample point by the average plot carbon concentration of each component (%), then expanding it to the hectare level (Mg ha−1) and averaging it for each plot. The total forest floor carbon stock (FFC) was finally estimated by summing the carbon stocks of the organic horizons and the coarse woody debris estimated from each plot.
2.4. Soil Properties and Organic Carbon Sampling and Calculations
Estimates of the organic carbon stock in the mineral soil (up to 1 m deep) were obtained by taking composite samples from 20 points distributed systematically within each plot. These composite samples were obtained using a soil auger at three depths: 0–20 cm, 20–40 cm, and 40–100 cm. A total of 180 composite mineral soil samples were obtained from all the evaluated sites in the study. The soil samples were air-dried at 30 °C, passed through a 2 mm sieve to remove all root and plant debris, and a 10 g subsample aliquot was taken and dried at 65 °C for 24 h, and then ground. Finally, a 5 g aliquot was obtained to determine the organic carbon (C) and total nitrogen (N) concentrations using an Infrared Mass Spectrometer analyzer (IRMS, SERCON Scientific Inc., Cheshire, UK) (
Table 2).
At each site, a 1 m depth soil pit was dug to describe the visual characteristics of the soil profile. Bulk density (BD) samples were obtained using a 100 cm
3 metal cylinder at depths of 0–20 cm, 20–40 cm, and 40–100 cm. Each soil sample was stored and taken to the laboratory, where it was dried at 65 °C until it reached a constant weight. A total of 60 soil bulk density samples were obtained from all the sampled sites. Additionally, a 0.5 kg bulk soil sample was taken at each sampled depth for subsequent Boyoucos soil texture analyses and to determine the soil organic matter (O.M.) content [
44]. This sample was also used to estimate the permanent wilting point (PWP) and field capacity (FC) moisture retention curve points using a pressure plate apparatus (Soil Moisture Inc., Goleta, CA, USA). The soil water holding capacity (SWHC) for each soil sample was estimated as the difference between the PWP and the FC. Analyses of both forest floor and the soil were carried out in the Soil, Water, and Forest Research Laboratory (LISAB) at the Faculty of Forest Sciences of the University of Concepción, Chile.
The soil organic carbon stock at each depth (SOC
d, Mg ha
−1) was calculated by using Equation (2) [
45]:
where SOC
d is the soil organic carbon stock at depth d (Mg ha
−1), C
d is the soil organic carbon concentration at depth d (g kg
−1), D
d is the soil thickness at depth d (cm), BD
d is the bulk density at depth d (g cm
−3), and δ
d is the proportion of rock fragments at depth d (>2 mm).
The total soil organic carbon stock (SOC) until 1 m of depth for each plot was calculated by summing the SOCd estimates at each depth.
2.5. Climate Data
Temperature, precipitation, and solar radiation data from 1995 to 2023 were acquired using Google Earth Engine (
https://earthengine.google.com) (accessed on 15 May 2025) from the ERAS5 climate dataset [
46]. This data was previously correlated and validated with climate stations close to each sampling site belonging to the Chilean government agencies, the Dirección General de Aguas (
https://snia.mop.gob.cl/BNAConsultas/reportes) (accessed on 15 May 2025), and the Instituto de Investigaciones Agropecuarias, (
https://agrometeorologia.cl/) (accessed on 15 May 2025). For precipitation we calculated cumulative annual precipitation (Pp). Vapor pressure deficit (VPD, mBar) was estimated using Equation (3) [
47]:
where VPD is the vapor pressure deficit (mBar), T
max is the maximum daily temperature (°C), and T
min is the minimum daily temperature (°C).
We estimated the reference evapotranspiration for each site using the method described by Hargreaves and Samani [
48]. Finally, we calculated the soil water deficit index (SWDI) using Equation (4):
where SWDI is the water deficit index during the year (mm year
−1), P
p is the cumulative annual precipitation (mm year
−1), ET is the evapotranspiration during a year (mm year
−1), and SWHC is the soil water holding capacity (mm).
2.6. Canopy Estimates
The leaf area index (LAI) is a key ecophysiological parameter that is closely related to stand growth, productivity, and the physiological processes occurring within stand canopies [
12,
49,
50]. Due to the strong correlation between LAI and the spectral vegetation indices (VIs) [
51], we estimated VIs using Sentinel-2 images. We downloaded a one Level-1C Sentinel-2 image with less than 10% cloud cover from the Copernicus Open Access Hub online repository, that was acquired close to the carbon sampling date on each site.
We converted digital numbers (DN) from Sentinel-2 images to at-sensor spectral radiance [
52] and then we calculated the top-of-atmosphere (TOA) reflectance [
53]. Only bands 4 (Red: 0.665 µm) and 8 (near infrared band, NIR: 0.834 µm) of the multispectral images with a 10 m spatial resolution were used in this analysis. For each plot, we calculated two commonly used VIs: the simple ratio index (SR), which is calculated as (NIR/Red) [
54], and the normalized difference vegetation index (NDVI), which is calculated as ((NIR − Red)/(NIR + Red)) [
55].
2.7. Data Analysis
The total carbon stock (TCS) for each plot was estimated as the sum of the evaluated components: total biomass carbon (TBC), soil organic carbon (SOC), and forest floor carbon (FFC) stocks. We evaluated carbon stocks, stand volume, and soil properties for normality using a Shapiro–Wilk test (PROC UNIVARIATE) and a Levene’s test for heteroscedasticity. Data that were not normally distributed were transformed using a Box–Cox transformation (PROC TRANSREG). An analysis of variance (ANOVA) was performed (PROC GLM) to evaluate the effects of soil type on each above- and belowground component, total carbon stock, stand volume, and in the soil properties at each depth. When significant differences were detected (p < 0.05), a Tukey’s Honestly Significant Difference (HSD) test was applied for multiple comparisons among soil type means (LSMEANS was applied with the ADJUST = TUKEY option).
In the regression analysis, the carbon stocks (TBC, SOC, FFC, and TCS) were considered the dependent variables. The independent variables were grouped as follows:
Climate: solar radiation (Rad), mean annual precipitation (Pp), potential evapotranspiration (ETP), soil water deficit (SWD), soil water deficit index (SWDI), minimum temperature (Tmin), mean temperature (Tmean), maximum temperature (Tmax), mean vapor pressure deficit (VPDmean), and summer vapor pressure deficit (VPDsum).
Stand attributes: stand volume (VHA), simple ratio (SR), and normalized difference vegetation index (NDVI).
Soil properties: nitrogen content at 20 cm depth (N20); C:N ratio at 20 cm depth (CN20), C:N ratio at 1 m depth (CNm), percentage of clay at 20 cm depth (Clay20), percentage of sand at 20 cm depth (Sand20), percentage of sand at 1m depth (Sandm), and soil water holding capacity (SWHC).
Site: altitude (Alt), and distance from sea (Dist).
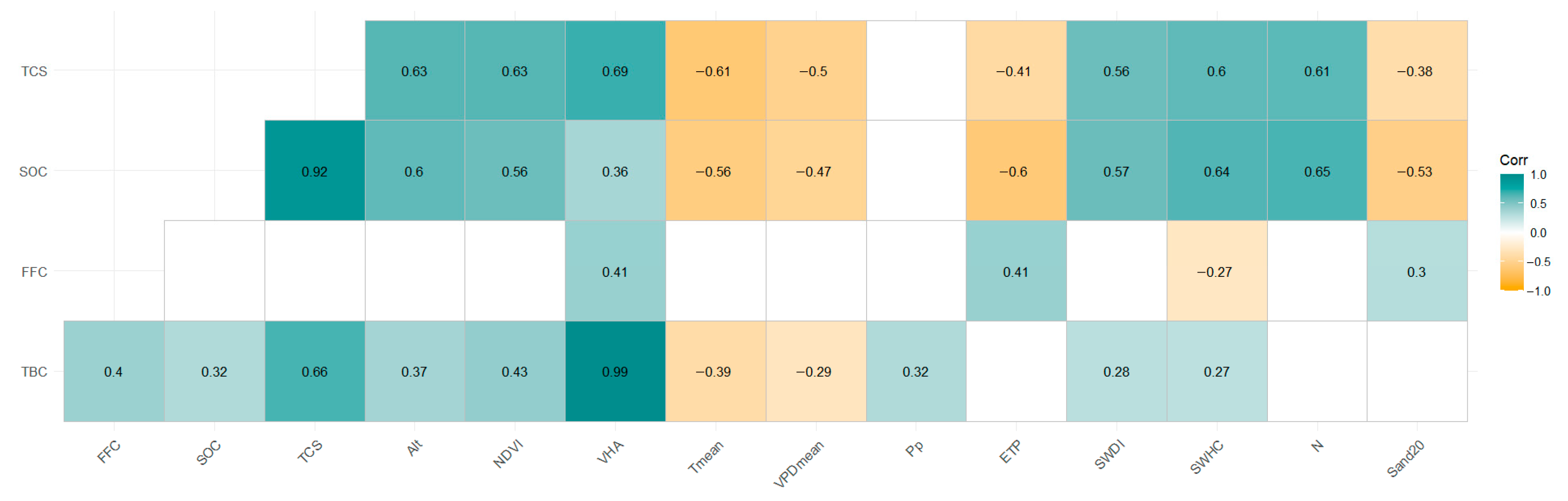
An exploratory analysis was performed to identify and remove outliers, defined as observations more than 2.5 times the standard deviation from the mean. A principal component analysis (PCA) was performed to avoid multicollinearity among possible predictor variables and to identify those that contributed significantly to variability (PROC PRINCOMP was applied with the STD option to standardize the data). Then, we performed a correlation analysis to evaluate the effect of the most relevant independent variables from the PCA on each dependent variable (carbon stock) (PROC CORR).
We then performed multiple linear regressions to examine the effects of the independent variables (climate, stand attributes, soil properties, and site) on each carbon pool (TBC, SOC, and FFC) and the total carbon stock (TCS). For each carbon pool, we adjusted the linear models in two steps. First, we fitted a complete model using all the variables selected from PCA and correlation analysis (PROC REG). Then, we used a stepwise process to fit a reduced model that included only significant variables (p < 0.05).
We evaluated and diagnosed the fitted models through graphical and analytical analyses to verify the assumptions of linearity (graphical analysis), normality (Kolmogorov–Smirnov test), homoscedasticity (Breusch–Pagan test), and residual independence (Durbin–Watson test). To identify the best model and compare the performance of each approach, we calculated the adjusted coefficient of determination (adj-R2), root mean square error (RMSE), and Akaike’s information criterion (AIC) values during the fitting process (PROC GLMSELECT). All statistical analyses and fitting procedures were carried out using SAS software (version 9.4, SAS Institute, Inc., Cary, NC, USA). All tests were considered significant at a level of α = 0.05.
4. Discussion
Similar to other studies, we found that high carbon stocks were present in the radiata pine plantations at pre-harvesting age, particularly in recent ash and metamorphic soils compared to sandy soil [
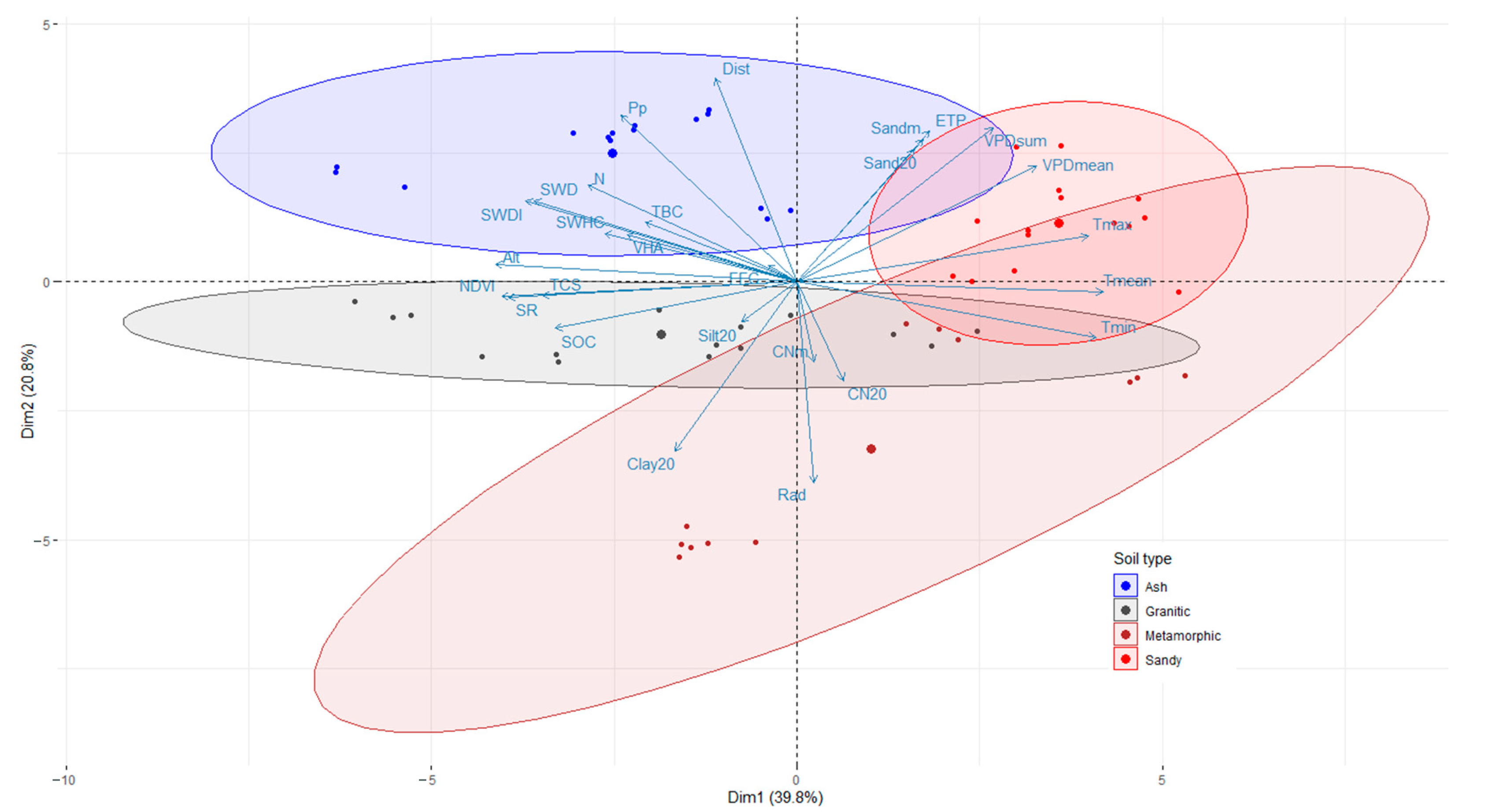
4]. Our results showed that the stand carbon stocks in radiata pine plantations are strongly related to climatic variables and soil properties (
Figure 2 and
Figure 3). As in other studies, sites with lower water and nutritional limitations tended to have the highest SOC and total carbon stock [
4,
56].
Precipitation (Pp) and soil water available showed a high positive correlation with stand growth, biomass, and soil organic carbon (SOC) [
10,
12,
25]. Water stress was a clear limiting factor in the accumulation of carbon accumulation in the evaluated radiata pine stands. Studies evaluating the productivity and growth of
Pinus radiata D. Don stands in Chile have shown that environmental variables, such as Pp and available soil water, directly affect growth and leaf area index (LAI) across different sites, while the soil water deficit inversely affects both variables [
10,
12]. This is related to the correlation analyses for TBC (
Figure 3 and
Table 5), in which Pp, SWHC, and SWDI had a positive coefficient of correlation, indicating that an increase in water availability increases stand growth and biomass.
Conversely, the variables related to water stress, such as evapotranspiration (ETP), temperature (Tmean), and VPDmean, presented negative coefficients of correlation. These results are consistent with those of Olmedo et al. [
4], who found that the most restrictive sites in terms of water availability had the lowest carbon stocks in above- and belowground biomass when evaluated by climatic zones. This indicates that, as observed in other studies, reduced water availability decreases LAI, and consequently, growth and biomass accumulation [
10]. Temperature affects tree growth and decomposition and respiration rates. These factors impact on the forest productivity and the carbon capture into biomass, mineral soil, and the forest floor [
8,
26,
57,
58]. In a radiata pine plantation, a direct relationship was observed between an increase in soil respiration (CO
2 flux) and temperature [
59]. This could be related to the negative correlation between SOC and temperature in our study, which could be due to an increase in organic matter decomposition caused by this environmental variable [
18].
The type of soil, specifically related to texture, has been described as a key factor in the stand growth [
60], affecting the soil water available to the trees, and consequently, the accumulation of carbon in above- and belowground biomass. Similar to what was found by Olmedo et al. [
4], soil organic carbon content and soil organic stock (SOC) were higher in metamorphic and volcanic ash soils than in granitic or sandy soils (
Table 2 and
Table 3). Our results, from both the correlation analyses and the fitted linear model (
Figure 3 and
Table 7), clearly indicate that SOC is positively influenced by variables related to soil nutrients and water storage (e.g., nitrogen, clay, and silt content), precipitation, and stand productivity (e.g., positive correlation with stand volume and LAI). According to our analyses, soil texture affects soil attributes such as water storage capacity (
Table 2 and
Table 4), soil carbon stock, and the stand growth, which is related to the total carbon biomass of the stand. Key characteristics that affect soil carbon in mineral soil include the aggregation of its particles, clay and silt content, and the mineralogy and specific surface area of clays [
8,
28,
61]. These factors have different capacities for accumulation, stabilization, and protection of soil organic matter (SOM) [
18,
21,
62]. Furthermore, clay content contributes to the retention and availability of water and nutrients in the soil, aggregation and formation of micropores, which promote the long-term accumulation of organic matter in the soil and improve stand productivity and soil carbon stock [
24,
63]. Our results and those of Olmedo et al. [
4] and Crovo et al. [
22], who evaluated the SOC in radiata pine plantations in Chile, suggest that due to the clay type and higher content of clay + silt content in recent volcanic ash, those soils store and maintain high levels of organic matter and available water over time [
20,
61,
64]. Soils with recent volcanic ash soils, such as Andisols, contain secondary minerals, including short-range-order (SRO) minerals, such as allophane and imogolite [
19]. These clay type and soil aggregates have a high specific surface area and variable charge. They provide physical and chemical protection and stabilization for SOM [
8,
21]. This evidence shows that Andisols are among the most productive soils in the world [
65]. Conversely, soils with a high sand content have a lower potential for storing high soil carbon stocks due to their coarse texture, larger pore size, and lower water and nutrient availability, which affects stand productivity, as well as the speed of decomposition and storage of organic matter [
4,
66,
67].
Accurately estimating this carbon stock is important because, in our study, it was the most important carbon pool for granitic, metamorphic, and recent ash soil sites, and the second most important carbon stock for sandy soil sites. Furthermore, many studies have found that SOC is the component with the highest proportion of the carbon pool in forests [
4,
68,
69]. Therefore, a model that considers stand volume, fertility, and climate characteristics would be helpful in quantifying SOC up to one meter deep (
Table 7 and
Table 8,
Figure 4). In our fitted models for SOC estimation, which combine soil characteristics, climate, and stand productivity, the predictor variables are easy and quick to obtain, allowing for good estimates of this carbon stock. As Paula et al. [
28] indicated, climatic variables are important for estimating of carbon, but soil characteristics must also be considered to improve SOC estimates.
The lowest carbon stock in our study was found in the forest floor (FFC), and significant differences were observed among soil types (
Table 3). In our study, the highest forest floor carbon stock was found in the granitic and sandy soil sites. Although these sites have higher mean temperatures than metamorphic and recent ash soil sites, they have less available water (
Table 4). This could be related to the fact that greater aridity decreases the litter decomposition rates [
70]. Therefore, in our evaluated sites, the higher SWHC and Pp in the metamorphic and recent ash sites could have affected the decomposition rates of the FFC, resulting in a lower carbon stock than in the granitic and sandy soil sites. Lower humidity and higher temperatures have been observed to decrease litter decomposition rates [
71], due to reduced biological activity of soil organisms, which could explain the higher biomass in our granitic and sandy soil sites. In metamorphic and recent ash soil sites, organic matter can more quickly incorporate into mineral soil and become associated with and protected within aggregates or mineral clay particles [
18]. López-Senespleda et al. [
31] estimated forest floor carbon stocks in Spain, using linear and Random Forest models. They found significant climate and environmental variables similar to those in our study, such as precipitation and temperature. These variables have been reported to significantly impact decomposition rates of the forest floor [
72,
73,
74]. Many studies have reported an increase in litter decomposition with increasing temperature and precipitation [
72,
75].
Furthermore, this carbon stock was the only one that weakly correlated with the stand productivity [
5,
6]. In many studies [
4,
31,
76,
77], the forest floor has the lowest proportion of carbon stock in adult stands, accounting for about 5% of the total carbon stock in the world’s forests [
2]. However, the forest floor is a key component in the site carbon and nutrient dynamics and contributes to soil mineral carbon and acts as a nutrient reservoir for successive rotations [
18,
30].
We acknowledge the limitations of our study and recommend exercising caution when using carbon stock estimates from the models we have developed. Our models are limited to Pinus radiata plantations that are close to harvest age, and they may not be accurate when applied to younger stands with the same productivity. Similarly, as our models were developed using four soil parent materials, their application to other soils (e.g., marine sediments or red clay) may not provide accurate estimates of soil and stand carbon stocks. Future work will consider measurement and sampling in other soil types to cover a wide range of Pinus radiata plantations and eroded soils to compare with our results. Measurement and sampling will also be conducted at sites with characteristics similar to those in our study to validate the generated models. In addition, we will consider other factors, such as different silvicultural practices, to assess their potential impact on soil carbon stocks.
5. Conclusions
Soil type was a key factor in carbon stocks in the adult radiata pine stands evaluated. We found significantly more total carbon stock in the metamorphic and recent ash soils than in the granitic or sandy soil sites. In all soil types, a high carbon stock was found in the mineral soil up to 1 m deep. The forest floor represented the lowest carbon pool of the stands in the four soil types. The climate, soil, and stand productivity variables were highly related to all carbon stocks in our radiata pine stands, being good predictors in our adjusted models, with high significance estimating each carbon stock (biomass, forest floor, mineral soil, and total carbon stock). Those variables related to soil water availability (minor sand content and more soil water holding capacity), fertility (nitrogen content), annual precipitation, and stand productivity (stand volume and NDVI) were strongly and directly related to all carbon stocks, while those variables related to poor-nutrients sites, and drought and water stress, such as temperature, VPD, soil water deficit index, evapotranspiration, and higher sand content, decreased the carbon stock of stands. Those results indicate that increasing growth and stand productivity could increase the potential capacity of forests to capture and store carbon. Our developed models to estimate carbon stocks presented good fits, with variables that were easy to acquire, measure, and sample, which could help in the analysis of estimating the effects of different scenarios of climate change on forests.