1. Introduction
The spotted lanternfly,
Lycorma delicatula (White) (SLF), an invasive phloem-feeding fulgorid (Hemiptera), was first discovered in the USA in Berks County, PA, in 2014 [
1]. Originally native to Asia, it has spread to 18 states [
2], where its feeding habits have caused both direct and indirect adverse effects for host plants and surrounding plant communities.
L. delicatula remove plant resources through feeding, which causes wilting, puncture wounds, and branch dieback, while excreting excess honeydew and sap. This in turn promotes the growth of sooty mold, reducing overall photosynthesis in neighboring plants [
3,
4,
5]. These effects pose a potential risk to a wide range of industries including vineyards, nursery, timber, and even tourism [
3].
One of the unique challenges with implementing an effective management strategy for SLF is its wide host range [
5].
L. delicatula is known to feed on at least 103 host plants, with over 56 host plants occurring in the United States [
6]. SLF’s host range is well documented and subject to change as it expands into new areas and develops throughout its life cycle [
7]. Kim et al. [
8] investigated the behavioral characteristics of SLF and described their cyclic feeding behavior as a continuous period in which nymphs ascend trees and feed often until physical factors such as wind dislodge them to the ground. This cyclic behavior matches with the nymph’s broader host range, which starts out wide and becomes narrower during the adult stage [
8].
To date, all spotted lanternfly traps used for detection and monitoring rely on taking advantage of the cyclic behavior and negative geotaxis of the lanternflies walking up tree trunks or poles. Traps are typically deployed on
Ailanthus altissima (Miller) Swingle (Sapindales: Simaroubaceae) (TOH),and intercept walking SLF as they climb up and down the tree. The first spotted lanternfly traps consisted of glued bands wrapped around tree trunks [
8,
9,
10]. However, due to the high amount of nontargets and vertebrate bycatch, including birds and mammals, new trapping options were required. BugBarrier
® tree bands (Envirometrics Systems, Victor, NY, USA) consist of a glue-coated surface facing inward toward the trunk while being separated from the tree surface by a layer of fiber batting. This represented a major improvement to increasing catch [
11] but was susceptible to trap saturation from trap catch and trap degradation, as adhesives can become less effective when exposed to rain and excess moisture. In turn, oversaturated bands can become less effective and difficult to process. During insect monitoring programs, trap processing often takes place back at the lab, so additional care must be taken with glue traps to keep samples separated. Glue traps are often bulky to carry into and out of field sites. Rolls of material must be brought into the field site to custom-cut traps, and then all used trapping material must be carried out of field sites at the end of each trapping period. Circle traps, created from modified pecan weevil traps (Great Lakes IPM, Vestaburg, MI, USA) and fitted with screw top rectangular storage jars, increased spotted lanternfly catch further and greatly reduced vertebrate bycatch [
11,
12,
13].
In most surveys, traps are typically placed on
A. altissima. While TOH may be the preferred host, SLF utilize other hosts during their development, which may also be suitable for trap placement. Nixon et al. [
13] found significantly higher
L. delicatula capture for traps deployed on TOH but noted other hosts, including walnut and maple
Acer spp. L. (Sapindales: Sapindalaceae) species, yielding consistent SLF captures. Multiple studies have been conducted to investigate SLF’s host range and preferences, as they change throughout its life cycle. Murman et al. [
14] found that, on average, more SLF were captured on TOH than on other species; however, 34 other species still helped to capture SLF. They also found that during the early instar life stage, detection rates (the ability to find a single target at low densities) on TOH were equal to or even lower compared to other species. In enclosed choice tests, nymphs showed no consistent preference for TOH when compared to black walnut,
Juglans nigra L. (Fagales: Juglandaceae). After reaching 4th instar and during the adult stage, sticky band detection rates on TOH remained higher. Choice tests remained higher on TOH, as well. Nixon et al. [
15] investigated the host suitability of various SLF life stages based on the 2-week survivorship on wild and cultivated host plants. They demonstrated that SLF were able to complete development on single hosts, including black walnut and tree of heaven, in addition to other hosts. Development rates changed depending on the hosts, with SLF developing faster when feeding on multiple hosts and not a singular species.
While trap catch is important in determining optimal designs, detection is just as important to regulatory surveys. Having sensitive tools to detect new infestations is necessary for a survey’s success [
16,
17]. To date, lure development for the spotted lanternfly has proven extremely challenging because SLF communication and attraction is multi-faceted and linked to a combination of host plant odors, species-specific pheromone blends, and substrate vibrations [
18]. Currently, the only recommended lure available is host plant-derived kairomone, methyl salicylate, that when placed on host plants, has been shown to be attractive to SLF at varying life stages [
10,
18,
19]. However, under field conditions, the lure alone has had mixed levels of success and is less able to outcompete natural signals such as wild SLF aggregating [
12].
A major component of most invasive species management programs is having dependable detection tools that can be implemented in the most efficient way. Improving on survey tools is a balancing act of managing material costs, labor intensity, ease of use, and overall effectiveness. Our goal was to improve survey tools and methods through modifications to the circle trap, as well as an exploration into the use of alternative hosts for trap placement locations. While Ailanthus is the preferred host, having alternative hosts available for trap placement could offer more flexibility when placing survey traps, especially as SLF continues to spread. We also conducted trapping assays using secondary hosts to investigate if the methyl salicylate lure provided improved trap catch and/or detection to the traps.
2. Materials and Methods
2.1. General Trapping Methods
All traps used in the following studies were set up approximately 1.5 m above the ground and were attached to the trunks of host trees. Each trapping pair, or trio of trees, was treated as a replicate, and trees in each replicate were selected to have similar DBH and positions within 10 m of each other. Replicates were placed at least 15 m apart. Traps were fastened with 1.43 cm long staples to secure them to the trunk of the tree. A single dichlorvos-impregnated insecticide strip (Vaportape II, Hercon, York, PA, USA) was placed inside the circle trap collection device and functioned as the killing agent. Insecticide strips were replaced every four to six weeks. For studies conducted after 2021 (
Section 2.5 and
Section 2.6 below), insecticide strips were not used because their presence in the traps was found to not significantly affect trap catch (JAF, unpublished data). All traps (except control traps in the lure study) were baited with methyl salicylate lures (Alpha Scents Inc, Canby, OR, USA). Lures were attached to the outer wooden strip on circle traps with a push pin and replaced every 3–4 weeks, as directed by the manufacturer’s guidelines. BugBarrier
® tree bands were set 1.5 m up from the ground, as described in Francese et al. [
11], with lures attached just above the band. Traps were typically monitored throughout the SLF active season (April to November) to account for all mobile life stages. All traps were checked on a 2–3-week cycle based on staffing availability. This trap-checking duration is typical of survey guidelines and preserves sample quality. During monitoring, bags were removed from the trap and replaced during each check, and for assays testing BugBarrier
®, the bands were replaced during each trap check. Samples were sorted and counted based on SLF life stages. Traps within each replicate were rotated for the collection device comparison study (
Section 2.2), but due to logistical issues, no traps were rotated in other studies.
2.2. Circle Trap Collection Device Comparison: Jar vs. Bag
Circle traps with two different modified collection devices were compared in a paired block design. Each replicate consisted of both a circle trap fitted with a 1.4 L plastic jar container (
Figure 1a), using methods described in Francese et al. [
11], and a circle trap modified to fit a gusseted plastic bag (Bag trap) (
Figure 1b). Circle traps fitted with bags, similarly to the jar traps, were also modified from a pecan weevil trap (Great lakes IPM, Vestaburg, MI, USA) by widening the opening from 0.7 cm to 1.5 cm to accommodate the larger body size of SLF.
The end of a pecan weevil trap collection cup was removed, and a plastic “tongue” was then attached with a ring of hot-melt glue (
Figure 2a). Clear gusseted bags (Uline 38.1 cm × 20.3 cm × 10.2 cm × 3 mil) were attached using a reusable, plastic zip tie (
Figure 2b). The gusseted bag design provides a ridged structure, which holds its shape. The clear plastic tongue functions as a backup means to ensure the bag refrains from closing the entrance funnel as it fills up and weighs down the bag. Twenty replicates were placed in a high-SLF-density site on 20 June 2019 but were only checked during the adult flight season (10 September to 1 October) at Trexler Nature Preserve (Schnecksville, Pennsylvania) on tree of heaven.
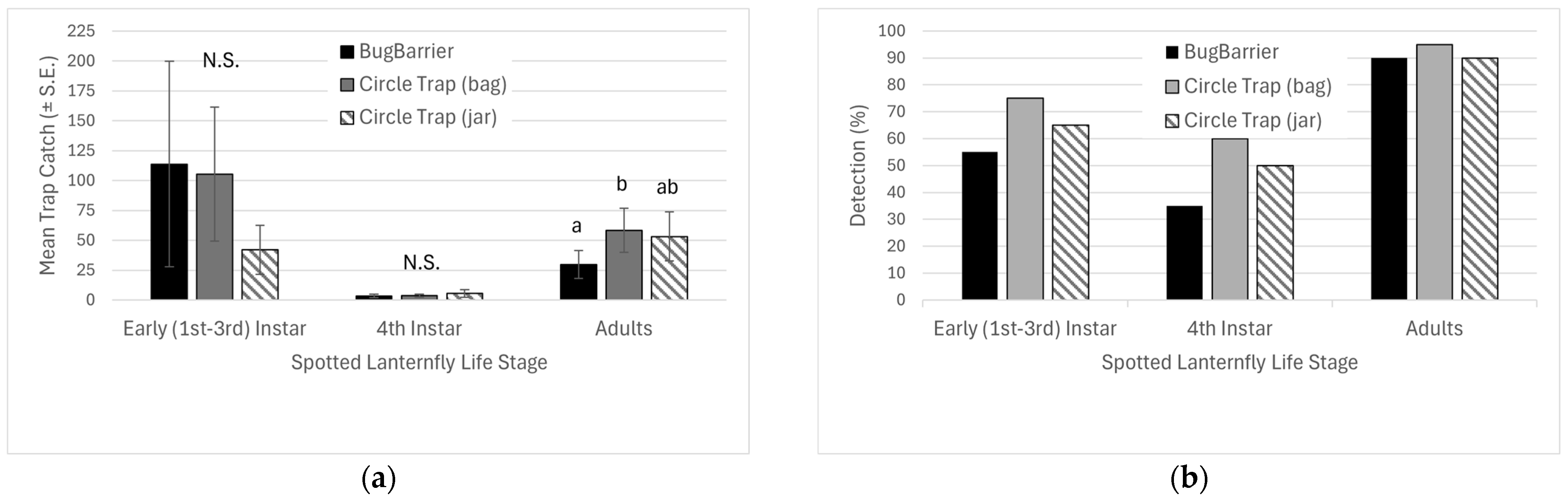
2.3. Multi-State Detection Tools Study: BugBarrier® vs. Circle Trap (Jar) vs. Circle Trap (Bag)
To compare SLF trap catch and detection across a population density gradient, three SLF trap types—(a) circle traps fitted with jar containers, (b) circle traps fitted with bags and, (c) BugBarrier®—were evaluated in a season-long multi-state comparison. Each replicate consisted of the three trap types placed in areas where surveyors, based on prior visual inspections, predicted finding low-density SLF sites. Thirty-four replicates were placed across three states: Maryland (n = 10), New Jersey (n = 14), and West Virginia (n = 10). Of these replicates, only 20 were positive for SLF and were included in the analysis. Depending on the locations, traps were placed to coincide with the full active period of spotted lanternfly (April to November).
2.4. Trap Host Comparison: Juglans nigra vs. Ailanthus altissima
In 2021, a trapping assay was conducted to compare SLF circle trap catch on the preferred host, tree of heaven, vs. an alternative host, black walnut (Juglans nigra). Twenty-three replicates were placed across four states: Maryland (n = 6), New Jersey (n = 12), Pennsylvania (n = 1), and West Virginia (n = 4). Depending on the locations, traps were placed to coincide with the full active period of spotted lanternfly (April to November). Seventeen replicates were positive for spotted lanternfly, and so only these were analyzed.
2.5. Trap Host Comparison: Acer spp. s. Ailanthus altissima
In 2022, a trapping assay was conducted in a paired block design to compare trap catch on tree of heaven (Ailanthus altissima) and maple (Acer spp.). A total of twenty-seven replicates were placed across three states: Indiana (n = 5), (Pennsylvania (n = 12), and West Virginia (n = 10). The Acer species used in trapping assays varied based on local availability. Species utilized included red maple (Acer rubrum), sugar maple (Acer saccharum), and boxelder (Acer negundo).
2.6. Lure Comparison: Methyl Salicylate Baited vs. Non-Baited Traps on Three Hosts
In 2023, paired comparisons of baited and non-baited traps set on neighboring trees of either A. altissima, J. nigra, or Acer spp. were conducted across multiple states: Ohio, Pennsylvania, and West Virginia. Fourteen replicates were placed on Acer spp., twenty-four were set on A. altissima, and eighteen were set on J. Nigra. These tree species were chosen based on host-plant trapping results from previous years. Depending on the locations, traps were placed to coincide with the full active period of spotted lanternfly (April to November). A total of five replicates (one Acer, two A. altissima, and two J. nigra) were not included in the final analysis.
2.7. Statistical Analysis
Spotted lanternfly trap catch was summed over the entire sampling period for each study. The number of trap checks for reps varied across sites due to cooperator and staffing availability and downed traps. When one of the traps within a replicate experienced issues or had fallen, both collections from that replicate were discarded for that that trapping period. Additionally, since some replicates were set on the outer range of known SLF infestations in which both traps failed to detect SLF, it was assumed to be in areas without active SLF populations, and they were removed from statistical analysis; so, analyses only included replicates where at least one SLF is reported.
For each assay described above, the distribution of the data was tested for normality in JMP 15 (SAS Institute, Cary, NC, USA, 2020). Only one data set tested was normally distributed: the proportion of males collected in the 2019 circle trap collection comparison. To compare the main effects of the 2020 detection tools study, a Kruskal–Wallis test was performed to determine if the trap type had a significant effect on the catch. Additionally, pair-wise Wilcoxon tests of ranked sums were conducted to determine if there were differences among treatments (α = 0.05). For all other data sets, Wilcoxon tests of summed ranks (α = 0.05) were performed to determine if there were significant differences between treatments. To analyze the proportion of male catch in the 2019 circle trap collection comparison, a paired Student’s t-test was performed (α = 0.05). Detection was defined as finding at least a single L. delicatula in a trap, and this was recorded as a binary response (yes or no) over the entire field season for the purposes of analysis. Pairwise χ2 tests (α = 0.05) were used to compare detection rates between traps for the overall results in studies, while Fisher’s exact tests (α = 0.05) were used to compare rates at three population density levels, when there were four or more replicates of each density level. Population density was determined post hoc and was based on the trap catch: high (≥101 individuals caught during the trapping season), medium (31–100 individuals caught during the trapping season), and low (≤30 individuals caught during the trapping season). Both types of tests were performed using Excel for Microsoft 365 (2019).
4. Discussion
Circle traps with wider openings and fitted with the bag collection devices were an improvement over the traps fitted with jars. The difference in total seasonal catch between collection devices was potentially caused by the collection jar inhibiting trap catch over time. The entrance of the jar can become obstructed by dead and dying insects, limiting further SLF from entering the trap, in higher-density SLF areas, and especially during the larger-bodied adult stage. The bag-fitted traps avoid inlet obstruction by allowing the insects that pass through the funnel to fall away from the entrance and into the lower gusseted portions of the bag. The plastic tongue maintains the entryway openness, keeping the bag from closing in on itself as heavier insects weigh down the bag. Additionally, in higher-SLF-density areas, where excess feeding in the upper canopy above the trap occurs, the understory including the collection jars can become coated in honeydew and eventually sooty mold. This sooty mold can form a dark coating on the outside of the traps, preventing light from entering the collection area. SLF ascending the trap trees may view the darkened collection container as more of a barrier instead of a passageway to the upper canopy, thereby avoiding the collector. This coating is mitigated by the bags being changed every 2–3 weeks. In addition, for large-scale-survey usage, the jar collectors do use a significant amount of storage space.
While both circle trap types and BugBarrier
® were equally effective at catching nymphs, the non-glue-coated traps were more effective during the adult stage. BugBarrier
®’s initial success during nymphal stages is consistent with other work that suggested sticky traps were an effective tool for monitoring nymphs [
8]; however, adults are capable of escaping from tree bands [
11,
12]. Desko et al. [
20], confirmed that the SLF escape probability was directly correlated with the size of the life stage when tested on other glue-coated tree bands. Catch between the two circle trap types was similar. Overall catch (and potentially population density) was lower than in the previous assay, potentially leading to the similarities in catch between the treatments, as the jar fitting did not overfill, so it did not cause trap catch inhibition.
In side-by-side comparisons of traps set in 2021, traps on
A. altissima vs.
J. nigra, as well as in 2022, with traps set on
A. altissima vs.
Acer, we found no significant difference in trap catch in the nymphal stage in either the 1st−3rd early instar group or the 4th instar group; however, the adult trap catch was significantly higher in traps set on tree of heaven (
Figure 4a and
Figure 5). These findings are consistent with documented narrowing host ranges observed in SLF life stages over the season [
7,
8,
21,
22]. SLF usage of black walnut and maple species is reported in the literature, with varying uses listed, including oviposition sites. If trap catch on a particular host tree is a measure of the relative use of that host, nymphal stages may be less selective than the adult stages and frequently utilize
J. nigra and
Acer spp.
Circle traps are a passive trap, relying on intercepting SLF as they move through the landscape, so it is important to recognize that trap catch on a particular host tree may not be a clear representation of the selection for a particular host but instead could be explained by other dispersal factors driven by morphological or physiological changes. The only stage of SLF that is not mobile is the egg stage. SLF frequently lay their eggs in clusters on the underside of branches and along the trunks of trees, and while commonly on
Ailanthus, many other species are used, as well [
23]. Egg masses laid on non-hosts, stone, rusty metal, cinder block, rail cars, pallets, and other manufactured substrates have also been reported [
3]. Since many of these sites are not suitable food sources, as eggs hatch, the nymphs must disperse and begin their search for food. Nymphal dispersal is often aggregate [
24] and is usually limited to only tens of meters over a 7-day period or less when preferred hosts are present [
10,
25]. Considering this relatively low dispersal distance in comparison to the trap tree distance, especially in early instars, higher trap catch in these first stages could be more of a measure of proximity to original oviposition sites as opposed to selection of a preferred host tree.
As nymphs develop, the stylet length increases exponentially [
22], and tarsal claws become several times larger [
8]. Stylet length is often attributed to host plant use and can be linked to the types of plant tissues used for feeding. Longer stylets are better suited for piercing further into the host and likely suggests more stem/trunk feeding. Shorter stylets would be better suited for feeding on the newer growth or leaves. Based on these assumptions, early instar nymphs are not able to feed through the thicker bark at the base of trees where circle traps are generally set, so SLF nymphs are more likely to be using the trunks as pathways to new feeding sites. Trap catch could also be affected by the number of times an individual becomes displaced from a canopy feeding site and attempts to reascend the tree. This would be affected by tarsal claw sizes, since the size of tarsal claws is correlated to SLF’s ability to cling to hosts, thereby reducing the frequency of becoming displaced [
8]. This may explain why trap catch and the detection of 4th instar nymphs were relatively low compared to other stages.
In 2023, during the lure comparison study, methyl salicylate lures had no impact on the detection or overall trap catch for any of the life stages on either black walnut or maple replicates. Similarly, 4th instar nymph and adult SLF stages on tree of heaven were not significantly attracted to baited traps. However, early instar nymphs did show a significant preference for tree of heaven baited with the lures. Cooperband et al. [
10] found significant attraction in third instar nymphs to methyl salicylate lures placed on
A. altissima, but for other stages, field results were mixed. In a mark–release–recapture field study, 1st and 2nd instars showed a preference for
Ailanthus trees baited with methyl salicylate lures when controlling for tree size and existing SLF densities, which were both major factors guiding SLF orientation [
26]. Nixon et al. [
12] did not find methyl salicylate lures to increase trap catch when placed on hosts or even posts, suggesting that the surrounding habitat and host–plant interactions and competition could be impacting this response. Benefits to removing lures from the trapping protocol include the increased time, labor, and cost savings associated with placing and replacing the lure every 28 days.
Limiting the spread of a newly established invasive species relies heavily on the ability to detect new infestations while also being able to monitor existing populations. This is often a laborious and costly process that involves coordinated efforts from surveyors and programs. Therefore, continued work aimed at developing and improving upon available survey tools, as well as refining surveying methods, works to reduce and alleviate this burden. There are still many critical knowledge gaps that should be further explored to improve overall SLF management practices. For instance, current SLF traps are greatly limited in that they are only passive traps and rely heavily on SLF movement around and between hosts. Until a more effective attractant becomes available and new modes of attraction can be exploited, circle traps may be limited in their effectiveness. Until then, continued efforts to better understand the biology, behaviors, and host selection of this species will help to develop new plans and tactics for mitigating its impacts.