Genotypic Variability in Growth and Leaf-Level Physiological Performance of Highly Improved Genotypes of Pinus radiata D. Don Across Different Sites in Central Chile
Abstract
1. Introduction
2. Materials and Methods
2.1. Sites
2.2. Leaf-Level Physiological Measurements
2.3. Chlorophyll Fluorescence
2.4. Statistical Analyses
3. Results
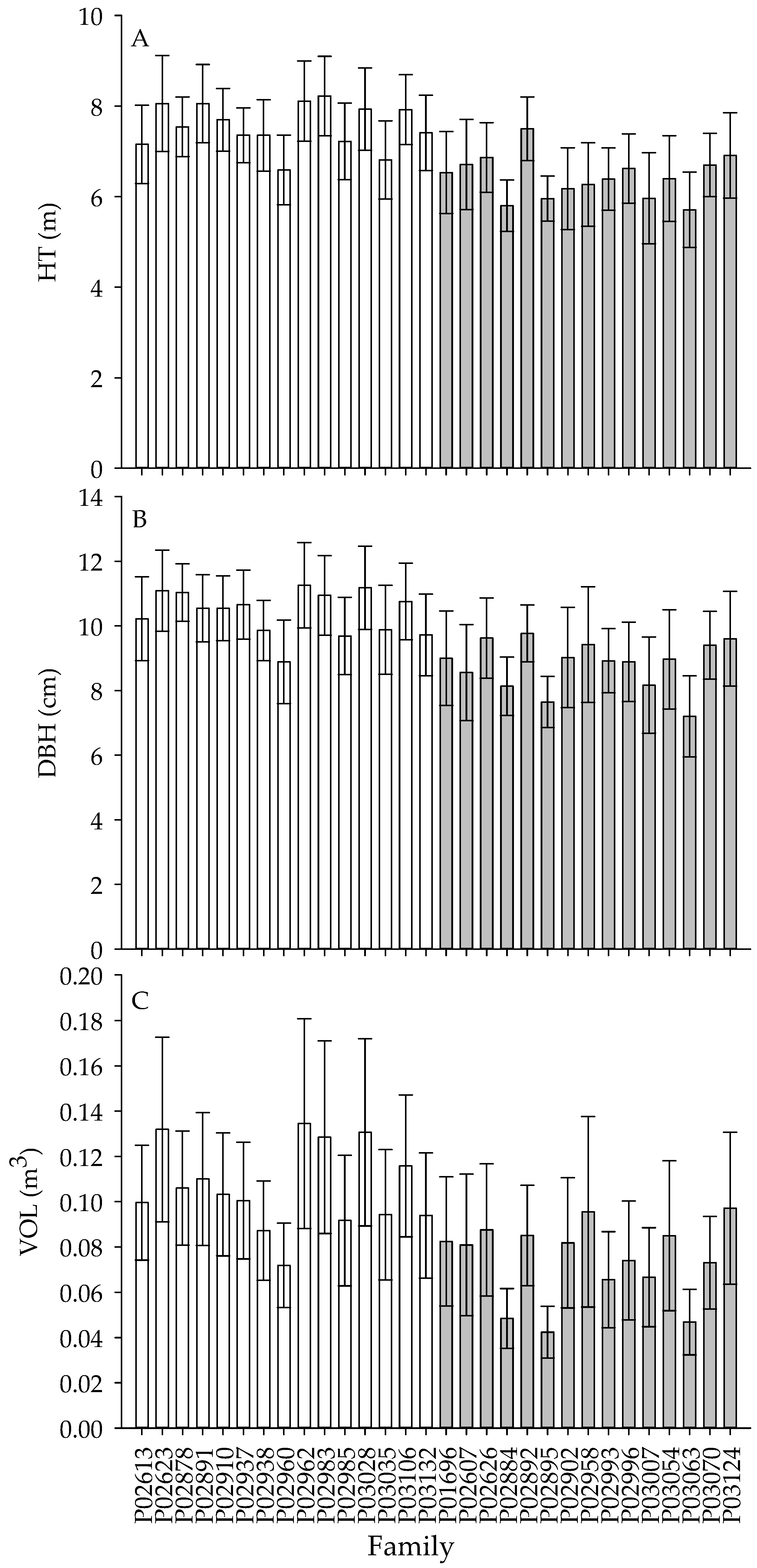
3.1. Differences in Growth Across Genotypes
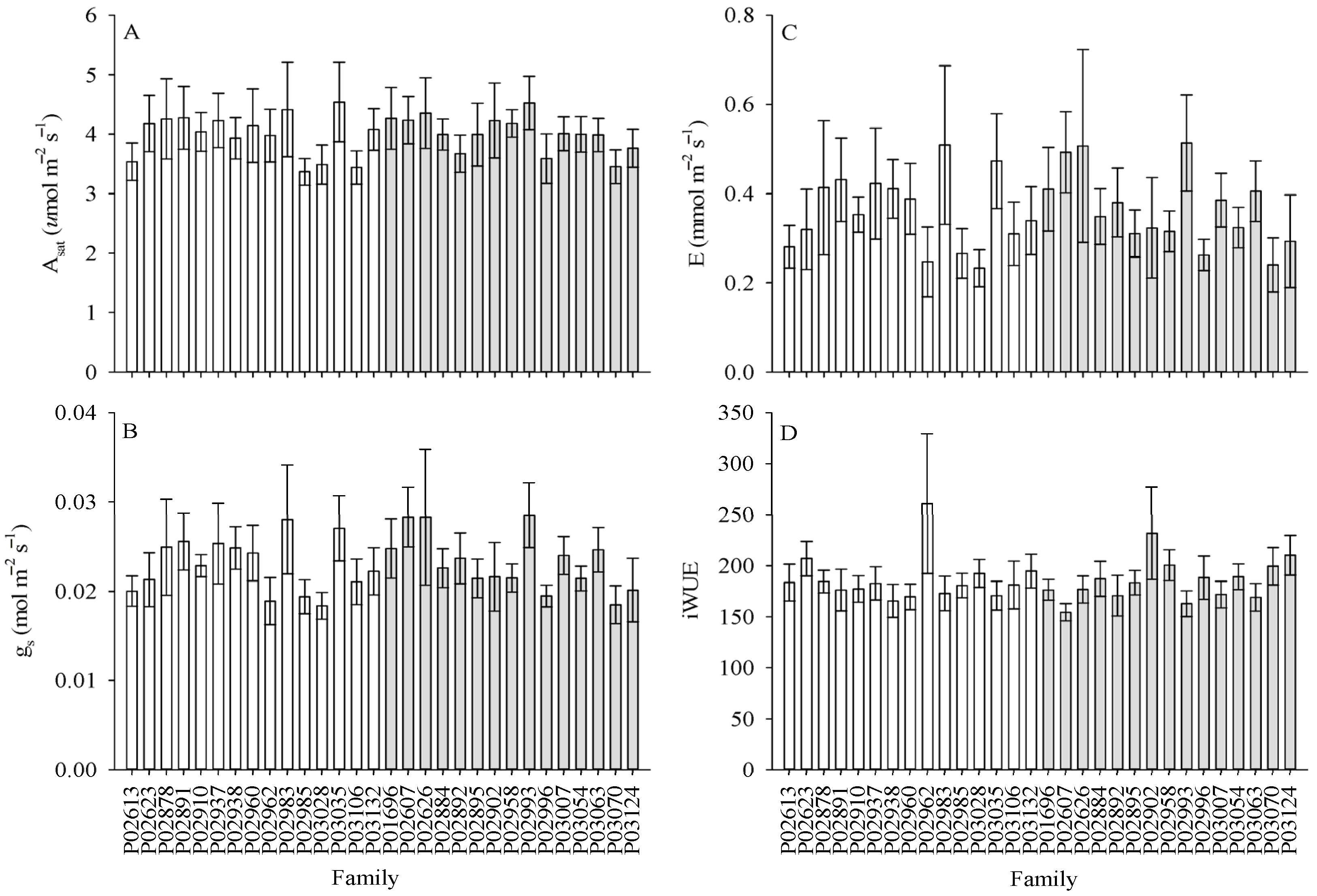
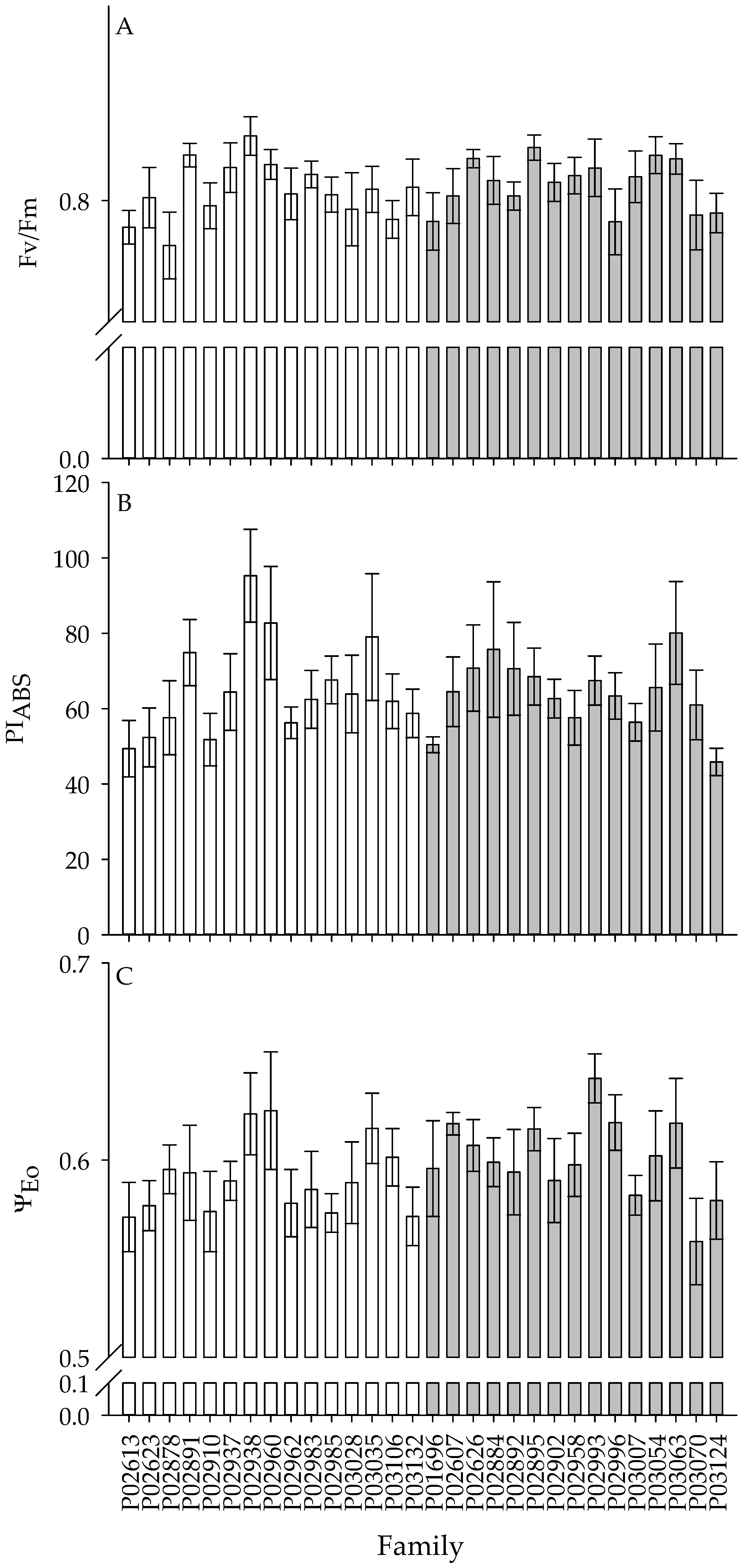
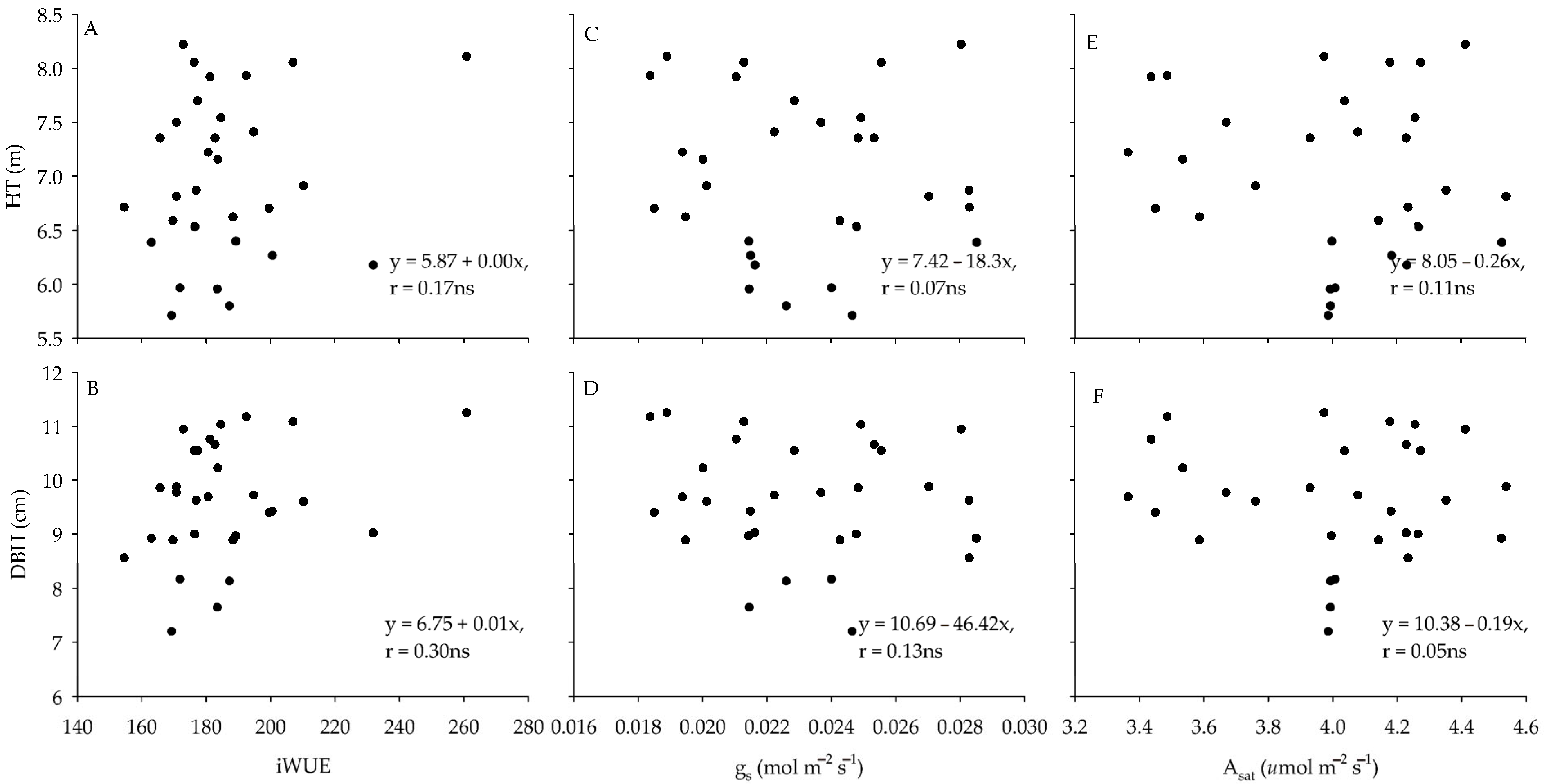
3.2. Differences in Gas Exchange and Chl a Fluorescence for Genotypes Across Sites
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HT | Height (m) |
| DBH | Diameter at the breast height (cm) |
| VOL | Stem index volume (m3) |
| MAP | Mean annual precipitation (mm) |
| MAT | Mean annual temperature (°C) |
| Asat | Light-saturated photosynthesis (μmol m−2 s−1) |
| gs | Stomatal conductance (mol m−2 s−1) |
| E | Transpiration (mmol m−2 s−1) |
| iWUE | Intrinsic water use efficiency (μmol m−2 s−1 mol m−2 s−1) |
| Fv/Fm | Quantum yield of primary PSII photochemistry |
| PIABS | Potential for energy conservation from photons absorbed by PSII to the reduction of intersystem electron acceptors |
| ΔVIP | Efficiency of electron flux through PSI to reduce the final acceptors of the electron transport chain |
| ψEo | Probability that a photon trapped by the PSII reaction center enters the electron transport chain |
References
- McDonald, P.M.; Laacke, R.J. Pinus radiata D. Don. Monterey Pine. In Silvics of North America. Volume 1, Conifers. Agriculture Handbook; Burns, R., Honkala, B., Eds.; Department of Agriculture, Forest Service: Washington, DC, USA, 1990; pp. 433–441. [Google Scholar]
- Mead, D.J. Sustainable Management of Pinus radiata Plantations; Food and Agriculture Organization of the United Nations (FAO): Roma, Italy, 2013. [Google Scholar]
- Contesse, G.D. Apuntes y consideraciones para la historia del pino radiata en Chile. Bol. Acad. Chil. Hist. 1987, 97, 351–373. [Google Scholar]
- INFOR. Anuario Forestal 2024. Instituto Forestal, Chile. Boletín Estadístico N° 199. 2025. Available online: https://wef.infor.cl/index.php/publicaciones/boletines-estadisticos/anuario-forestal (accessed on 19 May 2025).
- IPCC. Summary for Policymakers. In Climate Change 2013: The Physical Science Basis; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 1–27. [Google Scholar]
- Carrasco, G.; Almeida, A.C.; Falvey, M.; Olmedo, G.F.; Taylor, P.; Santibañez, F.; Coops, N.C. Effects of climate change on forest plantation productivity in Chile. Glob. Change Biol. 2022, 28, 7391–7409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zang, R.; Li, C. Population differences in physiological and morphological adaptations of Populus davidiana seedlings in response to progressive drought stress. Plant Sci. 2004, 166, 791–797. [Google Scholar] [CrossRef]
- Rae, A.M.; Robinson, K.M.; Street, N.R.; Taylor, G. Morphological and physiological traits influencing biomass productivity in short rotation coppice poplar. Can. J. For. Res. 2004, 34, 1488–1498. [Google Scholar] [CrossRef]
- Will, R.E.; Barron, G.A.; Burkes, E.C.; Shiver, B.; Teskey, R.O. Relationship between intercepted radiation, net photosynthesis, respiration, and rate of stem volume growth of Pinus taeda and Pinus elliottii stands of different densities. For. Ecol. Manag. 2001, 154, 155–163. [Google Scholar] [CrossRef]
- Jansson, G.; Li, B. Genetic gains of full-sib families from disconnected diallels in loblolly pine. Silvae Genet. 2004, 53, 60–64. [Google Scholar] [CrossRef]
- Isik, F.; Goldfarb, B.; LeBude, A.; Li, B.; McKeand, S. Predicted genetic gains and testing efficiency from two loblolly pine clonal trials. Can. J. For. Res. 2005, 35, 1754–1766. [Google Scholar] [CrossRef]
- Bettinger, P.; Clutter, M.; Siry, J.; Kane, M.; Pait, J. Broad implications of southern United States pine clonal forestry on planning and management of forests. Int. For. Rev. 2009, 11, 331–345. [Google Scholar] [CrossRef]
- Major, J.E.; Johnsen, K.H. Family variation in photosynthesis of 22-year-old black spruce: A test of two models of physiological response to water stress. Can. J. For. Res. 1996, 26, 1922–1933. [Google Scholar] [CrossRef]
- Major, J.E.; Johnsen, K.H. Shoot water relations of mature black spruce families displaying a genotype × environment interaction in growth rate. II. Temporal trends and response to varying soil moisture conditions. Tree Physiol. 1999, 19, 375–382. [Google Scholar] [CrossRef]
- Johnsen, K.H.; Major, J.E. Shoot water relations of mature black spruce families displaying a genotype × environment interaction in growth rate. I. Family and site effects over three growing seasons. Tree Physiol. 1999, 19, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Aspinwall, M.J.; King, J.S.; McKeand, S.E.; Domec, J.C. Leaf-level gas-exchange uniformity and photosynthetic capacity among loblolly pine (Pinus taeda L.) genotypes of contrasting inherent genetic variation. Tree Physiol. 2011, 31, 78–91. [Google Scholar] [CrossRef] [PubMed]
- McKeand, S.E.; Jokela, E.J.; Huber, D.A.; Byram, T.D.; Allen, H.L.; Li, B.; Mullin, T.J. Performance of improved genotypes of loblolly pine across different soils, climates, and silvicultural inputs. For. Ecol. Manag. 2006, 227, 178–184. [Google Scholar] [CrossRef]
- Baltunis, B.S.; Brawner, J.T. Clonal stability in Pinus radiata across New Zealand and Australia. I. Growth and form traits. New For. 2010, 40, 305–322. [Google Scholar] [CrossRef]
- Espinoza, S.E.; Magni, C.R.; Rubilar, R.A.; Yañez, M.A.; Santelices, R.E.; Cabrera, A.M.; Ivković, M. Field performance of various Pinus radiata breeding families established on a drought-prone site in central Chile. N. Z. J. For. Sci. 2017, 47, 12. [Google Scholar] [CrossRef]
- Bown, H.E.; Mason, E.G.; Clinton, P.W.; Watt, M.S. Chlorophyll fluorescence response of Pinus radiata clones to nitrogen and phosphorus supply. Cienc. Investig. Agrar. 2009, 36, 451–464. [Google Scholar] [CrossRef]
- Havaux, M.; Lannoye, R. Drought resistance of hard wheat cultivars measured by a rapid chlorophyll fluorescence test. J. Agric. Sci. 1985, 104, 501–504. [Google Scholar] [CrossRef]
- Araus, J.L.; Amaro, T.; Voltas, J.; Nakkoul, H.; Nachit, M.M. Chlorophyll fluorescence as a selection criterion for grain yield in durum wheat under Mediterranean conditions. Field Crops Res. 1998, 55, 209–223. [Google Scholar] [CrossRef]
- Swoczyna, T.; Kalaji, H.M.; Bussotti, F.; Mojski, J.; Pollastrini, M. Environmental stress- what can we learn from chlorophyll a fluorescence analysis in woody plants? A review. Front. Plant Sci. 2023, 13, 1048582. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- CIREN. Estudio Agrológico, VII Región. Descripciones de Suelos Materiales y Símbolos; Centro de Información de Recursos Naturales (CIREN): Santiago, Chile, 1997. [Google Scholar]
- CIREN. Descripciones de Suelos Materiales y Símbolos Estudio Agrológico VIII Región; Centro de Información de Recursos Naturales (CIREN): Santiago, Chile, 1999. [Google Scholar]
- King, N.T.; Seiler, J.R.; Fox, T.R.; Johnsen, K.H. Post-fertilization physiology and growth performance of loblolly pine clones. Tree Physiol. 2008, 28, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Ginn, S.E.; Seiler, J.R.; Cazell, B.H.; Kreh, R.E. Physiological and growth responses of eight-year-old loblolly pine stands to thinning. For. Sci. 1991, 37, 1030–1040. [Google Scholar] [CrossRef]
- Johnson, J.D. A rapid technique for estimating total surface area of pine needles. For. Sci. 1984, 30, 913–921. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, the Netherlands, 2004; pp. 321–362. [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor & Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dabrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef]
- Roth, B.E.; Jokela, E.J.; Martin, T.A.; Huber, D.A.; White, T.L. Genotype × environment interactions in selected loblolly and slash pine plantations in the Southeastern United States. For. Ecol. Manag. 2007, 238, 175–188. [Google Scholar] [CrossRef]
- Seiler, J.R.; Johnson, J.D. Physiological and morphological responses of three half-sib families of loblolly pine to water-stress conditioning. For. Sci. 1988, 34, 487–495. [Google Scholar] [CrossRef]
- Yang, W.Q.; Murthy, R.; King, P.; Topa, M.A. Diurnal changes in gas exchange and carbon partitioning in needles of fast- and slow- growing families of loblolly pine (Pinus taeda). Tree Physiol. 2002, 22, 489–498. [Google Scholar] [CrossRef]
- Cregg, B.M. Carbon allocation, gas exchange, and needle morphology of Pinus ponderosa genotypes known to differ in growth and survival under imposed drought. Tree Physiol. 1994, 14, 883–898. [Google Scholar] [CrossRef]
- Zhang, J.W.; Marshall, J.D. Variation in carbon isotope discrimination and photosynthetic gas exchange among populations of Pseudotsuga menziesii and Pinus ponderosa in different environments. Funct. Ecol. 1995, 9, 402–412. [Google Scholar] [CrossRef]
- Monson, R.K.; Grant, M.C. Experimental studies of ponderosa pine. III. Differences in photosynthesis, stomata1 conductance, and water-use efficiency between two genetic lines. Am. J. Bot. 1989, 76, 1041–1047. [Google Scholar]
- Mehta, P.; Jajoo, A.; Mathur, S.; Bharti, S. Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiol. Biochem. 2010, 48, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Stirbet, A.; Lazár, D.; Kromdijk, J.; Govindjee, G. Chlorophyll a fluorescence induction: Can just a one second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Marron, N.; Villar, M.; Dreyer, E.; Delay, D.; Boudouresque, E.; Petit, J.-M.; Delmotte, F.M.; Guehl, J.M.; Brignolas, F. Diversity of leaf traits related to productivity in 31 Populus deltoides × Populus nigra clones. Tree Physiol. 2005, 25, 425–435. [Google Scholar] [CrossRef]
- Monclus, R.; Dreyer, E.; Delmotte, F.M.; Villar, M.; Delay, D.; Boudouresque, E.; Petit, J.-M.; Maroron, N.; Brédnet, C.; Brignolas, F. Productivity, leaf traits and carbon isotope discrimination in 29 Populus deltoides × P. nigra clones. New Phytol. 2005, 167, 53–62. [Google Scholar] [CrossRef]
- Bonhome, L.; Barbaroux, C.; Monclus, R.; Morabito, D.; Berthelot, A.; Villar, M.; Dreyer, E.; Brignolas, F. Genetic variation in productivity, leaf traits and carbon isotope discrimination in hybrid poplars cultivated on contrasting sites. Ann. For. Sci. 2008, 65, 503. [Google Scholar] [CrossRef]
- Boltz, B.A.; Bongarten, B.C.; Teskey, R.O. Seasonal patterns of net photosynthesis of loblolly pine from diverse origins. Can. J. For. Res. 1986, 16, 1063–1068. [Google Scholar] [CrossRef]
- Bongarten, B.C.; Teskey, R.O. Dry weight partitioning and its relationship to productivity in loblolly pine seedlings from seven sources. For. Sci. 1987, 33, 255–267. [Google Scholar] [CrossRef]
- Leech, J.W. Estimating crown width from diameter at breast height for open grown radiata pine trees in South Australia. Aust. For. Res. 1984, 14, 333–337. [Google Scholar]
- Johnsen, K.H.; Seiler, J.R.; Major, J.E. Growth, shoot phenology and physiology of diverse seed sources of black spruce: II. 24-year-old trees. Tree Physiol. 1996, 16, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.D.; Rehfeldt, G.; Monserud, R.A. Family differences in height growth and photosynthetic traits in three conifers. Tree Physiol. 2001, 21, 727–734. [Google Scholar] [CrossRef] [PubMed]
- de Miguel, M.; Sánchez-Gómez, D.; Cervera, M.T.; Aranda, I. Functional and genetic characterization of gas exchange and intrinsic water use efficiency in a full-sib family of Pinus pinaster Ait. in response to drought. Tree Physiol. 2012, 32, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Pollastrini, M.; Holland, V.; Brüggemann, W.; Koricheva, J.; Jussila, I.; Scherer-Lorenzen, M.; Berger, S.; Bussotti, F. Interactions and competition processes among tree species in young experimental mixed forests, assessed with chlorophyll fluorescence and leaf morphology. Plant Biol. 2014, 16, 323–331. [Google Scholar] [CrossRef]
- Masek, J.G.; Hayes, D.J.; Hughes, M.J.; Healey, S.P.; Turner, D.P. The role of remote sensing in process-scaling studies of managed forest ecosystems. For. Ecol. Manag. 2015, 355, 109–123. [Google Scholar] [CrossRef]
- Čepl, J.; Holá, D.; Stejskal, J.; Korecký, J.; Kočová, M.; Lhotáková, Z.; Lstibůrek, M. Genetic variability and heritability of chlorophyll a fluorescence parameters in Scots pine (Pinus sylvestris L.). Tree Physiol. 2016, 36, 883–895. [Google Scholar] [CrossRef]
- Xue, J.; Clinton, P.W.; Davis, M.R.; Siddiqui, T.; Beets, P.N.; Leckie, A.C. Genotypic variation in foliar nutrient concentrations, δ13C, and chlorophyll fluorescence in relation to tree growth of radiata pine clones in a serpentine soil. J. Plant Nutr. Soil. Sci. 2013, 176, 724–733. [Google Scholar] [CrossRef]
- Stirbet, A.; Govindjee, G. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Banks, J.M. Chlorophyll fluorescence as a tool to identify drought stress in Acer genotypes. Environ. Exp. Bot. 2018, 155, 118–127. [Google Scholar] [CrossRef]
- Lambeth, C.C.; Van Buijtenen, J.P.; Duke, S.D.; McCullough, R.B. Early selection is effective in 20-year-old genetic tests of loblolly pine. Silvae Genet. 1983, 32, 210–215. [Google Scholar]
| Feature | Site | ||
|---|---|---|---|
| PC1801 | PC1802 | PC1803 | |
| Soil taxonomy— Subgroup | Mollic Endoaquepts (Inceptisol) | Typic Xeropsamments (Entisol) | Typic Vitrixerands (Andisol) |
| Parent material | Metamorphic | Alluvial sands | Volcanic sands |
| Previous land use | P. radiata plantation | P. radiata plantation. Burned in 2016 | P. radiata plantation |
| MAP (mm) | 875 | 1162 | 1303 |
| MAT (°C) | 14.0 | 13.3 | 12.4 |
| Latitude (S) | 35°57′11″ | 37°14′31″ | 37°28′27″ |
| Longitude (W) | 72°08′36″ | 72°37′14″ | 72°02′00″ |
| Altitude (m) | 161 m | 78 m | 300 m |
| Water holding capacity (mm m−1) | 270 | 45 | 69 |
| Number of blocks | 15 | 15 | 15 |
| Number of families | 197 | 212 | 195 |
| Spacing | 4.1 m × 2.2 m | 3.5 m × 2.6 m | 3.6 m × 2.5 m |
| Number of trees | 3150 | 3375 | 3150 |
| Planting date | 27 June 2018 | 17 July 2018 | 3 July 2018 |
| Average height (m) | 7.1 | 8.9 | 4.8 |
| Average DBH (cm) | 9.2 | 13.4 | 7.1 |
| Trait | Effect | ||
|---|---|---|---|
| Site | Family (Ranking) | Family (Ranking) × Site | |
| DBH (cm) | 6.0 * | 1.8 * | 0.9 ns |
| HT (m) | 11.3 ** | 2.5 *** | 0.7 ns |
| VOL (m3) | 4.1 ns | 1.5 * | 1.1 ns |
| Trait | Effect | ||
|---|---|---|---|
| Site | Family (Ranking) | Family (Ranking) × Site | |
| Asat (μmol m−2 s−1) | 0.5 ns | 0.7 ns | 1.0 ns |
| gs (mol m−2 s−1) | 3.3 ns | 1.0 ns | 1.1 ns |
| E (mmol m−2 s−1) | 5.6 * | 0.9 ns | 1.2 ns |
| iWUE | 4.0 ns | 1.2 ns | 1.0 ns |
| Fv/Fm | 0.0 ns | 1.9 ** | 0.9 ns |
| PIABS | 1.0 ns | 2.0 ** | 1.2 ns |
| ψEo | 5.3 * | 1.5 * | 1.0 ns |
| ΔVIP | 17.3 ** | 1.2 ns | 1.0 ns |
| Trait | Family Ranking | |||
|---|---|---|---|---|
| Top-15 | Bottom-15 | |||
| Mean | Coefficient of Variation (%) | Mean | Coefficient of Variation (%) | |
| Asat (μmol m−2 s−1) | 3.99 | 36 | 4.01 | 30 |
| gs (mol m−2 s−1) | 0.22 | 43 | 0.23 | 42 |
| E (mmol m−2 s−1) | 0.35 | 78 | 0.36 | 75 |
| iWUE | 186 | 38 | 184 | 31 |
| Fv/Fm | 0.80 | 3 | 0.80 | 3 |
| PIABS | 65 | 42 | 63 | 41 |
| ψEo | 0.59 | 8 | 0.60 | 8 |
| ΔVIP | 0.16 | 31 | 0.17 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza, S.; Yáñez, M.; Magni, C.; Martínez-Herrera, E.; Peña-Rojas, K.; Donoso, S.; Carrasco-Benavides, M.; Ortega-Farias, S. Genotypic Variability in Growth and Leaf-Level Physiological Performance of Highly Improved Genotypes of Pinus radiata D. Don Across Different Sites in Central Chile. Forests 2025, 16, 1108. https://doi.org/10.3390/f16071108
Espinoza S, Yáñez M, Magni C, Martínez-Herrera E, Peña-Rojas K, Donoso S, Carrasco-Benavides M, Ortega-Farias S. Genotypic Variability in Growth and Leaf-Level Physiological Performance of Highly Improved Genotypes of Pinus radiata D. Don Across Different Sites in Central Chile. Forests. 2025; 16(7):1108. https://doi.org/10.3390/f16071108
Chicago/Turabian StyleEspinoza, Sergio, Marco Yáñez, Carlos Magni, Eduardo Martínez-Herrera, Karen Peña-Rojas, Sergio Donoso, Marcos Carrasco-Benavides, and Samuel Ortega-Farias. 2025. "Genotypic Variability in Growth and Leaf-Level Physiological Performance of Highly Improved Genotypes of Pinus radiata D. Don Across Different Sites in Central Chile" Forests 16, no. 7: 1108. https://doi.org/10.3390/f16071108
APA StyleEspinoza, S., Yáñez, M., Magni, C., Martínez-Herrera, E., Peña-Rojas, K., Donoso, S., Carrasco-Benavides, M., & Ortega-Farias, S. (2025). Genotypic Variability in Growth and Leaf-Level Physiological Performance of Highly Improved Genotypes of Pinus radiata D. Don Across Different Sites in Central Chile. Forests, 16(7), 1108. https://doi.org/10.3390/f16071108








