Effects of Fire on Soil Bacterial Communities and Nitrogen Cycling Functions in Greater Khingan Mountains Larch Forests
Abstract
1. Introduction
2. Materials and Methods
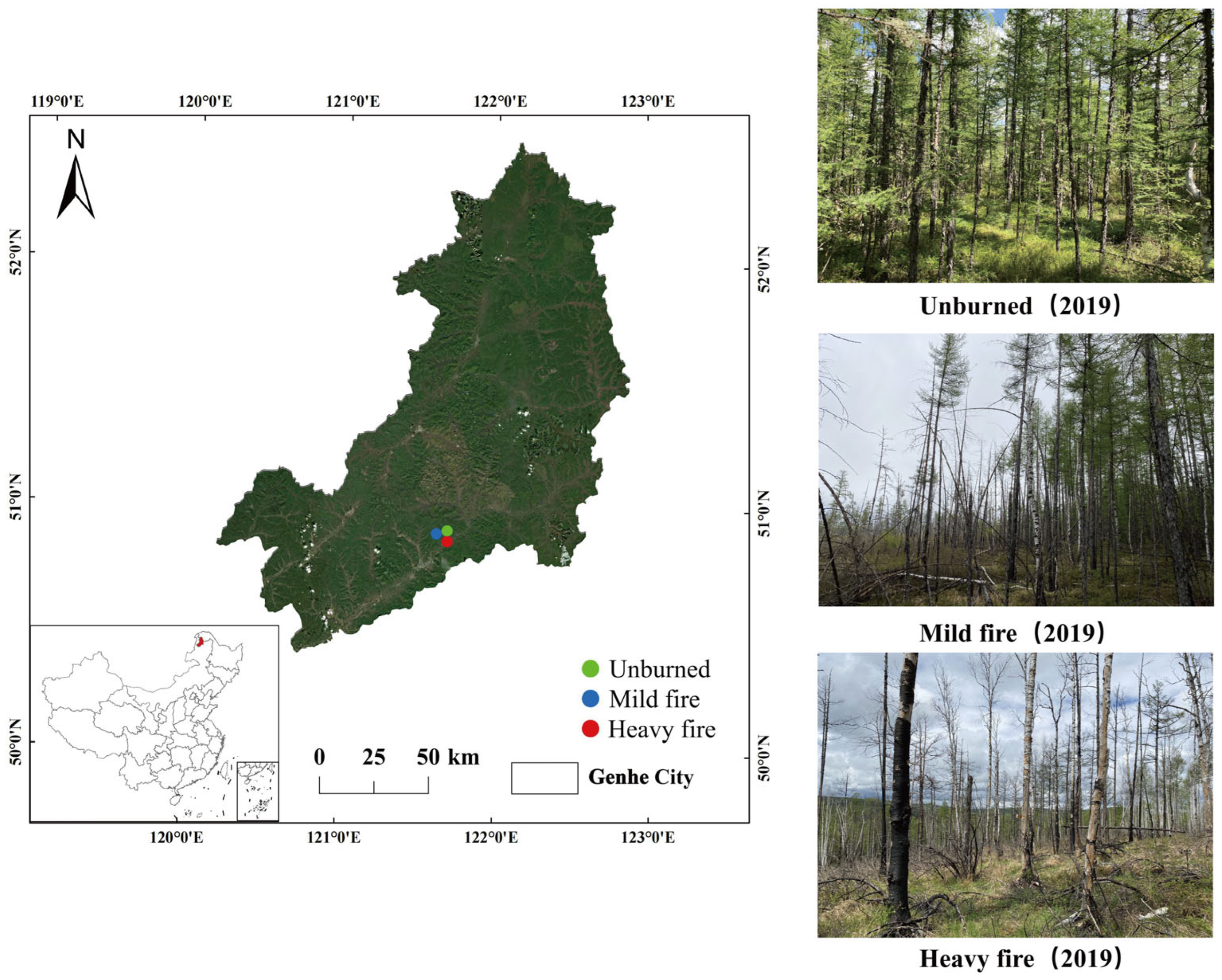
2.1. Overview of the Study Area
2.2. Research Methodology
2.2.1. Sample Collection
2.2.2. DNA Extraction and Illumina MiSeq Sequencing
2.2.3. Statistical Analysis
3. Results
3.1. Effects of Fire Intensity and Soil Physicochemical Properties
3.2. Effect of Fire Intensity on Soil Bacterial Community Diversity
3.2.1. Soil Bacterial Alpha Diversity Index Analysis
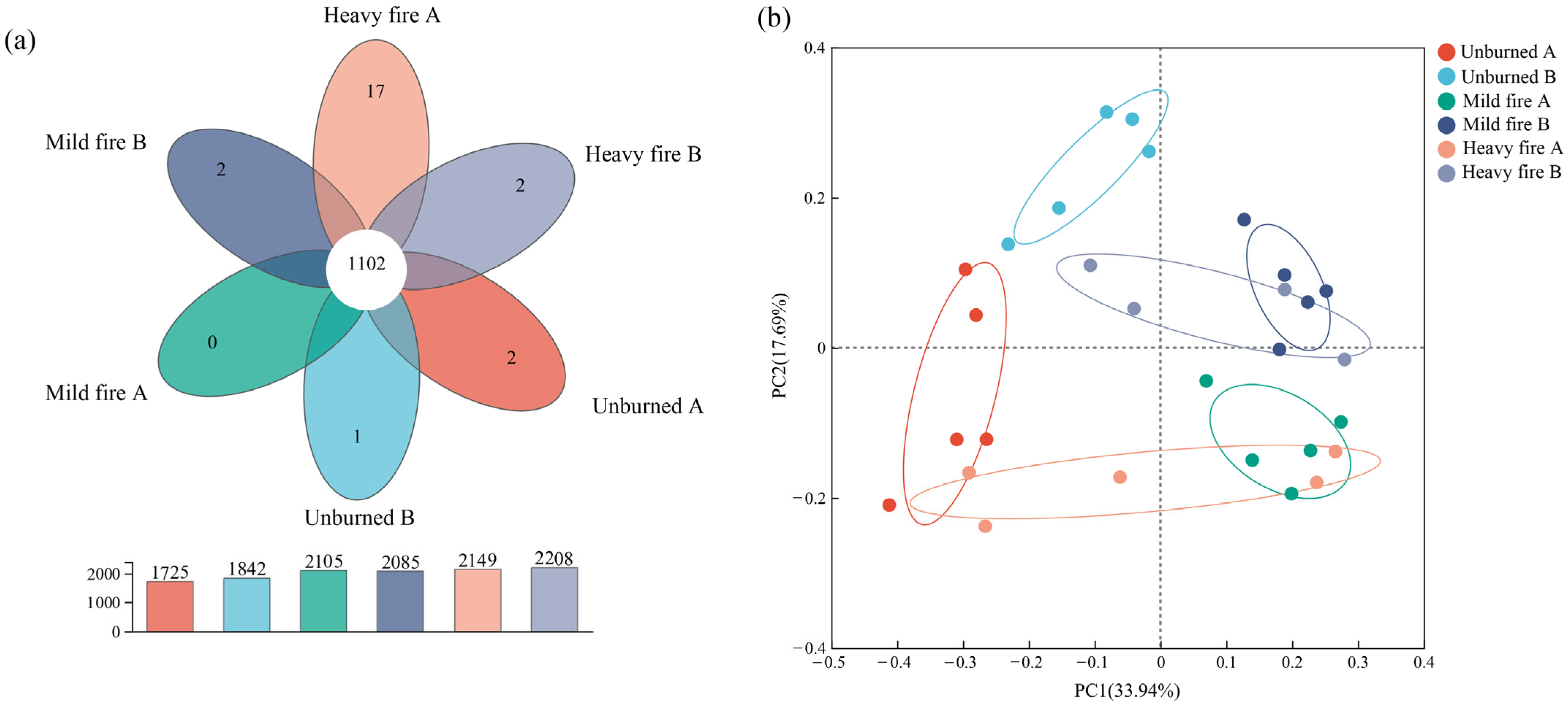
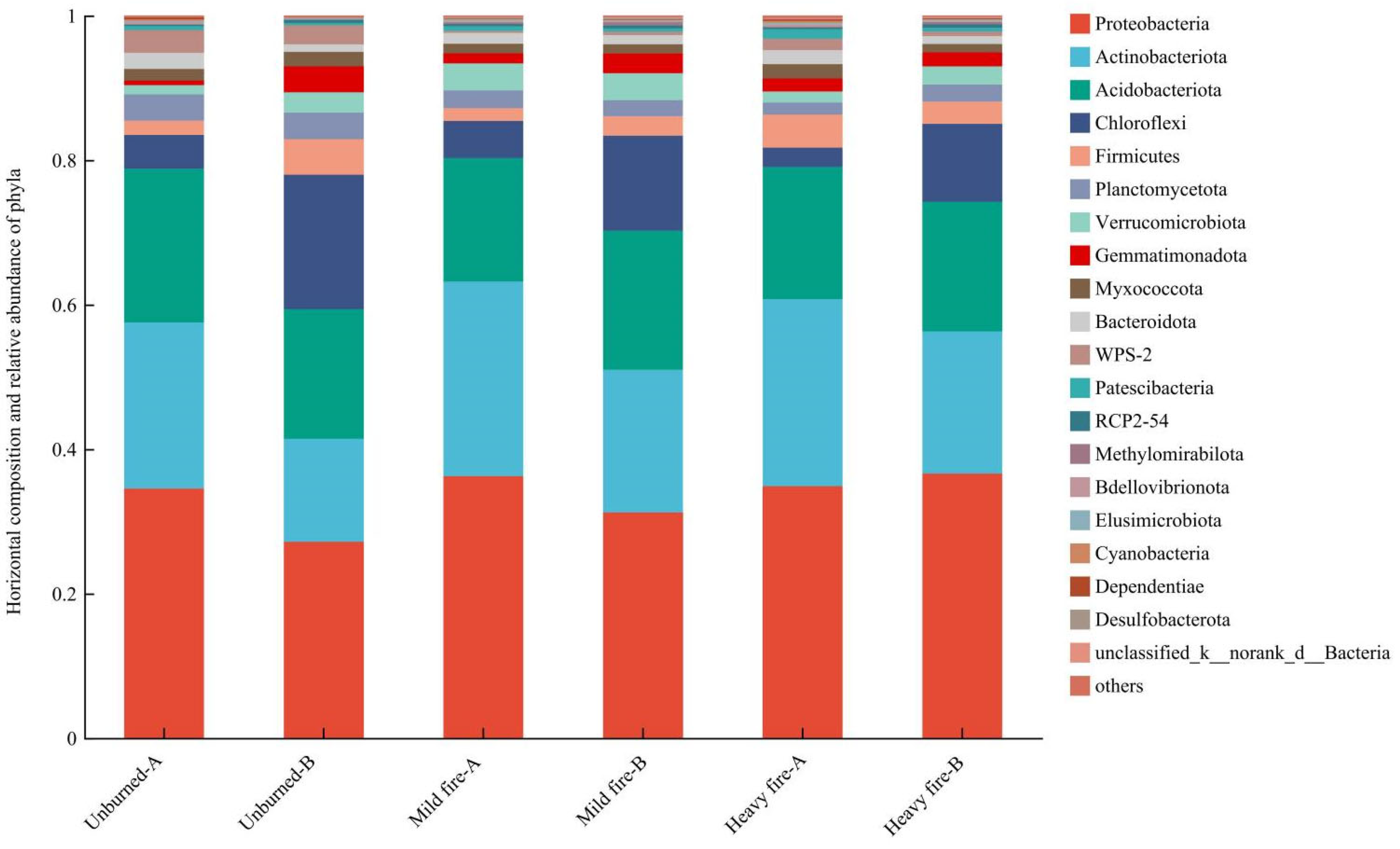
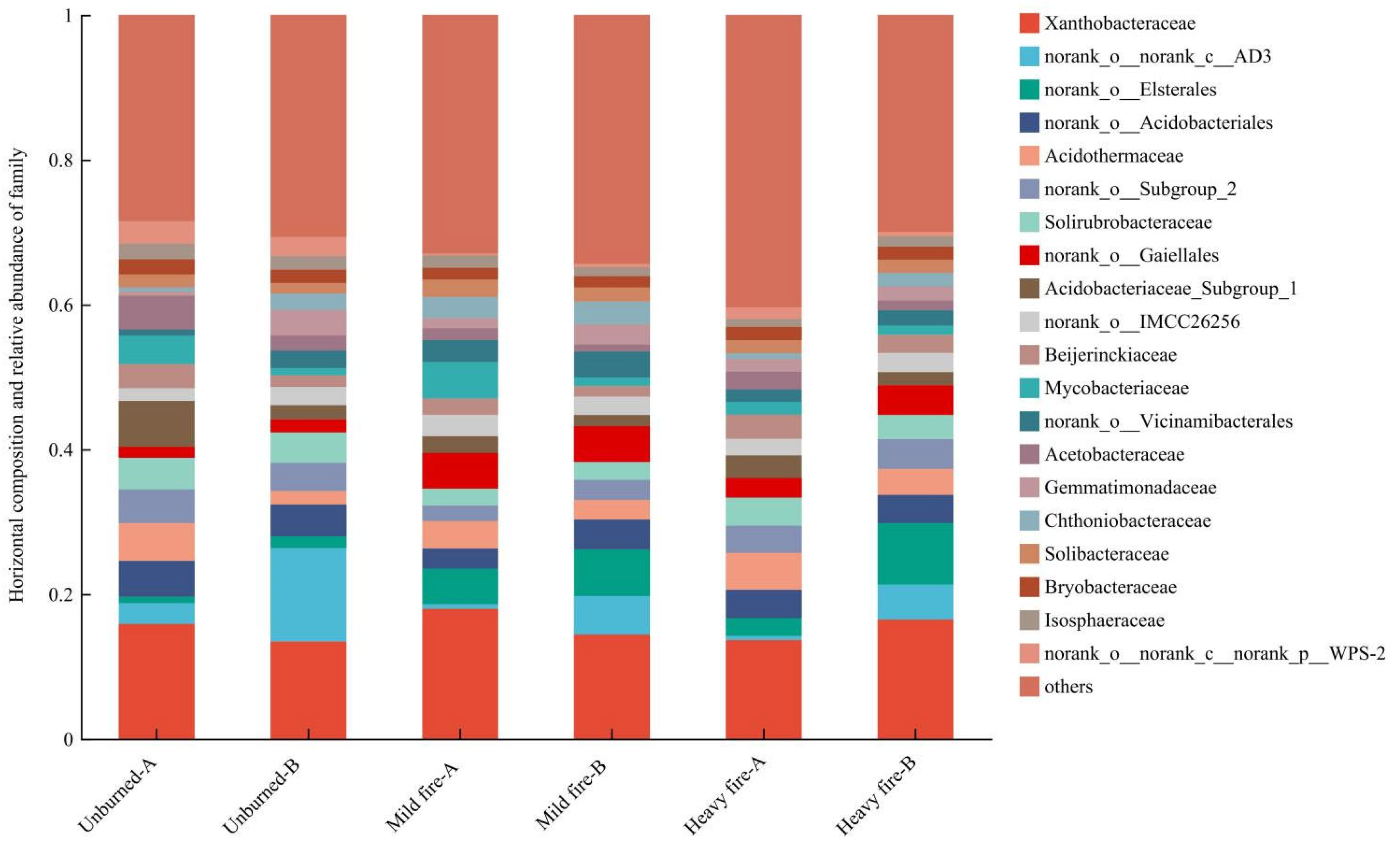
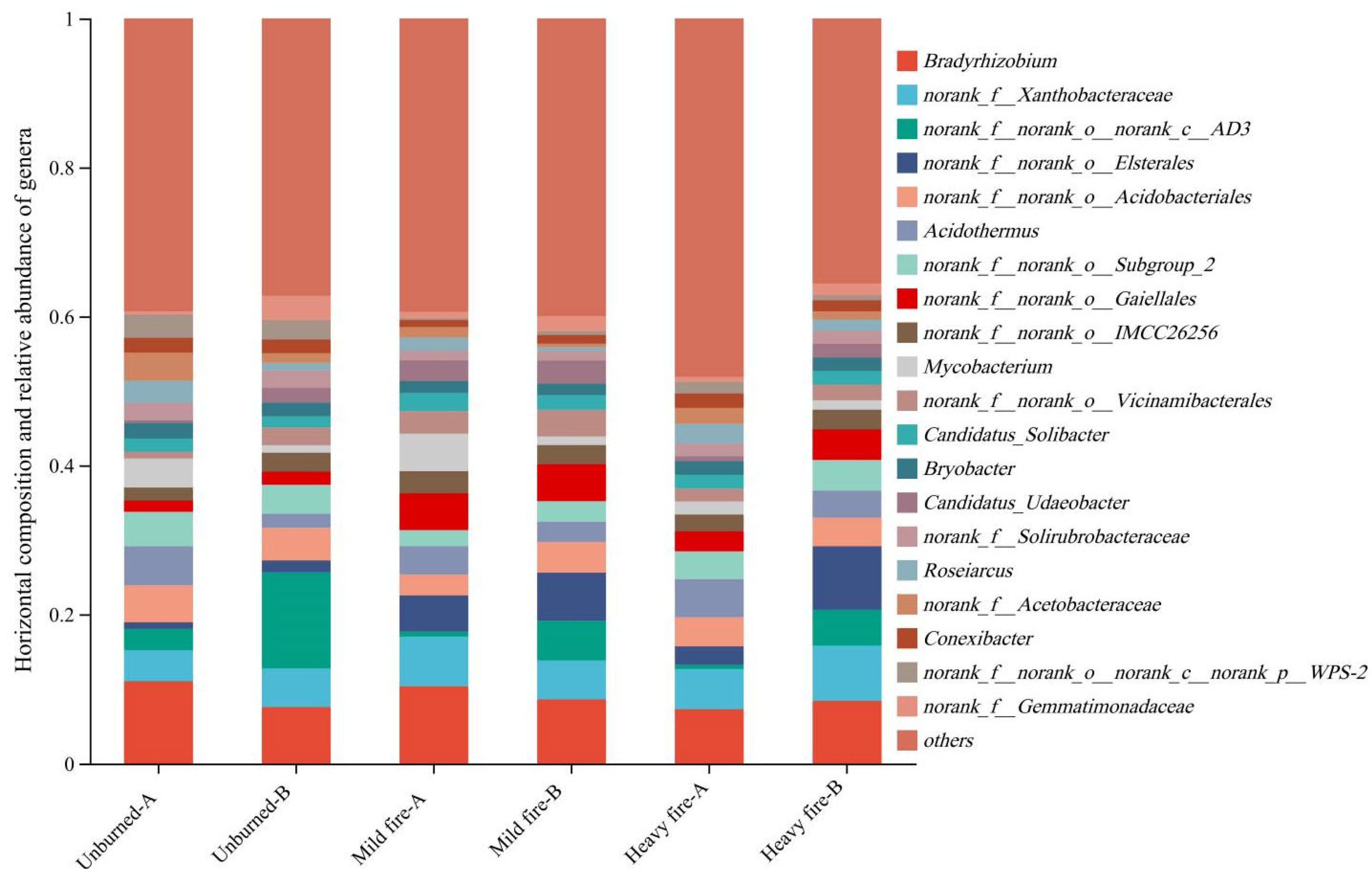
3.2.2. Structural Composition and Differential Analysis of Soil Bacterial Communities
3.3. Effect of Flaming Intensity on Functional Genes of Soil Microbial Nitrogen Cycling
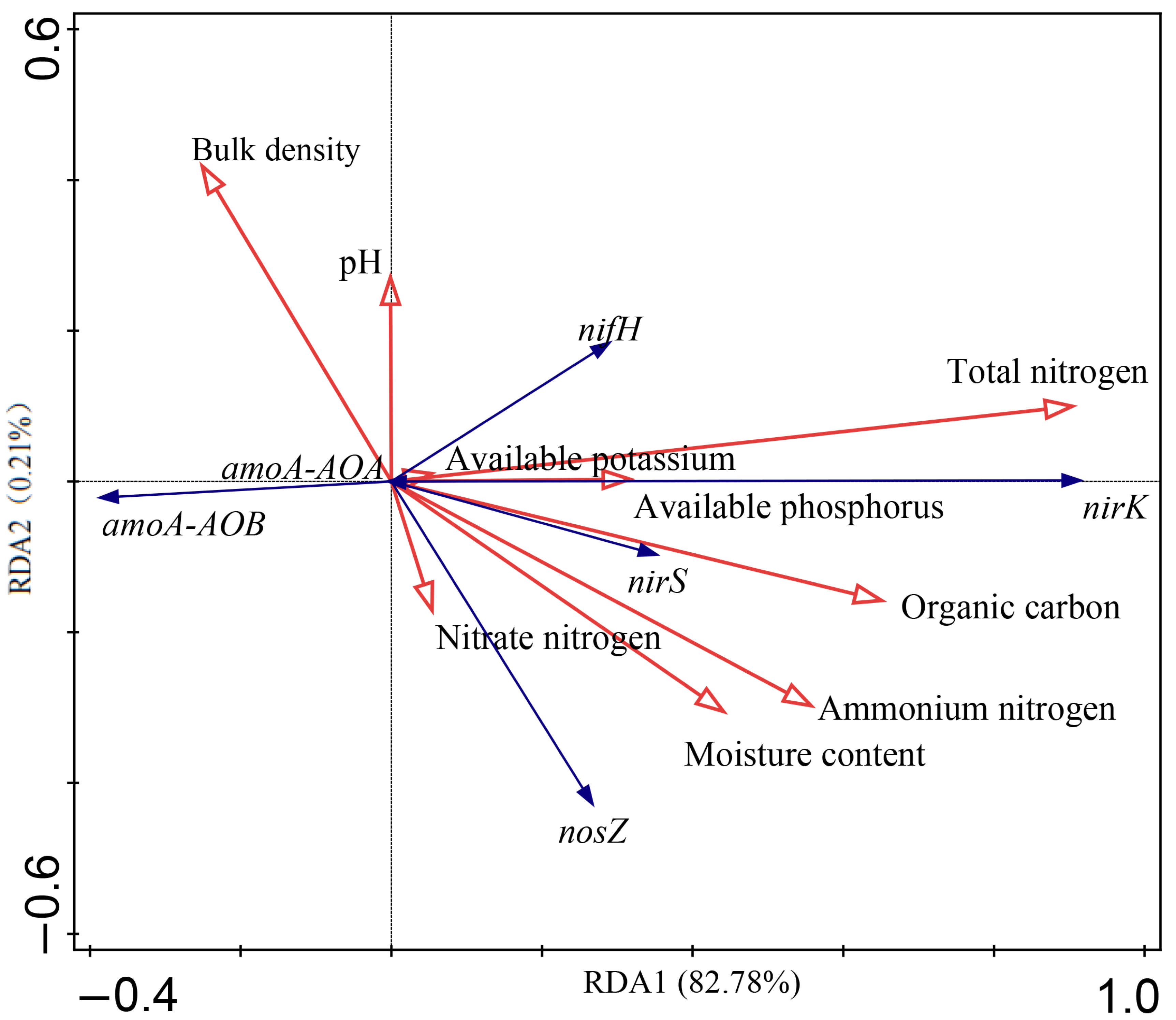
3.4. Soil Microbial Communities and Environmental Factors Correlate with Functional Genes of the Nitrogen Cycle
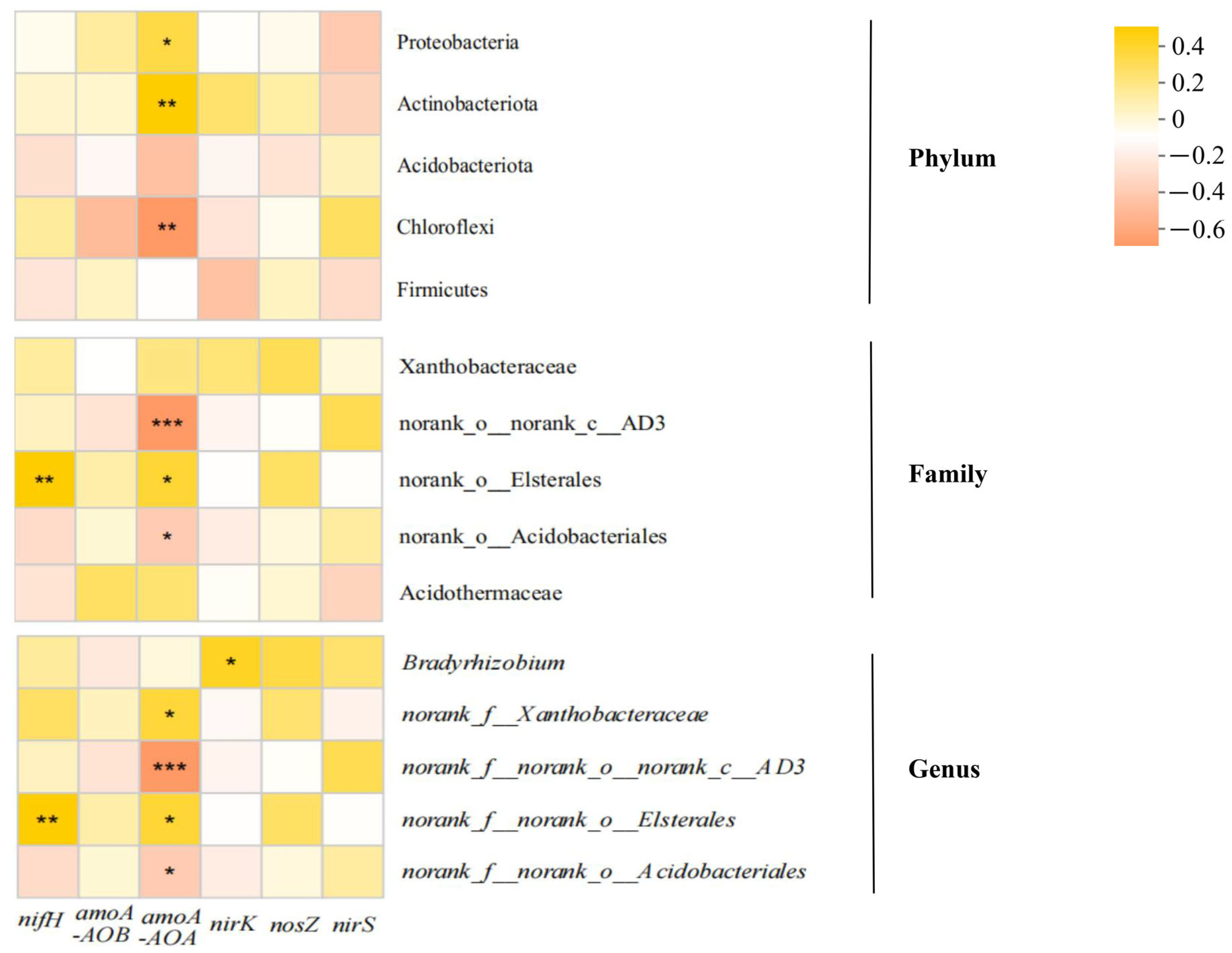
3.4.1. Soil Microbial Communities and Functional Gene Correlations for Microbial Nitrogen Cycling
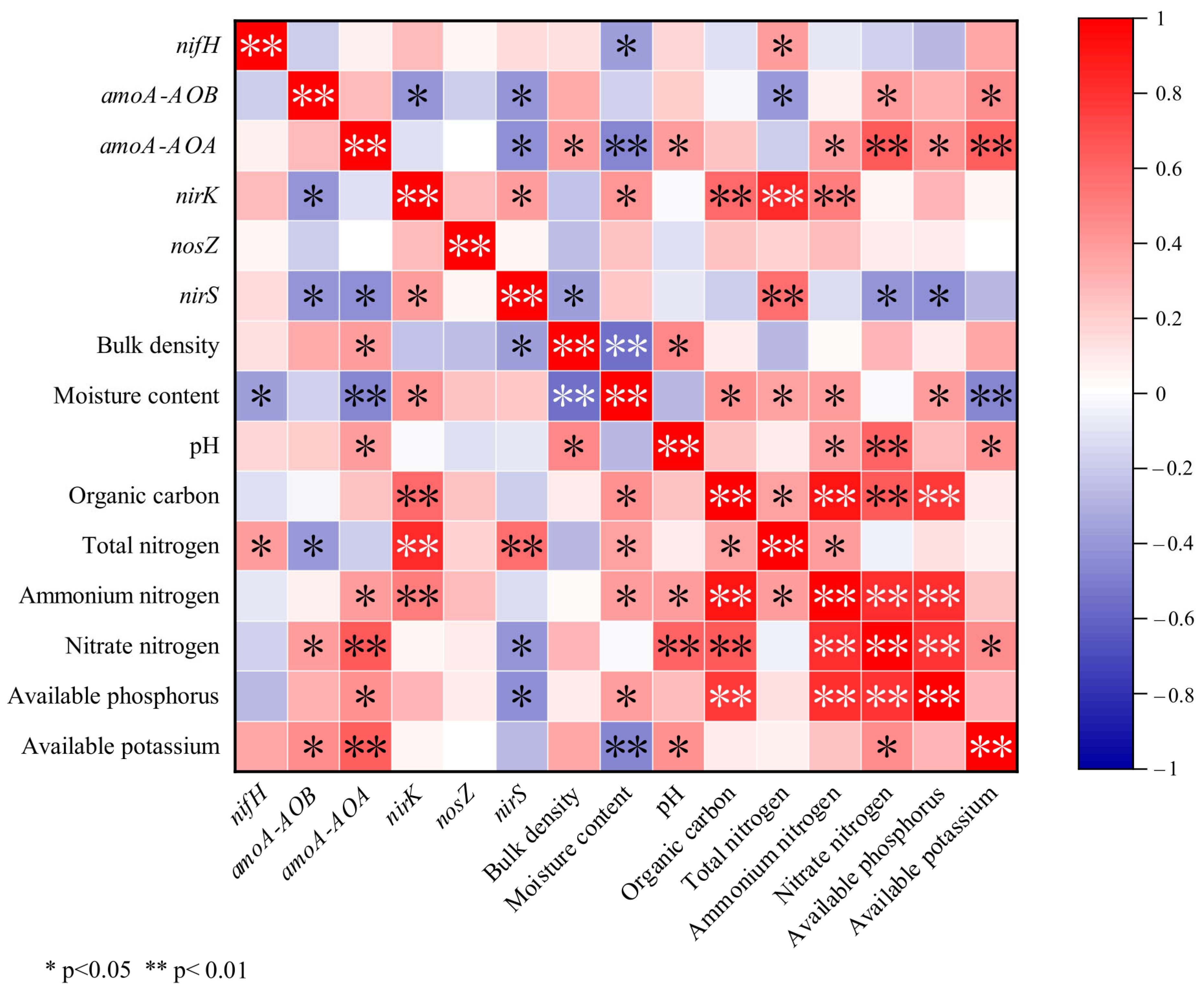
3.4.2. Correlation Between Soil Environmental Factors and Functional Genes of Microbial Nitrogen Cycling in Soils
4. Discussion
4.1. Analysis of the Effect of Different Burning Intensities on the Physicochemical Properties of Soil
4.2. Analysis of the Effect of Different Fire Intensities on the Diversity of Soil Microbial Communities
4.3. Analysis of the Effect of Different Fire Intensity on the Functional Genes of Soil Microbial Nitrogen Cycling
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wilkinson, S.L.; Andersen, R.; Moore, P.A.; Davidson, S.J.; Granath, G.; Waddington, J.M. Wildfire and degradation accelerate northern peatland carbon release. Nat. Clim. Change 2023, 13, 456–461. [Google Scholar] [CrossRef]
- Zhou, Y.; Biro, A.; Wong, M.Y.; Batterman, S.A.; Staver, A. Fire decreases soil enzyme activities and reorganizes microbially mediated nutrient cycles: A meta-analysis. Ecology 2022, 103, e3807. [Google Scholar] [CrossRef] [PubMed]
- Jolly, W.M.; Cochrane, M.A.; Freeborn, P.H.; Holden, Z.A.; Brown, T.J.; Williamson, G.J.; Bowman, D.M.J.S. Climate-induced variations in global wildfire danger from 1979 to 2013. Nat. Commun. 2015, 6, 7537. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.W.; Abatzoglou, J.T.; Veraverbeke, S.; Andela, N.; Lasslop, G.; Forkel, M.; Le Quéré, C. Global and regional trends and drivers of fire under climate change. Rev. Geophys. 2022, 60, e2020RG000726. [Google Scholar] [CrossRef]
- Jones, M.W.; Veraverbeke, S.; Andela, N.; Doerr, S.H.; Kolden, C.; Mataveli, G.; Abatzoglou, J.T. Global rise in forest fire emissions linked to climate change in the extratropics. Science 2024, 386, eadl5889. [Google Scholar] [CrossRef]
- Canadell, J.G.; Meyer, C.P.; Cook, G.D.; Dowdy, A.; Briggs, P.R.; Knauer, J.; Haverd, V. Multi-decadal increase of forest burned area in Australia is linked to climate change. Nat. Commun. 2021, 12, 6921. [Google Scholar] [CrossRef]
- Parks, S.A.; Abatzoglou, J.T. Warmer and drier fire seasons contribute to increases in area burned at high severity in western US forests from 1985 to 2017. Geophys. Res. Lett. 2020, 47, e2020GL089858. [Google Scholar] [CrossRef]
- Kazeev, K.; Vilkova, V.; Shkhapatsev, A.; Bykhalova, O.; Rudenok, Y.; Nizhelskiy, M.; Rajput, V.D. Consequences of the catastrophic wildfire in 2020 for the soil cover of the Utrish State Nature Reserve. Sains Tanah-J. Soil Sci. Agroclimatol. 2022, 19, 52–59. [Google Scholar] [CrossRef]
- Maksimova, E.; Abakumov, E. Micromorphological characteristics of sandy forest soils recently impacted by wildfires in Russia. Solid Earth 2017, 8, 553–560. [Google Scholar] [CrossRef]
- Babur, E.; Dindaroglu, T.; Danish, S.; Häggblom, M.M.; Ozlu, E.; Gozukara, G.; Uslu, O.S. Spatial responses of soil carbon stocks, total nitrogen, and microbial indices to post-wildfire in the Mediterranean red pine forest. J. Environ. Manag. 2022, 320, 115939. [Google Scholar] [CrossRef]
- O’Donnell, J.A.; Harden, J.W.; McGUIRE, A.D.; Kanevskiy, M.Z.; Jorgenson, M.T.; Xu, X. The effect of fire and permafrost interactions on soil carbon accumulation in an upland black spruce ecosystem of interior Alaska: Implications for post-thaw carbon loss. Glob. Change Biol. 2011, 17, 1461–1474. [Google Scholar] [CrossRef]
- Pérez-Izquierdo, L.; Clemmensen, K.E.; Strengbom, J.; Granath, G.; Wardle, D.A.; Nilsson, M.C.; Lindahl, B.D. Crown-fire severity is more important than ground-fire severity in determining soil fungal community development in the boreal forest. J. Ecol. 2021, 109, 504–518. [Google Scholar] [CrossRef]
- Doblas-Miranda, E.; Martínez-Vilalta, J.; Lloret, F.; Álvarez, A.; Ávila, A.; Bonet, F.J.; Brotons, L.; Castro, J.; Yuste, J.C.; Díaz, M.; et al. Reassessing global change research priorities in mediterranean terrestrial ecosystems: How far have we come and where do we go from here? Glob. Ecol. Biogeogr. 2015, 24, 25–43. [Google Scholar] [CrossRef]
- Vourlitis, G.L.; Hentz, C.S. Chronic N addition alters the carbon and nitrogen storage of a post-fire Mediterranean-type scrubland. J. Geophys. Res. Biogeosci. 2016, 121, 1265–1267. [Google Scholar] [CrossRef]
- Pellegrini, A.F.; Ahlström, A.; Hobbie, S.E.; Reich, P.B.; Nieradzik, L.P.; Staver, A.C.; Jackson, R.B. Fire frequency drives decadal changes in soil carbon and nitrogen and ecosystem productivity. Nature 2018, 553, 194–198. [Google Scholar] [CrossRef]
- Kolka, R.K.; Sturtevant, B.R.; Miesel, J.R.; Singh, A.; Wolter, P.T.; Fraver, S.; Townsend, P.A. Emissions of forest floor and mineral soil carbon, nitrogen and mercury pools and relationships with fire severity for the Pagami Creek Fire in the Boreal Forest of northern Minnesota. Int. J. Wildland Fire 2017, 26, 296–305. [Google Scholar] [CrossRef]
- Koyama, A.; Stephan, K.; Kavanagh, K.L. Fire effects on gross inorganic N transformation in riparian soils in coniferous forests of central Idaho, USA: Wildfires v. prescribed fires. Int. J. Wildland Fire 2012, 21, 69–78. [Google Scholar] [CrossRef]
- Palviainen, M.; Pumpanen, J.; Berninger, F.; Ritala, K.; Duan, B.; Heinonsalo, J.; Köster, K. Nitrogen balance along a northern boreal forest fire chronosequence. PLoS ONE 2017, 12, e0174720. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, T.H.; MacKenzie, M.D.; Gundale, M.J.; Holben, W.E. Wildfire-produced charcoal directly influences nitrogen cycling in ponderosa pine forests. Soil. Sci. Soc. Am. J. 2006, 70, 448–453. [Google Scholar] [CrossRef]
- Miesel, J.; Reiner, A.; Ewell, C.; Maestrini, B.; Dickinson, M. Quantifying Changes in Total and Pyrogenic Carbon Stocks Across Fire Severity Gradients Using Active Wildfire Incidents. Front. Earth Sci. 2018, 6, 41–48. [Google Scholar] [CrossRef]
- Knelman, J.E.; Graham, E.B.; Trahan, N.A.; Schmidt, S.K.; Nemergut, D.R. Fire severity shapes plant colonization effects on bacterial community structure, microbial biomass, and soil enzyme activity in secondary succession of a burned forest. Soil Biol. Biochem. 2015, 90, 161–168. [Google Scholar] [CrossRef]
- Graham, E.B.; Knelman, J.E. Implications of soil microbial community assembly for ecosystem restoration: Patterns, process, and potential. Microb. Ecol. 2023, 85, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Labouyrie, M.; Ballabio, C.; Romero, F.; Panagos, P.; Jones, A.; Schmid, M.W.; Orgiazzi, A. Patterns in soil microbial diversity across Europe. Nat. Commun. 2023, 14, 3311. [Google Scholar] [CrossRef]
- Pérez-Valera, E.; Verdú, M.; Navarro-Cano, J.A.; Goberna, M. Soil microbiome drives the recovery of ecosystem functions after fire. Soil Biol. Biochem. 2020, 149, 107948. [Google Scholar] [CrossRef]
- Jiang, F.; Bennett, J.A.; Crawford, K.M.; Heinze, J.; Pu, X.; Luo, A.; Wang, Z. Global patterns and drivers of plant–soil microbe interactions. Ecol. Lett. 2024, 27, e14364. [Google Scholar] [CrossRef] [PubMed]
- Revillini, D.; David, A.S.; Menges, E.S.; Main, K.N.; Afkhami, M.E.; Searcy, C.A. Microbiome-mediated response to pulse fire disturbance outweighs the effects of fire legacy on plant performance. New Phytol. 2022, 233, 2071–2082. [Google Scholar] [CrossRef]
- Li, J.; Pei, J.; Liu, J.; Wu, J.; Li, B.; Fang, C.; Nie, M. Spatiotemporal variability of fire effects on soil carbon and nitrogen: A global meta-analysis. Glob. Change Biol. 2021, 27, 4196–4206. [Google Scholar] [CrossRef]
- Warneke, C.R.; Yelenik, S.G.; Brudvig, L.A. Fire modifies plant–soil feedbacks. Ecology 2023, 104, e3994. [Google Scholar] [CrossRef]
- Su, W.Q.; Yu, M.; Lin, J.; Tang, C.; Xu, J.; Xu, J. Fire decreases gross mineralization rate but does not alter gross nitrification rate in boreal forest soils. Soil Biol. Biochem. 2022, 175, 108838. [Google Scholar] [CrossRef]
- Sun, H.; Santalahti, M.; Pumpanen, J.; Köster, K.; Berninger, F.; Raffaello, T.; Heinonsalo, J. Bacterial community structure and function shift across a northern boreal forest fire chronosequence. Sci. Rep. 2016, 6, 32411. [Google Scholar] [CrossRef]
- Taş, N.; Prestat, E.; McFarland, J.W.; Wickland, K.P.; Knight, R.; Berhe, A.A.; Jansson, J.K. Impact of fire on active layer and permafrost microbial communities and metagenomes in an upland Alaskan boreal forest. ISME J. 2014, 8, 1904–1919. [Google Scholar] [CrossRef]
- Liu, X.; Chen, C.R.; Hughes, J.M.; Wang, W.J.; Lewis, T. Temporal changes rather than long-term repeated burning predominately control the shift in the abundance of soil denitrifying community in an Australian sclerophyll forest. Microb. Ecol. 2017, 73, 177–187. [Google Scholar] [CrossRef]
- Docherty, K.M.; Balser, T.C.; Bohannan, B.J.; Gutknecht, J.L. Soil microbial responses to fire and interacting global change factors in a California annual grassland. Biogeochemistry 2012, 109, 63–83. [Google Scholar] [CrossRef]
- Shu, Y.; Shi, C.; Yi, B.; Zhao, P.; Guan, L.; Zhou, M. Influence of climatic factors on lightning fires in the primeval forest region of the Northern Daxing’an Mountains, China. Sustainability 2022, 14, 5462. [Google Scholar] [CrossRef]
- Sun, L.; Li GX, H.U.T.X. Advance in Carbon Loss of Peatlands Caused by Wildfire. Wetl. Sci. 2021, 19, 615–622. [Google Scholar]
- Bao, S.D. Soil Agricultural Chemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000; pp. 265–267. [Google Scholar]
- Liu, C.; Zhao, D.; Ma, W.; Guo, Y.; Wang, A.; Wang, Q.; Lee, D.J. Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonassp. Appl. Microbiol. Biotechnol. 2016, 100, 1421–1426. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Weber, C.F. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Barberan, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef]
- Chen, X.W.; Liu, F.L.; Han, Y.M. Soil physical and chemical properties of different soil layers in Liquidambar formosana secondary forest after fire disturbance. J. Nat. Disasters 2020, 29, 45–53. [Google Scholar]
- Hubbert, K.R.; Preisler, H.K.; Wohlgemuth, P.M.; Graham, R.C.; Narog, M.G. Prescribed burning effects on soil physical properties and soil water repellency in a steep chaparral watershed, southern California, USA. Geoderma 2006, 130, 284–298. [Google Scholar] [CrossRef]
- Verma, S.; Singh, D.; Singh, A.K.; Jayakumar, S. Post-fire soil nutrient dynamics in a tropical dry deciduous forest of Western Ghats, India. For. Ecosyst. 2019, 6, 6. [Google Scholar] [CrossRef]
- Mikita-Barbato, R.A.; Kelly, J.J.; Tate, R.L., III. Wildfire effects on the properties and microbial community structure of organic horizon soils in the New Jersey Pinelands. Soil. Biol. Biochem. 2015, 86, 67–76. [Google Scholar] [CrossRef]
- Weninger, T.; Filipović, V.; Mešić, M.; Clothier, B.; Filipović, L. Estimating the extent of fire induced soil water repellency in Mediterranean environment. Geoderma 2019, 338, 187–196. [Google Scholar] [CrossRef]
- Granged, A.J.; Zavala, L.M.; Jordán, A.; Bárcenas-Moreno, G. Post-fire evolution of soil properties and vegetation cover in a Mediterranean heathland after experimental burning: A 3-year study. Geoderma 2011, 164, 85–94. [Google Scholar] [CrossRef]
- Mataix-Solera, J.; Cerdà, A.; Arcenegui, V.; Jordán, A.; Zavala, L.M. Fire effects on soil aggregation: A review. Earth-Sci. Rev. 2011, 109, 44–60. [Google Scholar] [CrossRef]
- Moya, D.; González-De Vega, S.; Lozano, E.; García-Orenes, F.; Mataix-Solera, J.; Lucas-Borja, M.E.; de Las Heras, J. The burn severity and plant recovery relationship affect the biological and chemical soil properties of Pinus halepensis Mill. stands in the short and mid-terms after wildfire. J. Environ. Manag. 2019, 235, 250–256. [Google Scholar] [CrossRef]
- Dzwonko, Z.; Loster, S.; Gawroński, S. Impact of fire severity on soil properties and the development of tree and shrub species in a Scots pine moist forest site in southern Poland. For. Ecol. Manag. 2015, 342, 56–63. [Google Scholar] [CrossRef]
- Albert-Belda, E.; Hinojosa, M.B.; Laudicina, V.A.; García-Ruiz, R.; Pérez, B.; Moreno, J.M. Previous fire occurrence, but not fire recurrence, modulates the effect of charcoal and ash on soil C and N dynamics in Pinus pinaster Aiton forests. Sci. Total Environ. 2022, 802, 149924. [Google Scholar] [CrossRef]
- Fernández-García, V.; Marcos, E.; Fernández-Guisuraga, J.M.; Taboada, A.; Suárez-Seoane, S.; Calvo, L. Impact of burn severity on soil properties in a Pinus pinaster ecosystem immediately after fire. Int. J. Wildland Fire 2019, 28, 354–364. [Google Scholar] [CrossRef]
- Lu, X.Y.; Cui, X.Y. Effects of fire on soil habitat quality in the Daxing’anling Mountains. Chin. J. Agric. Resour. Reg. Plan. 2016, 37, 76–78. [Google Scholar]
- Cui, F.X. Effects of Fire Disturbance on Soil Microbial Diversity and Greenhouse Gas Emissions in Xing’an Larch Forest. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2022. [Google Scholar]
- Li, W.K. Effects of Fire on Soil Microbial Community Characteristics and Dynamic Changes in Pinus Tabulaeformis Stands; Beijing Forestry University: Beijing, China, 2019. [Google Scholar]
- Wang, Y.; Xu, X.; Liu, T.; Wang, H.; Yang, Y.; Chen, X. Analysis of bacterial and fungal communities in continuous-cropping ramie (Boehmeria nivea L. Gaud) fields in different areas in China. Sci. Rep. 2020, 10, 3264. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Duo, Y.; Deng, J.; Cheng, J.; Cheng, J.M.; Peng, C.H.; Guo, L. Influences of warming and snow reduction in winter on soil nutrients and bacterial communities composition in a typical grassland of the Loess Plateau. Chin. J. Plant Ecol. 2021, 45, 891–902. [Google Scholar] [CrossRef]
- Kang, B.T.; Hou, F.J.; Bowatte, S. Characterization of soil bacterial communities in alpine and desert grasslands in the Qilian Mountain range. Pratacultural Sci. 2020, 37, 10–19. [Google Scholar]
- Li, M.L.; Song, Z.P.; Liu, Y.H.; Wang, H.L. Effects of fire intensity on leaf functional traits and functional diversity of Larix gmelinii community. Chin. J. Appl. Ecol. 2019, 30, 4021–4030. [Google Scholar]
- She, R.; Yang, X.Y.; Xiao, W. Research status on forest soil microbes under fire disturbance. J. Sichuan For. Sci. Technol. 2021, 42, 94–101. [Google Scholar]
- Zhang, M.; Wang, W.; Wang, D.; Heenan, M.; Xu, Z. Short-term responses of soil nitrogen mineralization, nitrification and denitrification to prescribed burning in a suburban forest ecosystem of subtropical Australia. Sci. Total Environ. 2018, 642, 879–886. [Google Scholar] [CrossRef]
- García-Carmona, M.; Marín, C.; García-Orenes, F.; Rojas, C. Contrasting Organic Amendments Induce Different Short-Term Responses in Soil Abiotic and Biotic Properties in a Fire-Affected Native Mediterranean Forest in Chile. J. Soil. Sci. Plant Nutr. 2021, 21, 2105–2114. [Google Scholar] [CrossRef]
- Cao, L.H.; Liu, C.X.; Liu, M.; Fang, W.; Liang, C.F.; Qin, H.; Chen, J.F.; Xu, Q.F. Effects of Intensive Management Practice on Functional Gene Abundance of Denitrifying Bacteria in the Soil of Moso Bamboo(Phyllostachys heterocycle)Plantation. Acta Pedol. Sin. 2020, 57, 710–720. [Google Scholar]
- Li, H.; Shu, Y.; Wei, J.S.; Zhao, P.W.; Zhou, M.; Jia, W.J. Changes in the Soil Microbial Community Structure and Driving Factors during Post-Fire Recovery of the Larix gmelinii Rupr. Forest in Northern China. Forests 2024, 15, 664. [Google Scholar] [CrossRef]
| Diagnostic Property | Fire Intensity | ||
|---|---|---|---|
| Unburned | Mild Fire | Heavy Fire | |
| Type of fire disturbance | / | Surface fire | Surface and crown fires |
| Victimization of standing timber | / | ≤30% | ≥70% |
| Severity | / | Bark and stems disturbed and scorched, tree still covered with green leaves | Crown burned, no green leaf cover |
| Fire disturbance level | / | The organic matter layer is well preserved and charred to a depth of a few millimeters | Ash deposits and charred organic matter up to several centimeters thick |
| Plot Type | Latitude and Longitude | Elevation | Aspect | Slope | Vegetation Type | Soil Type | C/N |
|---|---|---|---|---|---|---|---|
| Unburned | 121°41′19″ E–50°52′49″ N | 1146.4 | Southwestern | 4° | Larix gmelinii—Rhododendron simsii | Brown coniferous forest soils | 19.27 |
| Mild fire | 121°41′19″ E–50°52′49″ N | 1130.7 | Southwestern | 6° | Larix gmelinii—Rhododendron simsii | Brown coniferous forest soils | 15.07 |
| Heavy fire | 121°41′19″ E–50°52′49’ N | 1144.9 | Southwestern | 4° | Larix gmelinii—Rhododendron simsii | Brown coniferous forest soils | 21.36 |
| Target Gene | Primer |
|---|---|
| nirS | Cd3aF: GTSAACGTSAAGGARACSGG |
| R3cd:GASTTCGGRTGSGTCTTGA | |
| nirK | nirk876: ATYGGCGGVCAYGGCGA |
| nirk1040: GCCTCGATCAGRTTRTGGT | |
| nosZ | nosz2F:CGCRACGGCAASAAGGTSMSSGT |
| nosz2R: CAKRTGCAKSGCRTGGCAGAA | |
| nifH | MMF2: TNATCACCKCNATCACTTCC |
| MMR1: CGCCGGACKWGACGATGTAG | |
| amoA-AOB | amoa1F: GGGGTTTCTACTGGTGGT |
| amoa2R: CCCCTCKGSAAAGCCTTCTTC | |
| amoA-AOA | Arch-AmoAF:STAATGGTCTGGCTTAGACG |
| Arch-AmoAR:GCGGCCATCCATCTGTATGT |
| Fire Intensity | Soil Depth (cm) | Heavy Fire | Mild Fire | Unburned | |
|---|---|---|---|---|---|
| Soil Properties | |||||
| Bulk density (g/cm3) | A (0–10) | 0.91 ± 0.04 Aa | 0.88 ± 0.03 ABa | 0.85 ± 0.06 Ba | |
| B (10–20) | 0.92 ± 0.07 Aa | 0.90 ± 0.04 Aa | 0.83 ± 0.04 Ba | ||
| Moisture content (%) | A (0–10) | 34.21 ± 3.20 Ca | 37.38 ± 2.39 Ba | 40.03 ± 0.64 Aa | |
| B (10–20) | 31.42 ± 2.65 Ca | 34.46 ± 3.32 Ba | 38.99 ± 1.95 Aa | ||
| pH | A (0–10) | 5.64 ± 0.20 Aa | 5.40 ± 0.17 Ba | 5.13 ± 0.12 Ca | |
| B (10–20) | 5.13 ± 0.23 Ab | 5.25 ± 0.07 Ab | 5.14 ± 0.09 Aa | ||
| Organic carbon (g/kg) | A (0–10) | 82.66 ± 8.32 Ba | 102.54 ± 17.15 Aa | 94.44 ± 14.98 Aa | |
| B (10–20) | 46.33 ± 9.45 Ab | 35.17 ± 7.70 Ab | 43.70 ± 13.03 Ab | ||
| Total nitrogen (g/kg) | A (0–10) | 3.06 ± 0.26 Aa | 4.68 ± 0.33 Ba | 3.71 ± 0.45 Ca | |
| B (10–20) | 2.95 ± 0.27 Aa | 4.28 ± 0.29 Ba | 3.34 ± 0.34 Ca | ||
| Ammonium nitrogen (mg/kg) | A (0–10) | 57.73 ± 4.85 Ca | 64.91 ± 2.55 Aa | 60.46 ± 6.12 Ba | |
| B (10–20) | 21.19 ± 3.59 Bb | 25.77 ± 7.85 Ab | 22.21 ± 7.94 Bb | ||
| Nitrate nitrogen (mg/kg) | A (0–10) | 5.58 ± 0.26 Aa | 4.33 ± 0.36 Ba | 3.77 ± 0.28 Ca | |
| B (10–20) | 2.98 ± 0.14 Ab | 2.37 ± 0.18 Bb | 2.21 ± 0.15 Cb | ||
| Available phosphorus (mg/kg) | A (0–10) | 26.68 ± 5.81 Aa | 19.83 ± 6.06 Ba | 18.61 ± 3.19 Ba | |
| B (10–20) | 16.02 ± 4.56 Ab | 11.80 ± 3.92 Ab | 14.38 ± 3.32 Ab | ||
| Available potassium (mg/kg) | A (0–10) | 297.70 ± 62.97 Aa | 276.01 ± 28.24 Aa | 265.23 ± 41.50 Aa | |
| B (10–20) | 325.74 ± 31.59 Aa | 312.05 ± 77.47 Aa | 252.71 ± 40.02 Aa | ||
| Soil Depth (cm) | Fire Intensity | Ace | Chao1 | Shannon | Simpson |
|---|---|---|---|---|---|
| A (0–10) | Heavy fire | 1464.34 ± 167.97 Ba | 1442.82 ± 170.70 Ba | 5.38 ± 0.23 ABa | 0.016 ± 0.006 Aa |
| Mild fire | 1713.57 ± 124.22 Aa | 1721.07 ± 120.85 Aa | 5.43 ± 0.16 Aa | 0.019 ± 0.004 Aa | |
| Unburned | 1401.52 ± 212.35 Ba | 1413.62 ± 203.37 Ba | 5.09 ± 0.23 Ba | 0.015 ± 0.005 Aa | |
| B (10–20) | Heavy fire | 1490.60 ± 112.24 Aa | 1489.29 ± 119.23 Aa | 5.38 ± 0.13 ABa | 0.018 ± 0.002 Aa |
| Mild fire | 1743.42 ± 100.79 Aa | 1753.87 ± 95.51 Aa | 5.60 ± 0.04 Aa | 0.021 ± 0.002 Aa | |
| Unburned | 1464.76 ± 107.23 Ba | 1477.74 ± 129.78 Ba | 5.14 ± 0.27 Ba | 0.016 ± 0.006 Aa |
| Classification | Name | Fire Intensity | Soil Depth | ||
|---|---|---|---|---|---|
| F | p | F | p | ||
| Phylum | Proteobacteria | 1.40 | p = 0.25 | 2.19 | p = 0.15 |
| Actinobacteriota | 1.89 | p = 0.17 | 11.56 | p < 0.05 | |
| Acidobacteriota | 0.21 | p = 0.81 | 0.05 | p = 0.82 | |
| Chloroflexi | 5.08 | p < 0.05 | 64.01 | p < 0.05 | |
| Firmicutes | 0.60 | p = 0.56 | 0.39 | p = 0.54 | |
| Family | Xanthobacteraceae | 0.34 | p = 0.71 | 0.46 | p = 0.50 |
| norank_o_norank_c_AD3 | 18.19 | p < 0.05 | 64.12 | p < 0.05 | |
| norank_o_Elsterales | 25.06 | p < 0.05 | 22.94 | p < 0.05 | |
| norank_o_Acidobacteriales | 1.23 | p = 0.31 | 0.13 | p = 0.72 | |
| Acidothermaceae | 0.68 | p = 0.52 | 6.02 | p < 0.05 | |
| Genus | Bradyrhizobium | 0.86 | p = 0.44 | 1.48 | p = 0.24 |
| norank_f_Xanthobacteraceae | 2.17 | p = 0.14 | 0.56 | p = 0.46 | |
| norank_f_norank _o_norank_c_AD3 | 18.19 | p < 0.05 | 64.12 | p < 0.05 | |
| norank_f_norank _o_Elsterales | 25.06 | p < 0.05 | 22.94 | p < 0.05 | |
| norank_f_norank _o_Acidobacteriales | 1.23 | p = 0.31 | 0.13 | p = 0.72 | |
| Soil Nitrogen Fraction | Soil Depth | HF | LF | UC |
|---|---|---|---|---|
| nifH | 0–10 cm | 2.91 × 103 ± 8.09 × 102 Ba | 8.44 × 103 ± 3.56 × 102 ABa | 1.13 × 104 ± 4.49 × 103 Aa |
| 10–20 cm | 5.68 × 103 ± 2.46 × 103 Aa | 7.55 × 103 ± 2.27 × 103 Aa | 7.91 × 103 ± 4.23 × 103 Aa | |
| amoA-AOA | 0–10 cm | 1.25 × 102 ± 2.37 × 101 Aa | 1.73 × 101 ± 1.65 × 100 Bb | 1.28 × 101 ± 2.26 × 100 Bb |
| 10–20 cm | 1.04 × 102 ± 1.51 × 101 Aa | 8.40 × 101 ± 6.71 × 100 Aa | 4.30 × 101 ± 9.58 × 100 Ba | |
| amoA-AOB | 0–10 cm | 2.33 × 105 ± 7.75 × 104 Aa | 7.70 × 102 ± 5.12 × 101 Ba | 2.02 × 102 ± 1.04 × 102 Ba |
| 10–20 cm | 3.92 × 105 ± 2.04 × 105 Aa | 6.51 × 102 ± 2.39 × 102 Ba | 5.01 × 102 ± 1.74 × 102 Ba | |
| nirK | 0–10 cm | 5.14 × 106 ± 1.93 × 106 Ba | 6.41 × 107 ± 2.69 × 107 Aa | 5.24 × 107 ± 2.56 × 107 Aa |
| 10–20 cm | 2.04 × 106 ± 7.78 × 105 Ba | 4.13 × 107 ± 1.22 × 107 Aa | 2.74 × 107 ± 9.55 × 106 Aa | |
| nirS | 0–10 cm | 6.15 × 104 ± 4.40 × 104 Aa | 3.75 × 105 ± 7.41 × 104 Aa | 3.55 × 105 ± 8.99 × 104 Aa |
| 10–20 cm | 6.41 × 104 ± 3.27 × 104 Ba | 4.25 × 105 ± 4.78 × 104 Aa | 3.72 × 105 ± 6.48 × 105 ABa | |
| nosZ | 0–10 cm | 1.10 × 106 ± 1.91 × 105 Ca | 6.61 × 106 ± 2.15 × 105 Aa | 2.75 × 106 ± 3.26 × 105 Ba |
| 10–20 cm | 1.64 × 106 ± 7.06 × 105 Aa | 3.90 × 106 ± 8.07 × 105 Ab | 2.66 × 106 ± 9.74 × 105 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, Y.; Jia, W.; Zhao, P.; Zhou, M.; Zhang, H. Effects of Fire on Soil Bacterial Communities and Nitrogen Cycling Functions in Greater Khingan Mountains Larch Forests. Forests 2025, 16, 1094. https://doi.org/10.3390/f16071094
Shu Y, Jia W, Zhao P, Zhou M, Zhang H. Effects of Fire on Soil Bacterial Communities and Nitrogen Cycling Functions in Greater Khingan Mountains Larch Forests. Forests. 2025; 16(7):1094. https://doi.org/10.3390/f16071094
Chicago/Turabian StyleShu, Yang, Wenjie Jia, Pengwu Zhao, Mei Zhou, and Heng Zhang. 2025. "Effects of Fire on Soil Bacterial Communities and Nitrogen Cycling Functions in Greater Khingan Mountains Larch Forests" Forests 16, no. 7: 1094. https://doi.org/10.3390/f16071094
APA StyleShu, Y., Jia, W., Zhao, P., Zhou, M., & Zhang, H. (2025). Effects of Fire on Soil Bacterial Communities and Nitrogen Cycling Functions in Greater Khingan Mountains Larch Forests. Forests, 16(7), 1094. https://doi.org/10.3390/f16071094







