Plant-Dwelling Spider Assemblages in Managed and Protected Primeval Deciduous Stands of Białowieża Forest, Poland
Abstract
1. Introduction
2. Materials and Methods
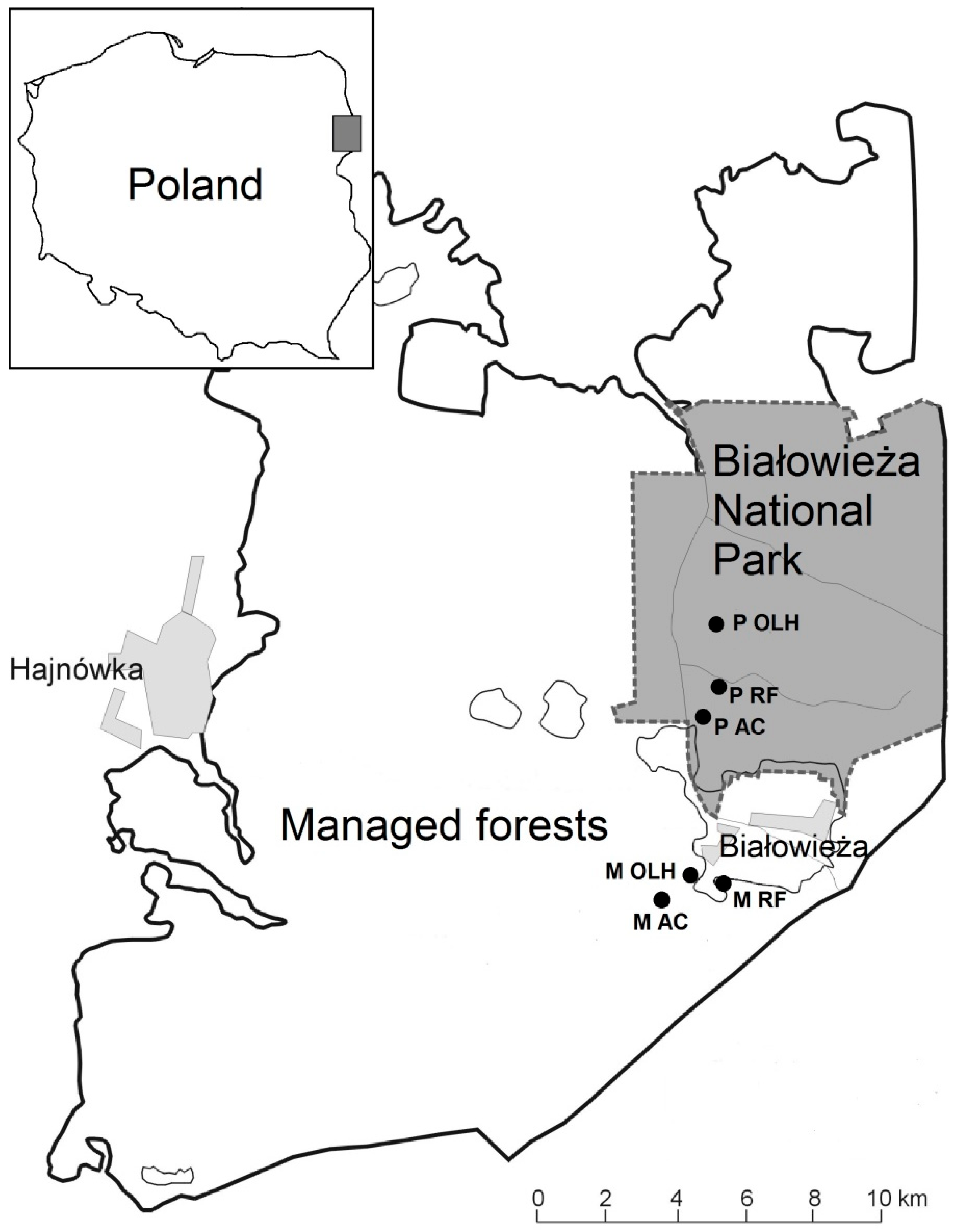
2.1. Study Site
2.2. Vegetation Measurements and Spider Sampling
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kujawa, A.; Orczewska, A.; Falkowski, M.; Blicharska, M.; Bohdan, A.; Buchholz, L.; Chylarecki, P.; Gutowski, J.M.; Latałowa, M.; Mysłajek, R.W.; et al. The Białowieża Forest—A UNESCO Natural Heritage Site–protection priorities. For. Res. Pap. 2016, 77, 302–323. [Google Scholar] [CrossRef]
- Wesołowski, T.; Kujawa, A.; Bobiec, A.; Bohdan, A.; Buchholz, L.; Chylarecki, P.; Engel, J.; Falkowski, M.; Gutowski, J.M.; Jaroszewicz, B.; et al. Dispute over the Future of the Białowieża Forest: Myths and Facts. A Voice in the Debate. 2016. Available online: https://www.researchgate.net/publication/303389991_Dispute_over_the_future_of_the_Bialowieza_Forest_myths_and_facts_A_voice_in_the_debate (accessed on 12 May 2025).
- Niedziałkowski, K. Why do foresters oppose the enlargement of the Białowieża National Park? The motivation of the State Forest Holding employees as perceived by social actors engaged in the conflict over the Białowieża Forest. For. Res. Pap. 2016, 77, 358–370. [Google Scholar] [CrossRef]
- Mikusiński, G.; Bubnicki, J.W.; Churski, M.; Czeszczewik, D.; Walankiewicz, W.; Kuijper, D.P.J. Is the impact of loggings in the last primeval lowland forest in Europe underestimated? The conservation issues of Białowieża Forest. Biol. Conserv. 2018, 227, 266–274. [Google Scholar] [CrossRef]
- Blicharska, M.; Angelstam, P.; Giessen, L.; Hilszczański, J.; Hermanowicz, E.; Holeksa, J.; Jacobsen, J.B.; Jaroszewicz, B.; Konczal, A.; Konieczny, A.; et al. Between biodiversity conservation and sustainable forest management–A multidisciplinary assessment of the emblematic Białowieża Forest case. Biol. Conserv. 2020, 248, 108614. [Google Scholar] [CrossRef]
- Walankiewicz, W.; Czeszczewik, D.; Mitrus, C.; Bida, E. Znaczenie martwych drzew dla zespołu dzięciołów w lasach liściastych Puszczy Białowieskiej [Snag importance for woodpeckers in deciduous stands of the Białowieża Forest]. Notatki Ornitol. 2002, 43, 61–71. [Google Scholar]
- Wesołowski, T.; Czeszczewik, D.; Rowiński, P. Effects of forest management on Three-toed Woodpecker Picoides tridactylus distribution in the Białowieża Forest (NE Poland): Conservation implications. Acta Ornithol. 2005, 40, 53–60. [Google Scholar] [CrossRef]
- Wesołowski, T. Value of Białowieża Forest for the conservation of white-backed woodpecker Dendrocopos leucotos in Poland. Biol. Conserv. 1995, 1, 69–75. [Google Scholar] [CrossRef]
- Czeszczewik, D.; Walankiewicz, W. Logging affects the white-backed woodpecker Dendrocopos leucotos distribution in the Białowieża Forest. Ann. Zool. Fenn. 2006, 43, 221–227. [Google Scholar]
- Jaworski, T.; Plewa, R.; Tarwacki, G.; Sućko, K.; Hilszczański, J.; Horák, J. Ecologically similar saproxylic beetles depend on diversified deadwood resources: From habitat requirements to management implications. For. Ecol. Manag. 2019, 449, 17462. [Google Scholar] [CrossRef]
- Tomiałojć, L. Characteristics of old growth in the Białowieża Forest, Poland. Nat. Areas J. 1991, 11, 7–18. [Google Scholar]
- Tomiałojć, L.; Wesołowski, T. Diversity of the Białowieża Forest avifauna in space and time. J. Ornithol. 2004, 145, 8–92. [Google Scholar] [CrossRef]
- Sławski, M.; Sławska, M. Seven Decades of Spontaneous Forest Regeneration after Large-Scale Clear-Cutting in Białowieża Forest do not Ensure the Complete Recovery of Collembolan Assemblages. Forests 2019, 10, 948. [Google Scholar] [CrossRef]
- Skłodowski, J. Consequence of the transformation of a primeval forest into a managed forest for carabid beetles (Coleoptera: Carabidae)—A case study from Białowieża (Poland). Eur. J. Entomol. 2014, 111, 639–648. [Google Scholar] [CrossRef]
- Stańska, M. Rare and threatened spider species (Araneae) in selected types of deciduous forests in the Białowieża Forest. Nat. Conserv. 2007, 64, 13–29. [Google Scholar]
- Stańska, M.; Stański, T.; Bartos, M. Spider Assemblages of Tree Branches in Managed and Primeval Deciduous Stands of the Białowieża Forest. Forests 2022, 13, 5. [Google Scholar] [CrossRef]
- Wesołowski, T. Virtual Conservation: How the European Union is turning a blind eye to its vanishing primeval forests. Conserv. Biol. 2005, 19, 1349–1358. [Google Scholar] [CrossRef]
- Prieto-Benítez, S.; Méndez, M. Effects of land management on the abundance and richness of spiders (Araneae): A meta-analysis. Biol. Conserv. 2011, 144, 683–691. [Google Scholar] [CrossRef]
- Shochat, E.; Stefanov, W.L.; Whitehouse, M.E.A.; Faeth, S.H. Urbanization and spider diversity: Influences of human modification of habitat structure and productivity. Ecol. Appl. 2004, 14, 268–280. [Google Scholar] [CrossRef]
- Stańska, M.; Stański, T.; Gładzka, A.; Bartos, M. Spider assemblages of hummocks and hollows in a primeval alder carr in the Białowieża National Park–Effect of vegetation structure and soil humidity. Pol. J. Ecol. 2016, 64, 564–577. [Google Scholar] [CrossRef]
- Lafage, D.; Djoudi, A.; Perrin, G.; Gallet, S.; Pétillon, J. Responses of ground-dwelling spider assemblages to changes vegetation from wet oligotrophic habitats of Western France. Arthropod-Plant Interact. 2019, 13, 653–662. [Google Scholar] [CrossRef]
- Ramberg, E.; Burdon, F.J.; Sargac, J.; Kupilas, B.; Rȋşnoveanu, G.; Lau, D.C.P.; Johnson, R.K.; McKie, B.G. The structure of riparian vegetation in agricultural landscapes influences spider communities and aquatic-terrestrial linkages. Water 2020, 12, 2855. [Google Scholar] [CrossRef]
- Oxbrough, A.G.; Gittings, T.; O’Halloran, J.; Giller, P.S.; Kelly, T.C. The influence of open space on ground-dwelling spider assemblages within plantation forests. For. Ecol. Manag. 2006, 237, 404–417. [Google Scholar] [CrossRef]
- Košulič, O.; Michalko, R.; Hula, V. Impact of canopy openness on spider communities: Implications for conservation management of formerly coppiced oak forests. PLoS ONE 2016, 11, e0148585. [Google Scholar] [CrossRef]
- Samu, F.; Lengyel, G.; Szita, É.; Bidló, A.; Ódor, P. The effect of forest stand characteristics on spider diversity and species composition in deciduous-coniferous mixed forests. J. Arachnol. 2014, 42, 135–141. [Google Scholar] [CrossRef][Green Version]
- Esquivel-Gómez, L.; Abdala-Roberts, L.; Pinkus-Rendón, M.; Parra-Tabla, V. Effects of tree species diversity on a community of weaver spiders in a tropical forest plantation. Biotropica 2017, 49, 63–70. [Google Scholar] [CrossRef]
- Stańska, M.; Stański, T.; Hawryluk, J. Spider assemblages on tree trunks in primeval deciduous forests of the Białowieża National Park in eastern Poland. Entomol. Fenn. 2018, 29, 75–85. [Google Scholar] [CrossRef]
- Gallé, R.; Gallé-Szpisjak, N.; Zsigmond, A.R.; Könczey, B.; Urák, I. Tree species and microhabitat affect forest bog spider fauna. Eur. J. For. Res. 2021, 140, 691–702. [Google Scholar] [CrossRef]
- Castro, A.; Wise, D.H. Influence of fine woody debris on spider diversity and community structure in forest leaf litter. Biodivers. Conserv. 2009, 18, 3705–3731. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Lobo, J.M. Determinants of local spider (Araneidae and Thomisidae) species richness on a regional scale: Climate and altitude vs. habitat structure. Ecol. Entomol. 2007, 32, 113–122. [Google Scholar] [CrossRef]
- Štokmane, M.; Spuņģis, V. The influence of vegetation structure on spider species richness, diversity and community organization in the Apšuciems calcareous fen, Latvia. Anim. Biodivers. Conserv. 2016, 39, 221–236. [Google Scholar] [CrossRef]
- Zhukovets, E.M. Spiders (Arachnida, Aranei) of Belovezhskaya Pushcha; Riftur Print: Minsk, Belarus, 2017. [Google Scholar]
- Hortal, J.; Borges, P.A.V.; Gaspar, C. Evaluating the performance of species richness estimators: Sensitivity to sample grain size. J. Anim. Ecol. 2006, 75, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Valverde, A.; Lobo, J.M. Establishing reliable spider (Araneae, Araneidae and Thomisidae) assemblage sampling protocols: Estimation of species richness, seasonal coverage and contribution of juvenile data to species richness and composition. Acta Oecol. 2006, 30, 21–32. [Google Scholar] [CrossRef]
- Colwell, R.K.; Mao, C.X.; Chang, J. Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 2004, 85, 2717–2727. [Google Scholar] [CrossRef]
- Colwell, R.K.; Chao, A.; Gotelli, N.J.; Lin, S.Y.; Mao, C.X.; Chazdon, R.L.; Longino, J.T. Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J. Plant Ecol. 2012, 5, 3–21. [Google Scholar] [CrossRef]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples, Version 9.1.0; Robert K. Colwell: Boulder, CO, USA, 2019. [Google Scholar]
- Tews, J.; Brose, U.; Grimm, V.; Tielbörger, K.; Wichmann, M.; Schwager, M.; Jeltsch, F. Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef]
- Hatley, C.L.; MacMahon, J.A. Spider community organization: Seasonal variation and the role of vegetation architecture. Environ. Entomol. 1980, 9, 632–639. [Google Scholar] [CrossRef]
- Scheidler, M. Influence of habitat structure and vegetation architecture on spiders. Zool. Anz. 1990, 225, 333–340. [Google Scholar]
- Halaj, J.; Ross, D.W.; Moldenke, A.R. Importance of habitat structure to the arthropod food-web in Douglas-fir canopies. Oikos 2000, 90, 139–152. [Google Scholar] [CrossRef]
- McDonald, B. Effects of vegetation structure on foliage dwelling spider assemblages in native and non-native Oklahoma grassland habitats. Proc. Okla. Acad. Sci. 2007, 87, 85–88. [Google Scholar]
- Diehl, E.; Mader, V.L.; Wolters, V.; Birkhofer, K. Management intensity and vegetation complexity affect web-building spiders and their prey. Oecologia 2013, 173, 579–589. [Google Scholar] [CrossRef]
- Gómez, J.E.; Lohmiller, J.; Joern, A. Importance of vegetation structure to the assembly of an aerial web-building spider community in North American open grassland. J. Arachnol. 2016, 44, 28–35. [Google Scholar] [CrossRef]
- Platen, R.; Berger, G. The impact of structural and landscape features of set-asides on the spiders (Araneae) of the herb layer. J. Arachnol. 2013, 41, 143–150. [Google Scholar] [CrossRef]




| Plot | Main Tree Species | Tree Stand Structure | Herbaceous Vegetation |
|---|---|---|---|
| M OLH | Quercus robur L., Populus tremula L., Carpinus betulus L. | Most trees were of similar age (around 70 years). Older trees were cut down and dead or dying trees were removed. Dense canopy cover (about 95%). | Anemone nemorosa L., Aegopodium podagraria L., Galeobdolon luteum (L.) L., Impatiens noli-tangere L. |
| P OLH | Carpinus betulus, Quercus robur, Picea abies (L.) H. Karst, Pinus silvestris L., Tilia cordata Mill. | Trees were diverse in size and age (up to 200 years). Considerable volume of dead wood. Canopy cover of about 70%. | Anemone nemorosa, Stellaria holostea L., Hepatica nobilis Mill, Oxalis acetosella L., Maianthemum bifolium (L.) F. W. Schmidt, Calamagrostis arundinacea (L.) Roth., Pteridium aquilinum (L.) Kuhn, Convallaria majalis L., Vaccinium myrtillus L., Trientalis europaea (L.) U. Manns & Anderb. |
| M RF | Fraxinus excelsior L., Alnus glutinosa (L.) Gaertn. | The oldest, thickest trees were cut down here, which caused a significant disturbance of the forest structure and thinning of the stand. Age of oldest trees were up to 50 years. Small amount of dead wood. Canopy cover of about 40%. | Rubus caesius L., Urtica dioica L., Aegopodium podagraria, Filipendula ulmaria (L.) Maxim., Lysimachia vulgaris L., L. nummularia L. |
| P RF | Alnus glutinosa, Fraxinus excelsior, Picea abies. | Trees were diverse in size and age (mainly 120 years, with the oldest trees up to 200 years old). Considerable volume of dead wood. Canopy cover of about 80%. | Ficaria verna huds., Impatiens noli-tangere, Mercurialis perennis L., Aegopodium podagraria, Lamium maculatum L., Urtica dioica. |
| M AC | Alnus glutinosa, Fraxinus excelsior, Picea abies. | After the oldest trees were cut down, the stand regenerated from regrowth. The hummock–hollow structure of the forest floor was preserved, although the hummocks were smaller than in the BNP. Trees at 70 years of age. Canopy cover of about 70%. | Filipendula ulmaria, Solanum dulcamara L., Carex elongate L., Galium palustre L., Iris pseudacorus L., Urtica dioica, Maianthemum bifolium, Oxalis acetosella. |
| P AC | Alnus glutinosa Fraxinus excelsior Picea abies | Hummock–hollow structure in the forest floor. The hummocks were large, which was related to the age and size of the root systems of the trees that formed them. Trees were diverse in size and age (averaging at 120 years, with the oldest trees up to 200 years old). Considerable volume of dead wood. Canopy cover of about 70%. | Carex elongata, Thelypteris palustris Schott, Solanum dulcamara, Lycopus europaeus L., Carex pseudocyperus L., Cicuta virosa L., Galium palustre, Iris pseudacorus, Scutellaria hastifolia L., Sium latifolium L., Peucedanum palustre (L.) Moench, Phalaris arundinacea L., Maianthemum bifolium, Oxalis acetosella. |
| Family/Genus/Species | M OLH ad./juv. | P OLH ad./juv. | M RF ad./juv. | P RF ad./juv. | M AC ad./juv. | P AC ad./juv. |
|---|---|---|---|---|---|---|
| Family Agelenidae | 0.08% | |||||
| Coelotes atropos (Walckener, 1830) | 0/1 | |||||
| Family Anyphaenidae | 2.71% | 0.39% | 1.36% | 1.52% | 0.70% | 0.85% |
| Anyphaena accentuata (Walckener, 1802) | 1/31 | 0/4 | 0/6 | 8/6 | 1/15 | 5/15 |
| Family Araneidae | 7.04% | 3.33% | 3.23% | 1.06% | 3.51% | 1.40% |
| Agalenatea redii (Scopoli, 1763) | 0/1 | 0/2 | ||||
| Araneidae un. | 0/8 | 0/2 | 0/2 | |||
| Araneus alsine (Walckener, 1802) | 0/2 | |||||
| Araneus diadematus Clerck, 1757 | 0/5 | 0/3 | 0/1 | 1/2 | 0/1 | |
| Araneus marmoreus Clerck, 1757 | 0/1 | 3/2 | 2/0 | 1/0 | ||
| Araneus quadratus Clerck, 1757 | 2/3 | |||||
| Araneus sp. Clerck, 1757 | 0/4 | 0/2 | 0/1 | |||
| Araneus sturmi (Hahn, 1831) | 0/2 | 0/3 | 0/1 | |||
| Araniella cucurbitina (Clerck, 1757) | 1/0 | 1/0 | 3/0 | 1/0 | ||
| Araniella sp. Chamberlin & Ivie, 1942 | 0/13 | 0/1 | 0/3 | 0/11 | 0/4 | |
| Cyclosa conica (Pallas, 1772) | 2/48 | 2/24 | 2/4 | 2/8 | 1/5 | 3/10 |
| Hypsosinga pygmaea (Sundevall, 1831) | 0/2 | |||||
| Mangora acalypha (Walckener, 1802) | 0/2 | 2/8 | 1/0 | |||
| Nuctenea umbratical (Clerck, 1757) | 0/1 | |||||
| Family Clubionidae | 1.36% | 1.57% | 1.62% | 2.35% | 1.76% | 2.01% |
| Clubiona comta C. L. Koch, 1839 | 1/0 | |||||
| Clubiona lutescens Westring, 1851 | 1/0 | 1/0 | 11/1 | 1/0 | 3/0 | |
| Clubiona pallidula (Clerck, 1757) | 1/0 | |||||
| Clubiona sp. Latreille, 1804 | 0/14 | 0/15 | 0/18 | 0/18 | 0/14 | 1/29 |
| Clubiona stagnatilis Kulczyński, 1897 | 1/0 | |||||
| Family Dictynidae | 0.08% | 0.20% | 0.09% | |||
| Dictyna arundinacea (Linnaeus, 1758) | 1/0 | |||||
| Dictyna sp. Sundevall, 1833 | 0/1 | 0/1 | 0/1 | |||
| Family Dysderidae | 0.10% | |||||
| Harpactea sp. Bristowe, 1939 | 0/1 | |||||
| Family Linyphiidae | 44.53% | 43.25% | 18.20% | 57.42% | 30.33% | 32.76% |
| Agyneta affinis (Kulczyński, 1898) | 1/0 | |||||
| Agyneta rurestris (C. L. Koch, 1836) | 1/0 | 1/0 | ||||
| Agyneta sp. Blackwall, 1859 | 0/1 | |||||
| Bathyphantes approximatus (O. Pickard-Cambridge, 1871) | 6/0 | 3/0 | ||||
| Bathyphantes gracilis (Blackwall, 1841) | 1/0 | 3/0 | ||||
| Bathyphantes nigrinus (Westring, 1851) | 39/0 | 8/0 | 12/0 | 239/0 | 45/0 | 103/0 |
| Bathyphantes parvulus (Westring, 1851) | 2/0 | |||||
| Bathyphantes sp. Menge, 1866 | 0/8 | 0/15 | 0/5 | 0/31 | 0/9 | |
| Centromerus sylvaticus (Blackwall, 1841) | 1/0 | |||||
| Ceratinella brevis (Wider, 1834) | 1/0 | |||||
| Diplocephalus picinus (Blackwall, 1841) | 1/0 | |||||
| Dismodicus bifrons (Blackwall, 1841) | 2/0 | 5/0 | ||||
| Drapetisca socialis (Sundevall, 1833) | 1/0 | 1/1 | ||||
| Entelecara congenera (O. Pickard-Cambridge, 1879) | 1/0 | |||||
| Entelecara media Kulczyński, 1887 | 1/0 | |||||
| Erigone atra Blackwall, 1833 | 2/0 | 2/0 | ||||
| Erigone dentipalpis (Wider, 1834) | 1/0 | |||||
| Floronia bucculenta (Clerck, 1757) | 1/0 | 4/0 | 2/1 | 10/0 | ||
| Gonatium rubellum (Blackwall, 1841) | 6/2 | 9/1 | ||||
| Gongylidiellum murcidium Simon, 1884 | 1/0 | 1/0 | ||||
| Gongylidium rufipes (Linnaeus, 1758) | 1/0 | 18/67 | 25/112 | 9/10 | 32/23 | |
| Helophora insignis (Blackwall, 1841) | 52/53 | 0/3 | 1/0 | 65/58 | 9/0 | 40/8 |
| Hypomma bituberculatum (Wider, 1834) | 3/0 | |||||
| Kaestneria dorsalis (Wider, 1834) | 1/2 | |||||
| Kaestneria pullata (O. Pickard-Cambridge, 1863) | 1/0 | |||||
| Linyphia hortensis Sundevall, 1830 | 1/0 | 3/0 | 9/1 | 4/1 | 4/0 | 3/0 |
| Linyphia sp. Latreille, 1804 | 0/26 | 0/61 | ||||
| Linyphia triangularis (Clerck, 1757) | 38/0 | 29/1 | 35/5 | 16/21 | 22/2 | 79/9 |
| Linyphiidae un. | 0/142 | 0/194 | 0/4 | 0/6 | ||
| Maso sundevalli (Westring, 1851) | 6/0 | |||||
| Microlinyphia pusilla (Sundevall, 1830) | 1/13 | 23/18 | 3/0 | 14/1 | ||
| Neriene clathrata (Sundevall, 1830) | 1/0 | 1/0 | 1/2 | 1/0 | 1/0 | |
| Neriene emphana (Walckenaer, 1841) | 1/0 | 0/1 | 3/0 | 1/0 | ||
| Neriene montana (Clerck, 1757) | 2/0 | 5/0 | 15/0 | 28/0 | 78/0 | |
| Neriene peltata (Wider, 1834) | 13/1 | 2/3 | 5/47 | 6/4 | 14/42 | |
| Neriene radiata (Walckenaer, 1841) | 1/2 | 1/0 | ||||
| Neriene sp. Blackwall, 1833 | 0/25 | 0/59 | 0/9 | 0/21 | 0/9 | 0/9 |
| Obscuriphantes obscurus (Blackwall, 1841) | 3/0 | |||||
| Oedothorax apicatus (Blackwall, 1850) | 1/0 | |||||
| Oedothorax gibbosus (Kulczyński, 1882) | 4/0 | |||||
| Oedothorax retusus (Westring, 1851) | 1/0 | 1/0 | ||||
| Oryphantes angulatus (O. Pickard-Cambridge, 1881) | 1/0 | |||||
| Pityohyphantes phrygianus (C. L. Koch, 1836) | 1/2 | |||||
| Porrhomma pygmaeum (Blackwall, 1834) | 1/0 | 2/0 | 63/0 | 16/5 | ||
| Savignia frontata Blackwall, 1833 | 1/0 | 1/0 | ||||
| Tenuiphantes alacris (Blackwall, 1853) | 1/0 | 15/0 | ||||
| Tenuiphantes cristatus (Menge, 1866) | 2/0 | 3/0 | 1/0 | 2/0 | 1/0 | |
| Tenuiphantes mengei (Kulczyński, 1887) | 1/0 | |||||
| Tenuiphantes sp. Saaristo & Tanasevitch 1996 | 0/8 | 0/14 | ||||
| Tenuiphantes tenebricola (Wider, 1834) | 3/0 | 1/0 | ||||
| Thyreostenius parasiticus (Westring, 1851) | 1/0 | |||||
| Trematocephalus cristatus (Wider, 1834) | 3/117 | 0/9 | 0/11 | 0/29 | 13/0 | 2/3 |
| Family Lycosidae | 4.08% | 0.12% | ||||
| Pardosa amentata (Clerck, 1757) | 1/45 | |||||
| Pardosa prativaga (L. Koch, 1870) | 1/1 | |||||
| Piratula hygrophila (Thorell, 1872) | 2/0 | |||||
| Family Mimetidae | 0.08% | 0.49% | 0.08% | |||
| Ero furcata (Villers, 1789) | 1/0 | 4/0 | 1/0 | |||
| Ero sp. (C. L. Koch, 1836) | 0/1 | |||||
| Family Philodromidae | 1.02% | 9.30% | 0.17% | 0.15% | 0.59% | 0.30% |
| Philodromus collinus (C. L. Koch, 1835) | 3/0 | 1/0 | ||||
| Philodromus dispar Walckenaer, 1826 | 1/0 | 1/0 | ||||
| Philodromus emarginatus (Schrank, 1803) | 1/0 | |||||
| Philodromus praedatus (O. Pickard-Cambridge, 1871) | 1/0 | |||||
| Philodromus sp. Walckenaer, 1826 | 0/9 | 0/91 | 0/2 | 0/1 | 0/4 | 0/4 |
| Tibellus oblongus (Walckenaer, 1802) | 1/0 | 1/0 | ||||
| Family Pisauridae | 0.76% | 12.59% | 0.30% | 2.58% | 0.85% | |
| Dolomedes fimbriatus (Clerck, 1757) | 1/2 | 7/137 | 0/4 | 3/19 | 3/10 | |
| Pisaura mirabilis (Clercka, 1757) | 0/6 | 0/4 | 1/0 | |||
| Family Salticidae | 0.17% | 0.23% | ||||
| Pseudicius encarpatus (Walckenaer, 1802) | 1/0 | 1/0 | ||||
| Salticidae un. | 0/1 | |||||
| Salticus cingulatus (Panzer, 1797) | 1/0 | |||||
| Family Segestriidae | 0.09% | 0.06% | ||||
| Segestria senoculata (Linnaeus, 1758) | 0/1 | 0/1 | ||||
| Family Tetragnathidae | 22.22% | 33.46% | 51.28% | 31.97% | 57.14% | 58.22% |
| Metellina mengei (Blackwall, 1869) | 8/0 | 34/0 | 5/1 | 12/0 | 0/21 | 12/34 |
| Metellina merianae (Scopoli, 1763) | 1/0 | |||||
| Metellina segmentata (Clerck, 1757) | 9/0 | 1/0 | 17/0 | 10/0 | 19/15 | 21/9 |
| Metellina sp. Chamberlin & Ivie, 1941 | 0/68 | 0/213 | 0/24 | 0/66 | 0/5 | 0/7 |
| Pachygnatha clercki Sundevall, 1823 | 39/20 | 67/13 | 135/4 | |||
| Pachygnatha degeeri Sundevall, 1830 | 2/0 | |||||
| Pachygnatha listeri Sundevall, 1830 | 62/5 | 71/0 | 21/13 | 129/32 | 18/27 | 66/26 |
| Pachygnatha sp. Sundevall, 1823 | 0/16 | 0/3 | ||||
| Tetragnatha dearmata Thorell, 1873 | 1/0 | 5/0 | 21/0 | 31/0 | ||
| Tetragnatha extensa (Linnaeus, 1758) | 1/0 | 0/0 | ||||
| Tetragnatha montana Simon, 1874 | 1/0 | 26/0 | 19/0 | 51/0 | 52/0 | |
| Tetragnatha pinicola L. Koch, 1870 | 1/- | |||||
| Tetragnatha sp. Latreille, 1804 | 0/93 | 0/19 | 0/436 | 0/148 | 0/231 | 9/547 |
| Family Theridiidae | 14.50% | 5.48% | 4.08% | 3.33% | 1.64% | 2.25% |
| Enoplognatha ovata (Clerck, 1757) | 49/100 | 13/34 | 28/12 | 18/21 | 8/4 | 23/0 |
| Episinus angulatus (Blackwall, 1836) | 0/7 | 1/1 | 0/1 | |||
| Neottiura bimaculata (Linnaeus, 1767) | 4/0 | 0/1 | ||||
| Paidiscura pallens (Blackwall, 1834) | 1/0 | 1/0 | 2/0 | |||
| Parasteatoda lunata (Clerck, 1757) | 1/0 | |||||
| Platnickina tincta (Walckenaer, 1802) | 0/1 | 1/0 | ||||
| Robertus neglectus (O. Pickard-Cambridge, 1871) | 13/0 | |||||
| Rougathodes instabilis (O. Pickard-Cambridge, 1871) | 1/0 | |||||
| Theridion sp. Walckenaer, 1805 | 0/6 | 0/2 | 0/3 | 0/9 | ||
| Theridion varians Hahn, 1833 | 2/0 | 1/0 | 1/0 | |||
| Family Theridiosomatidae | 0.30% | 0.12% | 0.49% | |||
| Theridiosoma gemmosum (L. Koch, 1877) | 1/3 | 1/0 | 8/0 | |||
| Family Thomisidae | 5.60% | 2.45% | 3.06% | 1.52% | 1.41% | 0.67% |
| Diaea dorsata (Fabricius, 1777) | 0/50 | 0/10 | 0/3 | 1/11 | 1/7 | 1/7 |
| Heriaeus graminicola (Doleschall, 1852) | 1/1 | |||||
| Misumena vatia (Clerck, 1757) | 0/1 | 0/18 | ||||
| Ozyptila sp. Simon, 1864 | 0/9 | 0/3 | ||||
| Psammitis sabulosa (Hahn, 1832) | 1/0 | |||||
| Xysticus cristatus (Clerck, 1757) | 2/0 | 1/0 | ||||
| Xysticus sp. C. L. Koch, 1835 | 0/6 | 0/12 | 0/13 | 0/5 | 0/2 | 0/2 |
| Xysticus ulmi (Hahn, 1831) | 1/0 | 1/0 | ||||
| Total no. of individuals | 301/878 | 217/805 | 259/917 | 637/683 | 429/425 | 816/826 |
| Total no. of species | 40 | 30 | 50 | 43 | 46 | 54 |
| No. of common species | 19 | 24 | 38 | |||
| M OLH | P OLH | M RF | P RF | M AC | P AC | |
|---|---|---|---|---|---|---|
| Observed species richness | 40 | 30 | 50 | 43 | 46 | 54 |
| Estimates | ||||||
| Chao1 ± SD | 113 ± 54 (35%) | 76 ± 30 (39%) | 61 ± 8 (82%) | 64 ± 16 (67%) | 91 ± 33 (51%) | 90 ± 24 (60%) |
| Chao2 ± SD | 155 ± 95 (26%) | 54 ± 19 (56%) | 62 ± 8 (81%) | 67 ± 17 (64%) | 65 ± 11 (71%) | 87 ± 20 (62%) |
| Jackknife1 ± SD | 61 ± 6 (66%) | 42 ± 3 (71%) | 65 ± 5 (77%) | 58 ± 4 (74%) | 64 ± 5 (72%) | 75 ± 5 (72%) |
| Jackknife2 | 79 (51%) | 51 (59%) | 71 (70%) | 68 (63%) | 73 (63%) | 87 (62%) |
| Michaelis–Menten | 46 (87%) | 39 (77%) | 61 (82%) | 49 (88%) | 56 (82%) | 61 (89%) |
| Effect | Wald χ2 | df | p |
|---|---|---|---|
| Spider abundance | |||
| Intercept | 6711.09 | 1 | <0.001 |
| Forest type | 1.18 | 2 | 0.572 |
| Managed/Primeval | 4.30 | 1 | 0.038 |
| Forest type x Managed/Primeval | 10.02 | 2 | 0.007 |
| Number of species | |||
| Intercept | 1380.93 | 1 | <0.001 |
| Forest type | 57.27 | 2 | <0.001 |
| Managed/Primeval | 0.001 | 1 | 0.973 |
| Forest type x Managed/Primeval | 14.71 | 2 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stańska, M.; Stański, T.; Patoleta, B. Plant-Dwelling Spider Assemblages in Managed and Protected Primeval Deciduous Stands of Białowieża Forest, Poland. Forests 2025, 16, 1093. https://doi.org/10.3390/f16071093
Stańska M, Stański T, Patoleta B. Plant-Dwelling Spider Assemblages in Managed and Protected Primeval Deciduous Stands of Białowieża Forest, Poland. Forests. 2025; 16(7):1093. https://doi.org/10.3390/f16071093
Chicago/Turabian StyleStańska, Marzena, Tomasz Stański, and Barbara Patoleta. 2025. "Plant-Dwelling Spider Assemblages in Managed and Protected Primeval Deciduous Stands of Białowieża Forest, Poland" Forests 16, no. 7: 1093. https://doi.org/10.3390/f16071093
APA StyleStańska, M., Stański, T., & Patoleta, B. (2025). Plant-Dwelling Spider Assemblages in Managed and Protected Primeval Deciduous Stands of Białowieża Forest, Poland. Forests, 16(7), 1093. https://doi.org/10.3390/f16071093







