Resprouting Ability and Carbon Allocation of Robinia pseudoacacia L. Trees After Cutting at Different Stem Heights
Abstract
1. Introduction
2. Materials and Methods
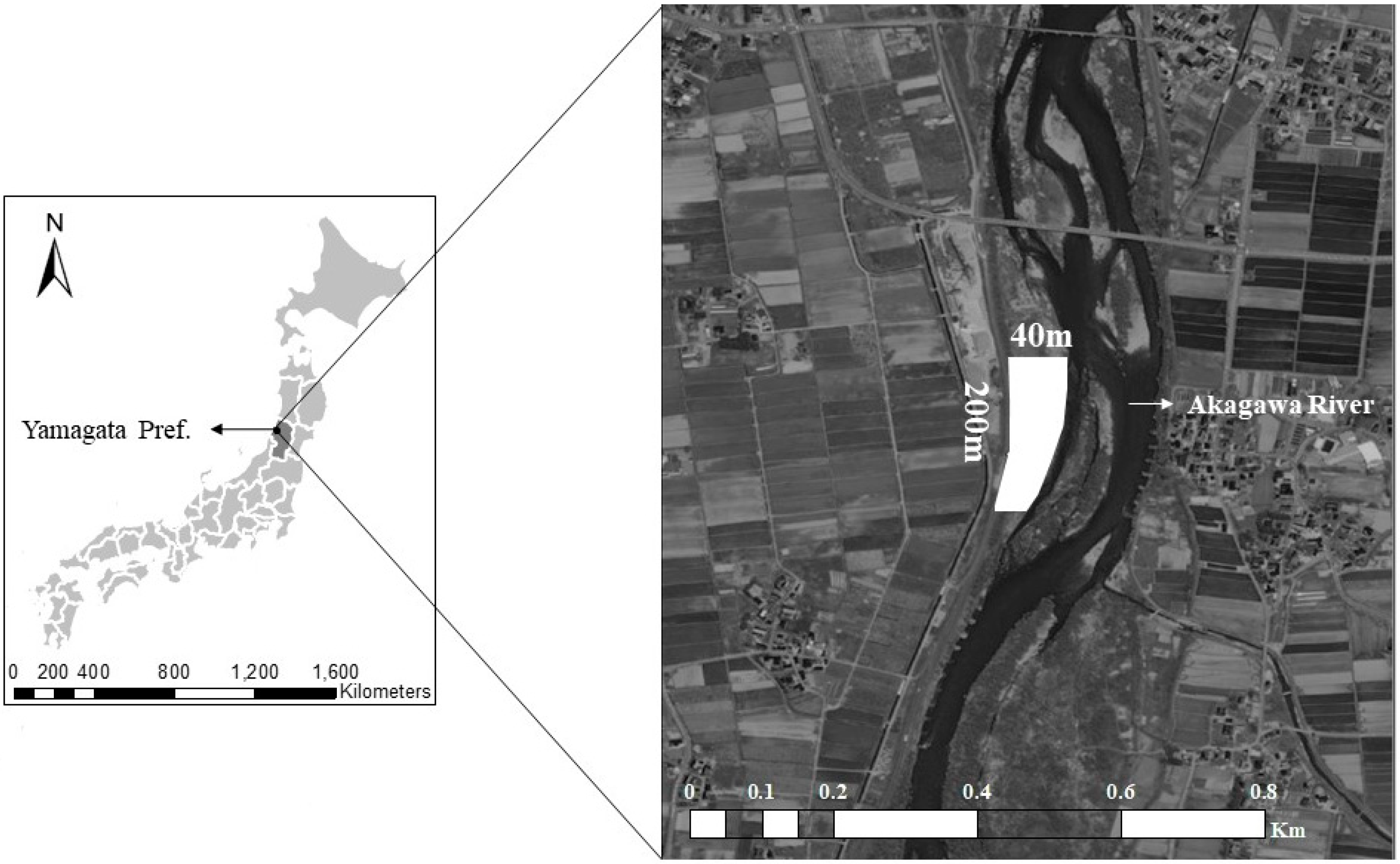
2.1. Study Site and Species
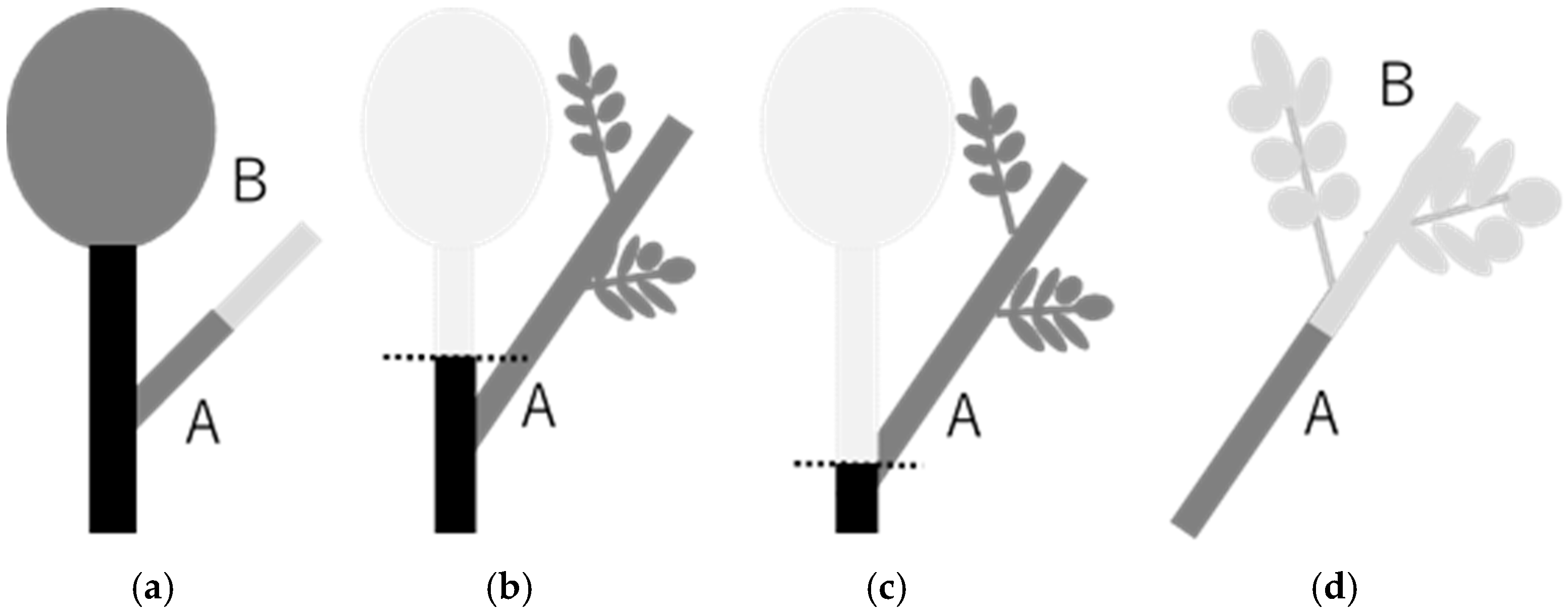
2.2. Treatment Application and Sampling Times
2.3. Organ Respiration Rates
2.4. NSCs
2.5. Data Analysis
3. Results
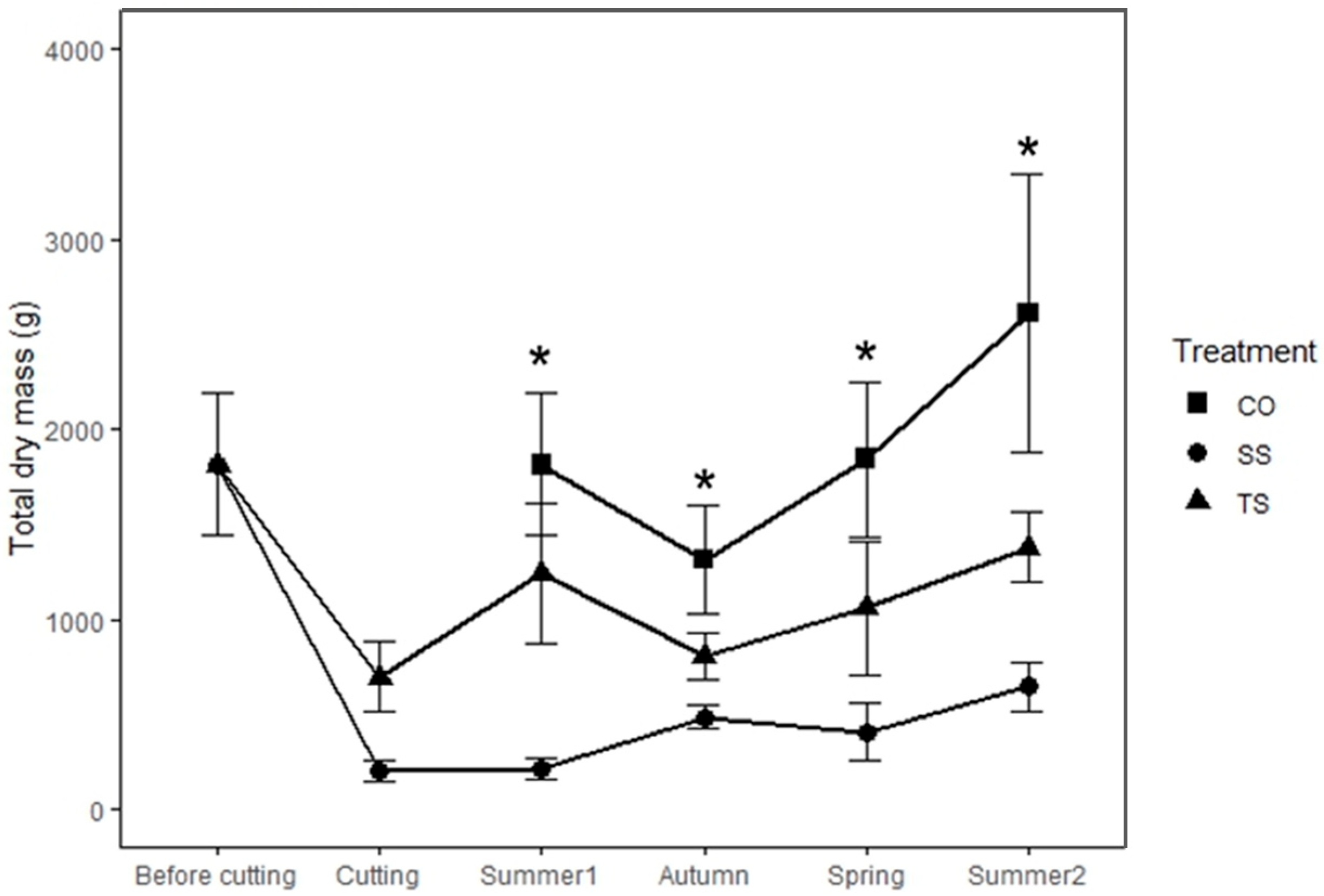
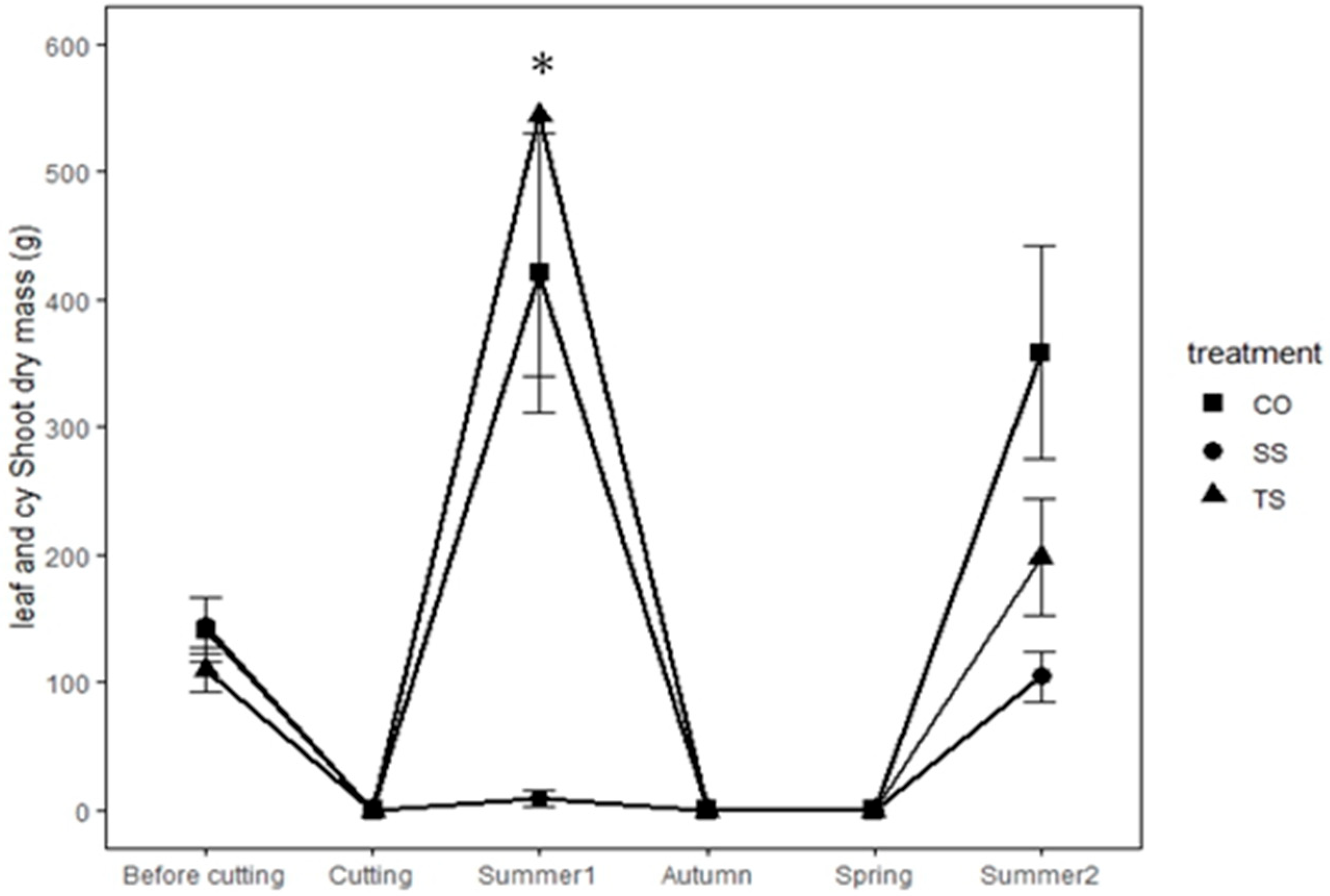
3.1. Biomass Production
3.2. Organ Dry Mass Proportion
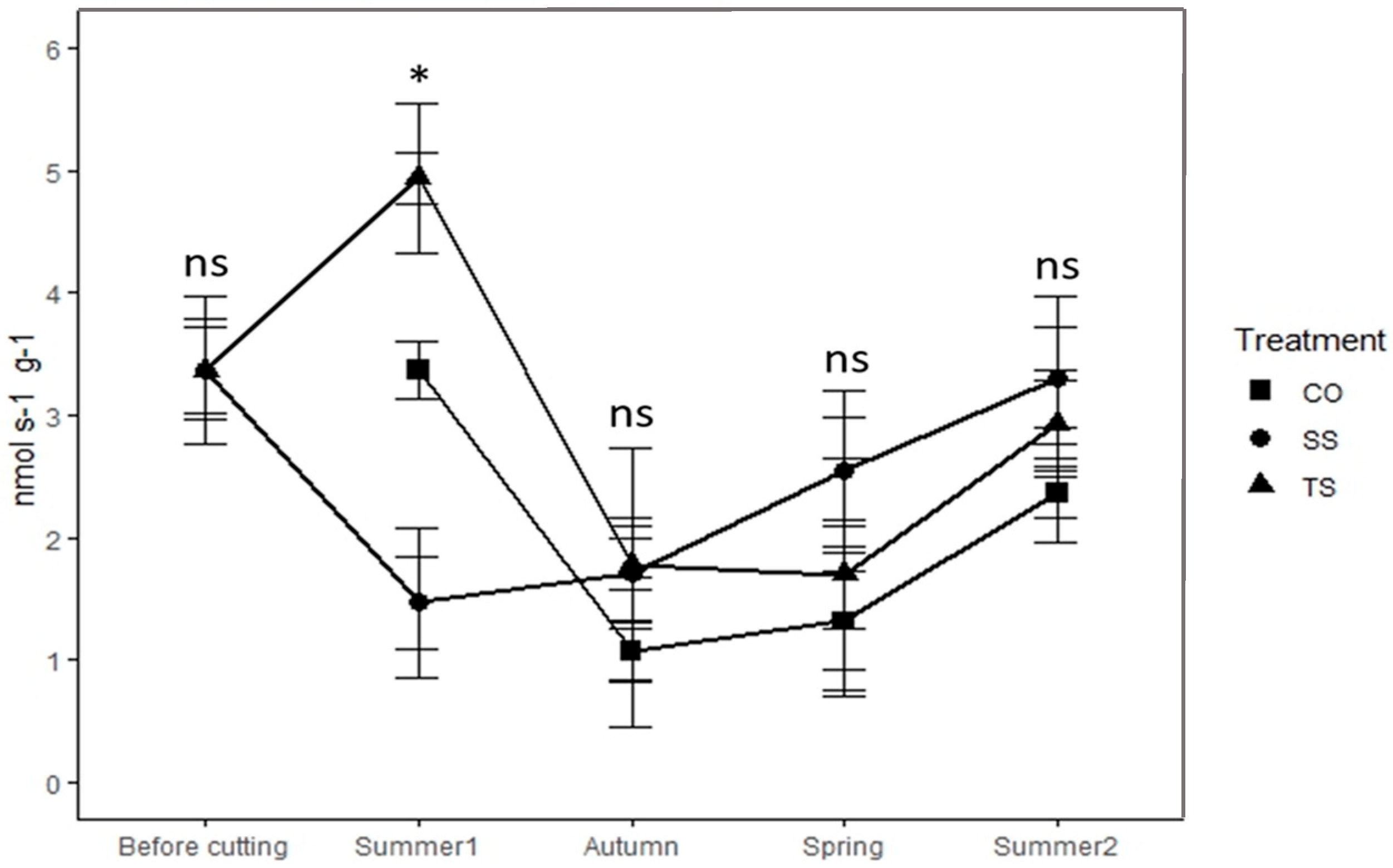
3.3. Whole-Plant and Organ Respiration Rates
3.4. NSCs at Whole-Plant and Organ Levels
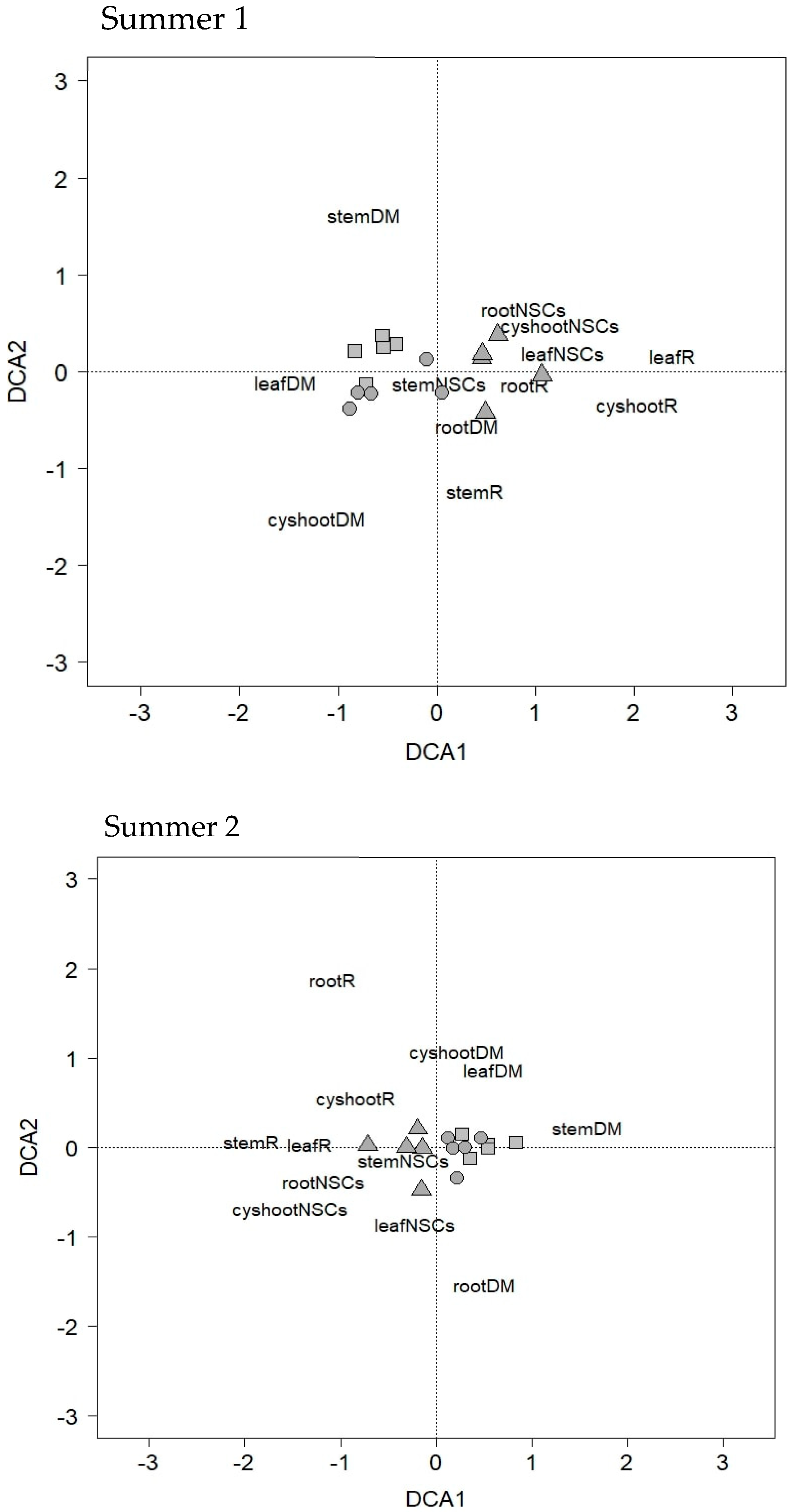
3.5. Detrended Correspondence Analysis (DCA)
4. Discussion
4.1. Resprouting Response and Biomass Recovery
4.2. Role of NSCs
4.3. Resource Regain
4.4. Resource Mobility and Organ Respiration During Resprouting
4.5. Resprouting and Resource Dynamics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CO | control trees |
| TS | tall-stump trees |
| SS | short-stump trees |
| NSCs | non-structural carbohydrates |
| DBH | diameter breast height |
| Ly-branch | last-year branch |
| Cy-shoot | current-year shoot |
| OR | organ respiration rates |
| R | respiration rates |
| DCA | detrended component analysis |
References
- Nzunda, E.F.; Griffiths, M.E.; Lawes, M.J. Sprouting by remobilization of above-ground resources ensures persistence after disturbance of coastal dune forest trees. Funct. Ecol. 2008, 22, 577–582. [Google Scholar] [CrossRef]
- Smith, M.G.; Miller, R.E.; Arndt, S.K.; Kasel, S.; Bennett, L.T. Whole-tree distribution and temporal variation of non-structural carbohydrates in broadleaf evergreen trees. Tree Physiol. 2018, 38, 570–581. [Google Scholar] [CrossRef]
- Bellingham, P.J.; Sparrow, A.D. Resprouting as a life history strategy in woody plant communities. Oikos 2000, 89, 409–416. [Google Scholar] [CrossRef]
- Teixeira, A.M.C.; Curran, T.J.; Jameson, P.E.; Meurk, C.D.; Norton, D.A. Post-fire resprouting in New Zealand Woody Vegetation: Implications for restoration. Forests 2020, 11, 269. [Google Scholar] [CrossRef]
- Rodgers, H.L.; Brakke, M.P.; Ewel, J.J. Shoot damage effects on starch reserves of Cedrela odorata. Biotropica 1995, 27, 71–77. [Google Scholar] [CrossRef]
- Latt, C.R.; Nair, P.K.R.; Kang, B.T. Interactions among cutting frequency, reserve carbohydrates, and post-cutting biomass production in Gliricidia sepium and Leucaena leucocephala. Agrof. Syst. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J.E. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Schulze, E.D.; Mooney, H.A. The ecology and economics of storage in plants. Ann. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
- Piper, F.I.; Paula, S. The role of nonstructural carbohydrates storage in forest resilience under climate change. Curr. For. Rep. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Lotscher, M.; Klumpp, K.; Schnyder, H. Growth and maintenance respiration for individual plants in hierarchically structured canopies of Medicago sative and Helianthus annuus: The contribution of current and old assimilates. New Phytol. 2004, 164, 305–316. [Google Scholar] [CrossRef]
- Weis, J.S. Respiration and metabolism. In Physiological, Developmental and Behavioral Effects of Marine Pollution; Springer: Dordrecht, The Netherlands, 2014; pp. 65–95. [Google Scholar]
- Zhang, X.; Wang, S. Joint control of plant ecological strategy by climate, regeneration mode, and ontogeny in Northeastern Chinese forests. Ecol. Evol. 2021, 11, 6703–6715. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Piper, F.; Hoch, G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 2010, 186, 274–281. [Google Scholar] [CrossRef]
- Yamada, K.; Masaka, K. Present distribution and historical background of the invasive alien species Robinia pseudoacacia on former coalmine land in Hokkaido. Jpn. J. Conser. Ecol. 2007, 12, 94–102. [Google Scholar]
- Morimoto, J.; Kominami, R.; Koike, T. Distribution and characteristics of the soil seed bank of the black locust (Robinia pseudoacacia) in a headwater basin in northern Japan. Lands Ecol. Eng. 2010, 6, 193–199. [Google Scholar] [CrossRef]
- Masaka, K.; Torita, H.; Kon, H.; Fukuchi, M. Seasonality of sprouting in the exotic tree Robinia pseudoacacia L. in Hokkaido, northern Japan. J. Res. 2015, 20, 386–395. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E. Evolutionary ecology of resprouting and seeding in fire-prone ecosystems. New Phytol. 2014, 102, 55–65. [Google Scholar] [CrossRef]
- Clarke, P.J.; Knox, K.; Wills, K.E.; Campbell, M. Landscape patterns of woody plant response to crown fire: Disturbance and productivity influence sprouting ability. J. Ecol. 2013, 93, 544–555. [Google Scholar] [CrossRef]
- Kramer, P.J.; Kozlowski, T.T. Physiology of Woody Plants; Academic Press: New York, NY, USA, 1979; Volume 7, pp. 258–281. [Google Scholar]
- Wiley, E.; Casper, B.B.; Helliker, B.R. Recovery following defoliation involves shifts in allocation that favour storage and reproduction over radial growth in black oak. J. Ecol. 2017, 105, 412–424. [Google Scholar] [CrossRef]
- Landhäusser, S.M.; Lieffers, V.J. Defoliation increases risk of carbon starvation in root systems of mature aspen. Trees 2012, 26, 653–661. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kato, M. Invasive characteristics of Robinia pseudoacacia and its impacts on species diversity. Diversity 2025, 16, 773. [Google Scholar] [CrossRef]
- Druege, U. Overcoming physiological bottlenecks of leaf vitality and root development in cuttings: A systemic perspective. Front. Plant Sci. 2020, 11, 907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Mu, Y.; Zhang, H.; Yu, C. Maintenance of stem cell activity in plant development and stress responses. Front. Plant Sci. 2023, 14, 1302046. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Sakai, S.; Akiyama, F. Do sprouting tree species on erosion-prone sites carry large reserves of resources? Ann. Bot. 1997, 79, 625–630. [Google Scholar] [CrossRef]
- Sakai, A.; Sakai, S. A test for the resource remobilization hypothesis: Tree sprouting using carbohydrates from above-ground parts. Ann. Bot. 1998, 82, 213–216. [Google Scholar] [CrossRef]
- Götmark, F.; Schott, K.M.; Jensen, A.M. Factors influencing presence–absence of oak (Quercus spp.) seedlings after conservation-oriented partial cutting of high forests in Sweden. Scand. J. For. Res. 2011, 26, 136–145. [Google Scholar] [CrossRef]
- Kabeya, D.; Sakai, A.; Matsui, K.; Sakai, S. Resprouting ability of Quercus crispula seedlings depends on the vegetation cover of their microhabitats. J. Plant Res. 2003, 116, 207–216. [Google Scholar] [CrossRef]
- Miranda, J.C.; Rodríguez-Calcerrada, J.; Pita, P.; Saurer, M.; Oleksyn, J.; Gil, L. Carbohydrate dynamics in a resprouting species after severe aboveground perturbations. Eur. J. For. Res. 2020, 139, 841–852. [Google Scholar] [CrossRef]
- Schutz, A.E.; Bond, W.J.; Cramer, M.D. Defoliation depletes the carbohydrate reserves of resprouting Acacia saplings in an African savanna. Plant Ecol. 2011, 212, 2047–2055. [Google Scholar] [CrossRef]
- Smith, A.M.; Stitt, M. Coordination of carbon supply and plant growth. Plant Cell Environ. 2007, 30, 1126–1149. [Google Scholar] [CrossRef]
- Zhang, Z.; Gong, J.; Li, X.; Ding, Y.; Wang, B.; Shi, J.; Liu, M.; Yang, B. Underlying mechanism on source-sink carbon balance of grazed perennial grass during regrowth: Insights into optimal grazing regimes of restoration of degraded grasslands in a temperate steppe. J. Environ. Manag. 2021, 277, 111439. [Google Scholar] [CrossRef]
- Sevanto, S.; Mcdowell, N.G.; Dickman, L.T.; Pangle, R.; Pockman, W.T. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ. 2014, 37, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Verdaguer, D.; Ojeda, F. Root starch storage and allocation patterns in seeder and resprouter seedlings of two Cape Erica (Ericaceae) species. Am. J. Bot. 2002, 89, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Shirke, P.A. Leaf photosynthesis, dark respiration and fluorescence as influenced by leaf age in an evergreen tree, Prosopis juliflora. Photosynth 2001, 39, 305–311. [Google Scholar] [CrossRef]
- Gersony, J.T.; Hochberg, U.; Rockwell, F.E.; Park, M.; Gauthier, P.P.G.; Holbrook, N.M. Leaf carbon export and nonstructural carbohydrates in relation to diurnal water dynamics in mature oak trees. Plant Physiol. 2020, 183, 1612–1621. [Google Scholar] [CrossRef]
- Li, Z.; Lai, Q.; Bao, Y.; Sude, B.; Bao, Z.; Liu, X. Carbon allocation to leaves and its controlling factors and impacts on gross primary productivity in forest ecosystems of Northeast China. Forests 2024, 15, 129. [Google Scholar] [CrossRef]
- Yamada, K.; Masaka, K. Dynamics in growth and survivals of Robinia pseudoacacia sprouts occurred after cutting conducted in different seasons. J. Jpn. Soc. 2009, 91, 42–45. [Google Scholar] [CrossRef]
- Nicolescu, V.N.; Redei, K.; Mason, W.L.; Vor, T.; Poetzelsberger, E.; Bastien, J.-C.; Brus, R.; Bancat, T.; Dodan, M.; Cvjetkovic, B.; et al. Ecology, growth and management of black locust (Robinia pseudoacacia L.), a non-native species integrated into European forests. J. Res. 2020, 31, 1081–1101. [Google Scholar] [CrossRef]
- Prevost, M.; Dumais, D.; Pothier, D. Growth and mortality following partial cutting in a trembling aspen–conifer stand: Results after 10 years. Can. J. For. Res. 2010, 40, 894–903. [Google Scholar] [CrossRef]



| Sampling | CO | TS | SS |
|---|---|---|---|
| p | p | p | |
| Total dry mass (g) | |||
| BC × Summer 1 | 0.515 ns | 0.143 ns | 0.030 * |
| BC × Autumn | 0.138 ns | 0.182 ns | 0.011 * |
| BC × Spring | 0.236 ns | 0.121 ns | 0.044 * |
| BC × Summer 2 | 0.164 ns | 0.381 ns | 0.012 * |
| Resprout (leaf and current-year shoot) (g) | |||
| BC × Summer 1 | NA | 0.101 ns | 0.002 * |
| BC × Summer 2 | 0.660 ns | 0.190 ns | 0.813 ns |
| Treatment | Number of New Shoots | Total Shoot Length (cm) | Volume of Shoot (cm3) | |||
|---|---|---|---|---|---|---|
| Summer 1 | Summer 2 | Summer 1 | Summer 2 | Summer 1 | Summer 2 | |
| CO | 23 ± 7 b | 140 ± 23 b | 560 ± 79 b | 1103 ± 172 a | 118 ± 26 a | 46 ± 10 a |
| TS | 15 ± 2 b | 112 ± 18 b | 658 ± 86 b | 1102 ± 167 a | 193 ± 41 a | 56 ± 11 a |
| SS | 4 ± 1 a | 29 ± 6 a | 263 ± 62 a | 859 ± 146 a | 113 ± 35 a | 63 ± 10 a |
| Treatments | Leaf | Current-Year Shoot | Stem | Root | Whole-Plant | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SE | p | Mean ± SE | p | Mean ± SE | p | Mean ± SE | p | Mean ± SE | p | ||
| Soluble sugar concentration (g/g) | |||||||||||
| Summer 1 | CO | 0.073 ± 0.004 | b | 0.030 ± 0.006 | a | 0.050 ± 0.006 | b | 0.046 ± 0.002 | b | 0.141 ± 0.010 | b |
| TS | 0.067 ± 0.009 | b | 0.030 ± 0.009 | a | 0.054 ± 0.007 | b | 0.049 ± 0.002 | b | 0.142 ± 0.008 | b | |
| SS | 0.024 ± 0.004 | a | 0.028 ± 0.004 | a | 0.012 ± 0.003 | b | 0.028 ± 0.001 | a | 0.066 ± 0.004 | a | |
| Autumn | CO | 0.058 ± 0.013 | a | 0.030 ± 0.013 | a | 0.029 ± 0.002 | a | 0.034 ± 0.003 | a | 0.128 ± 0.013 | a |
| TS | 0.070 ± 0.009 | a | 0.042 ± 0.009 | a | 0.029 ± 0.003 | a | 0.027 ± 0.002 | a | 0.142 ± 0.012 | a | |
| SS | 0.059 ± 0.013 | a | 0.041 ± 0.0 | a | 0.024 ± 0.003 | a | 0.041 ± 0.004 | a | 0.148 ± 0.095 | a | |
| Spring | CO | No leaf | 0.043 ± 0.003 | a | 0.056 ± 0.006 | b | 0.049 ± 0.024 | a | 0.145 ± 0.085 | a | |
| TS | No leaf | 0.048 ± 0.006 | a | 0.026 ± 0.002 | a | 0.049 ± 0.026 | a | 0.123 ± 0.086 | a | ||
| SS | No leaf | 0.049 ± 0.004 | a | 0.036 ± 0.002 | a | 0.046 ± 0.024 | a | 0.132 ± 0.044 | a | ||
| Summer 2 | CO | 0.127 ± 0.014 | b | 0.049 ± 0.003 | a | 0.074 ± 0.008 | a | 0.044 ± 0.005 | a | 0.265 ± 0.019 | b |
| TS | 0.073 ± 0.087 | a | 0.036 ± 0.003 | a | 0.051 ± 0.010 | a | 0.034 ± 0.005 | a | 0.182 ± 0.012 | a | |
| SS | 0.084 ± 0.016 | a | 0.039 ± 0.004 | a | 0.041 ± 0.006 | a | 0.034 ± 0.006 | a | 0.184 ± 0.017 | a | |
| Starch concentration (g/g) | |||||||||||
| Summer 1 | CO | 0.017 ± 0.007 | b | 0.015 ± 0.007 | b | 0.032 ± 0.00 | b | 0.089 ± 0.003 | c | 0.073 ± 0.009 | b |
| TS | 0.018 ± 0.005 | b | 0.019 ± 0.005 | b | 0.028 ± 0.008 | b | 0.057 ± 0.009 | b | 0.066 ± 0.019 | b | |
| SS | 0.008 ± 0.001 | a | 0.009 ± 0.001 | a | 0.003 ± 0.001 | a | 0.013 ± 0.003 | a | 0.019 ± 0.003 | a | |
| Autumn | CO | 0.014 ± 0.002 | a | 0.043 ± 0.002 | a | 0.053 ± 0.006 | b | 0.163 ± 0.009 | b | 0.091 ± 0.008 | a |
| TS | 0.016 ± 0.002 | a | 0.060 ± 0.002 | a | 0.038 ± 0.003 | a | 0.152 ± 0.011 | b | 0.109 ± 0.010 | b | |
| SS | 0.015 ± 0.002 | a | 0.035 ± 0.002 | a | 0.034 ± 0.003 | a | 0.054 ± 0.011 | a | 0.082 ± 0.008 | a | |
| Spring | CO | No leaf | 0.027 ± 0.004 | a | 0.034 ± 0.004 | b | 0.103 ± 0.007 | a | 0.171 ± 0.009 | a | |
| TS | No leaf | 0.026 ± 0.007 | a | 0.032 ± 0.007 | b | 0.083 ± 0.014 | a | 0.148 ± 0.019 | a | ||
| SS | No leaf | 0.026 ± 0.004 | a | 0.016 ± 0.005 | a | 0.103 ± 0.039 | a | 0.145 ± 0.005 | a | ||
| Summer 2 | CO | 0.008 ± 0.001 | a | 0.036 ± 0.005 | a | 0.029 ± 0.009 | a | 0.075 ± 0.006 | a | 0.070 ± 0.011 | a |
| TS | 0.008 ± 0.001 | a | 0.016 ± 0.005 | a | 0.021 ± 0.006 | a | 0.072 ± 0.009 | a | 0.048 ± 0.007 | a | |
| SS | 0.007 ± 0.001 | a | 0.023 ± 0.009 | a | 0.023 ± 0.011 | a | 0.084 ± 0.009 | a | 0.066 ± 0.018 | a | |
| NSCs (%dw) | |||||||||||
| Summer 1 | CO | 7.87 ± 0.71 | b | 4.49 ± 0.76 | a | 8.20 ± 0.55 | b | 13.49 ± 0.37 | b | 8.98 ± 0.53 | a |
| TS | 7.56 ± 0.70 | b | 6.95 ± 0.70 | b | 8.22 ± 1.03 | b | 10.58 ± 1.13 | b | 8.42 ± 0.35 | a | |
| SS | 3.44 ± 0.24 | a | 3.72 ± 0.45 | a | 1.18 ± 0.16 | a | 4.14 ± 0.63 | a | 7.48 ± 1.16 | a | |
| Autumn | CO | 5.66 ± 0.98 | a | 7.01 ± 0.39 | a | 8.24 ± 0.61 | b | 19.66 ± 0.92 | b | 14.11 ± 1.39 | a |
| TS | 5.89 ± 0.41 | a | 8.03 ± 0.64 | a | 6.73 ± 0.49 | ab | 17.84 ± 1.01 | b | 12.38 ± 0.97 | a | |
| SS | 5.52 ± 0.62 | a | 6.87 ± 0.82 | a | 5.73 ± 0.35 | a | 9.53 ± 1.29 | a | 15.30 ± 1.26 | a | |
| Spring | CO | No leaf | 6.73 ± 0.31 | a | 8.91 ± 0.84 | b | 15.14 ± 0.91 | a | 13.95 ± 0.87 | a | |
| TS | No leaf | 7.33 ± 0.67 | a | 5.86 ± 0.37 | a | 13.17 ± 1.45 | a | 11.76 ± 1.17 | a | ||
| SS | No leaf | 7.53 ± 0.45 | a | 5.26 ± 0.56 | a | 14.86 ± 0.55 | a | 14.50 ± 2.51 | a | ||
| Summer 2 | CO | 11.56 ± 0.84 | b | 8.54 ± 0.54 | a | 9.52 ± 1.39 | a | 11.89 ± 0.87 | a | 12.29 ± 1.32 | b |
| TS | 7.24 ± 0.71 | a | 5.26 ± 0.71 | a | 7.01 ± 1.42 | a | 10.64 ± 1.25 | a | 10.42 ± 1.13 | ab | |
| SS | 7.31 ± 0.70 | a | 7.57 ± 1.73 | a | 5.15 ± 0.61 | a | 11.82 ± 1.46 | a | 7.43 ± 1.12 | a | |
| Respiration rates (nmol·g−1·s−1) | |||||||||||
| Summer 1 | CO | 11.10 ± 2.07 | a | 4.89 ± 0.77 | a | 1.56 ± 0.27 | a | 1.94 ± 0.68 | a | 3.37 ± 0.61 | ab |
| TS | 15.02 ± 0.86 | a | 3.72 ± 0.50 | a | 2.75 ± 0.99 | a | 2.75 ± 1.16 | a | 4.94 ± 0.95 | b | |
| SS | 32.22 ± 3.52 | b | 5.53 ± 0.88 | a | 0.60 ± 0.20 | a | 0.82 ± 0.13 | a | 1.47 ± 0.45 | a | |
| Autumn | CO | 11.68 ± 3.05 | a | 0.80 ± 0.09 | a | 0.64 ± 0.09 | a | 1.18 ± 0.23 | a | 1.07 ± 0.19 | a |
| TS | 15.32 ± 2.08 | a | 0.87 ± 0.14 | a | 0.70 ± 0.11 | ab | 1.41 ± 0.19 | a | 1.78 ± 0.35 | a | |
| SS | 12.65 ± 1.39 | a | 0.88 ± 0.15 | a | 1.07 ± 0.11 | b | 1.10 ± 0.15 | a | 1.71 ± 0.41 | a | |
| Spring | CO | No leaf | 0.89 ± 0.37 | a | 0.56 0.14 | a | 2.14 ± 0.38 | a | 1.32 ± 0.23 | a | |
| TS | No leaf | 0.92 ± 0.20 | a | 0.82 ± 0.13 | a | 2.51 ± 0.26 | a | 1.70 ± 0.21 | ab | ||
| SS | No leaf | 1.66 ± 0.84 | a | 3.45 ± 1.07 | b | 2.65 ± 0.46 | a | 2.54 ± 0.38 | b | ||
| Summer 2 | CO | 9.37 ± 1.69 | a | 5.34 ± 1.93 | a | 1.22 ± 0.20 | a | 1.19 ± 0.20 | a | 2.36 ± 0.40 | a |
| TS | 13.69 ± 1.74 | a | 7.53 ± 1.03 | a | 1.42 ± 0.27 | a | 1.32 ± 0.28 | a | 2.93 ± 0.44 | a | |
| SS | 16.54 ± 2.77 | a | 6.31 ± 1.20 | a | 2.67 ± 0.40 | b | 1.14 ± 0.31 | a | 3.31 ± 0.66 | a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qurani, C.G.; Yoshimura, K.; Lee, B.; Maulidah, N.I. Resprouting Ability and Carbon Allocation of Robinia pseudoacacia L. Trees After Cutting at Different Stem Heights. Forests 2025, 16, 1084. https://doi.org/10.3390/f16071084
Qurani CG, Yoshimura K, Lee B, Maulidah NI. Resprouting Ability and Carbon Allocation of Robinia pseudoacacia L. Trees After Cutting at Different Stem Heights. Forests. 2025; 16(7):1084. https://doi.org/10.3390/f16071084
Chicago/Turabian StyleQurani, Citra G., Kenichi Yoshimura, Bora Lee, and Nur I. Maulidah. 2025. "Resprouting Ability and Carbon Allocation of Robinia pseudoacacia L. Trees After Cutting at Different Stem Heights" Forests 16, no. 7: 1084. https://doi.org/10.3390/f16071084
APA StyleQurani, C. G., Yoshimura, K., Lee, B., & Maulidah, N. I. (2025). Resprouting Ability and Carbon Allocation of Robinia pseudoacacia L. Trees After Cutting at Different Stem Heights. Forests, 16(7), 1084. https://doi.org/10.3390/f16071084







