Abstract
The Mixed Ombrophyllous Forest (MOF), inserted in the Atlantic Forest biome, is of great ecological value, with deficient management strategies. In this context, sustainable management helps to promote the regeneration and growth of individual trees and control others, while maintaining the natural forest structure. This study therefore aimed to discuss opportunities and limitations of biochar, produced from two species from the MOF, which are currently only utilized to a limited extent in the study area in southern Brazil. A slow pyrolysis process at a lab scale was designed, biochar was produced, and key properties were analyzed from Hovenia dulcis Thunb. (chosen as an invasive species) and Mimosa scabrella Benth. (chosen as a native, fast-growing species), including branches and stems. The results showed that branches of Mimosa scabrella (BMS) had the highest biochar yield (30.32 ± 0.3%) and the highest electrical conductivity (415.08 ± 24.75 mS cm−1). Stems of Mimosa scabrella (SMS) showed the highest higher heating value (HHV—31.76 ± 0.01 MJ kg−1), lower heating value (LHV—31.03 ± 0.01 MJ kg−1), and energy yield (49.1%), while the branches of Hovenia dulcis (BHD) showed the lowest values. For the elemental analysis, SMS showed the best results, with the highest amount of fixed carbon (78.62 ± 0.22%) and carbon content (85.87 ± 0.083%), and consequently the lowest amount of ash (3.52 ± 0.08%). BHD showed a better water-holding capacity (303.26 ± 15.21%) and higher pH value (7.65 ± 0.14). The investigations conducted on the biochar from both species indicate a strong suitability of these woods for producing high-quality biochar.
Keywords:
slow pyrolysis; biochar; Atlantic Forest; organic carbon; Hovenia dulcis; Mimosa scabrella 1. Introduction
The Mixed Ombrophyllous Forest (MOF—in Portuguese Floresta Ombrófila Mista) or Araucaria Forest is part of the Atlantic Forest biome in southern Brazil and covers an area of approximately 216,100 km2 [1]. Due to a very biodiverse environment and because of indigenous species such as Araucaria angustifolia Kuntze, this forest ecosystem has great ecological value [2,3]. However, the MOF is considered one of the most endangered forests [4]. It has been reduced to approximately 3 to 13% of its original area and divided into fragments due to urban and industrial growth and agricultural use [5,6]. The remaining areas of this are mainly regions with surface relief, legally protected areas, river margins, or private property [7]. As unmanaged or unprotected forests are more likely to be replaced by other land uses, one of the most significant levers for preserving biodiversity in natural forests is the implementation of sustainable management practices [1].
Currently, there is no legally binding policy instrument for native forests in Brazil [8]. However, landowners in Brazil have a historical obligation to maintain or restore native vegetation [5]. The first Brazilian Forest Code was approved in 1934 and established the concept of protected areas [9]. In 2012, the most recent version of the Forest Code came into force (law n° 12651/2012). Part of the Forest Code is the Rural Environmental Registry or ’CAR’ (Cadastro Ambiental Rural), requiring landowners to prepare a report (CAR) on boundaries, vegetation cover, permanent conservation areas, restricted use areas, and legal reserve areas of their property. This report is intended to help landowners comply with the requirements of the law [5,10].
However, the restrictions, the lack of sustainable management, and the partly elaborate requirements cause resentment among landowners and lead to a steady decline in native forest areas [11]. Due to the high terrestrial carbon stock in declining forest ecosystems, released by land-use change and deforestation, Brazil has become one of the largest emitters of carbon dioxide [12].
To effectively utilize the MOF as a carbon sink and keep its high ecological value, it is essential to provide landowners with greater financial incentives, enabling the adoption of more costly yet sustainable management practices. Preserving or restoring the natural forest structure and minimizing the spread of invasive species through appropriate management techniques are crucial steps. Sustainable management not only supports the regeneration of native species and the restoration of the ecoregion’s natural state, but also enhances the growth of existing trees, reduces natural mortality rates, and can generate additional income for farmers. Conversely, without proper management, invasive species such as Hovenia dulcis Thunb.—which are already prevalent in parts of the MOF—will continue to disrupt the natural forest structure [13]. Despite the increasing presence of H. dulcis, there are currently no mitigation measures in place to control exotic species [4].
In this context, biochar emerges as a promising tool for sustainable forest management and carbon sequestration that could address both the restoration needs of the MOF and Brazil’s carbon emission challenges. Biochar, produced through pyrolysis of biomass under oxygen-limited conditions, has demonstrated significant potential in forest ecosystem applications [14,15,16]. In Brazil, pioneering studies have demonstrated biochar’s effectiveness in recovering degraded pasturelands while increasing productivity by up to 27%. However, smallhold biochar production for ranching purposes would have to be supported by Payments for Ecosystem Services in order to be financially competitive [17]. From a carbon sequestration perspective, biochar represents a stable, long-term carbon storage solution, capable of sequestering CO2 for hundreds of years, depending on its quality [18,19]. For the MOF, biochar applications could offer landowners dual benefits: enhancing forest health and productivity, while simultaneously contributing to carbon sequestration objectives. These outcomes may create economic incentives that encourage the conservation of this endangered ecosystem, rather than its conversion to alternative land uses.
Therefore, this study investigates the valorization of invasive and underutilized tree species for biochar production through simplified pyrolysis methods applicable to smallholder operations in southern Brazil. Focusing on Hovenia dulcis (an invasive species requiring containment) and Mimosa scabrella Benth. (a native nitrogen-fixing species), this research employs lab-scale slow pyrolysis at 450 °C to assess biochar quality against European Biochar Certificate standards, while modeling scalable, low-tech production conditions [20,21,22,23]. The 450 °C temperature was strategically selected to balance biochar functionality with operational feasibility for rural producers, as higher temperatures (e.g., 700 °C) demand advanced reactors and energy inputs that are less accessible in decentralized settings [24,25]. In the traditionally employed charcoal kilns without mechanization and tar recovery systems, typical temperatures were found to be around 500 °C [26]. However, these temperatures can vary significantly depending on the kiln design, the type of wood used, and other factors such as ignition behavior. For example, García-Quezada et al. documented peak temperatures ranging from 420 to 596 °C across three trials of a kiln with a design analogous to the charcoal kilns prevalent in the region where the investigations were conducted [27].
By avoiding oxygen removal systems and prioritizing moderate thermal regimes, the approach aligns with smallholder-compatible techniques like double-barrel retorts or clay kilns that rely on passive airflow and self-sustaining gas combustion. These systems, demonstrated in studies using sunflower straw and food waste feedstock, achieve comparable biochar yields (28%–40%) at 300–500 °C while generating usable syngas byproducts [25,28].
This methodology intentionally mirrors real-world constraints—limited temperature control, batch processing, and basic emission management—to evaluate whether invasive biomass can yield marketable biochar with dual soil amendment and carbon sequestration benefits under practical field conditions. This temperature range also optimizes electrical conductivity (4.53–7.69 mS cm−1) and pH (8.04–10.75) parameters critical for agricultural applications, as evidenced in low-temperature food waste biochar studies [28]. By validating quality thresholds achievable without sophisticated reactors, this work provides a template for converting problematic biomass into circular economy resources through replicable, low-capital pyrolysis systems.
2. Materials and Methods
2.1. Subject of the Study and Sampling
The biomass sources to be analyzed for their characteristic properties as well as their corresponding abbreviations are as follows:
- Stems of Mimosa scabrella (SMS);
- Branches of Mimosa scabrella (BMS);
- Stems of Hovenia dulcis (SHD);
- Branches of Hovenia dulcis (BHD).
Each subject of this study was derived from the MOF, located in small rural properties, and provided by Universidade Estadual do Centro-Oeste (UNICENTRO). For a good representation of each species, the samples were chosen from three different trees of each of the two species. They ranged from 35.8 to 14.3 cm in breast height diameter and 14.3 to 11.9 m in total height for M. scabrella, and 33 to 11.2 cm breast height diameter and 20.3 to 15.3 m in total height for H. dulcis. Biomass from branches and stems were separated and the respective sections of each of the three trees were merged and milled. To maintain the low-cost approach regarding smallholder biochar production, no bark removal was performed on the samples. As a result, the bark content in the “Stem” samples is higher than in the “Branch” samples.
2.2. Pyrolysis-Based Biochar Production
The experimental setup for pyrolysis was based on the method and experimental setup used by Doumer et al. [29]. The biomass samples were milled (Forage Crusher TRF-400 Super, Trapp, Jaraguá do Sul, Brazil), screened with a mesh size of 4.75 mm, as well as air dried prior to pyrolysis, and transferred to a 1 L internal volume borosilicate glass reactor. At a heating rate of 5 °C min−1, the temperature in the electric furnace (EDGCON 3P, FC-2, EDG Equipamentos, São Carlos, Brazil) reached 450 °C, and the subsequent residence time of the reaction was 120 min. Cold water at 5 ± 2 °C was circulated in a Liebig condenser, where steam was condensed and separated into bio-oil and non-condensable gases. Pyrolysis reactions were conducted in quadruplicate. The schematic diagram is shown in Figure 1A, while a photograph of the setup is shown in Figure 1B. The biochar and bio-oil were collected to determine the mass yield and balance, while the non-condensable gases were determined by mass difference. Figure 2 illustrates the comparison between the initial material and the final product.
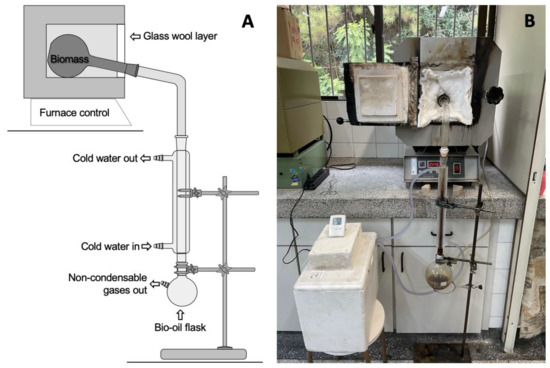
Figure 1.
(A) Schematic diagram of reactor layout for pyrolysis; (B) photo of the equipment used.

Figure 2.
Comparison between the initial material and final product of biomass samples.
2.3. Analysis of Key Properties of Target Species
The raw biomass and biochar were characterized using a series of laboratory tests. The choice of methods was based on the European guidelines for the sustainable production of biochar in the laboratories of UNICENTRO, the Federal University of Paraná and the University of Applied Forest Sciences in Rottenburg, Germany.
The higher heating value (HHV) for wood and biochar samples was determined in duplicate according to DIN 51900 using a C-5000 calorimeter (IKA Werke GmbH & Co. KG, Staufen, Germany). [30]. The lower calorific value (LHV) was calculated according to Equation (1).
LHV = HHV − 0.206 × H
The energy of vaporization (constant volume) for water at 25 °C is 41.53 kJ mol−1. This corresponds to 0.206 MJ kg−1 for 1% (by weight) of hydrogen content in the sample that was determined by elemental analysis. The net calorific value at a constant volume was derived from the corresponding higher heating value.
Ion chromatography was performed to determine the Cl− content on an 882 Compact IC plus, equipped with a model 863 Compact automatic sampler, Metrosep A supp5 column (150 × 4.0 mm; 5.0 µm), and Metrosep RP precolumn, and detection by conductivity and ultraviolet spectrophotometry was performed using a UV/Vis 887 Professional detector set at 205 nm (Metrohm AG, Herisau, Switzerland). Analyses were performed at 32 °C in an isocratic elution system using 0.85 mmol L−1 sodium carbonate and 0.9 mmol L−1 sodium bicarbonate (Na2CO3/NaHCO3 buffer). The flow rate was 1.0 mL min−1.
The bulk density was determined according to DIN EN ISO 17,828 and VDLUFA method A 13.2.1, as proposed in the EBC Guidelines (n = 4) [20,31,32]. A measuring cylinder (1 L) was dropped ten times from approximately 10 cm and both mass and volume were used to calculate the density. The bulk density of wood was measured on a received basis and converted to a dry basis, while the bulk density of biochar was measured after the pyrolysis process at zero moisture content (as dry basis).
The initial moisture content of the wood (sample size ≥ 4.75 mm, n = 3) was determined according to the Brazilian standard ABNT NBR 14929, using a KT900 drying oven (HERAEUS, Hanau, Germany) at 105 ± 2 °C for 24 h [33]. The raw moisture of the biochar was determined using an Alpha Life Science oven 100 L at 40 ± 2 °C for 24 h, after allowing 7 days of exposure to the environment (at a range in temperature of 18 ± 4 °C and in humidity of 50 ± 10%, n = 3). The hygroscopic moisture content of biochar (sample size between 250 and 425 µm) was estimated according to the EBC certificate by drying the sample at 106 ± 2 °C under a nitrogen atmosphere to mass constancy using a TGA instrument [20]. It was only determined once. A thermogravimetric analysis (TGA) was performed on a STA 449 F1 Jupiter (Erich NETZSCH B.V. & Co. Holding KG, Selb, Germany). Five mg of the sample between 250 and 450 μm was placed in an alumina crucible. The measurement was performed once per sample at a heating rate of 10 °C min−1 under a nitrogen atmosphere (40 mL min−1) with a temperature range from 25 to 950 °C.
The proximate analysis of biomass and biochar samples followed the DIN 51,719 standard for ash content, volatile matter (according to DIN 51720), and fixed carbon (by difference) [34,35]. The sample size used for wood was 355 µm (Forage Crusher TRF-400, TRAPP, Jaraguá do Sul, Brazil), while the test for biochar was continued with a sample size of <1.00 mm. A proximate analysis for each sample was performed in triplicate. In the first step for the measurement of the volatiles, the sample was weighed in a crucible with the lid (preheated) and placed in front of the muffle furnace for 3 min (door open). The crucible was placed in the muffle furnace (door closed) for 7 min and subsequently removed from the furnace, cooled to room temperature in a desiccator, and weighed. For the ash content, the uncovered crucible used for the volatile content was placed in the muffle furnace at 550 °C, with a rate temperature of 5 °C min−1 for 6 h. In the next step, the crucible was removed from the furnace and weighed after cooling to room temperature in a desiccator. Finally, the fixed carbon content was calculated using the 100% reduced by ash content and volatile matter on a dry basis.
An ultimate analysis was performed for carbon, hydrogen, nitrogen, sulfur, and oxygen (estimated by the difference method) using a Vario EL cube CHNS-O elemental analyzer (Elementar Analysensysteme GmbH, Langenselbold, Germany, according to DIN 51732, DIN 51724-3 and DIN 51733, n = 2) [36,37,38]. For wood, a sample size of 355 µm was used, and for biochar, a ground sample of <250 µm was used; the samples were analyzed using an A11 basic analytical mill (IKA Werke GmbH & Co. KG, Staufen, Germany). To determine the total organic carbon (TOC), total inorganic carbon (TIC) was analyzed in a Primacs SNC-100 (Skalar Analytical B.V., Breda, The Netherlands) and subtracted from the Ctot, whose determination is described above. This was performed in triplicate.
Mineral elements were measured in three replications for each sample by ICP-OES (Spectro blue, Spectro Analytical Instruments GmbH, Kleve, Germany) according to standard procedures (DIN EN ISO 11885) [39]. After weighing 100 mgDM of the sample into each of the 15 mL PTFE reaction vessels, 1 mL of H2O2 was added with a reaction time of 5 min. Then, 1 mL of HNO3 (69%) supra quality (Merck, Darmstadt, Germany) was added, and after a short waiting time, a further 2 mL of HNO3 was added. After 30 min, 3 mL of HCl supra quality (35%) (Roth, Germany) was added. In the next step, the containers were closed and left for 12 h before adding another 6 mL of HCl. Afterwards, the digestion was carried out using a microwave (Multiwave Go, Anton Paar GmbH, Graz, Austria) at 175 °C with a heating ramp of 7.75 °C min−1 and a holding time of 30 min. Then, the vessels were heated to 185 °C with an additional holding time of 5 min. The residues were placed into laboratory tubes and filled up to 50 mL with double-distilled water, before being filtered and measured.
To estimate the added value of soil amendments, an indicator for the cation exchange capacity of the biochar without the combination with corresponding soils was applied. Several approaches exist to determine this, albeit facing methodological challenges and often yielding non-reproducible results [40,41]. To roughly estimate the short- and medium-term nutrient supply by the biochar to different soils, a BaCl2 cation exchange experiment, as proposed by Gillman and Sumpter, was conducted to determine the plant-available portion of specific elements [42]. However, these calculations should be regarded as informative only, as this is not a standardized procedure for the applied method in the described experimental setting.
For this determination, the UNEP-UN/ECE Method 9106SA based on DIN ISO 11,260 was applied [43,44]. A total of 30 mL of 0.1 M BaCl2 solution was mixed with 2.5 g of each biochar sample in triplicate. After 2 h in a mechanical shaker, the tubes were centrifuged, and the solution was filtered through a pleated filter. Subsequently, the resulting liquid was measured via ICP-OES. As became apparent in the ICP-OES measurement of the pure 0.1 M BaCl2 solution, it was contaminated, likely due to incorporation in the production process, especially in higher concentrations with K, Ca, and Na (as well as high concentrations of Sr, which is not given in the respective result section). These concentrations were deducted from the sample results.
The pH and electrical conductivity were determined according to DIN ISO 10,390 and the EBC certificate, with a few adaptations [20,45]. The measurements were carried out in duplicate, each on the AK151 (Akso Produtos Eletrônicos Ltd.a, São Leopoldo, Brazil) equipment, which measures both properties. For pH, 5.00 g of biochar and 25.00 mL of 0.01 M CaCl2 solution were stirred for 1 h. The suspension was then filtered. For electrical conductivity, 5.00 g of biochar and 50.00 mL of deionized water were stirred for 1 h. The suspension was then filtered through filter paper and subsequently through a 0.22 µm PVDF membrane.
The water-holding capacity (WHC) of biochar was determined using the ISO 14,238 Annex A method, with minor adaptations [46]. A total of 5.0 g of biochar (<2.00 mm particle size and oven-dried at 40 °C) was placed in a #2 sintered glass crucible (40–100 µm of porosity, previously preheated and tared) and evenly distributed by gently tapping the crucible. The crucibles were then immersed in a water bath at room temperature with the water level lower than the biochar surface and left in this position overnight. After this period, the crucibles were removed from the water, placed on the sand tray, covered with wet paper to prevent water evaporation, and allowed to drain. The crucibles containing water-saturated biochar were weighed after 2 h. The biochar samples were then dried at 40 °C for 72 h. Finally, the oven-dried biochar samples were cooled to room temperature in a desiccator and weighed.
2.4. Statistical Analysis
Treatment effects were analyzed using a one-way analysis of variance (ANOVA) with post hoc Tukey tests to detect significant differences between means. All data are presented as mean ± standard derivation (SD). A p-value < 0.05 was considered significant. The statistical analyses were performed using STATISTICA 12 (TIBCO Software Inc., Palo Alto, CA, USA) software.
3. Results and Discussion
3.1. Pyrolysis Yields
The average pyrolysis yields of biochar, bio-oil, and non-condensable gases (produced in the experiments using the setup described in Section 2.2.) are presented in Figure 3. The highest yield of solid biochar was achieved with BMS (30.32 ± 0.34%), while generating the lowest amount of bio-oil (47.64 ± 0.89%). SHD and BHD produced the lowest yields of solid biochar (27.36 ± 0.33% and 27.53 ± 0.31%), while generating the highest amount of bio-oil (51.73 ± 0.08% and 51.70 ± 0.24%).
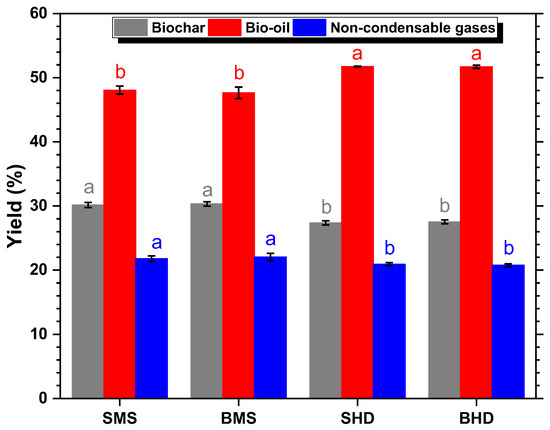
Figure 3.
Mass yields of pyrolysis products from SMS, BMS, SHD, and BHD. Different lowercase letters of the same color indicate significant differences between yields at p < 0.05 according to one-way ANOVA and Tukey’s test. Number of replicates = 4.
The aim was to achieve the maximum biochar yield for use in fertilizer or soil additive production. Panwar et al. reported a biochar yield range of 15%–35% under the provided conditions [47]. It is suggested in the literature that slow pyrolysis may yield higher biochar levels, potentially accounting for the results showcased in Figure 3, which fall towards the upper end of the range observed by Panwar et al. [47]. It can be concluded that pyrolysis was successful for all samples, as a biochar yield of approximately 30% was achieved. While no statistically significant differences were observed between stem and branch samples within each species, M. scabrella exhibited significantly higher biochar yields. The results are displayed in Figure 3, where different lowercase letters of the same color indicate significant differences between yields at p < 0.05 according to the one-way ANOVA and Tukey’s test. These results also correspond with the general findings from Spokas, who documented between approximately 25 and 65% in biochar yield from diverse biomass samples at pyrolysis temperatures of 400 °C [18].
Both species showed identical statistics by Tukey’s post hoc test regarding the output mass balances for stems and branches. The M. scabrella samples exhibited the highest yields of biochar and non-condensable gases, whereas the H. dulcis samples had lower yields of these products. In contrast, the H. dulcis samples demonstrated a higher bio-oil yield. Intra-species comparisons, i.e., between biochar produced from stems and branches, revealed no statistically significant differences.
Due to the implementation of a cooling system in the setup, condensable gases are also recorded as bio-oil output in Figure 3, whereas in traditional kilns, which are a main focus of this publication, a significant portion of these gases would be emitted into the surrounding area. As described by Gomes and Encarnação, this presents a substantial hazard to workers, necessitating measures to divert or cool these gases in practical applications. One method to reduce the proportion of acetic acids, methanol, aromatics, phenolics, aldehydes, and tar in the condensable gases as part of the exhaust gases, is the construction of an additional post-pyrolysis chamber for gas cooling, commonly referred to as the “Brochier” model [24].
To characterize the thermal behavior of samples, a thermogravimetric analysis (TGA) was carried out. The TGA and differential thermal gravimetric (DTG) curves of biomasses and biochar are shown in Figure 4 and Figure 5, respectively.
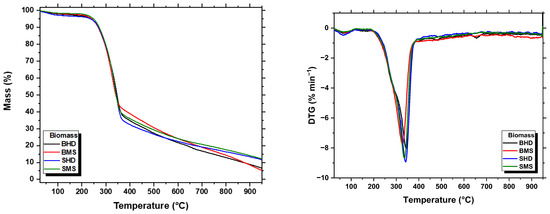
Figure 4.
TGA and DTG curves of the biomass materials.
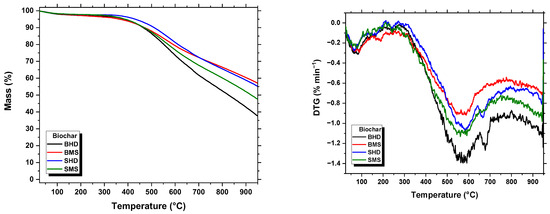
Figure 5.
TGA and DTG curves of the biochar samples.
There are three main stages of the thermal degradation process of biomass that are used to describe its thermal behavior, which are dehydration, devolatilization, and carbonization. In the first step, mass loss is observed due to the moisture content and volatiles in the structure of biomass for the temperature range between 25 and 120 °C. The second step, which occurs from 150 to 400 °C, showed a significant mass loss (about 70%) in all the biomass samples studied. This stage corresponds to the decomposition of hemicelluloses, cellulose, and lignin. The third stage begins at 400 °C and extends to 950 °C, where there is minimal mass loss due to the formation of carbonaceous solids and lignin degradation over a wide temperature range. Throughout the study, it was observed that a thermal degradation above 400 °C was almost constant [48,49,50].
In this study, all prepared biochar samples showed a similar thermal degradation profile (Figure 5) up to 450 °C. The first stage of mass loss is dehydration, which occurs at temperatures up to 150 °C, and corresponds to the moisture content of the biochar. The second stage of mass loss, (up to 625 °C) corresponds to devolatilization, and is considered the burning of the remaining volatile matter in the biochar samples. It is important to note that hemicellulose (220–315 °C) and cellulose (315–400 °C), the structural components of the biomass raw material, were practically degraded during the production of biochar in this study. However, lignin shows greater thermal stability; its mass loss occurs over a wide temperature range (160–900 °C), generating a large portion of solid residue [51,52,53]. Above this temperature (650 °C), a clear difference was observed between the biochar prepared from the branches and stems of H. dulcis and M. scabrella. It can be seen that the lowest weight loss is obtained in the case of BMS compared to SMS and the opposite is obtained in the case of SHD compared to BHD; this is due to the inorganic components, which showed the same behavior in the ash content results in the proximate analysis and biochar yield. Furthermore, the differences in the inherent structures and chemical composition of these three biomass components may explain the observed differences in thermal stability.
The stability of biochar as well as the success of the pyrolysis process be described and estimated based on the O/C and H/C atomic ratios. These ratios can be visualized using the Van Krevelen diagram (see Figure 6). To ensure the presence of abundant fused aromatic ring structures and the stability of carbon sequestered in biochar for over 100 years in soil, the guidelines of the International Biochar Initiative (IBI) and the European Biochar Certificate (EBC) define a rather conservative H/C ratio below 0.7, which is achieved by all samples tested in this study [20,54,55]. According to the correlation of the O/C ratio described by Spokas, this method suggests a half-life of over 1000 years for the pyrolyzed samples (ranging between 0.08 and 0.12) and falls within the lower ratio range for biochar produced at 400 °C (cf. Figure 5 and Figure 6 in Spokas [18]).
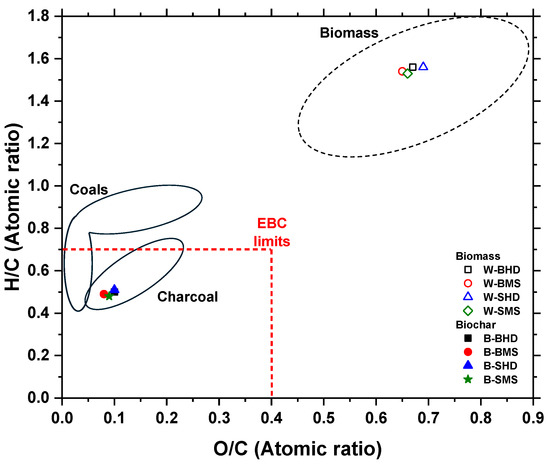
Figure 6.
Van Krevelen diagram with information from Wyn et al. [56] and EBC [20].
3.2. Physical Characteristics of Target Species
3.2.1. Higher and Lower Heating Value
The higher heating values (HHVs) and lower heating values (LHVs) of the biomass and biochar samples are given in Table 1. For the biomasses, the highest HHVs were identified with M. scabrella (19.54 ± 0.03 MJ kg−1 for BMS and 19.50 ± 0.01 MJ kg−1 for SMS), which is slightly lower than the calculated values from Panzarini Silva et al. for M. scabrella (20.08 MJ kg−1) [57]. Nevertheless, the high energy content, in conjunction with the higher bulk density (see Table 2), explains its use for energy purposes.

Table 1.
Higher and lower heating values (HHVs and LHVs of the biomass and biochar, each as dry basis).
Lower values were obtained for H. dulcis (19.10 ± 0.03 MJ kg−1 for SHD and 18.97 ± 0.01 MJ kg−1 for BHD), representing a value at the lower end of wooden biomass [58]. The HHV values for biomass ranged from 18.97 to 19.54 MJ kg−1 and were consistent with the reported values by Dhyani and Bhaskar for wood-based biomass (15.79 MJ kg−1 for furniture sawdust and 20.5 MJ kg−1 for wood bark) [59].
With pyrolysis, the HHV increased for all samples referred to as kgBiochar (between 60 and 65%), resulting in the highest HHV for SMS biochar (31.76 ± 0.01 MJ kg−1). The increase in fixed carbon content, as shown in Section 3.2.3., contributed to the specific rise in calorific values of biochar. This finding is also supported by various authors in the field, including Doumer et al. and Rathod et al. [29,60]. The range of HHVs achieved during the biochar production process commonly falls within the energy content range of 30 to 35 MJ kg−1 [61], and is also higher than reported LHV values of hard coal (29.7 MJ kg−1) [62].
With regard to the energy balance, however, it should be noted that the biochar yield (see Figure 3) only converts 27.4–30.3% of the original biomass into biochar. A total of 1 kg of biomass therefore only produces 8.5–9.6 MJ kg−1 of biochar (with an energy yield 44.9–49.1%), with the remaining energy being contained in the bio-oil and the non-condensable gases. A complete energy balance was not carried out in this study. The energy yield was calculated according to the Equation (2).
3.2.2. Bulk Density, Initial Moisture Content (Wood), and Raw Moisture Content (Biochar)
The bulk densities of the biomass of the different species, their respective biochar on a dry basis, as well as the initial moisture content of the biomasses and the raw moisture content of the biochar are given in Table 2. This is necessary information for the production of a consistent substrate mixture and for the trade of certified biochar (WBC & EBC Certificates). The bulk density of biochar also determines the transport properties (costs, greenhouse gas emissions) and bulk density of soil after biochar application (penetrability, drainage, aeration) [63].

Table 2.
Bulk density of the biomass and biochar (kg m−3 dry basis), initial moisture content of the biomass (%), and raw moisture content of the biochar (%).
Table 2.
Bulk density of the biomass and biochar (kg m−3 dry basis), initial moisture content of the biomass (%), and raw moisture content of the biochar (%).
| Material | Bulk Density (kg m−3) | Initial Moisture Content (%) | Raw Moisture Content (%) | Hygroscopic Moisture Content (%) | Water Content (%) |
|---|---|---|---|---|---|
| Biomass | |||||
| SMS | 239.38 ± 3.37 | 9.02 ± 0.05 | |||
| BMS | 200.77 ± 7.41 | 8.98 ± 0.22 | |||
| SHD | 218.57 ± 3.87 | 12.13 ± 1.39 | |||
| BHD | 175.25 ± 8.23 | 11.30 ± 1.26 | |||
| Biochar | |||||
| SMS | 165.62 ± 7.49 | 2.02 ± 0.16 | 2.46 | 4.43 | |
| BMS | 168.44 ± 10.81 | 2.50 ± 0.11 | 1.94 | 4.39 | |
| SHD | 159.44 ± 12.82 | 2.59 ± 0.06 | 1.88 | 4.56 | |
| BHD | 156.34 ± 5.50 | 2.69 ± 0.15 | 2.15 | 4.84 | |
For raw biomass, the highest density was obtained for SMS (239.38 ± 3.37 kg m−3) and the lowest for BHD (175.25 ± 8.23 kg m−3). For the biochar, the highest density was measured for BMS (168.44 ± 10.81 kg m−3) and the lowest for BHD (156.34 ± 5.50 kg m−3). The bulk density of biochar naturally correlates with the density of biomass (r = 0.68). The species with a high biomass density also turn into dense biochar. The highest initial moisture content inside the raw material was measured in SHD (12.13 ± 1.39%) and the lowest in BMS (8.98 ± 0.22%). The moisture content of the feedstock can influence the biochar yield and the energy needed to reach the pyrolysis temperature. A low water content is beneficial for biochar formation because it reduces the amount of thermal energy and time required for the pyrolysis process [64]. At the same time, Burhenne et al. showed that woody biomass with a higher initial moisture content produces less char and more condensable products than biomass with a lower initial moisture content [65]. The raw moisture content of biochar ranged from 2.51 ± 0.01% to 2.72 ± 0.03%. The lowest moisture content was found in BMS and the highest in BHD. When used as a fertilizer, the raw moisture content is a limiting factor, as the char absorbs water after application. If the soil is not watered sufficiently or there is a prolonged dry period, the biochar as a soil additive can extract additional water from the soil.
3.2.3. Proximate and Ultimate Analysis, TOC, and CHN
The results of the proximate analysis of the biomass and the biochar produced are given in Table 3.

Table 3.
Results for proximate analysis of the biomass and biochar in terms of the amounts (% dry basis) of fixed carbon, ash, volatile matter, and moisture content.
For the biomass, the fixed carbon content ranged from SHD 18.52 ± 0.34% to SMS 21.12 ± 0.10%, the ash content from SMS 2.25 ± 0.13% to BMS 2.85 ± 0.18%, and volatile matter from BMS 76.57 ± 0.25% to SHD 78.83 ± 0.30%. SHD also yielded a high amount of bio-oil (see Figure 3). This is consistent with the findings of Dhyani and Bhaskar, who stated that biomass with a higher volatile matter may be more suitable for bio-oil production compared to biochar production [59].
Higher fixed carbon contents were determined for biochar compared to biomass. It ranged from SHD 76.63 ± 0.35% to SMS 78.63 ± 0.22%. The same applies for the ash content. Volatile matter decreased and varied from SMS 17.86 ± 0.20% to BHD 19.46 ± 0.12%, due to the thermal treatment and release of volatiles during pyrolysis process [66].
The elemental contents of the biomass samples and their respective biochar are presented in Table 4. An expected increase in carbon content was observed for all species. While the C content of biomass ranged from SHD 48.71% to BMS 49.76%, the values for biochar varied from SHD 84.51% to SMS 85.87%. The concentration of elemental carbon could be due to decomposition of organic matter and volatilization of other elements, including H and O. The thermal treatment induces the breakdown of the biomass structure’s weakest bonds, providing a plausible explanation for the observed lower hydrogen content in the biomass (ranging from SMS 6.35% to BMS 6.45%) compared to the biochar (ranging from SMS 3.47% to SHD 3.59% [16]. The O content decreased for biochar compared to biomass, ranging from BMS 9.64% to SHD 11.37%. The initial O content of the biomass varied from BMS 43.17% to SHD 44.66%. In contrast, the N content of the biochar increased compared to the biomass, with the exception of SMS. This N enrichment was also found in the studies of Doumer et al., Al-Wabe et al. and Gaskin et al. [29,67,68]. The latter stated that this could be due to the incorporation of N into complex structures that are not volatilized. In the context of the agricultural use of biochar, the high N content could be beneficial, as it could provide nutrients to the soil, although they doubt that it is available to plants. The S content was negligible in both the biomass and biochar. It slightly decreased for SMS and increased for BMS, SHD, and BHD, which could also be beneficial for fertilizer use, since S determines plant growth and development [69].

Table 4.
Elemental contents of the woody biomass samples and their respective biochar (dry ash free basis).
The chloride content in the biomass determined in this study was 0.1, 0.05, 0.01, and 0.01% for BMS, SMS, BHD, and SHD samples, respectively. After the pyrolysis process, the chloride content was reduced in the prepared biochar samples.
Some researchers mentioned that this behavior is because the main form of Cl− released during biomass pyrolysis was HCl at temperatures between 250 and 500 °C. In other studies, chloride was released by the volatilization of methyl chloride, which occurs below 350 °C [70,71,72,73,74]. These studies indicated that Cl—released from biomass pyrolysis—can have a significant impact on the environment. The presence of high levels of Cl− can also cause corrosion problems and the formation of fouling and slagging in boilers. It can also contribute to the formation of dioxins (PCDDs) and furans (PCDFs), which are persistent organic pollutants (POPs) of concern because of their potential carcinogenicity [75,76].
3.3. Biochar Characterizations
3.3.1. Analysis of Nutrients and Potentially Toxic Elements and Plant Availability
The results of ICP-OES analysis are presented in Table 5. As Ca, K, and Mg elements are of high significance for biochar due to the effects on soil fertility and pH buffering of the soil systems, they are separately presented in Figure 7. BHD shows the highest values in Ca (10,384.94 mg kg−1 with plant availability of 8%). Second in line is SHD 8124.26 mg kg−1 (with plant availability of 11%). While H. dulcis branches and stems of H. dulcis do not show significant differences in Mg and K contents, M. scabrella uniformly shows higher contents for branches, presumably due to the increased bark content. For H. dulcis, this is only notable for Ca, while the plant-available content stays uniform in the intra-species comparison. Mimosa scabrella shows a notably higher K content in both batches, making it especially interesting for further examinations regarding K availability in different soil types.

Table 5.
Elemental analysis of biochar, determined by ICP-OES (mg kg−1).
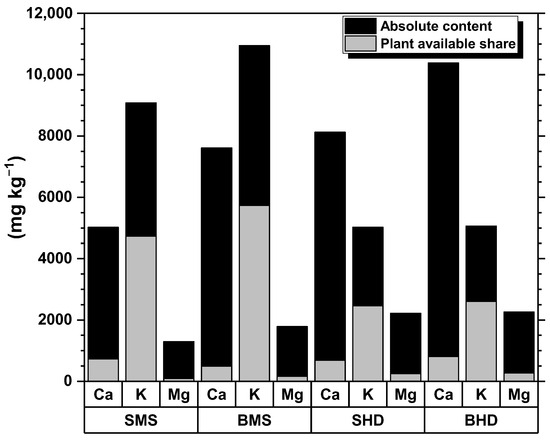
Figure 7.
Ca, K, and Mg contents of the biochar samples, with share of short or medium-term plant availability determined by BaCl2 cation exchange (see Section 2.3).
The contaminants specified in the German Fertilizer Ordinance (DüMV), with defined limits (As, Pb, Cd, Crtot, Ni), were either not detected or only found in concentrations that do not require reporting in any of the samples. The same applies to the heavy metal limits specified in the EBC guideline [20].
It should be noted that for Cd and Pb, the methodology employed—characterized by a low sample weight and high dilution (0.1 g to 50 mL solution), coupled with the established lower limit of detection—was not ideally suited for detecting the low threshold values stipulated by the EBC and their national annexes [20]. Despite these limitations, none of the elements were detected using ICP-OES, as is shown in Table 5.
3.3.2. Biochar pH, Electrical Conductivity, and Water-Holding Capacity
The pH values of the biochar samples are given in Table 6. The values ranged from BHD 7.65 ± 0.14 to BMS 8.05 ± 0.03. These results correlate with the oxygen content (r = −0.91) and the ash content (r = 0.84) of the biochar (n = 4, Pearson). The more oxygen functionalities are retained during pyrolysis, the lower the pH values. Conversely, the higher the ash content, the higher the pH of the biochar. These results are consistent with the findings of Ronsse et al. [77]. This study additionally demonstrated that as the treatment temperature increases, the pH value of the biochar increases, making it more alkaline. Understanding the pH of biochar is crucial for fertilizer use, as it affects soil properties and plant growth, since they respond to the soil characteristics. Its application increases soil pH, which is advantageous for acidic soils, but can also lead to yield reductions and micronutrient deficiencies in certain agricultural systems [78,79]. The carbonates in the biochar can increase the pH of acidic soils, promoting plant growth [63].

Table 6.
pH values (measured in 0.01 M CaCl2), electrical conductivity (mS cm−1), and water-holding capacity (%) of the biochar.
Biochar EC values are also given in Table 6 and ranged from SHD 91.83 ± 5.30 mS cm−1 to BMS 415.08 ± 24.75 mS cm−1. The values for branches and stems differ considerably, with the largest difference and highest EC values for biochar from M. scabrella. The ash contents for stems and branches of M. scabrella and stems and branches of H. dulcis are similar, but the volatile matter in SHD and BHD is comparatively higher, resulting in a diminished residue content. This provides a plausible explanation for the high EC values for SMS and BMS.
As can be seen in Table 6, water-holding capacities of samples with a higher bark content (branches) are slightly elevated. The achieved water-holding capacities are very similar to the findings of Yu et al., who used biochar produced from yellow pine at a pyrolysis temperature of 400 °C [80]. As Yu et al. and Basso et al. [81] conclude, biochar can significantly increase the water-holding capacity of sandy loam soils, up to 47.3% at a 5% biochar addition per % of soil (representing approximately 100 tons ha−1), even for longer retention times (91 days, see [81]). However, as Atkinson points out, there is only reasonable evidence for these effects for predominantly sandy soils, while there is only some evidence for an increased soil water content at saturation and field capacity in loamy/clay-type soils [82].
For energetic applications, M. scabrella demonstrates a higher energy yield, whereas its markedly elevated potassium content suggests increased particulate matter emissions. Nevertheless, silicon was not analyzed in this study; if present at higher concentrations, it could potentially mitigate the potassium-driven formation and release of particulate matter [83]. Regarding the ash content, which is a critical factor for large-scale energy applications, it is noteworthy that the stem section of H. dulcis exhibited a higher ash content compared to its branches.
Even if production temperatures and technical requirements were low, EBC standard limits were fulfilled by all samples. This, however, does not automatically imply positive effects in applications. The effective utilization of biochar in agricultural and forestry systems demands a precise understanding of soil properties and strategic fertilizer integration, as indiscriminate applications can yield counterproductive outcomes for both productivity and greenhouse gas (GHG) balance. Meta-analyses demonstrate that biochar effects vary dramatically based on the initial soil pH, with the greatest yield increases observed in very acidic soils (pH ≤ 5), while applications to alkaline soils can exacerbate pH stress and reduce crop performance [84,85]. The biochar application rate, initial soil pH, and soil sand content collectively explain the majority of the variance in soil chemical property responses, emphasizing that site-specific knowledge is paramount rather than universal application protocols [84].
Combined fertilizer–biochar applications can enhance crop yields by up to 179.6% compared to individual treatments, but only when properly calibrated to soil conditions [85]. However, excessive biochar rates (>20 t ha−1) in alkaline soils significantly increase pH and electrical conductivity, creating nutrient uptake barriers and growth inhibition [86]. Furthermore, the biochar’s complex effects on GHG emissions reveal critical application dependencies: while generally reducing N2O emissions [87,88], CO2 emissions can increase by 19% in limited time spans [89,90,91]. Without comprehensive soil characterization and tailored application strategies, biochar can paradoxically worsen both agronomic performance and climate impact, undermining its intended benefits.
4. Conclusions
The production method in combination with the raw materials used showed good biochar characteristics, even if the operated and proactively chosen pyrolysis temperature and technical requirements can be described as rather low, as they were deliberately chosen to closely replicate traditional production methods. The good output quality is also clearly evident from the atomic O/C and H/C ratios achieved. Mimosa scabrella showed a higher biochar output, while H. dulcis showed a 10% lower biochar output, in favor of an increased bio-oil share under the described operating conditions, whose properties were not investigated in depth in this study. The N content of the biochar samples were slightly higher in char from branches, while this is only statistically significant for H. dulcis. Potassium contents were notably higher in M. scabrella biochar, while for H. dulcis, Ca contents were higher. Therefore, demand-driven applications or blending may be considered and may be examined further. None of the samples were determined to be potentially harmful and therefore regulated trace elements are present in a critical concentration. The choice of wood from the stems or branches does not show a notable influence on product qualities, despite the higher share of bark.
However, the substantial share of bio-oil suggests the need for technical interventions to reduce emissions of gaseous pollutants, thereby contributing to improved climate resilience and carbon sequestration. Also, both the long-term impacts of the produced and incorporated biochar, especially the latter in field studies, and the production conditions in practical contexts, must continue to be thoroughly investigated, adjusted, and monitored.
The production of biochar may present a viable opportunity for producers reliant on wood pyrolysis and forest owners to diversify their income by adapting their production methods and include both invasive and native species. This approach could make targeted sustainable forest management economically feasible. Laws, management guidelines, and plans for the sustainable management of the MOF must be defined and reviewed in order to gain a full picture of the economic feasibility and potential to support the long-term goal of preserving biodiversity in MOF.
Author Contributions
Conceptualization S.P., D.A.d.S. and E.d.S.L.; Data curation F.A.F., F.E., M.S. and S.K.; formal analysis M.S., S.K. and F.E.; funding acquisition S.P. and E.d.S.L.; investigation M.S., S.K., A.T.d.A.G., F.A.F. and F.E.; methodology F.E., F.A.F. and A.T.d.A.G.; project administration S.P. and E.d.S.L.; resources A.T.d.A.G. and F.A.F.; supervision S.P., E.d.S.L. and D.A.d.S.; validation F.E. and F.A.F.; visualization M.S., S.K. and F.A.F.; writing—original draft F.E., M.S., S.K., L.H.N. and F.A.F.; writing—review and editing; A.T.d.A.G., F.A.F., D.A.d.S., E.d.S.L. and A.F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Coordination for the Improvement of Higher Education Personnel—Brazil (CAPES), a federal postgraduate support and evaluation agency of the Brazilian Ministry of Education under Grant Nr. 88881.701042/2022-01, and by the German Academic Exchange Service (DAAD) under Grant Nr. 57656251.
Institutional Review Board Statement
Both species used in this study were obtained from the Mixed Ombrophyllous Forest (MOF) located in Parana State and provided by Universidade Estadual do Centro-Oeste, in compliance with all relevant institutional, national, and international guidelines and Brazilian legislation. Hovenia dulcis has been classified as “Least Concern” by the IUCN, whereas Mimosa scabrella has not yet been assessed. The plant material was authenticated by Alexandre Techy de Almeida Garrett (one of the authors), and wood samples were deposited in the UNICENTRO Laboratory, Irati campus, for future reference.
Data Availability Statement
The data have been processed and presented in this publication for clarity. If there is interest in the raw data, they can be obtained from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MOF | Mixed Ombrophyllous Forest |
| EBC | European Biochar Certificate |
| SMS | Stem of Mimosa scabrella |
| BMS | Branches of Mimosa scabrella |
| SHD | Stem of Hovenia dulcis |
| BHD | Branches of Hovenia dulcis |
| HHV | Higher heating value |
| LHV | Lower heating value |
| TGA | Thermogravimetric analysis |
| TOC | Total organic carbon |
| TIC | Total inorganic carbon |
| ICP-OES | Inductively coupled plasma optical emission spectroscopy |
| EC | Electrical conductivity |
| WHC | Water-holding capacity |
| DTG | Differential thermal gravimetric |
| IBI | International Biochar Initiative |
References
- Lacerda, A.E.B.d.; Doetzer Rosot, M.A.; Figueiredo, A.; Cordeiro, M.; Roberta, E.; Kellermann, B.; Izabel, M.; Beimgraben, T.; Mattos, P.P.d.; Oliveira, Y.M.M.d. Sustainable Forest Management in Rural Southern Brazil: Exploring Participatory Forest Management Planning. In Sustainable Forest Management—Case Studies; Diez, J.J., Ed.; InTech: Sydney, Australia, 2012; ISBN 978-953-51-0511-4. [Google Scholar]
- Klipel, J.; Bergamin, R.S.; Esquivel-Muelbert, A.; de Lima, R.A.F.; de Oliveira, A.A.; Prado, P.I.; Müller, S.C. Climatic distribution of tree species in the Atlantic Forest. Biotropica 2022, 54, 1170–1181. [Google Scholar] [CrossRef]
- Rufino Vaz, D.; Dobner, M.; Callegari Scipioni, M.; Nicoletti, M.F.; Arce, J.E. Old-growth and secondary Araucaria Forest characterization. Trees For. People 2022, 9, 100306. [Google Scholar] [CrossRef]
- Fetter, D.; Putzke, J.; Ribas Moraes, J.; Forster, J. Avaliação por meio de monitoramento aéreo de espécie de árvore biologicamente invasora–Caso da proliferação da Hovenia dulcis Thunb junto ao Cinturão Verde da cidade de Santa Cruz do Sul /RS. Rev. Espac. 2015, 36, 1–6. [Google Scholar]
- Rosário, V.A.C.; Guimarães, J.C.; Viani, R.A.G. How Changes in Legally Demanded Forest Restoration Impact Ecosystem Services: A Case Study in the Atlantic Forest, Brazil. Trop. Conserv. Sci. 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Roque, R.H.; Sebbenn, A.M.; Boshier, D.H.; Filho, A.F.; Tambarussi, E.V. Logging Affects Genetic Diversity Parameters in an Araucaria angustifolia Population: An Endangered Species in Southern Brazil. Forests 2023, 14, 1046. [Google Scholar] [CrossRef]
- Da Mazza, C.A.S.; Mazza, M.C.M.; Almeida, D.; Santos, J.E.d.; Fushita, A.T. Land Use and Environmental Zoning of Mixed Ombrophilous Forests for Sustainable Use (Irati National Forest, Brazil Southern Region). Braz. Arch. Biol. Technol. 2016, 59, 1–11. [Google Scholar] [CrossRef]
- Faggin, J.M.; Behagel, J.H. Translating Sustainable Forest Management from the global to the domestic sphere: The case of Brazil. For. Policy Econ. 2017, 85, 22–31. [Google Scholar] [CrossRef]
- de Oliveira, A.L.; Borges, L.A.C.; Coelho, M.G., Jr.; de Barros, D.A.; Coelho, L.M., Jr. Forest Replacement in Brazil: A Fundamental Policy for Forestry. Floresta Ambient. 2020, 27, 1–12. [Google Scholar] [CrossRef]
- Luiz, C.H.P.; Steinke, V.A. Recent Environmental Legislation in Brazil and the Impact on Cerrado Deforestation Rates. Sustainability 2022, 14, 8096. [Google Scholar] [CrossRef]
- Mundstock Xavier de Carvalho, M. Os fatores do desmatamento da Floresta com Araucária: Agropecuária, lenha e indústria madeireira. Esboços 2012, 18, 32–52. [Google Scholar] [CrossRef][Green Version]
- David, H.C.; de Araújo, E.J.G.; Morais, V.A.; Scolforo, J.R.S.; Marques, J.M.; Péllico Netto, S.; MacFarlane, D.W. Carbon stock classification for tropical forests in Brazil: Understanding the effect of stand and climate variables. For. Ecol. Manag. 2017, 404, 241–250. [Google Scholar] [CrossRef]
- Figueiredo Filho, A.; Nauiack, C.H.B.; Roik, M.; Gomes, G.S. Inventário Das Florestas Nativas Em Pequenas Propriedades Rurais Na Bacia Do Imbituvão; Centro-Sul do Paraná Irati, Ed.; Centro-Sul do Paraná Irati: Irati, Brazil, 2013. [Google Scholar]
- Zhang, J.; Zhang, S.; Niu, C.; Jiang, J.; Sun, H. Positive Effects of Biochar on the Degraded Forest Soil and Tree Growth in China: A Systematic Review. Phyton 2022, 91, 1601–1616. [Google Scholar] [CrossRef]
- Sarauer, J.L.; Page-Dumroese, D.S.; Coleman, M.D. Soil greenhouse gas, carbon content, and tree growth response to biochar amendment in western United States forests. GCB Bioenergy 2019, 11, 660–671. [Google Scholar] [CrossRef]
- Bruckman, V.J.; Pumpanen, J. Biochar use in global forests: Opportunities and challenges. Global Change and Forest Soils; Elsevier: Amsterdam, The Netherlands, 2019; pp. 427–453. ISBN 9780444639981. [Google Scholar]
- Latawiec, A.E.; Strassburg, B.B.N.; Junqueira, A.B.; Araujo, E.; de Moraes, L.F.D.; Pinto, H.A.N.; Castro, A.; Rangel, M.; Malaguti, G.A.; Rodrigues, A.F.; et al. Biochar amendment improves degraded pasturelands in Brazil: Environmental and cost-benefit analysis. Sci. Rep. 2019, 9, 11993. [Google Scholar] [CrossRef] [PubMed]
- Spokas, K.A. Review of the stability of biochar in soils: Predictability of O:C molar ratios. Carbon Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef]
- Ghosh, D.; Page-Dumroese, D.S.; Han, H.-S.; Anderson, N. Role of biochar made from low-value woody forest residues in ecological sustainability and carbon neutrality. Soil. Sci. Soc. Am. J. 2025, 89, e20793. [Google Scholar] [CrossRef]
- EBC. European Biochar Certificate—Guidelines of the European Biochar Certificate; Version 10.3G (2012-2023); Ithaka Institute: Arbaz, Switzerland, 2023; Available online: http://www.european-biochar.org (accessed on 5 March 2025).
- Schwerz, F.; Neto, D.D.; Caron, B.O.; Nardini, C.; Sgarbossa, J.; Eloy, E.; Behling, A.; Elli, E.F.; Reichardt, K. Biomass and potential energy yield of perennial woody energy crops under reduced planting spacing. Renew. Energy 2020, 153, 1238–1250. [Google Scholar] [CrossRef]
- Domermuth, D. Small Scale Biochar Production. 2018. Available online: https://biochar-international.org/wp-content/uploads/2018/04/Small_Scale_Biochar_Production_Domermuth.pdf (accessed on 5 March 2025).
- Sagl, C. Small Scale Biochar Kiln. Available online: http://biochar.info/?p=en.small_scale_biochar_kiln (accessed on 6 June 2025).
- Faé Gomes, G.M.; Encarnação, F. The environmental impact on air quality and exposure to carbon monoxide from charcoal production in southern Brazil. Environ. Res. 2012, 116, 136–139. [Google Scholar] [CrossRef]
- Yue, Y.; Lin, Q.; Irfan, M.; Chen, Q.; Zhao, X.; Li, G. Slow Pyrolysis as a Promising Approach for Producing Biochar from Sunflower Straw. BioResources 2018, 13, 7455–7469. [Google Scholar] [CrossRef]
- Nogueira, L.A.H.; Coelho, S.T.; Uhlig, A. Sustainable charcoal production in Brazil. In Criteria and Indicators for Sustainable Woodfuels; FAO: Rome, Italy, 2009. [Google Scholar]
- García-Quezada, J.; Musule-Lagunes, R.; Prieto-Ruíz, J.A.; Vega-Nieva, D.J.; Carrillo-Parra, A. Evaluation of Four Types of Kilns Used to Produce Charcoal from Several Tree Species in Mexico. Energies 2023, 16, 333. [Google Scholar] [CrossRef]
- Świechowski, K.; Matyjewicz, B.; Telega, P.; Białowiec, A. The Influence of Low-Temperature Food Waste Biochars on Anaerobic Digestion of Food Waste. Materials 2022, 15, 945. [Google Scholar] [CrossRef]
- Doumer, M.E.; Arízaga, G.G.C.; Da Silva, D.A.; Yamamoto, C.I.; Novotny, E.H.; Santos, J.M.; dos Santos, L.O.; Wisniewski, A., Jr.; de Andrade, J.B.; Mangrich, A.S. Slow pyrolysis of different Brazilian waste biomasses as sources of soil conditioners and energy, and for environmental protection. J. Anal. Appl. Pyrolysis 2015, 113, 434–443. [Google Scholar] [CrossRef]
- DIN 51900:2023-12; Testing of Solid and Liquid Fuels—Determination of Gross Calorific Value by the Bomb Calorimeter and Calculation of Net Calorific Value. DIN Media GmbH: Berlin, Germany, 2023.
- DIN EN ISO 17828:2025-06; Solid Biofuels—Determination of Bulk Density (ISO 17828:2025); German Version EN ISO 17828:2025. DIN Media GmbH: Berlin, Germany, 2025.
- Verband Deutscher Landwirtschaftlicher Untersuchungs-und Forschungsanstalten. VDLUFA-Method A 13.2.1. Available online: https://www.methodenbuch.de/produkt/methodenbuch-band-i-boden/ (accessed on 5 March 2025).
- ABNT NBR 14929:2017; Wood—Determination of Moisture of Chips—Method by Drying in Oven-Dried. Associação Brasileira de Normas Técnicas: Sao Paulo, Brazil, 2017.
- DIN 51719:1997-07; Testing of Solid Fuels-Solid Mineral Fuels-Determination of Ash Content. DIN Media GmbH: Berlin, Germany, 1997.
- DIN 51720:2001-03; Testing of Solid Fuels—Determination of Volatile Matter Content. DIN Media GmbH: Berlin, Germany, 2001.
- DIN 51724-3:2012-07; Solid Mineral Fuels—Determination of Sulfur Content—Part 3: Instrumental Methods. DIN Media GmbH: Berlin, Germany, 2012.
- DIN 51732:2014-07; Testing of Solid Mineral Fuels—Determination of Total Carbon, Hydrogen and Nitrogen—Instrumental Methods. DIN Media GmbH: Berlin, Germany, 2014.
- DIN 51733:2016-04; Testing of Solid Mineral Fuels—Ultimate Analysis and Calculation of Oxygen Content. DIN Media GmbH: Berlin, Germany, 2016.
- DIN EN ISO 11885:2009-09; Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) (ISO 11885:2007); German Version EN ISO 11885:2009. DIN Media GmbH: Berlin, Germany, 2009.
- Rosa, J.M.; Miguel, M.; Knicker, H.E.; Boy, E.F. Testing Established Method for the Determination of the Cation Exchange Capacity in Soils for the Characterization of Biochars. In Proceedings of the 20th EGU General Assembly, EGU2018, Vienna, Austria, 4–13 April 2018; p. 5071. [Google Scholar]
- Munera-Echeverri, J.L.; Martinsen, V.; Strand, L.T.; Zivanovic, V.; Cornelissen, G.; Mulder, J. Cation exchange capacity of biochar: An urgent method modification. Sci. Total Environ. 2018, 642, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Gillman, G.P.; Sumpter, E.A. Modification to the compulsive exchange method for measuring exchange characteristics of soils. Soil. Res. 1986, 24, 61–66. [Google Scholar] [CrossRef]
- Gutachterausschuss Forstliche Analytik (Ed.) Handbuch Forstliche Analytik: Eine Loseblatt-Sammlung der Analysemethoden im Forstbereich; Ohann Heinrich von Thünen-Institut: Eberswalde, Germany, 2022. [Google Scholar]
- DIN EN ISO 11260:2018-11; Soil Quality—Determination of Effective Cation Exchange Capacity and Base Saturation Level Using Barium Chloride Solution (ISO 11260:2018); German Version EN ISO 11260:2018. DIN Media GmbH: Berlin, Germany, 2018.
- DIN EN ISO 10390:2022-08; Soil, Treated Biowaste and Sludge—Determination of pH (ISO 10390:2021); German version EN ISO 10390:2022. DIN Media GmbH: Berlin, Germany, 2022.
- DIN EN ISO 14238:2014-03; Soil Quality—Biological Methods—Determination of Nitrogen Mineralization and Nitrification in Soils and the Influence of Chemicals on These Processes (ISO 14238:2012); German Version EN ISO 14238:2013. DIN Media GmbH: Berlin, Germany, 2014.
- Panwar, N.L.; Pawar, A.; Salvi, B.L. Comprehensive review on production and utilization of biochar. SN Appl. Sci. 2019, 1, 168. [Google Scholar] [CrossRef]
- El-Sayed, S.A.; Khass, T.M.; Mostafa, M.E. Thermal degradation behaviour and chemical kinetic characteristics of biomass pyrolysis using TG/DTG/DTA techniques. Biomass Conv. Bioref. 2024, 14, 17779–17803. [Google Scholar] [CrossRef]
- Choudhary, M.; Kumar Jain, S.; Singh, D.; Srivastava, K.; Patel, A.K.; Mahlknecht, J.; Shekher Giri, B.; Kumar, M. Determination of thermal degradation behavior and kinetics parameters of chemically modified sun hemp biomass. Bioresour. Technol. 2023, 380, 129065. [Google Scholar] [CrossRef]
- Apaydın Varol, E.; Mutlu, Ü. TGA-FTIR Analysis of Biomass Samples Based on the Thermal Decomposition Behavior of Hemicellulose, Cellulose, and Lignin. Energies 2023, 16, 3674. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Panizio, R.; Castro, C.; Pacheco, N.; Assis, A.C.; Longo, A.; Vilarinho, C.; Teixeira, J.C.; Brito, P.; Gonçalves, M.; Nobre, C. Investigation of biochars derived from waste lignocellulosic biomass and insulation electric cables: A comprehensive TGA and Macro-TGA analysis. Heliyon 2024, 10, e37882. [Google Scholar] [CrossRef]
- Gorshkov, A.; Berezikov, N.; Kaltaev, A.; Yankovsky, S.; Slyusarsky, K.; Tabakaev, R.; Larionov, K. Analysis of the Physicochemical Characteristics of Biochar Obtained by Slow Pyrolysis of Nut Shells in a Nitrogen Atmosphere. Energies 2021, 14, 8075. [Google Scholar] [CrossRef]
- International Biochar Initiative. Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil: Product Definition and Specification Standards; (IBI-STD-2.1); IBI: Canandaigua, NY, USA, 2015. [Google Scholar]
- Leng, L.; Huang, H.; Li, H.; Li, J.; Zhou, W. Biochar stability assessment methods: A review. Sci. Total Environ. 2019, 647, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Wyn, H.K.; Zárate, S.; Carrascal, J.; Yermán, L. A Novel Approach to the Production of Biochar with Improved Fuel Characteristics from Biomass Waste. Waste Biomass Valor. 2020, 11, 6467–6481. [Google Scholar] [CrossRef]
- Panzarini Silva, I.; Moraes e Silva, M.; de Oliveira Machado, G.; Almeida de Araujo, V.; Aparecido Lopes Silva, D.; Luís Christoforo, A.; Antonio Rocco Lahr, F. Effect of Temperature and Time Torrefaction on the Energetic Properties of Bracatinga Wood. Int. J. Agric. For. 2017, 7, 111–114. [Google Scholar] [CrossRef]
- Kaltschmitt, M.; Stampfer, K. (Eds.) Energie aus Biomasse: Ressourcen und Bereitstellung, 4th ed.; Springer Vieweg: Wiesbaden/Heidelberg, Germany, 2024; ISBN 978-3-658-40828-2. [Google Scholar]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Rathod, N.; Jain, S.; Patel, M.R. Thermodynamic analysis of biochar produced from groundnut shell through slow pyrolysis. Energy Nexus 2023, 9, 100177. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. Chapter 2: A Review of Biochar and Its Use and Function in Soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Kaltschmitt, M.; Hofbauer, H.; Lenz, V. (Eds.) Energie aus Biomasse: Thermo-chemische Konversion, 4th ed.; Springer Vieweg: Wiesbaden/Heidelberg, Germany, 2024; ISBN 978-3-658-41216-6. [Google Scholar]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation, 2th ed; Routledge: London, UK, 2015; ISBN 9780203762264. [Google Scholar]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef]
- Burhenne, L.; Damiani, M.; Aicher, T. Effect of feedstock water content and pyrolysis temperature on the structure and reactivity of spruce wood char produced in fixed bed pyrolysis. Fuel 2013, 107, 836–847. [Google Scholar] [CrossRef]
- Almutairi, A.A.; Ahmad, M.; Rafique, M.I.; Al-Wabel, M.I. Variations in composition and stability of biochars derived from different feedstock types at varying pyrolysis temperature. J. Saudi Soc. Agric. Sci. 2023, 22, 25–34. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R.A. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.C.; Bibens, B. Effect of Low-Temperature Pyrolysis Conditions on Biochar for Agricultural Use. Trans. ASABE 2008, 51, 2061–2069. [Google Scholar] [CrossRef]
- Lisowska, A.; Filipek-Mazur, B.; Kalisz, A.; Gorczyca, O.; Kowalczyk, A. Changes in Soil Sulfate Sulfur Content as an Effect of Fertilizer Granules Containing Elemental Sulfur, Halloysite and Phosphate Rock. Agronomy 2023, 13, 1410. [Google Scholar] [CrossRef]
- Björkman, E.; Strömberg, B. Release of Chlorine from Biomass at Pyrolysis and Gasification Conditions. Energy Fuels 1997, 11, 1026–1032. [Google Scholar] [CrossRef]
- Lane, D.J.; van Eyk, P.J.; Ashman, P.J.; Kwong, C.W.; de Nys, R.; Roberts, D.A.; Cole, A.J.; Lewis, D.M. Release of Cl, S, P, K, and Na during Thermal Conversion of Algal Biomass. Energy Fuels 2015, 29, 2542–2554. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, W.; Rokni, E.; Chen, G.; Sun, R.; Levendis, Y.A. Release of Alkalis and Chlorine from Combustion of Waste Pinewood in a Fixed Bed. Energy Fuels 2019, 33, 1256–1266. [Google Scholar] [CrossRef]
- Ng, J.; DeMartini, N. Effect of Steam on the Release of K and Cl during Biomass and Black Liquor Combustion. Energy Fuels 2022, 36, 7733–7743. [Google Scholar] [CrossRef]
- Peng, B.; Li, X.; Luo, J.; Yu, X. Fate of Chlorine in Rice Straw under Different Pyrolysis Temperatures. Energy Fuels 2019, 33, 9272–9279. [Google Scholar] [CrossRef]
- Zhang, C.; Bai, L.; Yao, Q.; Li, J.; Wang, H.; Shen, L.; Sippula, O.; Yang, J.; Zhao, J.; Liu, J.; et al. Emission characteristics of polychlorinated dibenzo-p-dioxins and dibenzofurans from industrial combustion of biomass fuels. Environ. Pollut. 2022, 292, 118265. [Google Scholar] [CrossRef]
- Giudicianni, P.; Gargiulo, V.; Grottola, C.M.; Alfè, M.; Ferreiro, A.I.; Mendes, M.A.A.; Fagnano, M.; Ragucci, R. Inherent Metal Elements in Biomass Pyrolysis: A Review. Energy Fuels 2021, 35, 5407–5478. [Google Scholar] [CrossRef]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Zubairu, A.; Ngala, A.; Kwari, S.; Usman, K.; Tela Buba, M. Exploring the Effect of Biochar on Soil pH (A Review). In Proceedings of the 46th Conference of Soil Science Society of Nigeria, Sustaining Living Soil Ecosystem Through Adoption of Soil Management Practices for Mitigating Climate Change for National Development, Maiduguri, Nigeria, 14–18 March 2022. [Google Scholar]
- Singh, B.; MM, D.; Shen, Q.; Camps Arbestain, M. (Eds.) Biochar: A Guide to Analytical Methods. In Biochar pH, Electrical Conductivity and Liming Potential; CSIRO Publishing: Clayton, Australia, 2017; Chapter 3. [Google Scholar]
- Yu, O.-Y.; Raichle, B.; Sink, S. Impact of biochar on the water holding capacity of loamy sand soil. Int. J. Energy Environ. Eng. 2013, 4, 44. [Google Scholar] [CrossRef]
- Basso, A.S.; Miguez, F.E.; Laird, D.A.; Horton, R.; Westgate, M. Assessing potential of biochar for increasing water-holding capacity of sandy soils. GCB Bioenergy 2013, 5, 132–143. [Google Scholar] [CrossRef]
- Atkinson, C.J. How good is the evidence that soil-applied biochar improves water-holding capacity? Soil. Use Manag. 2018, 34, 177–186. [Google Scholar] [CrossRef]
- Feldmeier, S.; Wopienka, E.; Schwarz, M.; Schön, C.; Pfeifer, C. Applicability of Fuel Indexes for Small-Scale Biomass Combustion Technologies, Part 2: TSP and NO x Emissions. Energy Fuels 2019, 33, 11724–11730. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, Y.; Shi, L.; Li, G.; Pang, Z.; Liu, S.; Chen, Y.; Jia, B. Effects of biochar on soil chemical properties: A global meta-analysis of agricultural soil. Plant Soil. Environ. 2022, 68, 272–289. [Google Scholar] [CrossRef]
- Bai, S.H.; Omidvar, N.; Gallart, M.; Kämper, W.; Tahmasbian, I.; Farrar, M.B.; Singh, K.; Zhou, G.; Muqadass, B.; Xu, C.-Y.; et al. Combined effects of biochar and fertilizer applications on yield: A review and meta-analysis. Sci. Total Environ. 2022, 808, 152073. [Google Scholar] [CrossRef]
- Cong, M.; Hu, Y.; Sun, X.; Yan, H.; Yu, G.; Tang, G.; Chen, S.; Xu, W.; Jia, H. Long-term effects of biochar application on the growth and physiological characteristics of maize. Front. Plant Sci. 2023, 14, 1172425. [Google Scholar] [CrossRef]
- Song, X.; Pan, G.; Zhang, C.; Zhang, L.; Wang, H. Effects of biochar application on fluxes of three biogenic greenhouse gases: A meta-analysis. Ecosyst. Health Sustain. 2016, 2, e01202. [Google Scholar] [CrossRef]
- Cayuela, M.L.; van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.-Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil. Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Ventura, M.; Alberti, G.; Panzacchi, P.; Vedove, G.D.; Miglietta, F.; Tonon, G. Biochar mineralization and priming effect in a poplar short rotation coppice from a 3-year field experiment. Biol. Fertil. Soils 2019, 55, 67–78. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Laird, D.A.; Heaton, E.A.; Rathke, S.; Acharya, B.S. Soil carbon increased by twice the amount of biochar carbon applied after 6 years: Field evidence of negative priming. GCB Bioenergy 2020, 12, 240–251. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).