Is It Possible to Preserve the Full Diversity of Birds in Managed Oak–Lime–Hornbeam Forests?
Abstract
1. Introduction
2. Materials and Methods
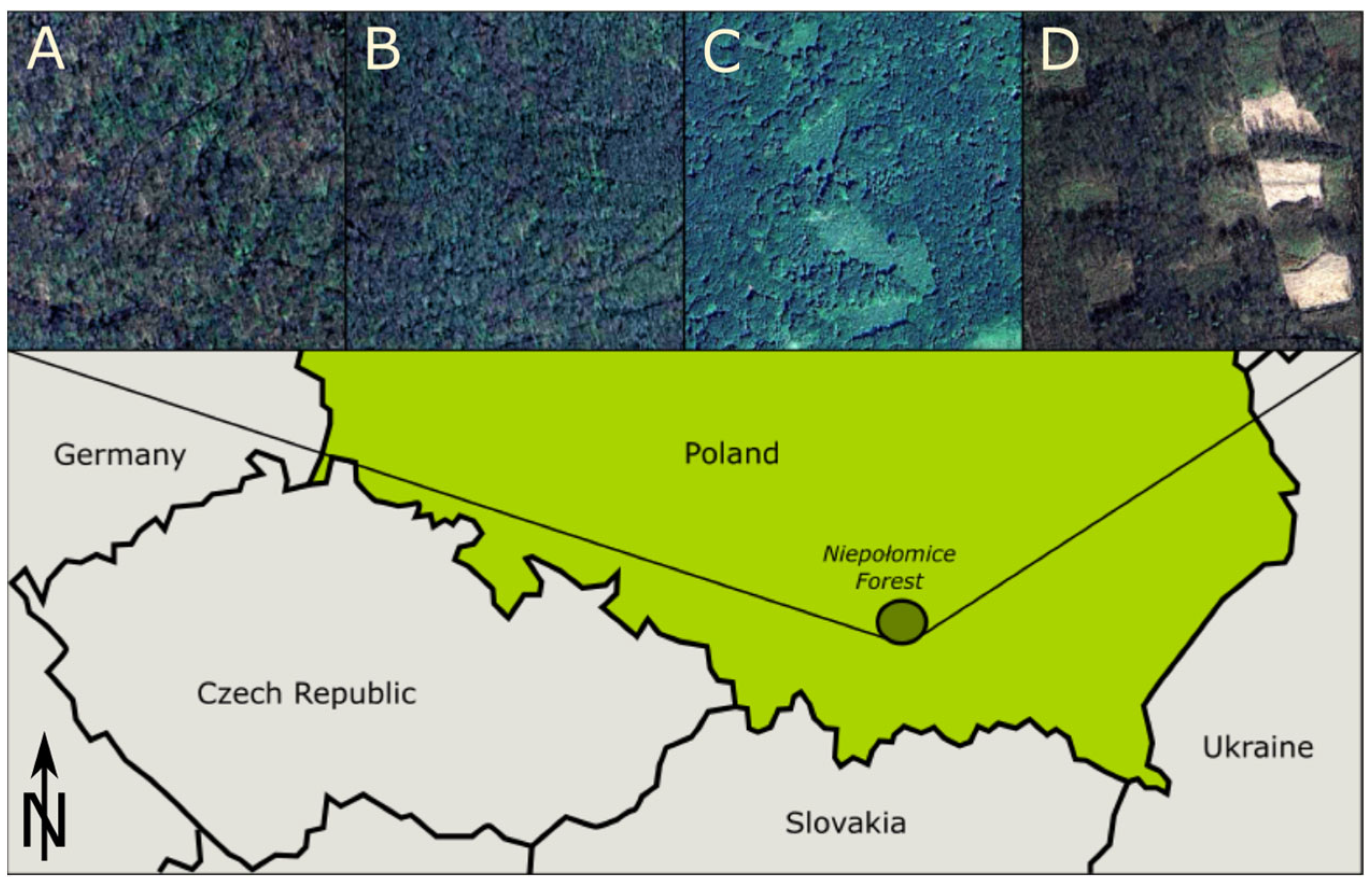
2.1. Study Area
2.2. Stand Measurements and Bird Counts
2.3. Data Analysis
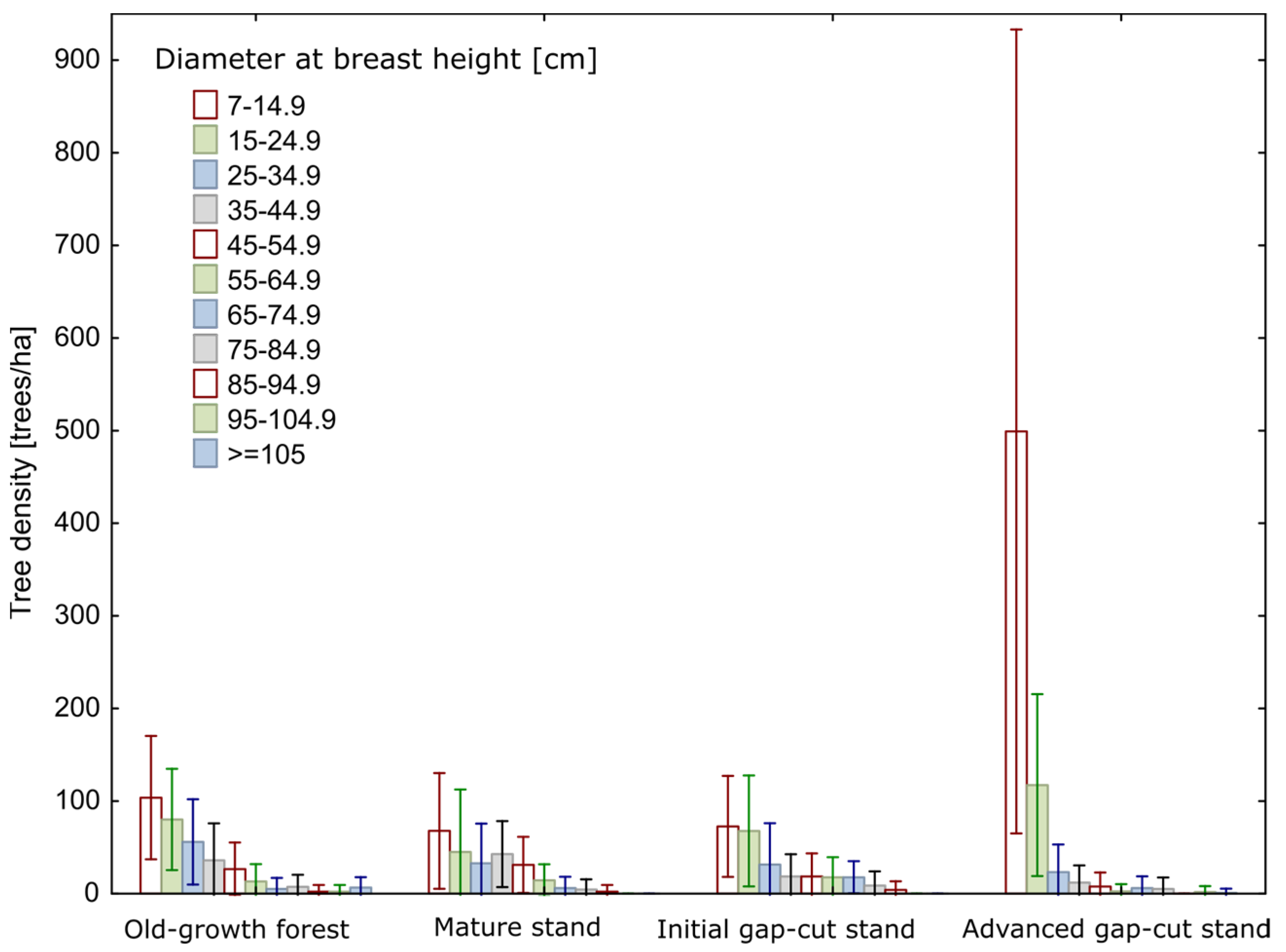
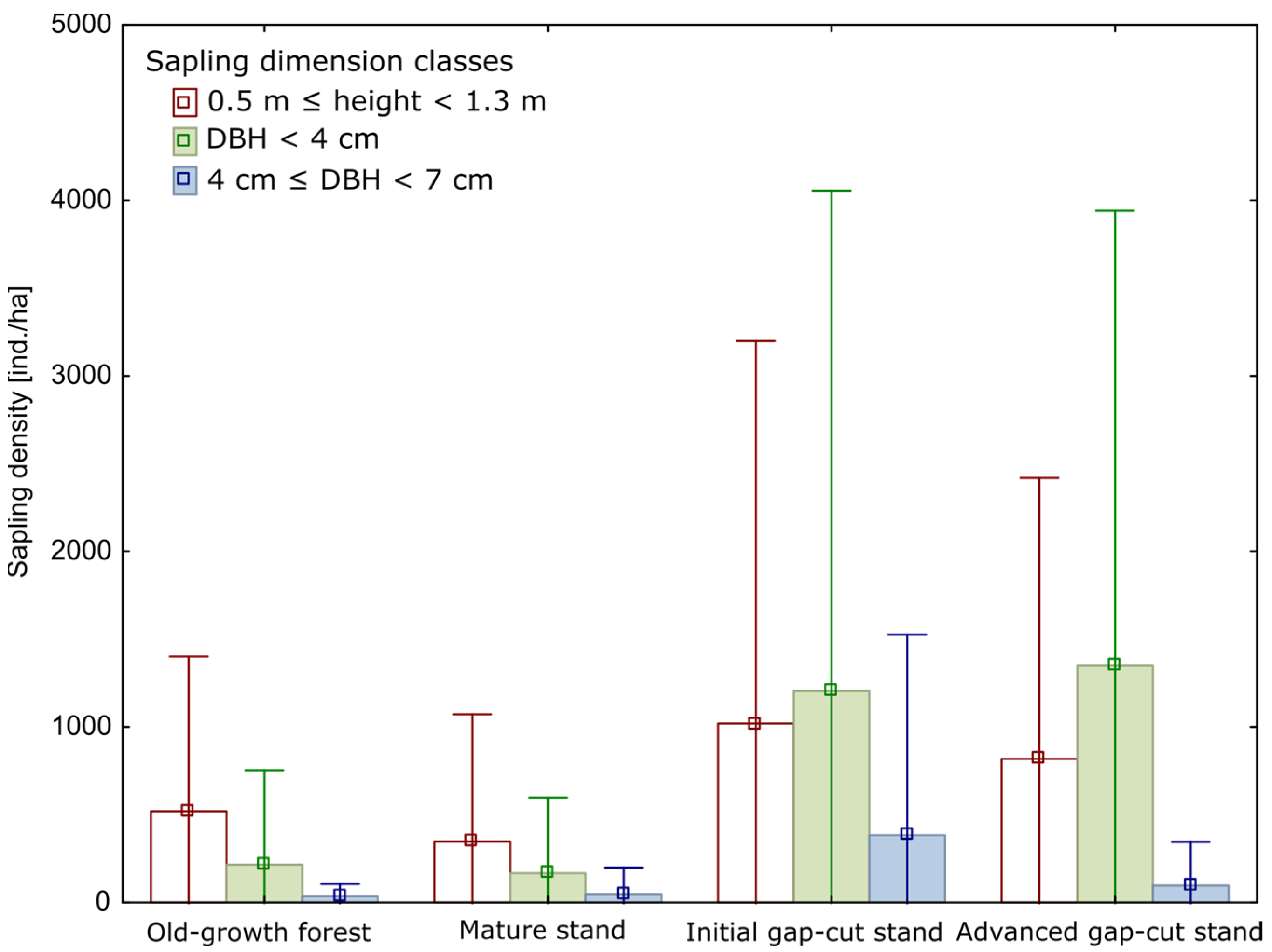
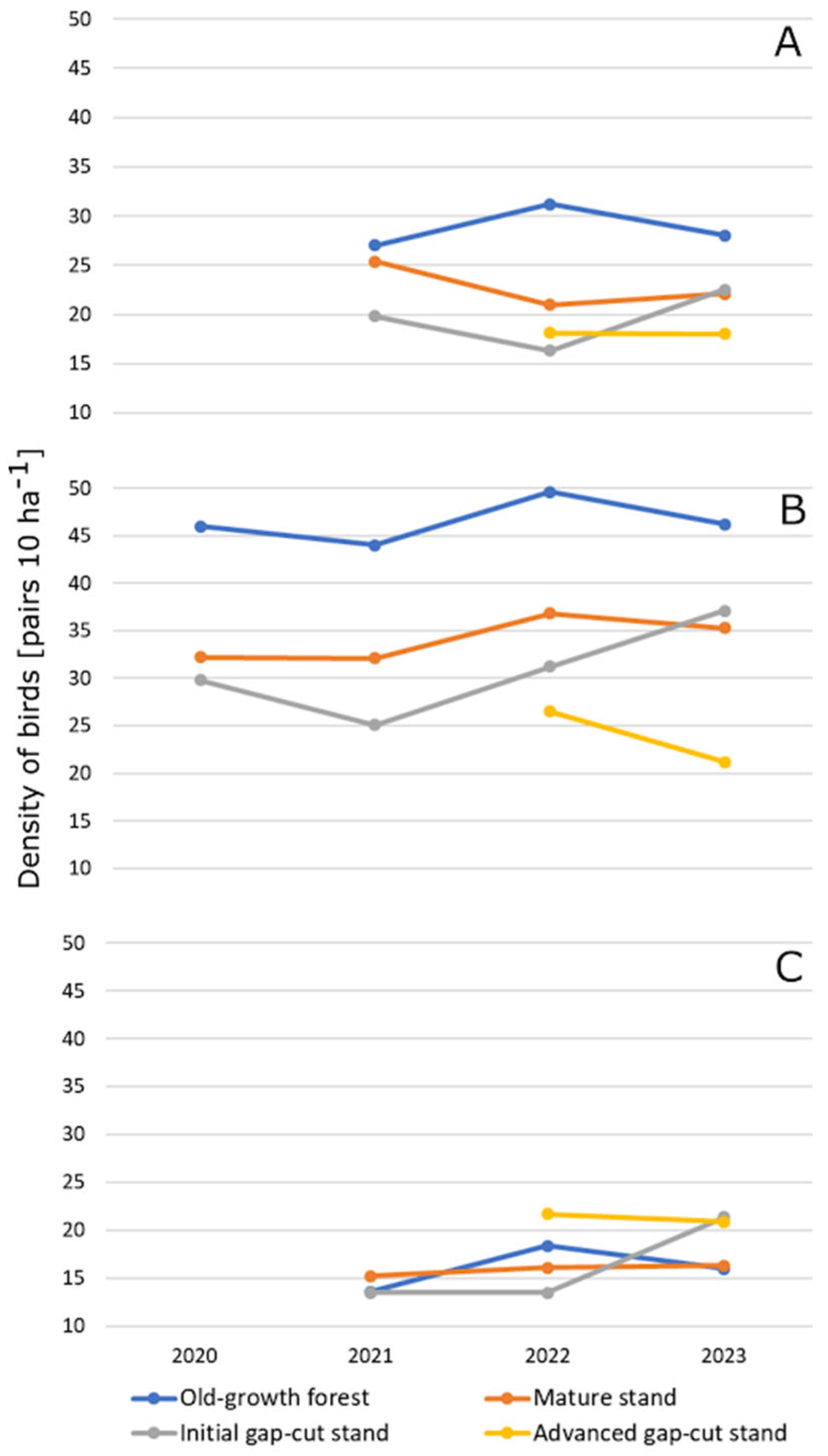
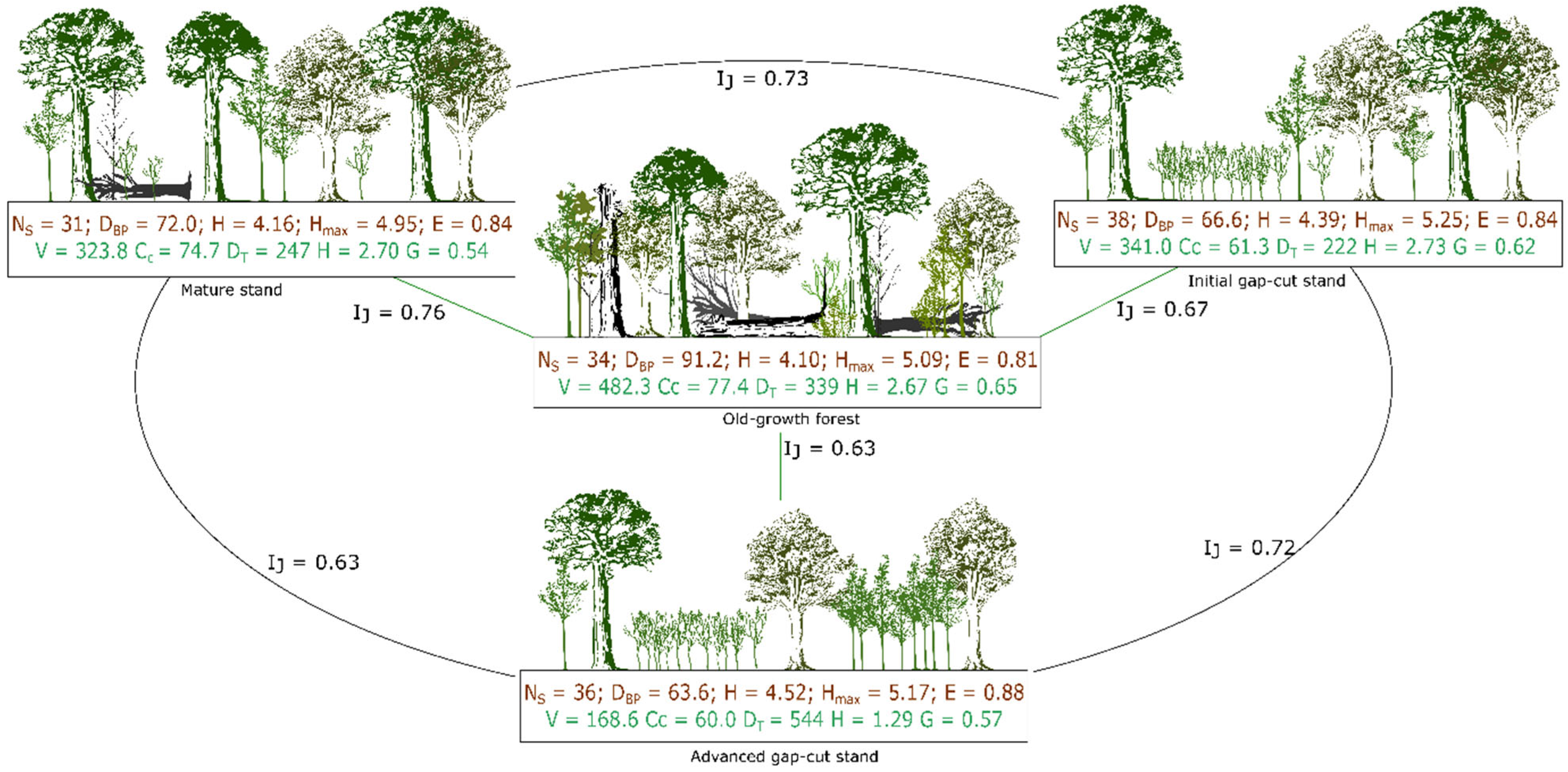
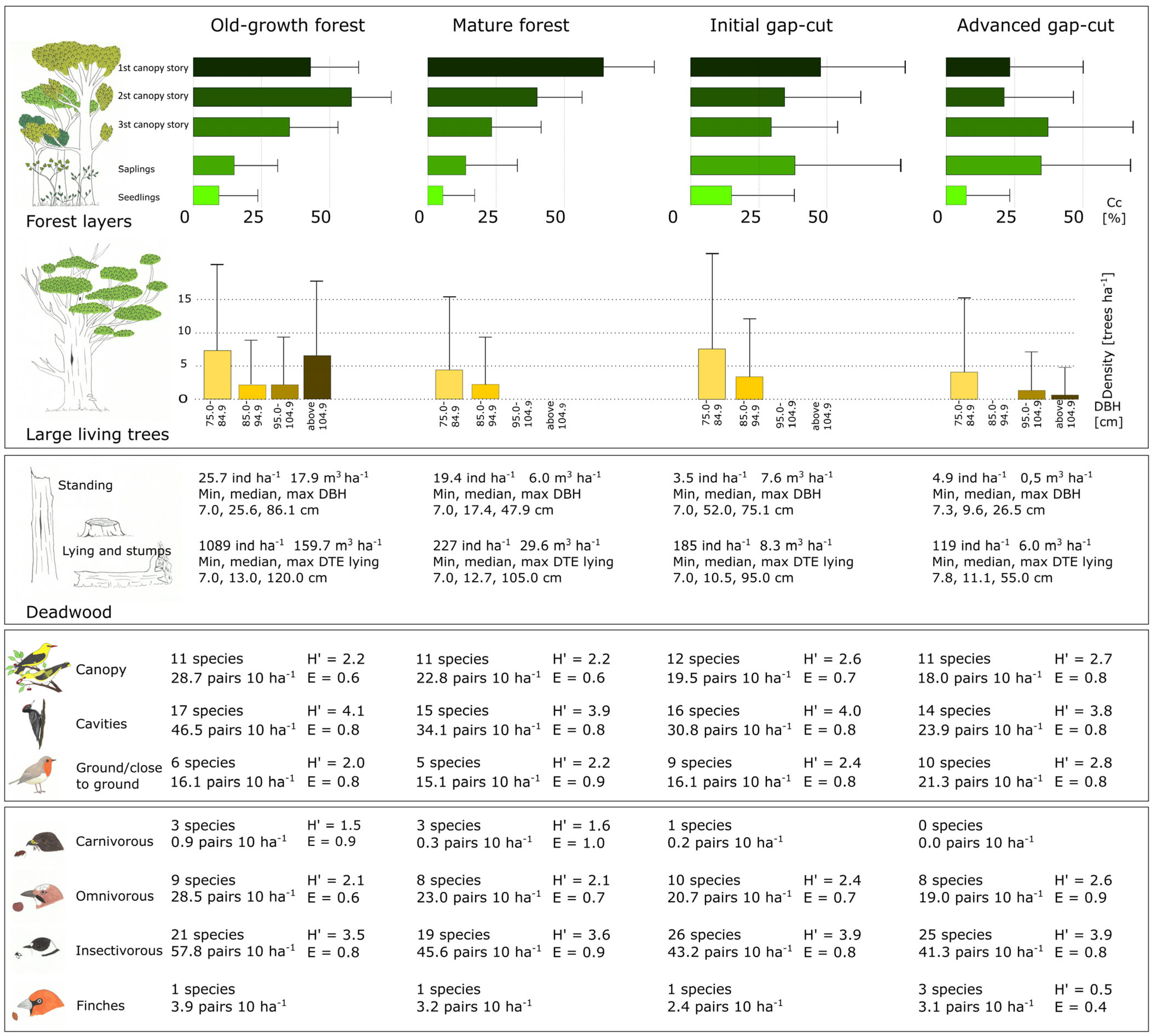
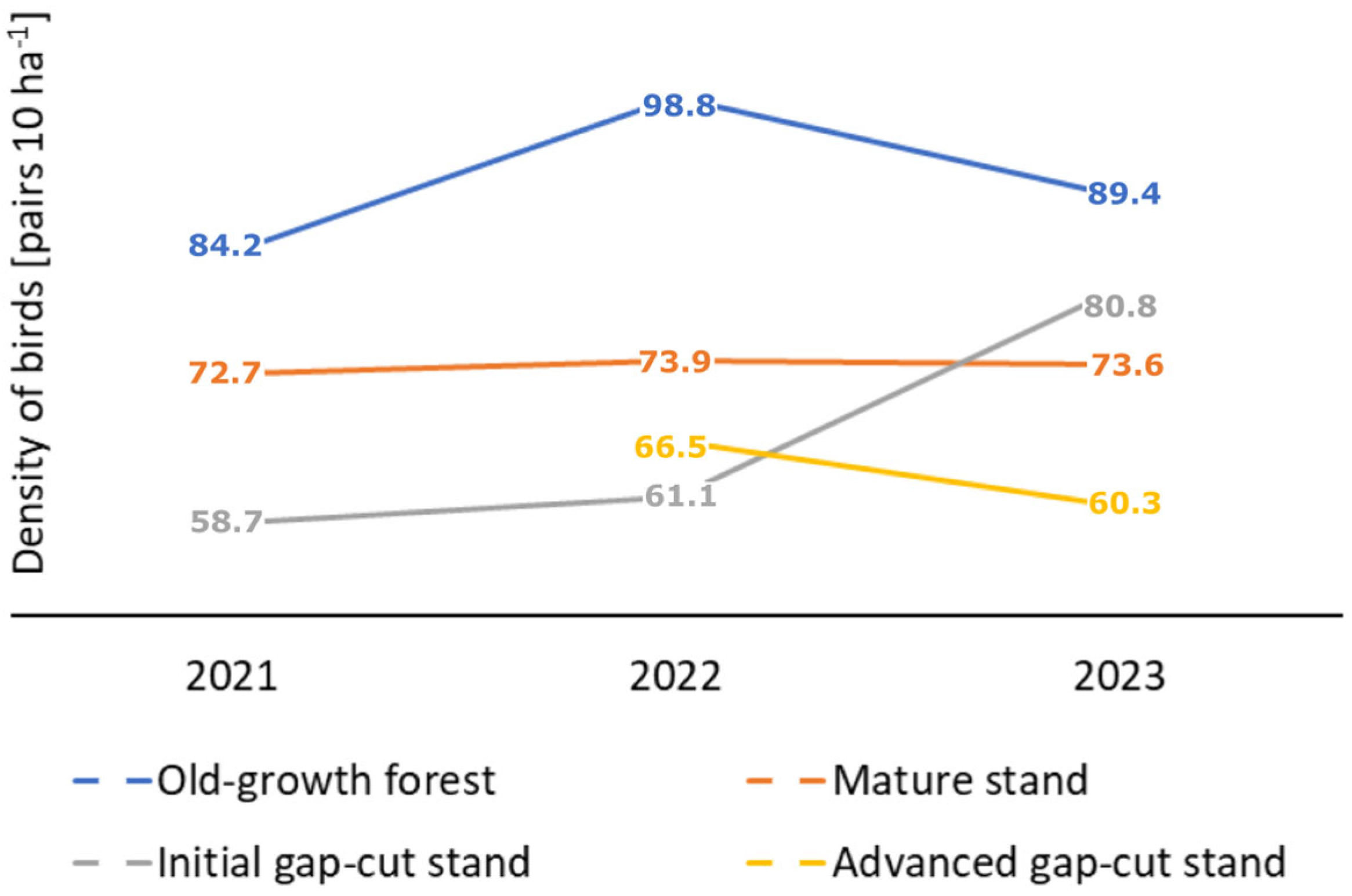
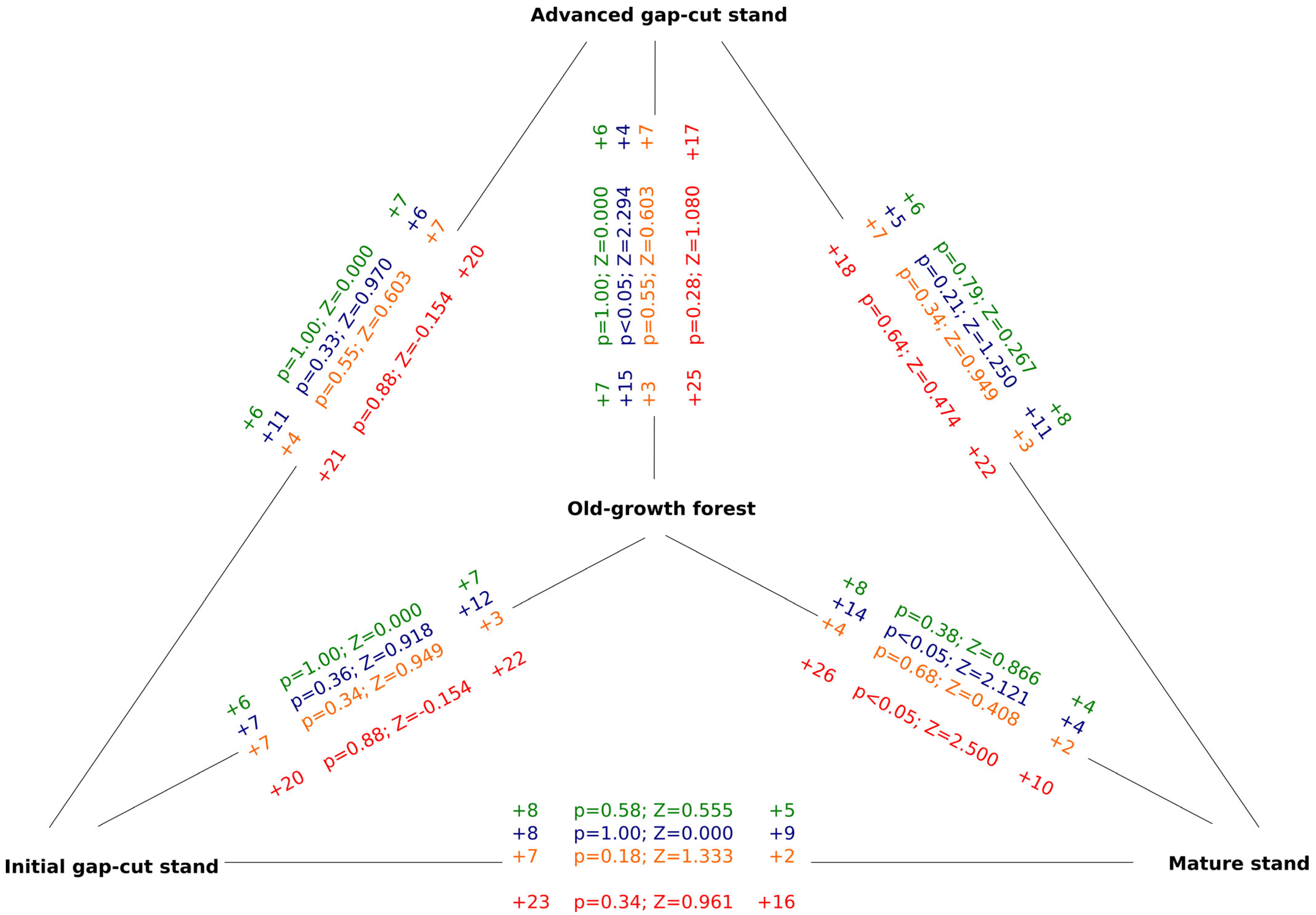
3. Results
3.1. Stand Characteristics
3.2. Characteristics of Birds Assemblages
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Old-Growth Forest (Reserve Lipówka) | Mature Stand | Initial Gap-Cut Stand | Advanced Gap-Cut Stand |
|---|---|---|---|---|
| Mean Density Throughout the Study Period [Pairs per 10 ha] | ||||
| Columba oenas L. | 0.1 | |||
| Strix aluco L. | 0.4 | 0.1 | ||
| Strix uralensis Pallas | 0.4 | |||
| Jynx torquilla L. | 0.1 | |||
| Picus canus J. F. Gmelin | 0.1 | 0.3 | ||
| Picus viridis L. | 0.1 | 0.2 | 0.1 | |
| Dryocopus martius L. | 0.2 | 0.1 | 0.0 | |
| Dendrocoptes medius L. | 2.8 | 2.1 | 2.3 | 1.3 |
| Dryobates minor L. | 0.1 | 0.2 | 0.3 | 0.4 |
| Dendrocopos major L. | 5.2 | 3.6 | 2.7 | 2.4 |
| Poecile palustris L. | 0.2 | 0.6 | 0.5 | 1.0 |
| Cyanistes caeruleus L. | 4.9 | 4.9 | 3.4 | 3.5 |
| Parus major L. | 10.5 | 7.3 | 6.3 | 5.1 |
| Sitta europaea L. | 3.9 | 3.2 | 2.9 | 1.4 |
| Certhia familiaris L. | 1.9 | 0.7 | 0.9 | 0.4 |
| Certhia brachydactyla C. L. Brehm | 1.5 | 1.0 | 1.5 | 0.4 |
| Sturnus vulgaris L. | 4.3 | 3.9 | 4.5 | 3.0 |
| Muscicapa striata Pallas | 0.5 | 0.3 | 0.2 | 0.4 |
| Ficedula hypoleuca Pallas. | 0.2 | |||
| Ficedula albicollis Temminck | 9.6 | 6.2 | 5.1 | 4.3 |
| Columba palumbus L. | 2.4 | 1.8 | 2.1 | 1.6 |
| Cuculus canorus L. | 0.1 | 0.4 | ||
| Pernis apivorus L. | 0.1 | |||
| Buteo buteo L. | 0.1 | 0.2 | ||
| Accipiter nisus L. | 0.1 | |||
| Oriolus oriolus L. | 0.5 | 0.1 | 0.4 | 0.4 |
| Garrulus glandarius L. | 0.4 | 0.6 | 0.9 | 1.2 |
| Corvus corax L. | 0.1 | |||
| Hippolais icterina Vieillot | 0.1 | 0.2 | 0.4 | |
| Acrocephalus palustris Bechstein | 0.4 | |||
| Phylloscopus sibilatrix Bechstein | 1.4 | 1.5 | 2.1 | 1.0 |
| Phylloscopus trochilus L. | 0.1 | 0.4 | ||
| Phylloscopus collybita Vieillot | 0.1 | 1.7 | 3.7 | 3.4 |
| Aegithalos caudatus L. | 0.4 | 0.4 | 0.7 | 0.7 |
| Sylvia atricapilla L. | 4.3 | 4.2 | 5.3 | 7.0 |
| Sylvia borin Boddaert | 0.5 | 0.6 | ||
| Curruca communis Latham | 0.3 | 2.5 | ||
| Troglodytes troglodytes L. | 5.1 | 3.3 | 0.3 | 0.4 |
| Erithacus rubecula L. | 5.1 | 4.4 | 3.4 | 3.2 |
| Turdus philomelos C. L. Brehm | 1.5 | 1.8 | 1.1 | 1.7 |
| Turdus merula L. | 3.7 | 3.1 | 2.7 | 2.1 |
| Anthus trivialis L. | 0.6 | |||
| Fringilla coelebs L. | 15.5 | 11.5 | 8.6 | 6.9 |
| Coccothraustes coccothraustes L. | 3.9 | 3.2 | 2.4 | 2.8 |
| Pyrrhyla pyrrhula L. | 0.2 | |||
| Chloris chloris L. | 0.2 | |||
| Carduelis carduelis L. | 0.1 | 0.1 | 0.2 | |
| Emberiza citrinella L. | 0.2 | 2.3 | ||
References
- Novák, P.; Willner, W.; Biurrun, I.; Gholizadeh, H.; Heinken, T.; Jandt, U.; Kollar, J.; Kozhevnikova, M.; Naqinezhad, A.; Onyshchenko, V.; et al. Classification of European oak–hornbeam forests and related vegetation types. Appl. Veg. Sci. 2023, 26, e12712. [Google Scholar] [CrossRef]
- Novák, P.; Willner, W.; Zukal, D.; Kollár, J.; Roleček, J.; Świerkosz, K.; Ewald, J.; Wohlgemuth, T.; Csiky, J.; Onyshchenko, V.; et al. Oak-hornbeam forests of Central Europe: A formalized classification and syntaxonomic revision. Preslia 2020, 92, 1–34. [Google Scholar] [CrossRef]
- Stefańska-Krzaczek, E.; Kącki, Z.; Szypuła, B. Coexistence of ancient forest species as an indicator of high species richness. For. Ecol. Manag. 2016, 365, 12–21. [Google Scholar] [CrossRef]
- Stański, T.; Czeszczewik, D.; Stańska, M.; Walankiewicz, W. Anvils of the Great Spotted Woodpecker (Dendrocopos major) in primeval oak-lime-hornbeam stands of the Białowieża National Park. Eur. Zool. J. 2021, 88, 1–8. [Google Scholar] [CrossRef]
- Stański, T.; Stańska, M.; Goławski, A.; Czeszczewik, D. Foraging Site Selection of the Middle Spotted Woodpecker (Leiopicus medius L.) in Primeval Oak-Lime-Hornbeam Forest of the Białowieża National Park: Comparison of Breeding and Non-Breeding Seasons. Forests 2021, 12, 837. [Google Scholar] [CrossRef]
- Jelinčić, A.; Vukelić, J.; Papković, D. Phytosociological research into the Illyrian oak-hornbeam forest on the limestone cliffs of Kupa river canyon (Kamanje, West-Central Croatia). Šumarski List 2024, 1–2, 7–18. [Google Scholar] [CrossRef]
- Fornal-Pieniak, B.; Ollik, M. Diversity of Flora in the Undergrowth of Park Afforestations, Rural Plantings and Oak-Hornbeam Forests. Folia For. Pol. Ser. A 2013, 55, 132–136. [Google Scholar] [CrossRef]
- Ławrynowicz, M. Higher macroscopic fungi in the oak-hornbeam forests of the central Poland. Acta Mycol. 1973, 9, 133–264. [Google Scholar] [CrossRef]
- Pugacewicz, E. Ptaki Lęgowe Puszczy Białowieskiej; Północnopodlaskie Towarzystwo Ochrony Ptaków: Białowieża, Poland, 1997. [Google Scholar]
- Czeszczewik, D.; Walankiewicz, W. Ecology and biology of birds in the Białowieża Forest: A 40-year perspective. For. Res. Pap. 2016, 77, 332–340. [Google Scholar] [CrossRef][Green Version]
- Cmoluch, Z.; Cmoluchowa, A.; Lechowski, L.; Łętowski, J.; Minda-Lechowska, A.; Stączek, Z. Insect fauna of a linden-oak-hornbeam association (Tilio-Carpinetum) in the Bachus reserve (The Lubelska Upland). Fragm. Faunist. 1990, 33, 337–347. [Google Scholar] [CrossRef]
- Holecová, M.; Krumpál, M.; Országh, I.; Krumpálová, Z.; Fedor, P. Biodiversity of selected invertebrate groups in oak-hornbeam forest ecosystem in SW Slovakia. Ekol. Bratisl. 2005, 24, 205–222. [Google Scholar]
- Holecová, M.; Christophoryová, J.; Mrva, M.; Roháčová, M.; Stašiov, S.; Štrichelová, J.; Šustek, Z.; Tirjaková, E.; Tuf, I.H.; Vďačný, P. Faunal richness of selected invertebrate groups in epigeon of oak-hornbeam forests on the territory of Bratislava. In Bio-Diversity of Soil Micro- and Macrofauna in Oak-Hornbeam Forest Ecosystem on the Territory of Bratislava; Comenius University in Bratislava: Bratislava, Slovakia, 2012; pp. 129–137. [Google Scholar]
- Bohn, U.; Neuhausl, R.; Gollub, G.; Hettwer, C.; Neuhauslova, Z.; Raus, T.; Schluter, H.; Weber, H. Map of the Natural Vegetation of Europe. Scale: 1:2.500.000; Landwirtschaftsverlag: Munster, Germany, 2003. [Google Scholar]
- Zerbe, S. Restoration of natural broad-leaved woodland in Central Europe on sites with coniferous forest plantations. For. Ecol. Manag. 2002, 167, 27–42. [Google Scholar] [CrossRef]
- Łaska, G. Ecological consequences of deforestation and afforestation on a post-arable land: Changes in the composition and structure of plant communities and transformations of oak-hornbeam habitats and soil. Ecol. Quest. 2014, 20, 9–21. [Google Scholar] [CrossRef]
- Eliáš, P. Water deficit of plants in an oak-hornbeam forest. Preslia 1978, 50, 173–188. [Google Scholar]
- Beloiu, M. Forest Response to Climate Warming and Drought in Europe, Bayreuth. Ph.D. Thesis, University of Bayreuth, Faculty of Biology, Chemistry and Earth Sciences, Bayreuth, Germany, 2022. [Google Scholar]
- Padoa-Schioppa, E.; Baietto, M.; Massa, R.; Bottoni, L. Bird communities as bioindicators: The focal species concept in agricultural landscapes. Ecol. Ind. 2006, 6, 83–93. [Google Scholar] [CrossRef]
- Wesołowski, T.; Mitrus, C.; Czeszczewik, D.; Rowiński, P. Breeding bird dynamics in a primeval temperate forest over thirty-five years: Variation and stability in the changing world. Acta Ornit. 2010, 45, 209–232. [Google Scholar] [CrossRef]
- Mekonen, S. Birds as Biodiversity and Environmental Indicator. J. Nat. Sci. Res. 2017, 7, 28–34. [Google Scholar]
- Chowfin, S.M.; Leslie, A.J. Using birds as biodindicators of forest restoration progress: A preliminary study. Trees For. People 2021, 3, 100048. [Google Scholar] [CrossRef]
- Bergner, A.; Avcı, M.; Eryiğit, H.; Jansson, N.; Niklasson, M.; Westerberg, L.; Milberg, P. Influences of forest type and habitat structure on bird assemblages of oak (Quercus spp.) and pine (Pinus spp.) stands in southwestern Turkey. For. Ecol. Manag. 2015, 336, 137–147. [Google Scholar] [CrossRef]
- Domokos, E.; Domokos, J. Bird communities of different woody vegetation types from the Niraj valley, Romania. Turk. J. Zool. 2016, 40, 734–742. [Google Scholar] [CrossRef]
- Głowaciński, Z. Secondary Succession of Birds in Maturing Forest Ecosystem (Synthesis); PWN: Warszawa, Kraków, 1981. [Google Scholar]
- ZHL. Zasady Hodowli Lasu; Państwowe Gospodarstwo Leśne Lasy Państwowe: Warszawa, Poland, 2023.
- Virkkala, R.; Rajasärkkä, A.; Väisänen, R.; Vickholm, M.; Virolainen, E. Conservation value of nature reserves: Do hole-nesting birds prefer protected forests in southern Finland? Ann. Zool. Fenn. 1994, 31, 173–186. [Google Scholar]
- Walankiewicz, W.; Czeszczewik, D.; Tumiel, T.; Stański, T. Woodpeckers abundance in the Białowieża Forest—A comparison between deciduous, strictly protected and managed stands. Ornis Pol. 2011, 52, 161–168. [Google Scholar]
- Lešo, P.; Kropil, R.; Kajtoch, Ł. Effects of forest management on bird assemblages in oak-dominated stands of the Western Carpathians—Refuges for rare species. For. Ecol. Manag. 2019, 453, 117620. [Google Scholar] [CrossRef]
- Godzik, B.; Piechnik, Ł. Puszcza Niepołomicka—Zrównoważona gospodarka leśna a ochrona bogactwa przyrodniczego. Zjazd PTB Przew. Ses. Teren. 2019, 58, 185–213. [Google Scholar]
- PUL. Plan Urządzenia Lasu Sporządzony na Lata od 2022 do 2031 dla Nadleśnictwa Niepołomice; KRAMEKO sp. z o. o.: Krakow, Poland, 2022. [Google Scholar]
- Paletto, A.; Tosi, V. Forest canopy cover and canopy closure: Comparison of assessment techniques. Eur. J. For. Res. 2009, 128, 265–272. [Google Scholar] [CrossRef]
- NFI. The National Forest Inventory; State Forests National Forest Holding: Stary Sękocin, Poland, 2015.
- Chmura, D.; Salachna, A.; Sierka, E. Porównanie oceny zwarcia drzewostanu za pomocą metody wizualnej i zwarciomerza. Sylwan 2016, 160, 475–481. [Google Scholar]
- Braun-Blanquet, J. Plant Sociology. The Study of Plant Communities; McGraw-Hill Book Co., Inc.: New York, NY, USA; London, UK, 1932. [Google Scholar]
- Wikum, D.A.; Shanholtzer, G.F. Application of the Braun-Blanquet cover-abundance scale for vegetation analysis in land development studies. Environ. Manag. 1978, 2, 323–329. [Google Scholar] [CrossRef]
- Tomiałojć, L. The combined version of the mapping method. In Bird Census Work and Nature Conservation, Proc. VI Intern. Conf. Bird Census and Atlas Work, Göttingen; Oelke, H., Ed.; DDA: Göttingen, Germany, 1980; pp. 92–106. [Google Scholar]
- Czuraj, M. Tablice Miąższości Kłód Odziomkowych i Drzew Stojących; PWRiL: Warszawa, Poland, 1990. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell: Oxford, UK, 2004. [Google Scholar]
- Chao, A.; Chazdon, R.L.; Colwell, R.K.; Shen, T.J. Abundance-based similarity indices and their estimation when there are unseen species in samples. Biometrics 2006, 62, 361–371. [Google Scholar] [CrossRef]
- Snow, D.W.; Perrins, C.M. The Birds of the Western Palearctic; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Bobiec, A.; van der Burgt, H.; Meijer, K.; Zuyderduyn, C.; Haga, J.; Vlaanderen, B. Rich deciduous forests in Białowieża as a dynamic mosaic of developmental phases: Premises for nature conservation and restoration management. For. Ecol. Manag. 2000, 130, 159–175. [Google Scholar] [CrossRef]
- Baláž, M.; Balážová, M. Diversity and abundance of bird communities in three mountain forest stands: Effect of the habitat heterogeneity. Pol. J. Ecol. 2012, 60, 629–634. [Google Scholar]
- Lin, C.W.; Hsu, F.H.; Ding, T.S. Applying a territory mapping method to census the breeding bird community composition in a montane forest of Taiwan. J. f For. Sci. 2011, 26, 267–285. [Google Scholar]
- Thompson, F., III; Fritzell, E.K. Bird Densities and Diversity in Clearcut and Mature Oak-Hickory Forest; Research Paper NC-293; U.S. Dept. of Agriculture, Forest Service, North Central Forest Experiment Station: St. Paul, MN, USA, 1990.
- Sutherland, W.J. (Ed.) Ecological Census Techniques: A Handbook; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Koch Widerberg, M.; Ranius, T.; Drobyshev, L.; Lindbladh, M. Oaks retained in production spruce forests help maintain saproxylic beetle diversity in southern Scandinavian landscapes. For. Ecol. Manag. 2018, 417, 257–264. [Google Scholar] [CrossRef]
- Kebrle, D.; Zasadil, P.; Hošek, J.; Barták, V.; Šťastný, K. Large trees as a key factor for bird diversity in spruce-dominated production forests: Implications for conservation management. For. Ecol. Manag. 2021, 496, 119460. [Google Scholar] [CrossRef]
- Komlós, M.; Botta-Dukát, Z.; Bölöni, J.; Aszalós, R.; Veres, K.; Winkler, D.; Ónodi, G. Tall, large-diameter trees and dense shrub layer as key determinants of the abundance and composition of bird communities in oak-dominated forests. J. For. Res. 2024, 35, 62. [Google Scholar] [CrossRef]
- Hebda, G.; Wesołowski, T.; Rowiński, P. Nest sites of a strong excavator, the Great Spotted Woodpecker Dendrocopos major, in a primeval forest. Ardea 2017, 105, 61–71. [Google Scholar] [CrossRef]
- Charman, E.C.; Smith, K.W.; Dodd, S.; Gruar, D.J.; Dillon, I.A. Pre-breeding foraging and nest site habitat selection by Lesser Spotted Woodpeckers Dendrocopos minor in mature woodland blocks in England. Ornis Fenn. 2012, 89, 182–196. [Google Scholar] [CrossRef]
- Hebda, G.; Wesołowski, T. Low flea loads in birds’ nests in tree cavites. Ornis Fenn. 2012, 89, 139–144. [Google Scholar] [CrossRef]
- IUL. Instrukcja Urządzania Lasu; Część I; Centrum Informacyjne Lasów Państwowych: Warszawa, Poland, 2012.
- ZHL. Zasady Hodowli Lasu; Państwowe Gospodarstwo Leśne Lasy Państwowe: Warszawa, Poland, 2012.
- Renner, S.C.; Gossner, M.M.; Ayasse, M.; Böhm, S.; Teuscher, M.; Weisser, W.W.; Jung, K. Forest structure, plants, arthropods, scale, or birds’ functional groups: What key factor are forest birds responding to? PLoS ONE 2024, 19, e0304421. [Google Scholar] [CrossRef]
- Walankiewicz, W.; Czeszczewik, D. Importance of Carpinus betulus for hole-nesting birds in the Białowieża National Park. Chrońmy Przyr. Ojczystą 2006, 62, 50–57. [Google Scholar]
- Piechnik, Ł. Carpinus betulus and occurrence of natural tree hollows in managed forests. Fragm. Florist. Geobot. Pol. 2020, 7, 33–43. [Google Scholar] [CrossRef]
| Variable | Old-Growth Forest (Reserve Lipówka) | Mature Stand | Initial Gap-Cut Stand | Advanced Gap-Cut Stand | Test |
|---|---|---|---|---|---|
| Mean (Variation Coefficient) (Min–Max) | |||||
| Volume of living trees [m3 ha−1] | 482.3 (56) a (85.5–1092.5) | 323.8 (43) a (41.3–670.5) | 341.0 (75) a (0.0–851.5) | 168.6 (118) b (0.0–838.5) | H = 32.0 p < 0.001 |
| Density of living trees [trees ha−1] | 339.0 (29) a (150.0–550.0) | 246.7 (49) b (75.0–725.0) | 221.5 (64) c (0.0–500.0) | 544.4 (86) abc (0.0–1600.0) | H = 20.8 p < 0.001 |
| Volume of lying deadwood [m3 ha−1] | 159.7 (81) a (12.1–506.5) | 29.6 (162) b (0.0–215.9) | 8.3 (224) c (0.0–90.3) | 6.0 (168) bc (0.0–60.7) | H = 73.8 p < 0.001 |
| Volume of standing deadwood [m3 ha−1] | 17.9 (209) a (0.0–195.6) | 6.0 (186) ab (0.0–41.5) | 7.6 (405) b (0.0–146.5) | 0.5 (443) b (0.0–12.0) | H = 20.8 p < 0.001 |
| Total volume of deadwood [m3 ha−1] | 177.6 (81) a (12.1–569.3) | 35.7 (149) b (0.0–224.3) | 15.9 (222) c (0.0–149.9) | 6.5 (159) bc (0.0–60.7) | H = 69.7 p < 0.001 |
| Sapling density [saplings ha−1] | 1247.1 (168) ab (0.0–10100.0) | 900.0 (162) a (0.0–8300.0) | 4852.8 (136) bc (0.0–26000.0) | 4466.7 (133) c (0.0–26000.0) | H = 20.7 p < 0.001 |
| Seedling cover [%] | 9.5 (151) a (1.0–70.0) | 5.6 (207) a (0.0–60.0) | 15.1 (153) a (0.0–80.0) | 7.5 (213)a (0.0–80.0) | F = 2.4 p = 0.07 |
| Total canopy cover [%] | 77.9 (22) a (20.0–95.0) | 74.7 (25) a (15.0–100.0) | 61.3 (61) a (0.0–100.0) | 60.0 (60) a (0.0–100.0) | H = 3.0 p > 0.5 |
| 1st-floor canopy cover [%] | 43.1 (44) ac (15.0–80.0) | 64.5 (29) b (8.0–100.0) | 47.8 (65) ab (0.0–85.0) | 23.5 (115) c (0.0–90.0) | H = 41.3 p < 0.001 |
| 2st-floor canopy cover [%] | 58.1 (25) a (20.0–85.0) | 40.2 (41) b (10.0–75.0) | 34.6 (81) bc (0.0–100.0) | 21.4 (119) c (0.0–80.0) | H = 39.9 p < 0.001 |
| 3st-floor canopy cover [%] | 35.4 (50) a (5.0–75.0) | 23.5 (77) a (0.0–80.0) | 29.7 (82) a (0.0–90.0) | 37.5 (84) a (0.0–100.0) | H = 7.8 p = 0.05 |
| Density of living trees with DBH ≥ 65 cm [trees ha−1] | 23.5 (94) a (0.0–75.0) | 12.8 (136) a (0.0–50.0) | 26.4 (113) a (0.0–100.0) | 11.1 (174) a (0.0–75.0) | H = 11.3 p = 0.01 |
| Density of living trees with DBH ≥ 75 cm [trees ha−1] | 18.4 (117) a (0.0–75.0) | 6.7 (186) a (0.0–50.0) | 11.1 (165) a (0.0–75.0) | 6.3 (260) a (0.0–75.0) | H = 11.3 p = 0.01 |
| Density of living trees with DBH ≥ 85 cm [trees ha−1] | 11.0 (150) a (0.0–50.0) | 2.2 (324) a (0.0–25.0) | 3.5 (253) a (0.0–25.0) | 2.1 (336) a (0.0–25.0) | H = 13.6 p = 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stąpór, K.; Bujoczek, M.; Bujoczek, L. Is It Possible to Preserve the Full Diversity of Birds in Managed Oak–Lime–Hornbeam Forests? Forests 2025, 16, 1060. https://doi.org/10.3390/f16071060
Stąpór K, Bujoczek M, Bujoczek L. Is It Possible to Preserve the Full Diversity of Birds in Managed Oak–Lime–Hornbeam Forests? Forests. 2025; 16(7):1060. https://doi.org/10.3390/f16071060
Chicago/Turabian StyleStąpór, Karolina, Małgorzata Bujoczek, and Leszek Bujoczek. 2025. "Is It Possible to Preserve the Full Diversity of Birds in Managed Oak–Lime–Hornbeam Forests?" Forests 16, no. 7: 1060. https://doi.org/10.3390/f16071060
APA StyleStąpór, K., Bujoczek, M., & Bujoczek, L. (2025). Is It Possible to Preserve the Full Diversity of Birds in Managed Oak–Lime–Hornbeam Forests? Forests, 16(7), 1060. https://doi.org/10.3390/f16071060





