Defoliation of Norway Spruce by Spruce Budworm (Lepidoptera: Tortricidae) and Protection Using Bacillus thuringiensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Evaluation of Spruce Budworm Defoliation on Host Species
2.3. Evaluation of Btk Treatments on Defoliation of Norway and White Spruce Plantation
2.4. Btk Formulation Applications
2.5. Btk Treatment Efficacy
2.6. Statistical Analysis
3. Results
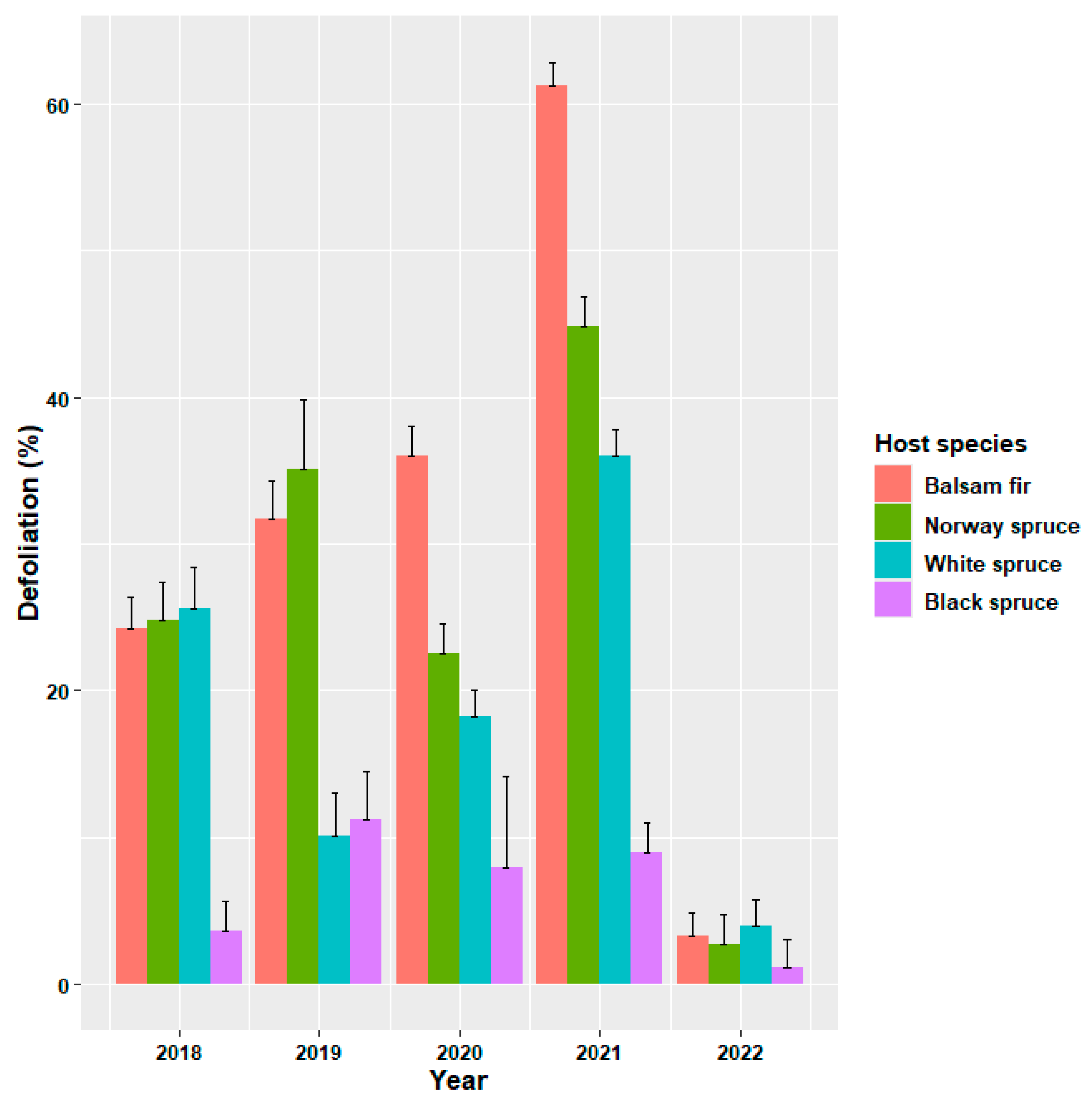
3.1. Annual Defoliation on Host Species
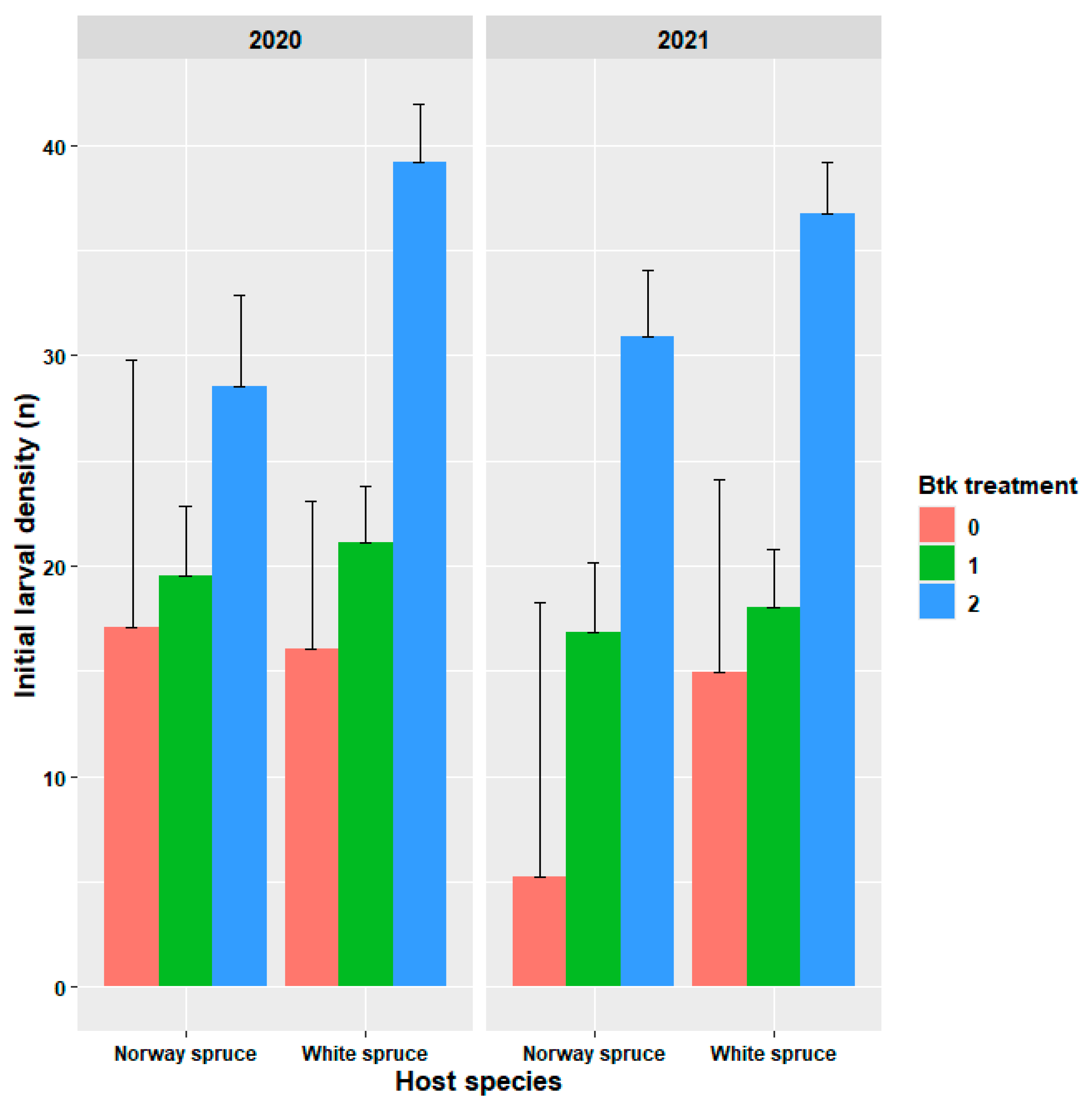
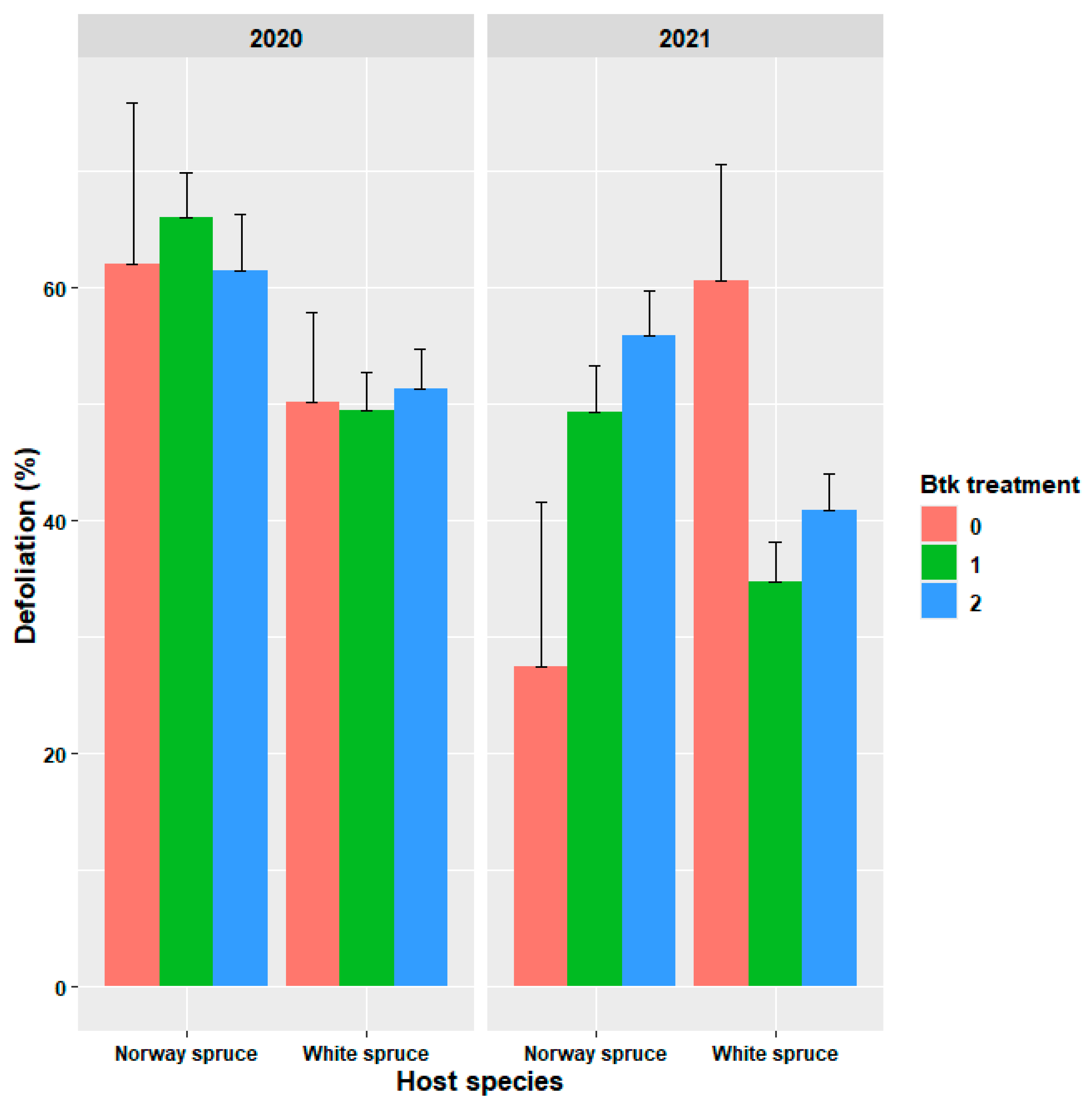
3.2. Evaluation of Treatment Efficacy
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farrar, J.L. Trees in Canada; Canadian Forest Service, Natural Resources Canada: Ottawa, ON, Canada, 1995; 502p.
- Jansson, G.; Danusevičius, D.; Grotehusman, H.; Kowalczyk, J.; Krajmerova, D.; Skrøppa, T.; Wolf, H. Norway Spruce (Picea abies (L.) H. Karst.). In Forest Tree Breeding in Europe; Pâques, L.E., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 123–176. [Google Scholar]
- Caudullo, G.; Tinner, W.; de Rigo, D. Picea Abies in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Eds.; Publications Office of the European Union: Luxembourg, 2016; pp. 114–116. [Google Scholar]
- Hosley, N.W. Norway spruce in the northeastern United States. Harv. For. Bull. 1936, 19, 80. [Google Scholar]
- MacArthur, J.D. Norway spruce plantations in Québec. In Forest Research Branch Publication No. 1059; Canadian Department of Forestry: Ottawa, ON, Canada, 1964; 44p. [Google Scholar]
- Holst, M.J.; Heimburger, C.C. Tree breeding and genetic of exotic conifers in Canada. For. Chron. 1969, 45, 434–440. [Google Scholar] [CrossRef]
- Mottet, M.-J.; Lambert, M.-C.; DeBlois, J. Natural regeneration of Norway spruce, an introduced species, in and around plantations in Quebec, Canada. For. Ecol. Manag. 2021, 498, 119553. [Google Scholar] [CrossRef]
- Ministère des Forêts, de la Faune et des Parcs (MFFP). Sommaire du Plan D’aménagement Forestier Intégré Tactique 2018–2023. Région du Bas-Saint-Laurent. Unité D’aménagement 011-71; 2018; 342p. Available online: https://cdn-contenu.quebec.ca/cdn-contenu/forets/documents/planification/Bas-Saint-Laurent/plans/PL_PAFIT_BSL_UA01171 (accessed on 7 March 2024).
- Berthiaume, R.; Hébert, C.; Dupont, A.; Charest, M.; Bauce, É. The spruce budworm, a potential threat for Norway spruce in eastern Canada? For. Chron. 2020, 96, 71–76. [Google Scholar] [CrossRef]
- MacLean, D.A. Impacts of insect outbreaks on tree mortality, productivity, and stand development. Can. Entomol. 2016, 148, S138–S159. [Google Scholar] [CrossRef]
- Ministère des Ressources Naturelles et Des Forêts (MRNF). Aires Infestées Par la Tordeuse des Bourgeons de L’épinette au Québec en 2024; Gouvernement du Québec, Direction de la Protection des Forêts: Quebec, QC, Canada, 2024; 34p. Available online: https://cdn-contenu.quebec.ca/cdn-contenu/forets/documents/insectes/RA_Aires_infesteesTBE_2024.pdf (accessed on 20 January 2025).
- Ministère des Forêts, de la Faune et des Parcs (MFFP). Insectes, Maladies et Feux Dans les Forêts du Québec en 2021; Direction de la Protection Des Forêts: Quebec, QC, Canada, 2022; 82p. Available online: https://mffp.gouv.qc.ca/documents/forets/RA_2021_DPF.pdf (accessed on 7 March 2024).
- Henningar, C.R.; MacLean, D.A.; Quiring, D.T.; Kershaw, J.A. Differences in spruce budworm defoliation among balsam fir and white, red, and black spruce. For. Sci. 2008, 54, 158–166. [Google Scholar]
- van Frankenhuyzen, K.; Lucarotti, C.; Lavallée, R. Canadian contributions to forest insect pathology and to the use of pathogens in forest pest management. Can. Entomol. 2016, 148, S210–S238. [Google Scholar] [CrossRef][Green Version]
- Höfter, H.; Whiteley, H.R. Insecticidal Crystal Proteins of Bacilllus thuringiensis. Microbiol. Mol. Biol. Rev. 1989, 53, 242–255. [Google Scholar]
- Bauce, É.; Carisey, N.; van Frankenhuyzen, K.; Dupont, A. Bacillus thuringiensis subsp. kurstaki (Btk) aerial spray prescriptions for balsam fir protection against spruce budworm, Choristoneura fumiferana (Lepidoptera: Tortricidae). J. Econ. Entomol. 2004, 97, 624–634. [Google Scholar] [CrossRef] [PubMed]
- SOPFIM. Programmes de Pulvérisation Aérienne D’insecticide Biologique (Btk) Contre la Tordeuse des Bourgeons de L’épinette. In Rapport de Réalisation des Travaux 2022; SOPFIM: Québec, QC, Canada, 2022; 127p. [Google Scholar]
- Carisey, N.; Bauce, É.; Miron, S.; Dupont, A. Effects of bud phenology and foliage chemistry of balsam fir and white spruce trees on the efficacy of Bacillus thuringiensis against the spruce budworm, Choristoneura fumiferana. Agric. For. Entomol. 2004, 6, 55–69. [Google Scholar] [CrossRef]
- Fuentealba, A.; Bauce, É.; Dupont, A. Bacillus thuringiensis efficacy in reducing spruce budworm damage as affected by host tree species. J. Pest Sci. 2015, 88, 593–603. [Google Scholar] [CrossRef]
- Fuentealba, A.; Pelletier-Beaulieu, É.; Dupont, A.; Hébert, C.; Berthiaume, R.; Bauce, É. Optimizing Bacillus thuringiensis (Btk) aerial spray prescriptions in mixed balsam fir-white spruce stands against the eastern spruce budworm. Forests 2023, 14, 1289. [Google Scholar] [CrossRef]
- Schönherr, J. Nun moth outbreak in Poland 1978–1984. Z. Angew. Entomol. 1985, 99, 73–76. [Google Scholar] [CrossRef]
- Bejer, B. Outbreaks of the nun moth (Lymantria monacha) in Denmark with remarks on their control. Anz. Schaedlingskd. Pflanz. Umweltschutz. 1986, 59, 86–89. [Google Scholar] [CrossRef]
- Volney, W.J.A.; Cerezke, H.F. The phenology of white spruce and the spruce budworm in northern Alberta. Can. J. For. Res. 1992, 22, 198–205. [Google Scholar] [CrossRef]
- Desaulniers, R. Épidémie de la Tordeuse des Bourgeons de L’épinette Dans la Région de Grand-mère en 1967 et Suggestions en Vue de Sa répression en 1968; Ministère des Terres et Forêts du Québec: Québec, QC, Canada, 1968; 11p.
- Fowler, D.P.; Coles, J.F. Provenance Trials of Norway Spruce in the Maritimes; Information Report M-X-101; Canadian Forestry Service, Maritimes Forest Research Centre: Fredericton, NB, Canada, 1979; 81p. [Google Scholar]
- Hennigar, C.R.; MacLean, D.A. Spruce budworm and management effects on forest and wood product carbon for an intensively managed forest. Can. J. For. Res. 2010, 40, 1736–1750. [Google Scholar] [CrossRef]
- Saucier, J.-P.; Robitaille, A.; Grondin, P. Cadre Bioclimatique du Québec. In Manuel de Foresterie, 2nd ed.; Doucet, R., Côté, M., Eds.; Editions Multimonde: Quebec, QC, Canada, 2009; pp. 186–205. [Google Scholar]
- MacLean, D.A.; Lidstone, R.G. Defoliation by spruce budworm: Estimation by ocular and shoot-count methods and variability among branches, trees, and stands. Can. J. For. Res. 1982, 12, 582–594. [Google Scholar] [CrossRef]
- Miller, C.A.; Kettela, E.G.; McDougall, G.A. A Sampling Technique for Overwintering Spruce Budworm and Its Applicability to Population Surveys; Rep. M-X-25; Canadian Forest Service, Department of Fisheries and Forestry: Ottawa, ON, Canada, 1971; 12p. [Google Scholar]
- Dorais, L.G.; Kettela, E. Revue, par Région, des Techniques D’inventaire Entomologique et D’évaluation Des Programmes de Pulvérisation à Grande Échelle Contre la Tordeuse des Bourgeons de L’épinette Choristoneura fumiferana (Clem.); Rapport du Comité pour la Standardisation des Techniques Entomologiques, Ministère de l’Énergie et des Ressources du Québec; Conseil de l’Est de la Tordeuse des Bourgeons de L’épinette: Quebec, QC, Canada, 1982; p. 51. [Google Scholar]
- Fettes, J.J. Investigations of Sampling Techniques for Population Studies of the Spruce Budworm on Balsam Fir in Ontario; Forest Insect Laboratory: Sault Ste. Marie, ON, Canada, 1950; Volume 4, pp. 163–401. [Google Scholar]
- Régnière, J.; St-Amant, R. BioSIM 9 User’s Manual; Information Report LAU-X-134; Natural Resources Canada, Canadian Forest Service, Laurentian Forestry Centre: Quebec, QC, Canada, 2008; 82p.
- SAS Institute Inc. SAS/STAT User’s Guide; Release 9.1 edn; SAS Institute Inc.: Cary, NC, USA, 2003. [Google Scholar]
- Wu, Y.; MacLean, D.A.; Hennigar, C.; Taylor, A.R. Interactions among defoliation, species, and soil richness determine foliage production during and after simulated spruce budworm attack. Can. J. Res. 2020, 50, 565–580. [Google Scholar] [CrossRef]
- Bellemin-Noël, B.; Bourassa, S.; Despland, E.; De Grandpré, L.; Pureswaran, D.S. Improved performance of the eastern spruce budworm on black spruce as warming temperatures disrupt phenological defences. Glob. Change Biol. 2021, 27, 3358–3366. [Google Scholar] [CrossRef]
- Campbell, D.; Campbell, M.; Moore, R.; Thompson, J.; Meating, J.H.; Bolan, P.M.; Francis, M.W. The Eastern Spruce Budworm in Saskatchewan—1999; Forest Pest Management Forum: Ottawa, ON, Canada, 1999; 9p. [Google Scholar]
- Sundaram, K.M.S. Influence of foliar morphology and crown geometry on insecticide deposition and dissipation following aerial spray over a mixed forest. J. Environ. Sci. Health 1991, 26, 601–629. [Google Scholar] [CrossRef]
- van Frankenhuyzen, K.; Nystrom, C.; Dedes, J.; Seligy, V. Mortality, feeding inhibition, and recovery of spruce budworm (Lepidoptera: Tortricidae) larvae following aerial application of a high-potency formulation of Bacillus thuringiensis subsp. kurstaki. Can. Entomol. 2000, 132, 505–518. [Google Scholar] [CrossRef]
- Barry, J.W.; Barber, L.R.; Kenney, P.A.; Overgaard, N. Feasibility of aerial spraying of southern pine seed orchards. South. J. Appl. For. 1984, 8, 127–131. [Google Scholar] [CrossRef]
- Himel, C.M.; Sundaram, A.; Sundaram, K.M.S.; Cadogan, B.L.; Villaveces, A. Assessment of aerial spray deposits in a spruce forest using inflight microencapsulation technique. J. Environ. Sci. Health 1987, 22, 195–219. [Google Scholar] [CrossRef]
- Varty, I.W.; Holmes, S.E. Heterogeneity of Spray Deposit and Efficacy Within a Single Swath Applied by Aircraft Over Forest Infested with Spruce Budworm, Choristoneura fumiferana (Clem.); Information Report No. M-X-168; Canadian Forestry Service, Maritimes Forest Research Centre: Fredericton, NB, Canada, 1988; 76p. [Google Scholar]
- Thistle, H.W.; Reardon, R.C.; Bonds, J.A.S.; Fritz, B.L.; Hoffmann, W.C.; Kees, G.J.; Grob, I.J.; Hewitt, A.J.; O’Donnell, C.C.; Felton, K.D.; et al. Aerially released spray penetration in a tall coniferous forest canopy. Trans. ASABE 2016, 59, 1221–1231. [Google Scholar]
- Colombo, M.; Grauso, L.; Lanzotti, V.; Incerti, G.; Adamo, A.; Storlazzi, A.; Gigliotti, S.; Mazzoleni, S. Self-DNA inhibition in Drosophila melanogaster development: Metabolomic evidence of the molecular determinants. Biology 2023, 12, 1378. [Google Scholar] [CrossRef]
- Alfaro, R.; King, J.N.; van Akker, L. Delivering Sitka spruce with resistance against white pine weevil in British Columbia, Canada. For. Chron. 2013, 89, 235–245. [Google Scholar] [CrossRef]
- Parent, G.J.; Méndez-Espinoza, C.; Giguère, I.; Mageroy, M.H.; Charest, M.; Bauce, É.; Bohlmann, J.; MacKay, J.J. Hydroxyacetophenone defenses in white spruce against spruce budworm. Evol. Appl. 2020, 13, 62–75. [Google Scholar] [CrossRef]
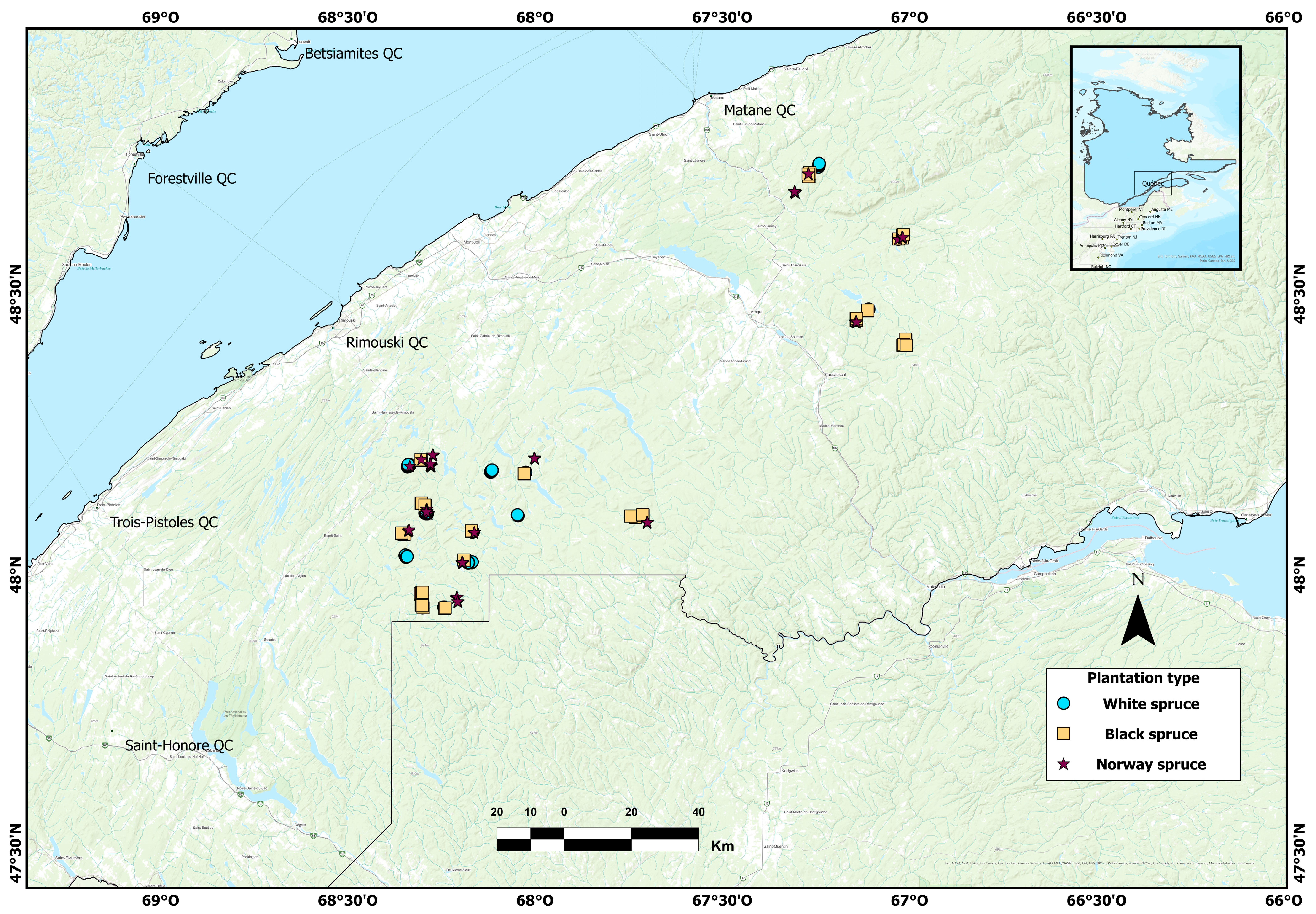
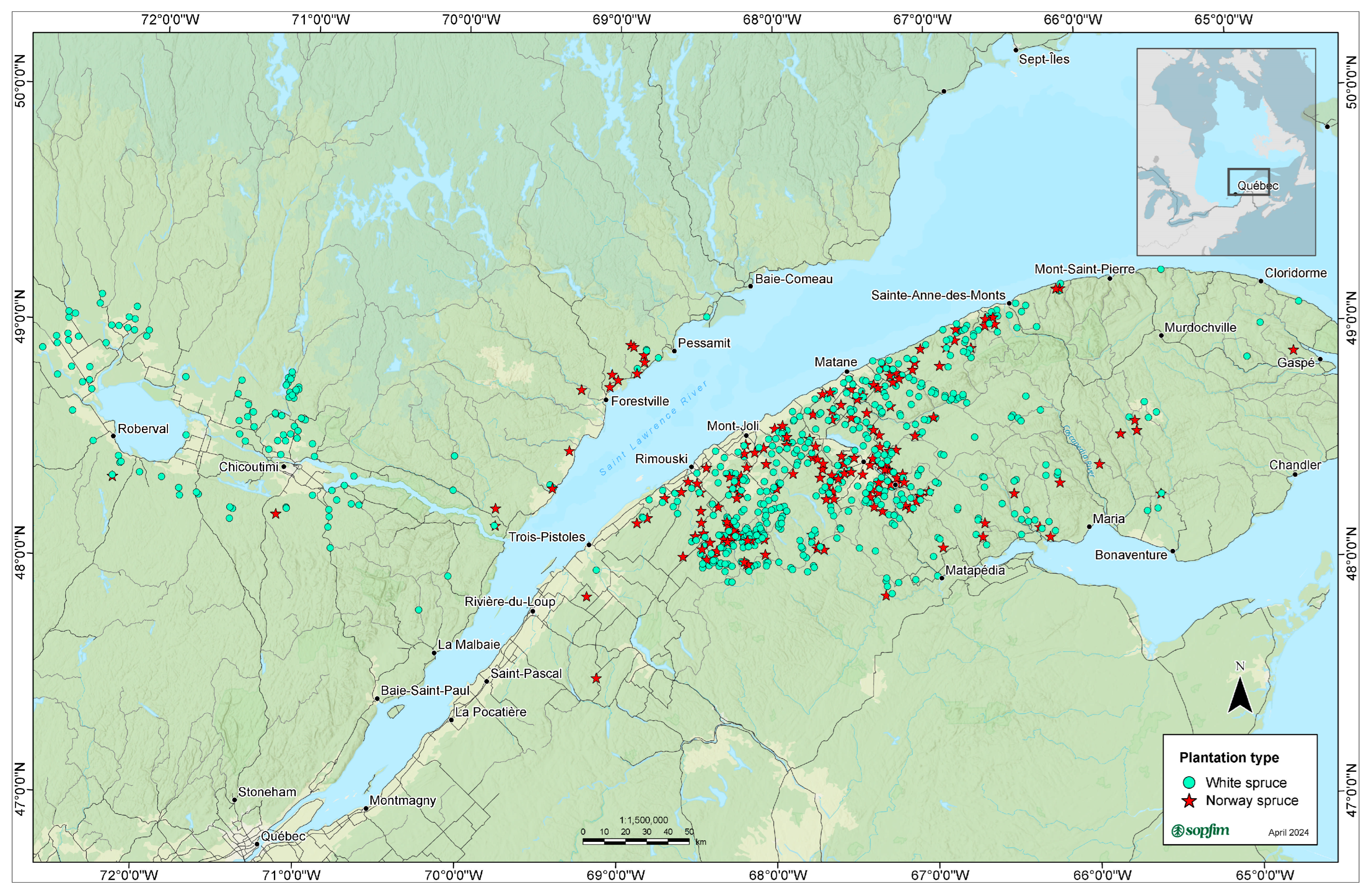
| Plantation | Year | |||||
|---|---|---|---|---|---|---|
| 2018 | 2019 | 2020 | 2021 | 2022 | Total | |
| White spruce | 39 | 34 | 90 | 82 | 82 | 327 |
| Black spruce | 66 | 26 | 0 | 75 | 75 | 242 |
| Norway spruce | 45 | 14 | 72 | 73 | 73 | 277 |
| Total plots per year | 150 | 74 | 162 | 230 | 230 | 846 |
| Year | 2020 | |||
|---|---|---|---|---|
| Btk treatment | ||||
| Plantation | Control | Single | Double | Total |
| Norway spruce | 30 | 48 | 22 | 100 |
| White spruce | 129 | 137 | 98 | 364 |
| Total | 159 | 185 | 120 | 464 |
| Year | 2021 | |||
| Btk treatment | ||||
| Plantation | Control | Single | Double | Total |
| Norway spruce | 2 | 47 | 68 | 117 |
| White spruce | 5 | 85 | 290 | 380 |
| Total | 7 | 132 | 358 | 497 |
| Source of Variation | F | df | p |
|---|---|---|---|
| Host species | 84.95 | 38,730 | <0.0001 |
| Year | 469.56 | 31,253 | <0.0001 |
| Host species × Year | 17.36 | 91,204 | <0.0001 |
| Initial Larval Density | Insect Development Index (IDI) | |||||
|---|---|---|---|---|---|---|
| Source of Variation | F | df | p | F | df | p |
| Btk treatment | 46.73 | 2688 | <0.0001 | 0.65 | 2692 | 0.52 |
| Host species | 5.25 | 1657 | 0.0223 | 0.02 | 1693 | 0.87 |
| Host species × Btk treatment | 1.90 | 2691 | 0.15 | 2.26 | 2691 | 0.105 |
| Year | 1.42 | 1692 | 0.23 | 0.01 | 1692 | 0.93 |
| Btk treatment × year | 0.54 | 2689 | 0.58 | 4.09 | 2692 | 0.0172 |
| Host species × year | 0.90 | 1677 | 0.34 | 3.21 | 1693 | 0.07 |
| Btk treatment × Host × Year | 0.26 | 2677 | 0.76 | 5.76 | 2692 | 0.0033 |
| Year | Host Species | ||
|---|---|---|---|
| Btk Treatment | White Spruce | Norway Spruce | |
| 2020 | Control | 4.59 ± 0.22 | 5.29 ± 0.38 |
| Single application | 4.51 ± 0.12 | 4.51 ± 0.13 | |
| Double application | 4.52 ± 0.12 | 4.47 ± 0.15 | |
| 2021 | Control | 4.84 ± 0.28 | 3.29 ± 0.39 |
| Single application | 4.66 ± 0.12 | 5.06 ± 0.13 | |
| Double application | 4.74 ± 0.11 | 4.77 ± 0.13 | |
| Source of Variation | F | dl | p |
|---|---|---|---|
| Initial larval density | 140.47 | 1691 | <0.0001 |
| Btk treatment | 0.84 | 2691 | 0.4337 |
| Host species | 2.05 | 1685 | 0.1524 |
| Host species × Btk treatment | 2.68 | 2691 | 0.0695 |
| Year | 8.94 | 1692 | 0.0029 |
| Btk treatment × year | 2.28 | 2691 | 0.1029 |
| Host species × year | 3.03 | 1689 | 0.082 |
| Btk treatment × Host × Year | 2.36 | 2688 | 0.0947 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentealba, A.; Berthiaume, R.; Fortier, S.; Morneau, L.; Bauce, É. Defoliation of Norway Spruce by Spruce Budworm (Lepidoptera: Tortricidae) and Protection Using Bacillus thuringiensis. Forests 2025, 16, 1056. https://doi.org/10.3390/f16071056
Fuentealba A, Berthiaume R, Fortier S, Morneau L, Bauce É. Defoliation of Norway Spruce by Spruce Budworm (Lepidoptera: Tortricidae) and Protection Using Bacillus thuringiensis. Forests. 2025; 16(7):1056. https://doi.org/10.3390/f16071056
Chicago/Turabian StyleFuentealba, Alvaro, Richard Berthiaume, Simon Fortier, Louis Morneau, and Éric Bauce. 2025. "Defoliation of Norway Spruce by Spruce Budworm (Lepidoptera: Tortricidae) and Protection Using Bacillus thuringiensis" Forests 16, no. 7: 1056. https://doi.org/10.3390/f16071056
APA StyleFuentealba, A., Berthiaume, R., Fortier, S., Morneau, L., & Bauce, É. (2025). Defoliation of Norway Spruce by Spruce Budworm (Lepidoptera: Tortricidae) and Protection Using Bacillus thuringiensis. Forests, 16(7), 1056. https://doi.org/10.3390/f16071056









