Potential Distribution of Tamarix boveana Bunge in Mediterranean Coastal Countries Under Future Climate Scenarios
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition and Preparation
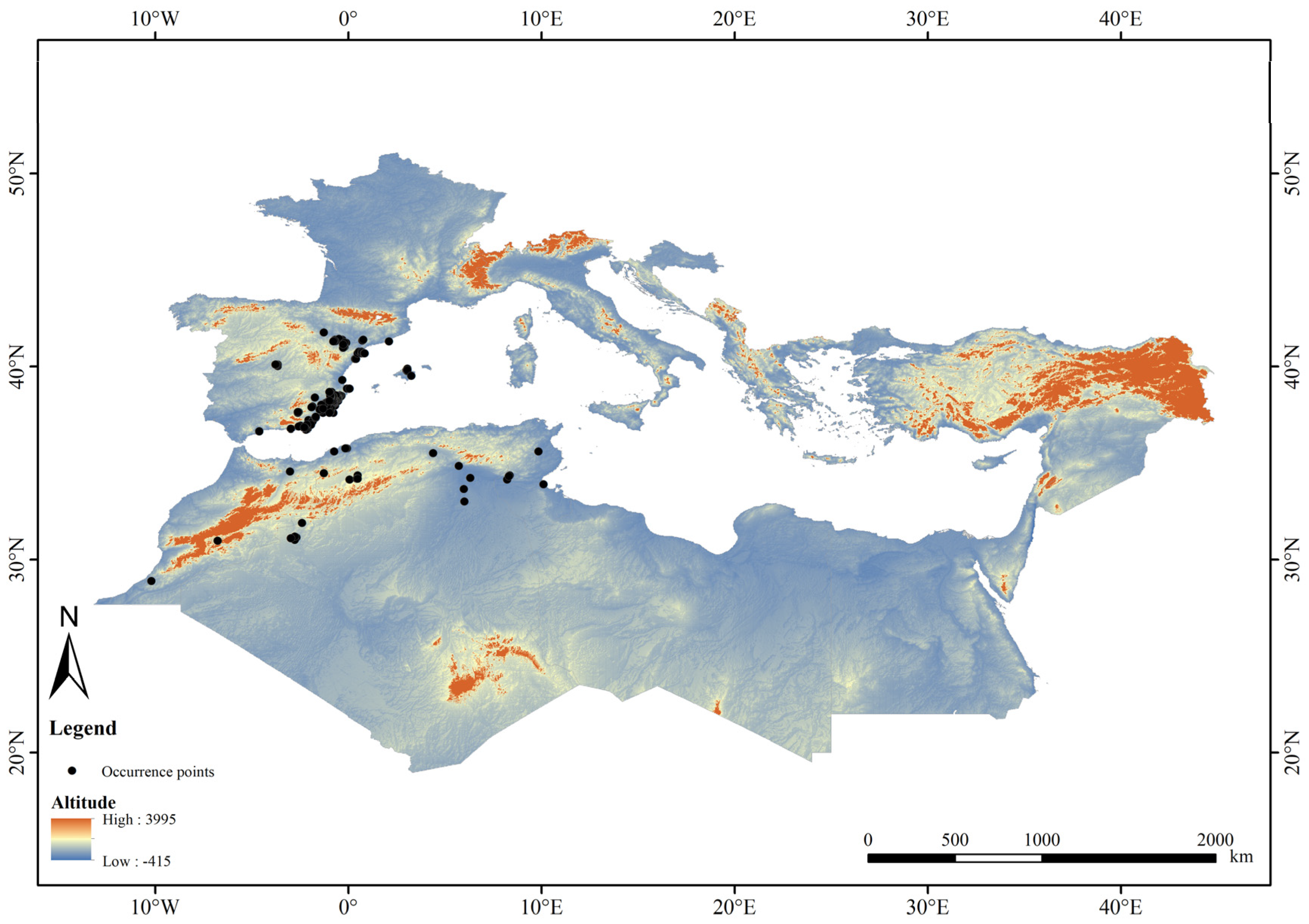
2.1.1. Species Distribution Data of Tamarix boveana
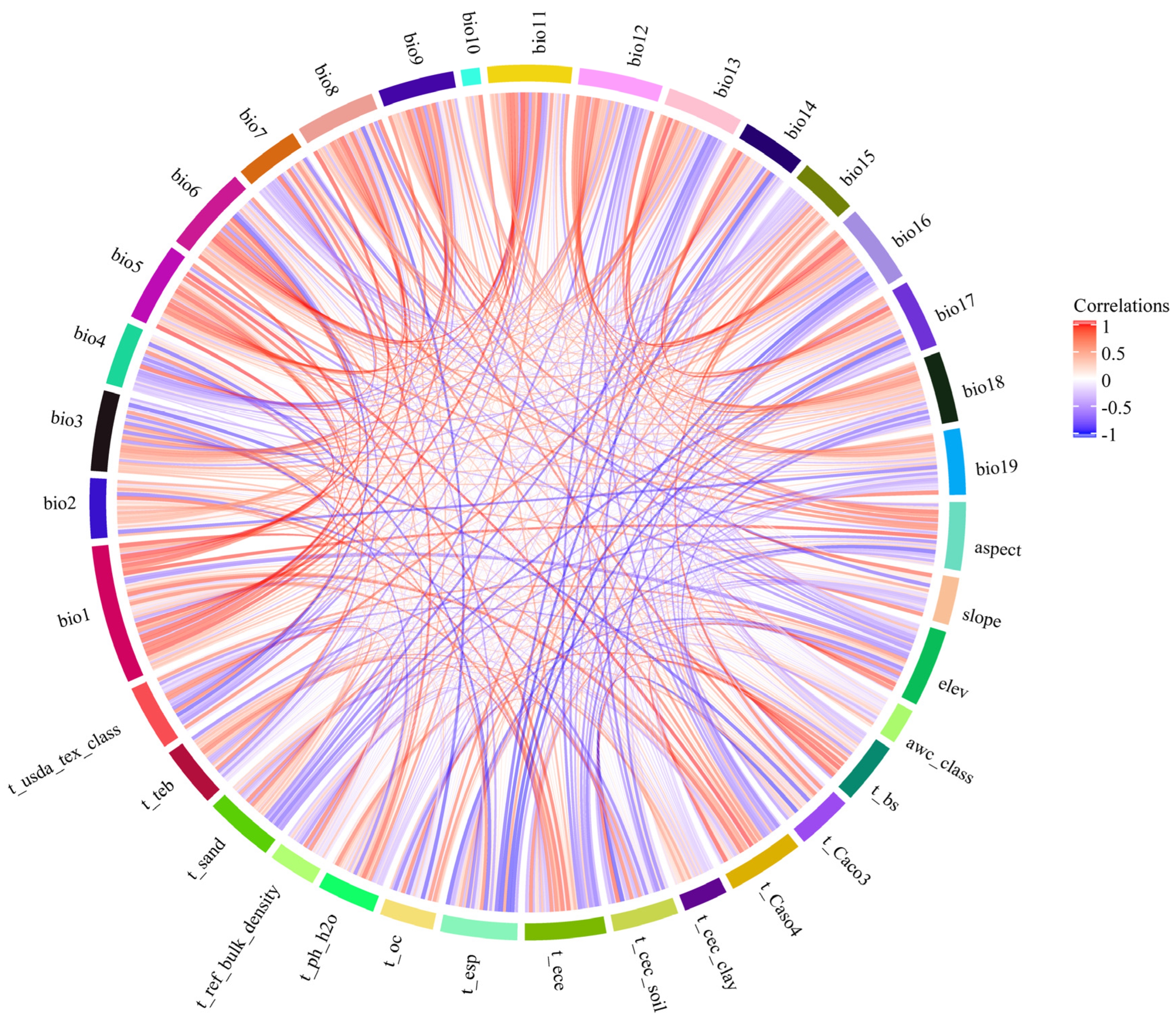
2.1.2. Sources and Processing of Environmental Data
2.2. Model Parameter Optimization
2.3. Model Construction and Evaluation
2.4. Tamarix boveana Habitat Classification, Patterns, and Changes in Centroid Migration
3. Results
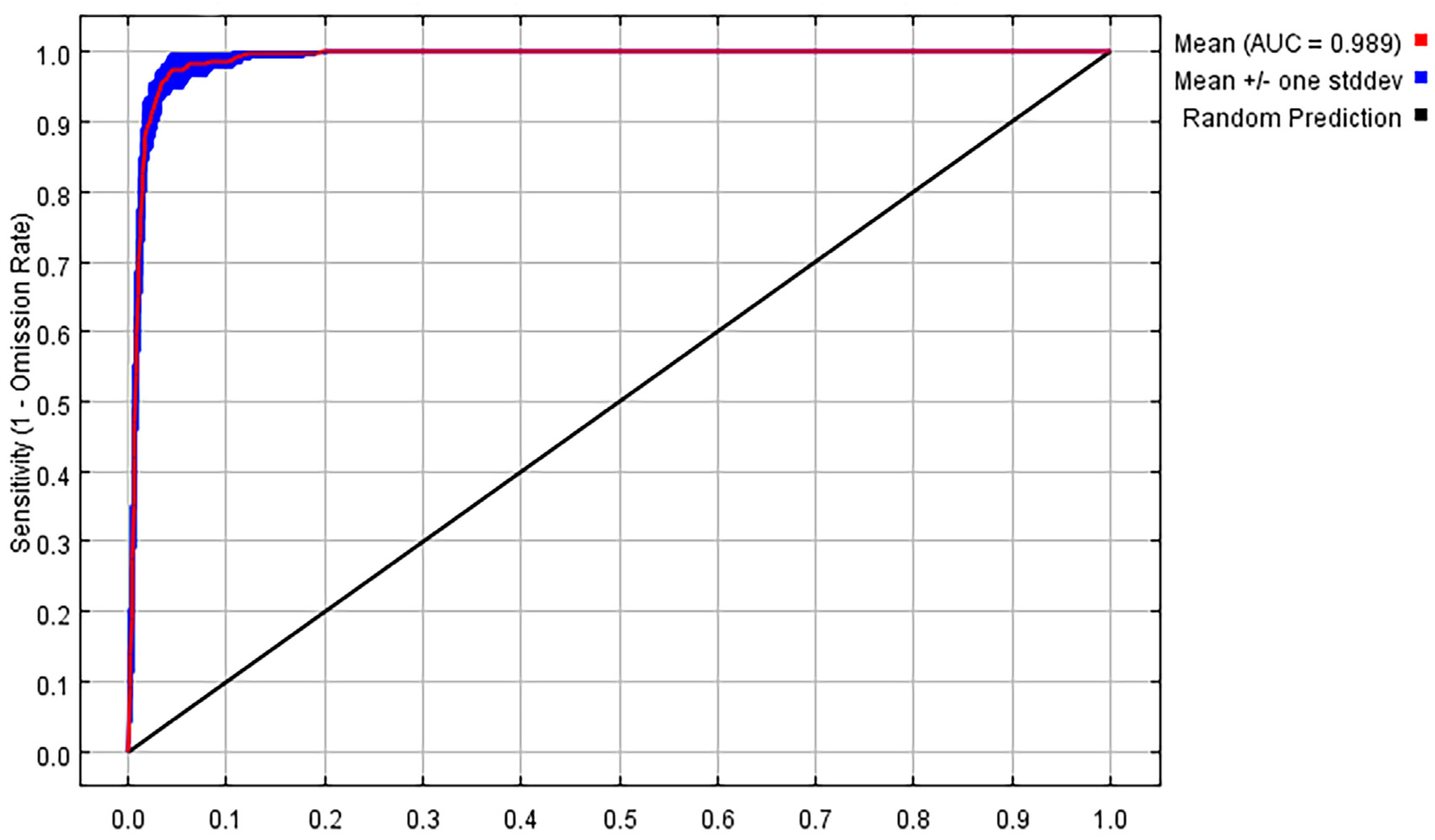
3.1. Model Accuracy Assessment
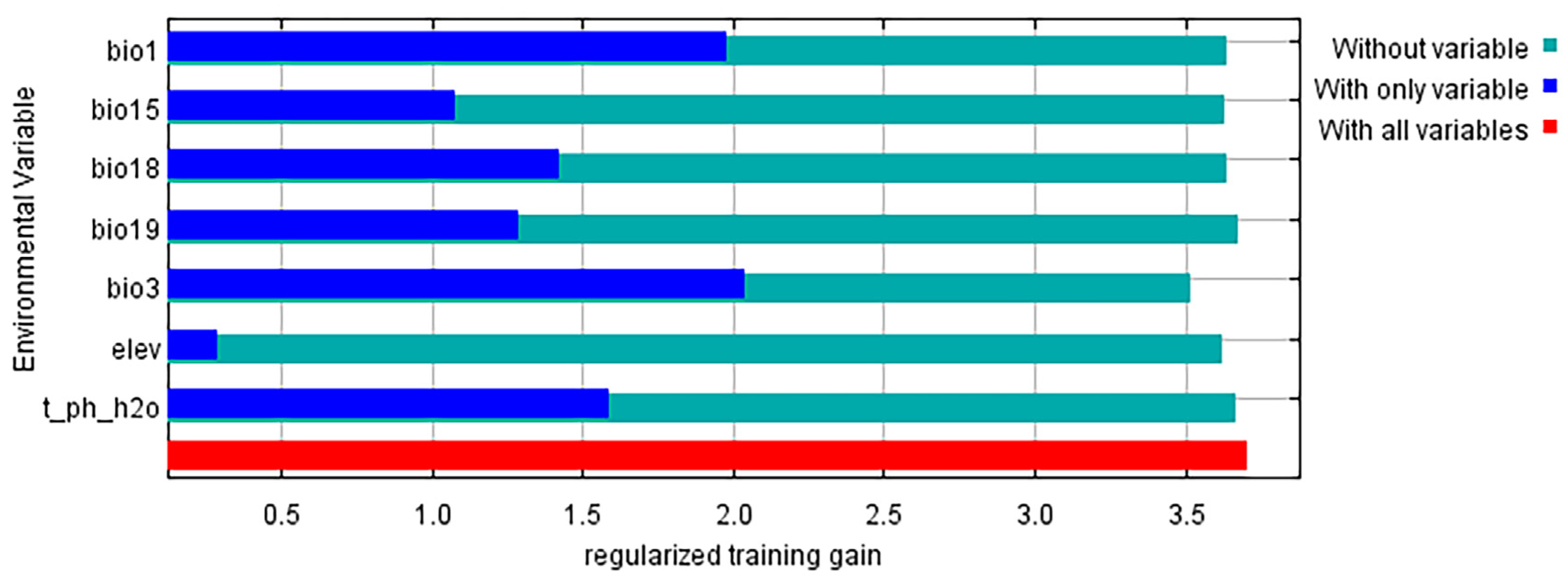
3.2. Main Environmental Variables Affecting the Distribution of Tamarix boveana
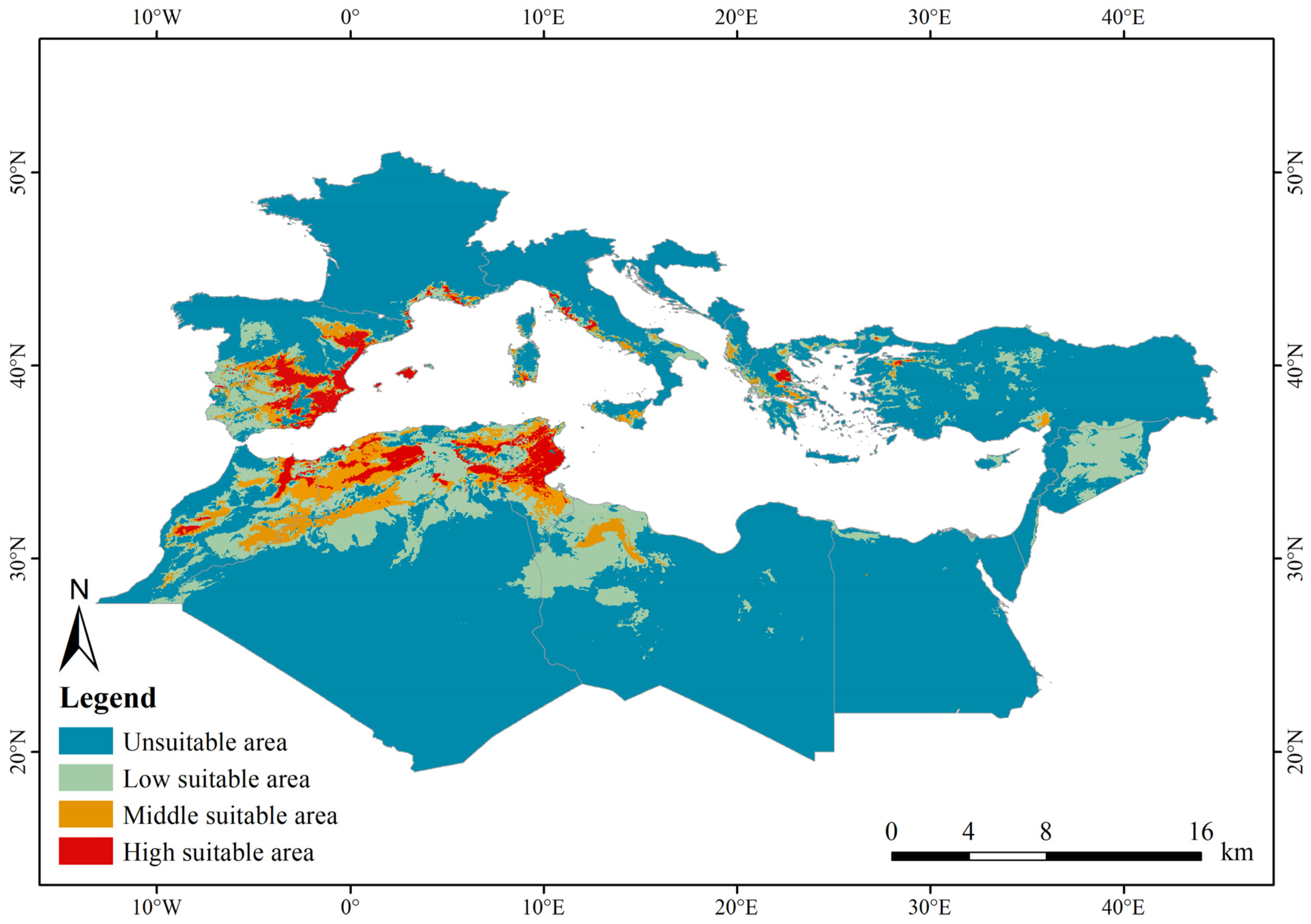
3.3. Results of Tamarix boveana Habitat Prediction During the Current Period
3.4. Modeling Results of Potential Tamarix boveana Habitat Areas in the Future
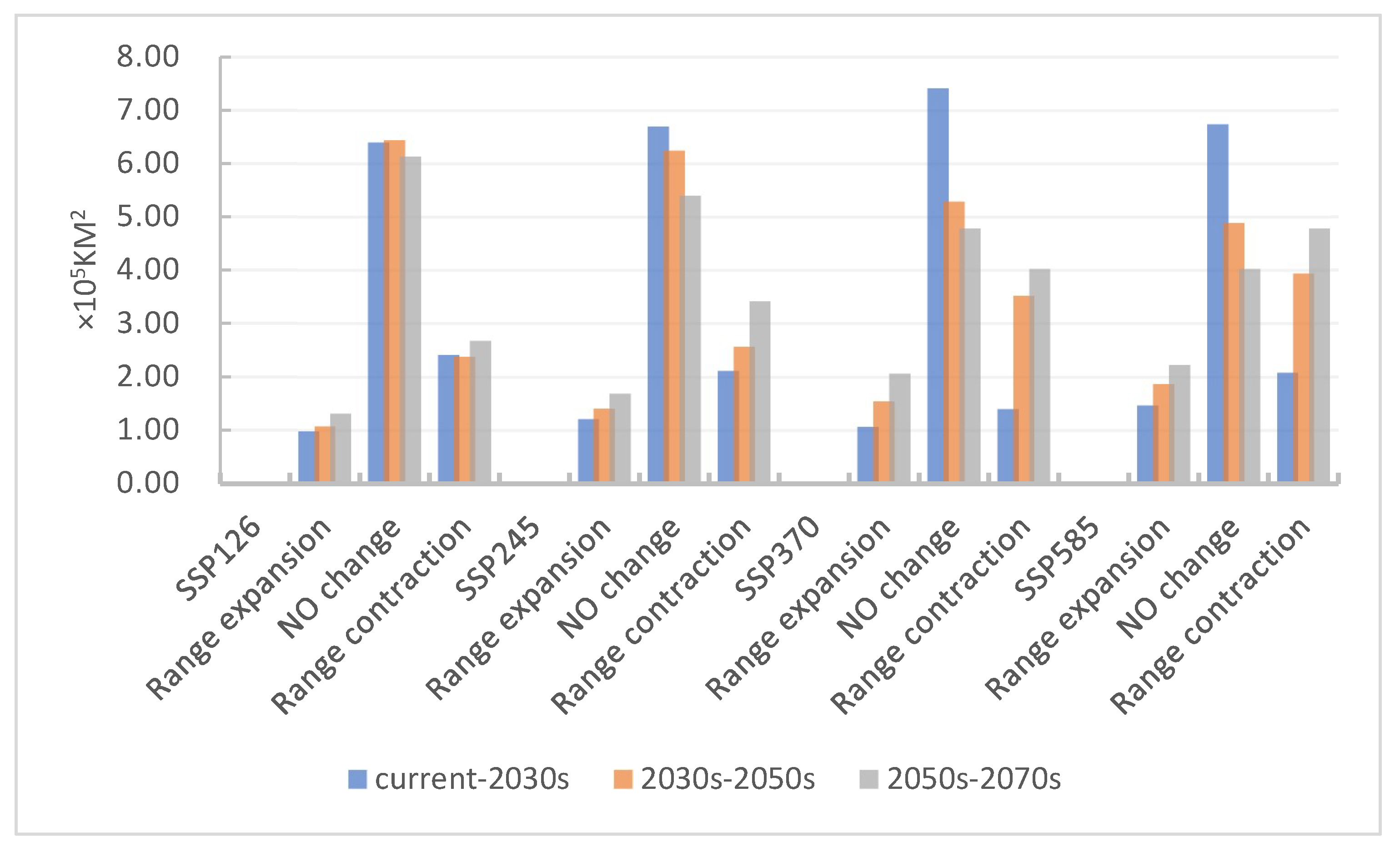
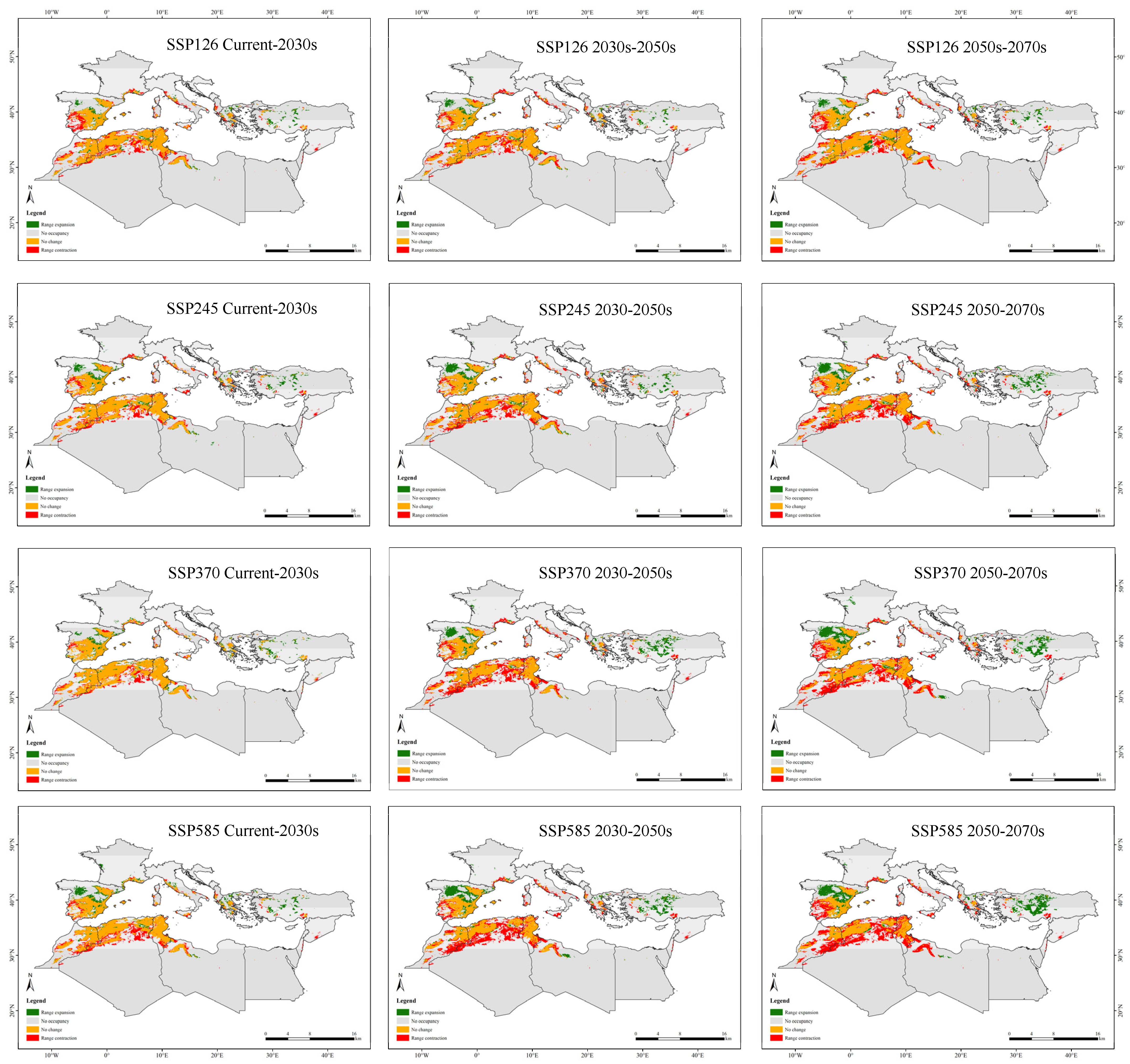
3.5. Changes in the Migration Patterns of Potentially Suitable Areas for Tamarix boveana over Time
3.6. Changes in the Centroid of Tamarix boveana over Time
4. Discussion
4.1. Model Prediction Accuracy
4.2. Environmental Factors Affecting the Distribution of Tamarix boveana
4.3. Distribution, Changes, and Analysis of Centroid Transfer in the Potential Fitness Zone of Tamarix boveana
4.4. Tamarix boveana Conservation Status and Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arocena, J.M.; van Mourik, J.M.; Schilder, M.L.M.; Faz, C.A. Initial soil development under pioneer plant species in metal mine waste deposits. Restor. Ecol. 2010, 18, 244–252. [Google Scholar] [CrossRef]
- Terrones, A.; Morano, J.; Agullo, J.; Villar, J.L.; Vicente, A.; Alonso, M.A.; Juan, A. Influence of salinity and storage on germination of Tamarix taxa with contrasted ecological requirements. J. Arid. Environ. 2016, 135, 17–21. [Google Scholar] [CrossRef]
- Beech, E. Tamarix boveana. IUCN Red List. Threat. Species 2018, 2018, e.T79928148A123870302. [Google Scholar]
- Ashori, A.; Nourbakhsh, A. Effect of press cycle time and resin content on physical and mechanical properties of particleboard panels made from the underutilized low-quality raw materials. Ind. Crops Prod. 2008, 28, 225–230. [Google Scholar] [CrossRef]
- Saïdana, D.; Mahjoub, M.A.; Boussaada, O.; Chria, J.; Chéraif, I.; Daami, M.; Mighri, Z.; Helal, A.N. Chemical composition and antimicrobial activity of volatile compounds of Tamarix boveana (Tamaricaceae). Microbiol. Res. 2008, 163, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Saidana, D.; Amel, B.; Boussaada, O. The antioxidant and free-radical scavenging activities of Tamarix boveana and Suaeda fruticosa fractions and related active compound. Eur. Sci. J. 2014, 10, 201–219. [Google Scholar]
- Saidana, D.; Halima, M.K.B.; Haouas, D.H.; Tiba, B. Insecticidal activities of Tunisian halophytic plant extracts against larvae and adults of Tribolium confusum. Tropicultura 2007, 25, 193–199. [Google Scholar]
- Kaddour, Z.; Khaled, M.T.O. Phytoremediation of Nutrient from Domestic Wastewater using Tamarix Boveana and Salsola Baryosma under salt stress. Asian J. Res. Chem. 2021, 14, 203–207. [Google Scholar] [CrossRef]
- Houtan, K.S.V.; Tanaka, K.R.; Gagné, T.O.; Becker, S. The geographic disparity of historical greenhouse emissions and projected climate change. Sci. Adv. 2021, 7, eabe4342. [Google Scholar] [CrossRef]
- Duan, R.; Kong, X.; Huang, M.; Varela, S.; Ji, X. The potential effects of climate change on amphibian distribution, range fragmentation and turnover in China. PeerJ 2016, 4, e2185. [Google Scholar] [CrossRef]
- Terence, P.D.; Stephen, T.J.; Joanna, I.H.; Iain, C.P.; Georgina, M.M. Beyond predictions: Biodiversity conservation in a changing climate. Science 2011, 332, 53–58. [Google Scholar]
- Nolan, C.; Overpeck, J.T.; Allen, J.R.M.; Anderson, P.M.; Betancourt, J.L.; Binney, H.A.; Brewer, S.; Bush, M.B.; Chase, B.M.; Cheddadi, R.; et al. Past and future global transformation of terrestrial ecosystems under climate change. Science 2018, 361, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.G.; Moody, H.; Hull, V.; Davis, S.J.; Gaskell, J.; Hertel, T.; Lubchenco, J.; Seto, K.C.; Gleick, P.; Kremen, C.; et al. Systems integration for global Sustainability. Science 2015, 347, 1258832. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Hanson, P.J.; Weltzin, J.F. Drought disturbance from climate change: Response of United States forests. Sci. Total Environ. 2000, 262, 205–220. [Google Scholar] [CrossRef]
- Ye, X.Z.; Zhang, M.Z.; La, W.F.; Yang, M.M.; Fan, H.H.; Zhang, G.F.; Chen, S.P.; Liu, B. Prediction of potential suitable distribution of Phoebe bournei based on MaxEnt optimization model. Acta Ecol. Sin. 2021, 41, 8135–8144. [Google Scholar]
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Giorgi, F. Climate change Hot-spots. Geophys. Res. Lett. 2006, 33, L08707. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Guo, Y.L.; Zhao, Z.F.; Qiao, H.J.; Wang, R.; Wei, H.Y.; Wang, L.K.; Gu, W.; Li, X. Challenges and development trend of species distribution model. Adv. Earth Sci. 2020, 35, 1292–1305. [Google Scholar]
- Philips, S.J.; Anderson, R.P.; Schapire, R.E. Maxinum entropy moedling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of MaxEnt. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Crimmins, S.M.; Dobrowski, S.Z.; Mynsberge, A.R. Evaluating ensemble forecasts of plant species distributions under climate change. Ecol. Model. 2013, 266, 126–130. [Google Scholar] [CrossRef]
- Smith, A.B.; Santos, M.J.; Koo, M.S.; Rowe, K.M.C.; Rowe, K.C.; Patton, J.L.; Perrine, J.D.; Beissinger, S.R.; Moritz, C. Evaluation of species distribution models by resampling of sites surveyed a century ago by Joseph Grinnell. Ecography 2013, 36, 1017–1031. [Google Scholar] [CrossRef]
- Zhu, G.P.; Peterson, A.T. Do consensus models outperform individual models? Transferability evaluations of diverse modeling approaches for an invasive moth. Biol. Invasions 2017, 19, 2519–2532. [Google Scholar] [CrossRef]
- Li, R.Z.; Hu, X.J.; Li, Q.Z.; Liu, L.Y.; He, Y.R.; Chen, C.Y. Gap analysis of Firmiana danxiaensis, a rare tree species endemic to southern China. Ecol. Indic. 2024, 158, 111606. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef]
- Gargano, M.L.; Mandracchia, G.; Venturella, G.; Calvo, R. A revision of Tamarix specimens (Tamaricaceae) kept in the BCN herbarium of Barcelona (Spain). Fl. Medit. 2018, 28, 393–397. [Google Scholar]
- Wu, H.; Liu, Y.X.; Zhang, T.T.; Xu, M.X.; Rao, B.Q. Impacts of soil Properties on Species Diversity and Structure in Alternanthera philoxeroides-Invaded and Native Plant Communities. Plants 2024, 13, 1196. [Google Scholar] [CrossRef]
- Ni, M.; Vellend, M. Soil effects on plant distributions and potential migration in Eastern North America. Res. Gate 2022, 1, 1–18. [Google Scholar]
- Wu, T.W.; Lu, Y.X.; Fang, Y.J.; Li, L.; Li, W.P.; Jie, W.H.; Zhang, J.; Liu, Y.M.; Zhang, L.; Zhang, F.; et al. The Beijing Climate Center Climate System Model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model. Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Zhou, R.B.; Gao, Y.; Chang, N.; Ma, D.L.; Li, C.; Wu, H.X.; Wang, J.; Liu, Q.Y. Potential distribution of Triatoma rubrofasciata under different climatic scenarios in China. Chin. J. Biol. Control 2022, 33, 125–132. [Google Scholar]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.M.; Carl, G.; Gabriel, C.; Diekotter, T.; Jaime, R.G.; Gruber, B.; Lafourcade, B.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Xie, B.Y.; Wan, F.H.; Xiao, Q.M.; Dai, L.Y. Application of ROC curve analysis in evaluating the performance of alien species’ potential distribution models. Biodivers. Sci. 2007, 15, 365–372. [Google Scholar]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Chen, X.M.; Lei, Y.C.; Zhang, X.Q.; Jia, H.Y. Effects of sample sizes on accuracy and stability of maximum entropy model in predicting species distribution. Sci. Silvae Sin. 2012, 48, 53–59. [Google Scholar]
- Liu, L.; Zheng, J.H.; Guan, J.Y.; Han, W.Q.; Liu, Y.J. Grassland cover dynamics and their relationship with climatic factors in China from 1982 to 2021. Sci. Total Environ. 2023, 905, 167067. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Guo, F.Y.; Liu, X.H.; Zhao, X.F.; Maimaiti, Z.; Wang, Y.G.; Lin, T.; Zhao, C.Y.; Xing, L.Y.; Wang, R.; Zhao, H.H.; et al. Impacts of climate change and human activities on saline vegetation-an example of Tamarix chinensis. Geol. China 2024, 1–16. [Google Scholar] [CrossRef]
- Liu, Y.J.; Ge, Q.S.; Dai, J.H. Research progress in crop phenology under global climate change. Acta Geogr. Sin. 2020, 75, 14–24. [Google Scholar]
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Duan, Q.; Zhu, Z.; Wang, B.; Chen, M. Recent Progress on the Salt Tolerance Mechanisms and Application of Tamarisk. Int. J. Mol. Sci. 2022, 23, 3325. [Google Scholar] [CrossRef]
- Shen, X.T.; TU, B.H.; LIU, C.Y.; Zhao, Y.; Ding, J.X.; Liang, Y.T. Mechanisms of rhizosphere microorganisms in regulating plant root system architecture in acidic soils. Environ. Sci. 2025, 46, 570–578. [Google Scholar]
- Francisco, G.M.; Fernando, M.T.; Esther, G.; Sergio, H.L. Salinity Tolerance of the Hygrophilous Plant Species in the Wetlands of the South of the Iberian Peninsula. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 18–28. [Google Scholar]
- Wu, T.G.; Wu, M.; Yu, M.K.; Xiao, J.H. Plant distribution in relation to soil conditions in hangzhou bay coastal wetlands, China. Pak. J. Bot. 2011, 43, 2331–2335. [Google Scholar]
- Boivin, N.L.; Zeder, M.A.; Fuller, D.Q.; Crowther, A.; Larson, G.; Erlandson, J.M.; Denham, T.; Petraglia, M.D. Ecological consequences of human niche construction: Examining long-term anthropogenic shaping of global species distributions. Proc. Natl. Acad. Sci. USA 2016, 113, 6388–6396. [Google Scholar] [CrossRef]
- Zhou, S.; Yu, B.F.; Zhang, Y. Global concurrent climate extremes exacerbated by anthropogenic climate change. Sci. Adv. 2023, 9, eabo1638. [Google Scholar] [CrossRef]
- Cramer, W.; Guiot, J.; Fader, M.; Garrabou, J.; Gattuso, J.P.; Lglesias, A.; Lange, M.A.; Lionello, P.; Liasat, M.C.; Paz, S.; et al. Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Clim. Change 2018, 8, 972–980. [Google Scholar] [CrossRef]
- Filonchyk, M.; Peterson, M.P.; Zhang, L.F.; Hurynovich, V.; He, Y. Greenhouse gases emissions and global climate change: Examining the influence of CO2, CH4, and N2O. Sci. Total Environ. 2024, 935, 173359. [Google Scholar] [CrossRef] [PubMed]
- Hernaiz-Huerta, P.P. The geology of Sierra Calderona (SE Iberian Cordillera, Spain), a review. Results of a new 1:25.000 scale geological mapping. J. Maps 2024, 20, 2302363. [Google Scholar] [CrossRef]
- Ding, W.N.; Ree, R.H.; Apicer, R.A.; Xing, Y.W. Ancient orogenic and monsoon-driven assembly of the world’s richest temperate alpine flora. Science 2020, 369, 578–581. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhao, R.X.; Zhou, X.Y.; Zhang, X.L.; Zhao, G.H.; Zhang, F.G. Prediction of potential distribution areas and priority protected areas of Agastache rugosa based on Maxent model and Marxan model. Front. Plant Sci. 2023, 14, 1200796. [Google Scholar] [CrossRef]
- Rodrigues, J.F.M.; Botero, C.A. The global determinants of climate niche breadth in birds. Nat. Commun. 2025, 16, 3685. [Google Scholar] [CrossRef] [PubMed]
- Tanarhte, M.; De Vries, A.J.; Zittis, G.; Chfadi, T. Severe droughts in North Africa: A review of drivers, impacts and management. Earth Sci. Rev. 2024, 250, 104701. [Google Scholar] [CrossRef]
- Bi, M.; Wan, L.; Zhang, Z.; Zhang, X.; Yu, C. Spatio-Temporal Variation Characteristics of North Africa’s Climate Potential Productivity. Land 2023, 12, 1710. [Google Scholar] [CrossRef]
- Nematollanis, S.; Fakheran, S.; Kienast, F.; Jafari, A. Application of invest habitat quality module in spatially vulnerability asseaament of natural habitats (case study: Chaharmahal and Bakhtiari province, Iran). Environ. Monit. Assess. 2020, 192, 487. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, S.; Wang, H.; Gao, C.; Yang, C. Potential Distribution of Tamarix boveana Bunge in Mediterranean Coastal Countries Under Future Climate Scenarios. Forests 2025, 16, 1053. https://doi.org/10.3390/f16071053
Dong S, Wang H, Gao C, Yang C. Potential Distribution of Tamarix boveana Bunge in Mediterranean Coastal Countries Under Future Climate Scenarios. Forests. 2025; 16(7):1053. https://doi.org/10.3390/f16071053
Chicago/Turabian StyleDong, Siqi, Hongfeng Wang, Caiqiu Gao, and Chengjun Yang. 2025. "Potential Distribution of Tamarix boveana Bunge in Mediterranean Coastal Countries Under Future Climate Scenarios" Forests 16, no. 7: 1053. https://doi.org/10.3390/f16071053
APA StyleDong, S., Wang, H., Gao, C., & Yang, C. (2025). Potential Distribution of Tamarix boveana Bunge in Mediterranean Coastal Countries Under Future Climate Scenarios. Forests, 16(7), 1053. https://doi.org/10.3390/f16071053





