Burn Severity Does Not Significantly Alter Pollen Abundance Across a Burn Matrix Four Years Post Wildfire in Sub-Boreal Forests of British Columbia, Canada
Abstract
1. Introduction
1.1. Importance of Fire in Sub-Boreal Ecosystems
1.2. Importance of Pollen Dispersal for Plant Reproduction
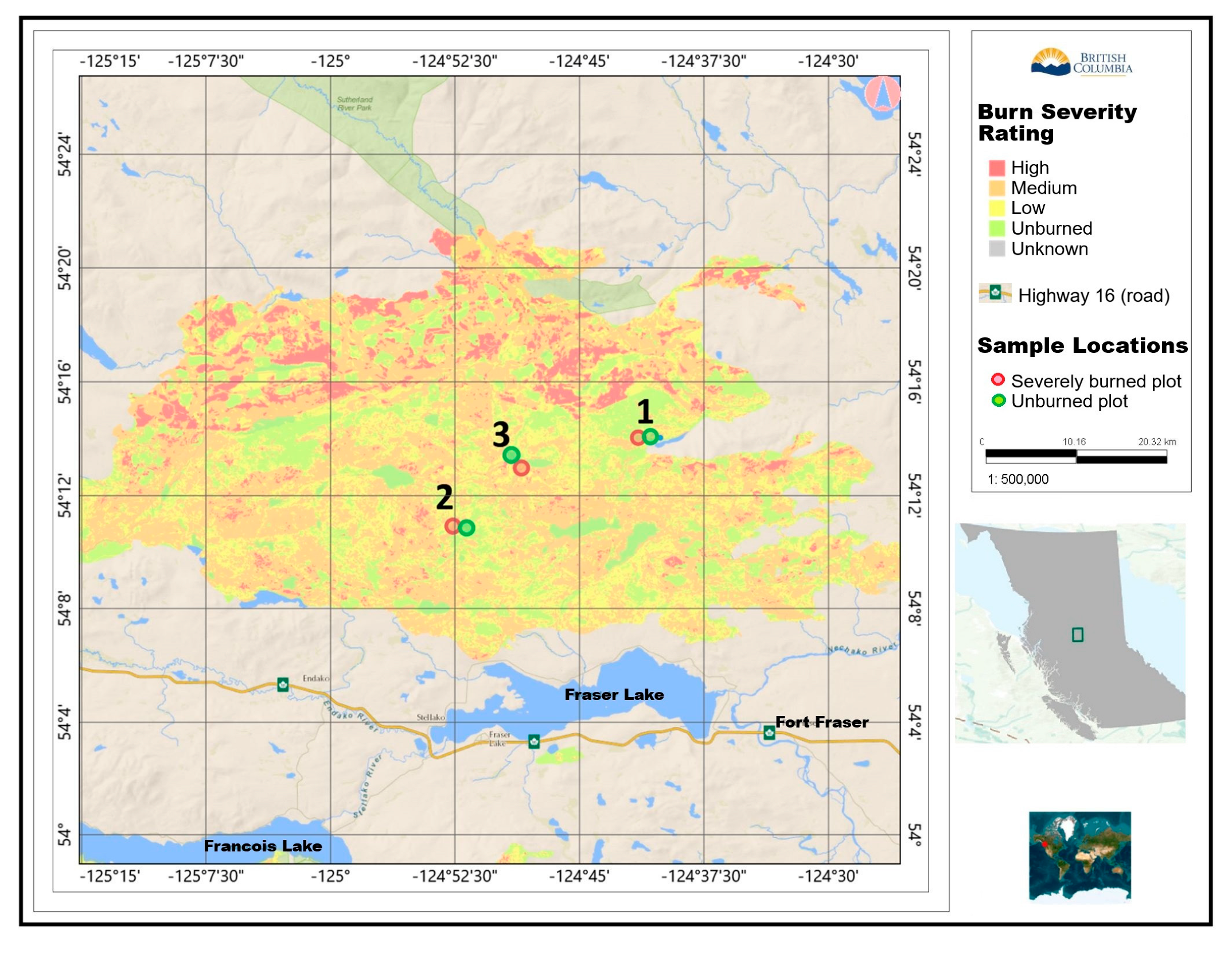
1.3. The Shovel Lake Wildfire Area
1.4. Research Objectives
- Whether the quantity and variability of pollen collected, summed from all species, differed between severely burned and unburned forest patches within the fire matrix;
- How the taxonomic composition of pollen samples varied, in terms of relative abundance and frequency of occurrence, between severely burned and unburned forest patches with focus on the four families of interest;
- Whether pollen composition (both frequency of occurrence and relative abundance) and plant community relative abundance follow the same patterns across space and burn severities, with focus on the four families of interest.
2. Materials and Methods
2.1. Study Sites
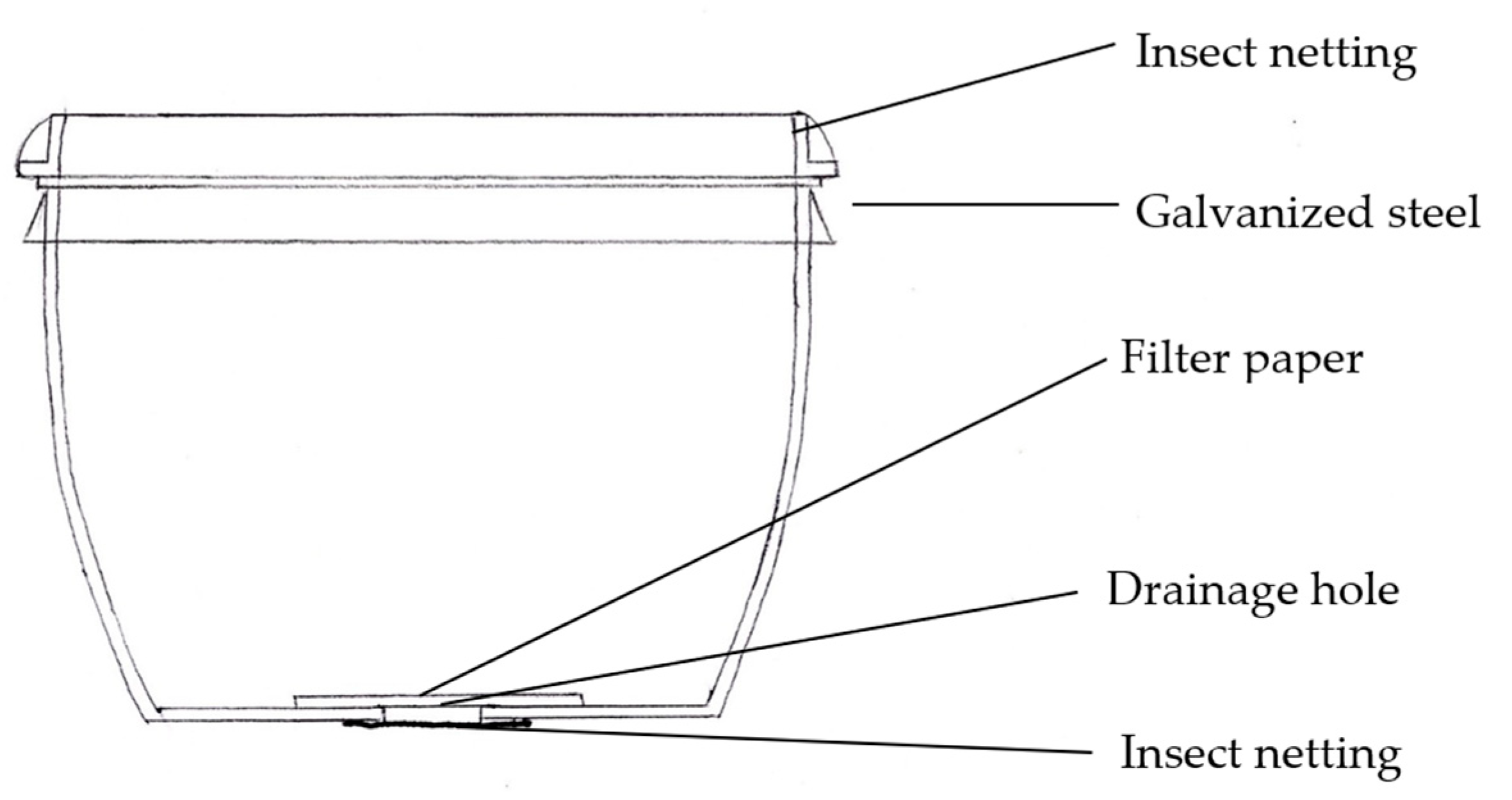
2.2. Pollen Sampling
2.3. Pollen Sample Processing
2.4. Plant Community Data Collection
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, D.F. Post-Fire Succession in the Sub-Boreal Spruce Forests of the Nechako Plateau, Central British Columbia. Master’s Thesis, University of Victoria, Victoria, BC, Canada, 1994. [Google Scholar]
- Kneeshaw, D.D. Tree Population Dynamics of Some Old-Growth Sub-Boreal Spruce Stands. Master’s Thesis, University of British Columbia, Vancouver, BC, Canada, 1992. [Google Scholar]
- Hamilton, E.H. Fire Effects and Post-Burn Vegetation Development in the Sub-Boreal Spruce Zone: Mackenzie (Windy Point) Site; British Columbia Ministry of Forests and Range: Victoria, BC, Canada, 2006.
- British Columbia Forest Practices Board. Fire Hazard Abatement and the Shovel Lake Wildfire: Complaint investigation #18061. 2019. Available online: https://www.bcfpb.ca/wp-content/uploads/2019/04/IRC221-Shovel-Lake.pdf (accessed on 1 April 2021).
- Kolden, C.A.; Abatzoglou, J.T.; Lutz, J.A.; Cansler, C.A.; Kane, J.T.; Van Wagtendonk, J.W.; Key, C.H. Climate contributors to forest mosaics: Ecological persistence following wildfire. Northwest Sci. 2015, 89, 219–238. [Google Scholar] [CrossRef]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Carleial, S.; van Kleunen, M.; Stift, M. Relatively weak inbreeding depression in selfing but also in outcrossing populations of North American Arabidopsis lyatra. J. Evol. Biol. 2017, 30, 1994–2004. Available online: https://doi-org.prxy.lib.unbc.ca/10.5061/dryad.6qj2n (accessed on 1 January 2023). [CrossRef]
- Wright, S.I.; Kalisz, S.; Slotte, T. Evolutionary consequences of self-fertilization in plants. Proc. Biol. Sci. 2013, 280, 20130133. [Google Scholar] [CrossRef]
- Regal, P.J. Pollination by wind and animals: Ecology of geographic patterns. Ann. Rev. Ecol. Syst. 1982, 13, 497–524. [Google Scholar] [CrossRef]
- Barrett, S.C.H.; Helenurm, K. The reproductive biology of boreal forest herbs I: Breeding systems and pollination. Can. J. Bot. 1986, 65, 2036–2046. [Google Scholar] [CrossRef]
- Owens, J.N.; Takaso, T.; Runions, C. Pollination in conifers. Trends Plant Sci. 1998, 3, 479–485. [Google Scholar] [CrossRef]
- Panchen, Z.A.; Johnston, M.O. Shifts in pollen release envelope differ between genera with non-uniform climate change. Am. J. Bot. 2018, 105, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Shwendemann, A.B.; Wang, G.; Mertz, M.L.; McWilliams, R.T.; Thatcher, S.L.; Osborn, J.M. Aerodynamics of saccate pollen and its implications for wind pollination. Am. J. Bot. 2007, 94, 1371–1381. [Google Scholar] [CrossRef]
- Fægri, K.; Iversen, J.; Kaland, P.E.; Krzywinski, K. Textbook of Pollen Analysis; John Wiley and Sons: Chichester, UK, 1989. [Google Scholar]
- Halbritter, H.; Ulrich, S.; Grímsson, F.; Weber, M.; Zetter, R.; Hesse, M.; Buchner, R.; Svojtka, M.; Frosch-Radivo, A. Illustrated Pollen Terminology, 2nd ed.; Springer International Publishing AG: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Ferguson, I.K.; Skvarla, J.J. Pollen morphology in relation to pollinators in Papilionoideae (Leguminosae). Bot. J. Linn. 1982, 84, 183–193. [Google Scholar] [CrossRef]
- Pacini, E.; Hesse, M. Pollenkitt—Its composition, forms, and functions. Ecol. Plants 2005, 200, 399–415. [Google Scholar] [CrossRef]
- Banza, P.; Macgregor, C.J.; Belo, A.D.F.; Fox, R.; Pocock, M.J.O.; Evans, D.M. Wildfire alters the structure and seasonal dynamics of nocturnal pollen-transport networks. Funct. Ecol. 2019, 33, 1882–1892. [Google Scholar] [CrossRef]
- Banza, P.; Evans, D.M.; Medeiros, R.; Macgregor, C.J.; Belo, A.D.F. Short-term positive effects of wildfire on diurnal insects and pollen transport in a Mediterranean ecosystem. Ecol. Entomol. 2021, 46, 1353–1363. [Google Scholar] [CrossRef]
- Shohami, D.; Nathan, R. Fire –induced population reduction and landscape opening increases gene flow via pollen dispersal in Pinus halepensis. Molec. Ecol. 2014, 23, 70–81. [Google Scholar] [CrossRef]
- Tadoumant, S.; Bouimetarhan, I.; Baqloul, A.; Hssaisoune, M.; Reddad, H.; Bouchaou, L. Modern pollen distribution and its relationship with environmental gradient in southern Morocco. Rev. Palaeobot. Palynol. 2022, 298, 104595. [Google Scholar] [CrossRef]
- Brown, K.J.; Hebda, N.J.; Conder, N.; Golinski, K.G.; Hawkes, B.; Schoups, G. Changing climate, vegetation, and fire disturbance in a sub-boreal pine-dominated forest, British Columbia, Canada. Can. J. For. Res. 2017, 47, 615–627. [Google Scholar] [CrossRef]
- Chileen, B.V.; McLauchlan, K.K.; Higuera, P.E.; Parish, M.; Shuman, B.N. Vegetation response to wildfire and climate in a Rocky Mountain lodgepole pine forest over the past 2500 years. Holocene 2020, 30, 1493–1503. [Google Scholar] [CrossRef]
- British Columbia Wildfire Service; Government of British Columbia: Victoria, BC, Canada, 2018. Available online: http://bcfireinfo.for.gov.bc.ca/hprScripts/WildfireNews/OneFire.asp?ID=712 (accessed on 14 November 2022).
- Shovel Lake Wildfire Ecosystem Restoration Plan; [SERNbc] Society for Ecosystem Restoration in Northern British Columbia: Vanderhoof, BC, Canada, 2019.
- Edwards, M.; Krawchuk, M.A.; Burton, P.J. Short-interval disturbance in lodgepole pine forests, British Columbia, Canada: Understory and overstory response to mountain pine beetle and fire. For. Ecol. Manag. 2015, 338, 163–175. [Google Scholar] [CrossRef]
- Key, C.H.; Benson, N.C. Landscape assessment (LA): Sampling and analysis methods. In FIREMON: Fire Effects Monitoring and Inventory System; Lutes, D.C., Keane, R.E., Caratti, J.F., Key, C.H., Benson, N.C., Sutherland, S., Gangi, L.J., Eds.; USDA Forest Service: Fort Collins, CO, USA, 2006; p. LA-1-55. Available online: https://www.fs.usda.gov/rm/pubs_series/rmrs/gtr/rmrs_gtr164/rmrs_gtr164_13_land_assess.pdf (accessed on 1 April 2021).
- Google Earth Pro, version 7.3.6.9345; Google: Mountain View, CA, USA, 2022.
- Gosling, W.D.; Mayle, F.E.; Killeen, T.J.; Siles, M.; Sanchez, L.; Boreham, S. A simple and effective methodology for sampling modern pollen rain in tropical environments. Holocene 2003, 13, 613–618. [Google Scholar] [CrossRef]
- Bush, M.B. A simple yet effective pollen trap for use in vegetation studies. J. Veg. Sci. 1992, 3, 275–276. [Google Scholar] [CrossRef]
- Agriculture and Agri-Food Canada’s Agri-Environmental Services Branch (AESB); Government of Canada Publications: Ottawa, ON, Canada, 2010. Available online: https://publications.gc.ca/collections/collection_2018/aac-aafc/A59-57-2010-eng.pdf (accessed on 1 January 2023).
- Crompton, C.W.; Wojtas, W.A. Pollen Grains of Canadian Honey Plants; Agriculture Canada Research Branch: Ottawa, ON, USA, 1993. [Google Scholar]
- Martin, A.C.; Harvey, W.J. The Global Pollen Project: A new tool for pollen identification and the dissemination of physical reference collections. Methods Ecol. Evol. 2017, 8, 892–897. [Google Scholar] [CrossRef]
- Lechowicz, K.; Wrońska-Pilarek, D.; Bocianowski, J.; Maliński, T. Pollen morphology of Polish species from the genus Rubus L. (Rosaceae) and its systematic importance. PLoS ONE 2020, 15, e0221607. [Google Scholar] [CrossRef]
- Xiong, X.-H.; Zhou, X.-m.; Li, M.; Xu, B.; Deng, H.-N.; Yu, Q.; Gao, X. Pollen morphology in Rubus (Rosaceae) and its taxonomic implications. Plant Syst. Evol. 2019, 305, 705–716. [Google Scholar] [CrossRef]
- Hebda, R.J.; Chinnappa, C.C. Studies on pollen morphology of Rosaceae. Acta Bot. Gall. 1994, 141, 183–193. [Google Scholar] [CrossRef]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation. R Package Version 1.1.4. 2023. Available online: https://github.com/tidyverse/dplyr (accessed on 1 November 2023).
- Wickham, H.; Bryan, J. readxl: Read Excel Files. 2023. Available online: https://github.com/tidyverse/readxl (accessed on 1 November 2023).
- MacKinnon, A.; Pojar, J.; Coupe, R. (Eds.) Plants of Northern British Columbia, 2nd ed.; B.C. Ministry of Forests and Lone Pine Media Productions (BC) Ltd.: Vancouver, BC, Canada, 2021. [Google Scholar]
- Straka, J.R.; Starzomski, B.M. Fruitful factors: What limits seed production of flowering plants in the alpine? Oecologica 2015, 178, 249–260. [Google Scholar] [CrossRef]
- Luijten, S.H.; Oostermeijer, J.G.B.; van Leeuwen, N.C.; den Nijs, H.C.M. Reproductive success and clonal genetic structure of the rare Arnica montana (Compositae) in the Netherlands. Plant Syst. Evol. 1996, 201, 15–30. [Google Scholar] [CrossRef]
- Clark, M.J.; Husband, B.C. Plasticity and timing of flower closure in response to pollination in Chamerion angustifolium (Onagraceae). Int. J. Plant Sci. 2007, 168, 619–625. [Google Scholar] [CrossRef]
- Spellman, K.V.; Schneller, L.C.; Mulder, C.P.H.; Carlson, M.L. Effects of non-native Melilotus albus on pollination and reproduction in two boreal shrubs. Oecologica 2015, 179, 495–507. [Google Scholar] [CrossRef]
- Usui, M.; Kevan, P.G.; Obbard, M. Pollination and breeding system of lowbush blueberries, Vaccinium angustifolium Ait. and V. myrtilloides Michx. (Ericaceae), in the boreal forest. Can. Field Nat. 2005, 119, 48–57. [Google Scholar] [CrossRef]
- Fægri, K.; Iversen, J. Textbook of Pollen Analysis, 3rd ed.; John Wiley and Sons: New York, NY, USA, 1975. [Google Scholar]
| Plot | Distance to Road (m) | Distance to Forest Edge (m) | Distance to Other Opening (m) | Approximate Slope Position | Relative Soil Moisture |
|---|---|---|---|---|---|
| U1 | 40 | 453 | 91 | Backslope (mid-slope) | Moderate |
| S1 | 47 | 175 | 54 | Crest | Moderate-high |
| U2 | 81 | 183 | 570 | Shoulder (upper-slope) | High |
| S2 | 21 | 27 | 27 | Foot/toe slope | Low |
| U3 | 34 | 75 | 29 | Flat at toe of slope | Moderate-high |
| S3 | 118 | 45 | 106 | Backslope, in gully | High |
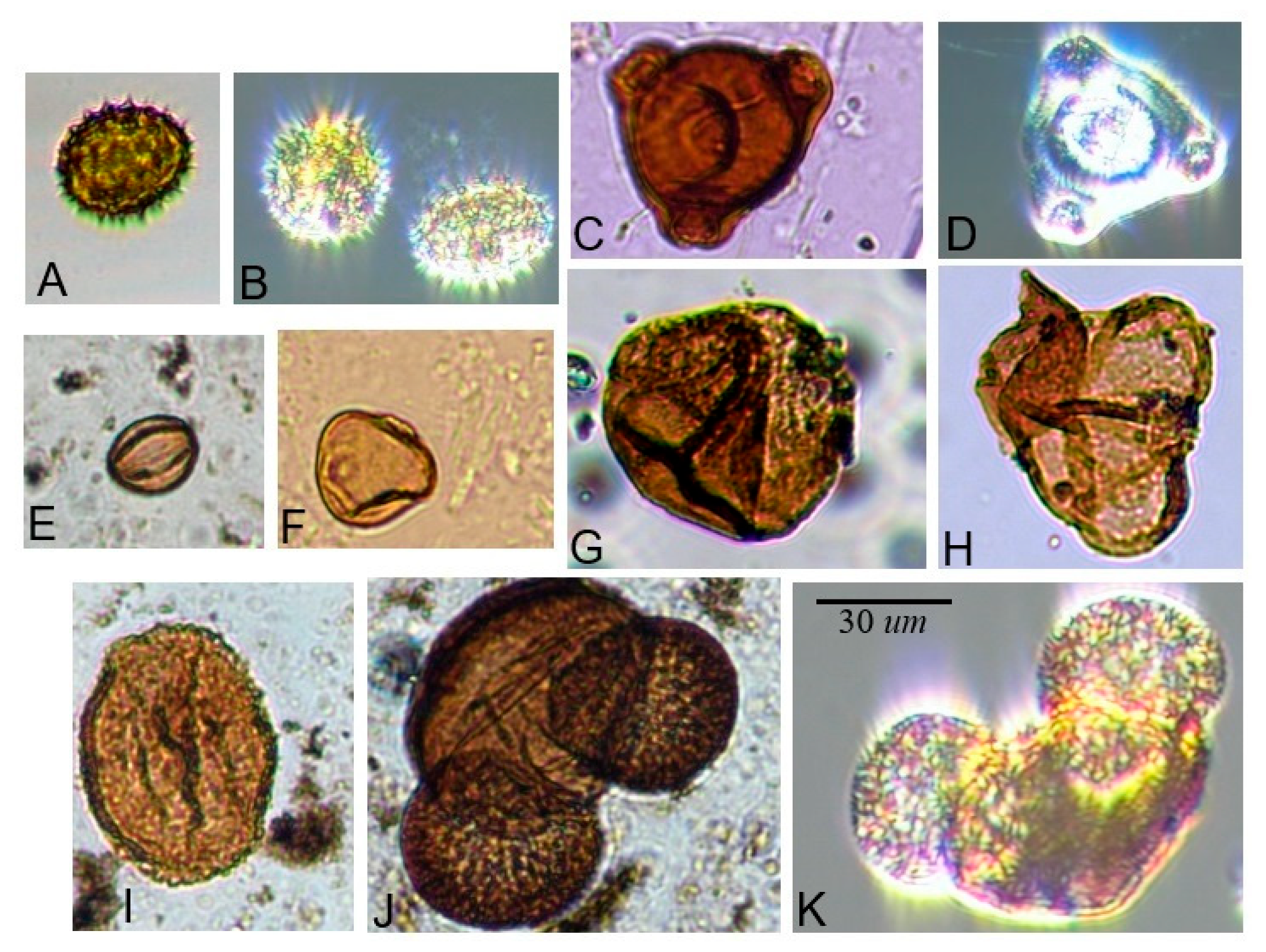
| Family | Diameter | Shape | Ornamentation | Aperture Type |
|---|---|---|---|---|
| Pinaceae | 70–100 µm | Saccate | Any | NA |
| Asteraceae | 20–30 µm | Oblate to suboblate or peroblate | Echinate (or echinolophate) | Tricolporate |
| Onagraceae | 55–65 µm | Spherical to sub-triangular | Psilate | Tetraporate |
| Ericaceae | 29–45.3 µm (entire tetrad) | Spherical, arranged in permanent tetrads | Any (Ericaceae is the only plant family with permanent pollen tetrads that is native to the region) | Any |
| Rosaceae | In Rubus: Average length of polar axis is 27.24 ± 7.51 μm and equatorial axis is 20.47 ± 4.58 μm [35] (Xiong et al. 2019) | Prolate to oblate ellipsoidal, most genera have subspherical pollen [36] (Hebda and Chinnapa 1994) | In Rubus: Perforations are large and often extending onto tectal ridges; smooth or verrucate sculpturing with pores [36] (Hebda and Chinnapa 1994) | Tricolporate (rarely tricolpate) [36] (Hebda and Chinnapa 1994) |
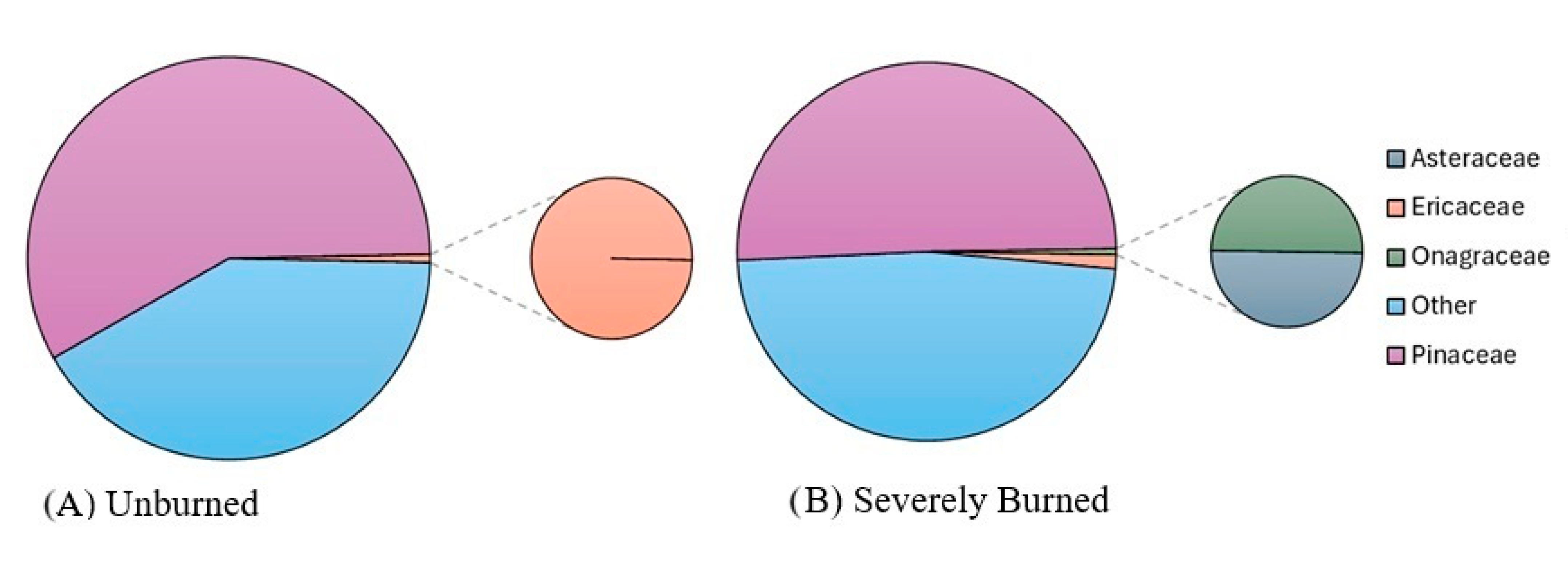
| Plant Relative Abundance (In Treatment) | Pollen Relative Abundance (In Treatment) | ||||
|---|---|---|---|---|---|
| Family | Treatment | Mean | Std. Deviation | Mean | Std. Deviation |
| Asteraceae | Severe | 1.985% | 1.911% | 0.081% | 0.141% |
| Ericaceae | Severe | 2.878% | 3.919% | 0.407% | 0.373% |
| Onagraceae | Severe | 9.089% | 2.893% | 0.081% | 0.141% |
| Other | Severe | 11.862% | 4.830% | 15.935% | 7.562% |
| Pinaceae | Severe | 1.177% | 1.143% | 16.829% | 12.577% |
| Asteraceae | Unburned | 0.962% | 1.401% | 0.000% | 0.000% |
| Ericaceae | Unburned | 10.252% | 3.367% | 0.219% | 0.190% |
| Onagraceae | Unburned | 0.216% | 0.143% | 0.000% | 0.000% |
| Other | Unburned | 12.451% | 8.087% | 13.816% | 10.542% |
| Pinaceae | Unburned | 8.068% | 7.148% | 19.298% | 10.492% |
| Site | Burn Severity | Asteraceae Pollen Frequency (% of Traps) | Ericaceae Pollen Frequency (% of Traps) | Onagraceae Pollen Frequency (% of Traps) |
|---|---|---|---|---|
| 1 | Unburned | 0 | 25 | 12.5 |
| Severe | 50 | 33.3 | 11.1 | |
| 2 | Unburned | 0 | 33.3 | 0 |
| Severe | 50 | 25 | 25 | |
| 3 | Unburned | 40 | 40 | 20 |
| Severe | 40 | 80 | 80 |
| Families of Interest | Genera in Unburned Sample Area | Genera in Severely Burned Sample Area |
|---|---|---|
| Asteraceae | Achillea | Anaphalis |
| Anaphalis | Arnica | |
| Antennaria | Hieracium | |
| Arnica | Petasites | |
| Aster | Taraxacum | |
| Petasites | ||
| Ericaceae | Arctostaphylos | Orthilia |
| Empetrum | Vaccinium | |
| Gaultheria | ||
| Orthilia | ||
| Rhododendron | ||
| Vaccinium | ||
| Onagraceae | Chamaenerion | Chamaenerion |
| Pinaceae | Abies | Picea |
| Picea | Pinus | |
| Pseudotsuga | ||
| Rosaceae | Amelanchier | Rosa |
| Fragaria | Rubus | |
| Rosa | Spirea | |
| Rubus | ||
| Spirea |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berg-Khoo, L.; Wilford, S.; Wood, L.J. Burn Severity Does Not Significantly Alter Pollen Abundance Across a Burn Matrix Four Years Post Wildfire in Sub-Boreal Forests of British Columbia, Canada. Forests 2025, 16, 1051. https://doi.org/10.3390/f16071051
Berg-Khoo L, Wilford S, Wood LJ. Burn Severity Does Not Significantly Alter Pollen Abundance Across a Burn Matrix Four Years Post Wildfire in Sub-Boreal Forests of British Columbia, Canada. Forests. 2025; 16(7):1051. https://doi.org/10.3390/f16071051
Chicago/Turabian StyleBerg-Khoo, Laurel, Stephanie Wilford, and Lisa J. Wood. 2025. "Burn Severity Does Not Significantly Alter Pollen Abundance Across a Burn Matrix Four Years Post Wildfire in Sub-Boreal Forests of British Columbia, Canada" Forests 16, no. 7: 1051. https://doi.org/10.3390/f16071051
APA StyleBerg-Khoo, L., Wilford, S., & Wood, L. J. (2025). Burn Severity Does Not Significantly Alter Pollen Abundance Across a Burn Matrix Four Years Post Wildfire in Sub-Boreal Forests of British Columbia, Canada. Forests, 16(7), 1051. https://doi.org/10.3390/f16071051






