Physiological Characteristics and Transcriptomic Analysis of Young Stems Differentiation in Adventitious Bud and Root Formation in Cinnamomum parthenoxylon
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Measurement of Endogenous Nutrient, Hormone, and Enzyme Activity
2.3. Transcriptome sequencing and Data Analysis
2.3.1. RNA Extraction and Transcriptome Sequencing
2.3.2. Transcriptome Assembly and Functional Annotation
2.3.3. Identification and Analysis of Differentially Expressed Genes (DEGs)
2.3.4. Validation of RNA-Seq by qRT-PCR
2.3.5. Data Processing
3. Results
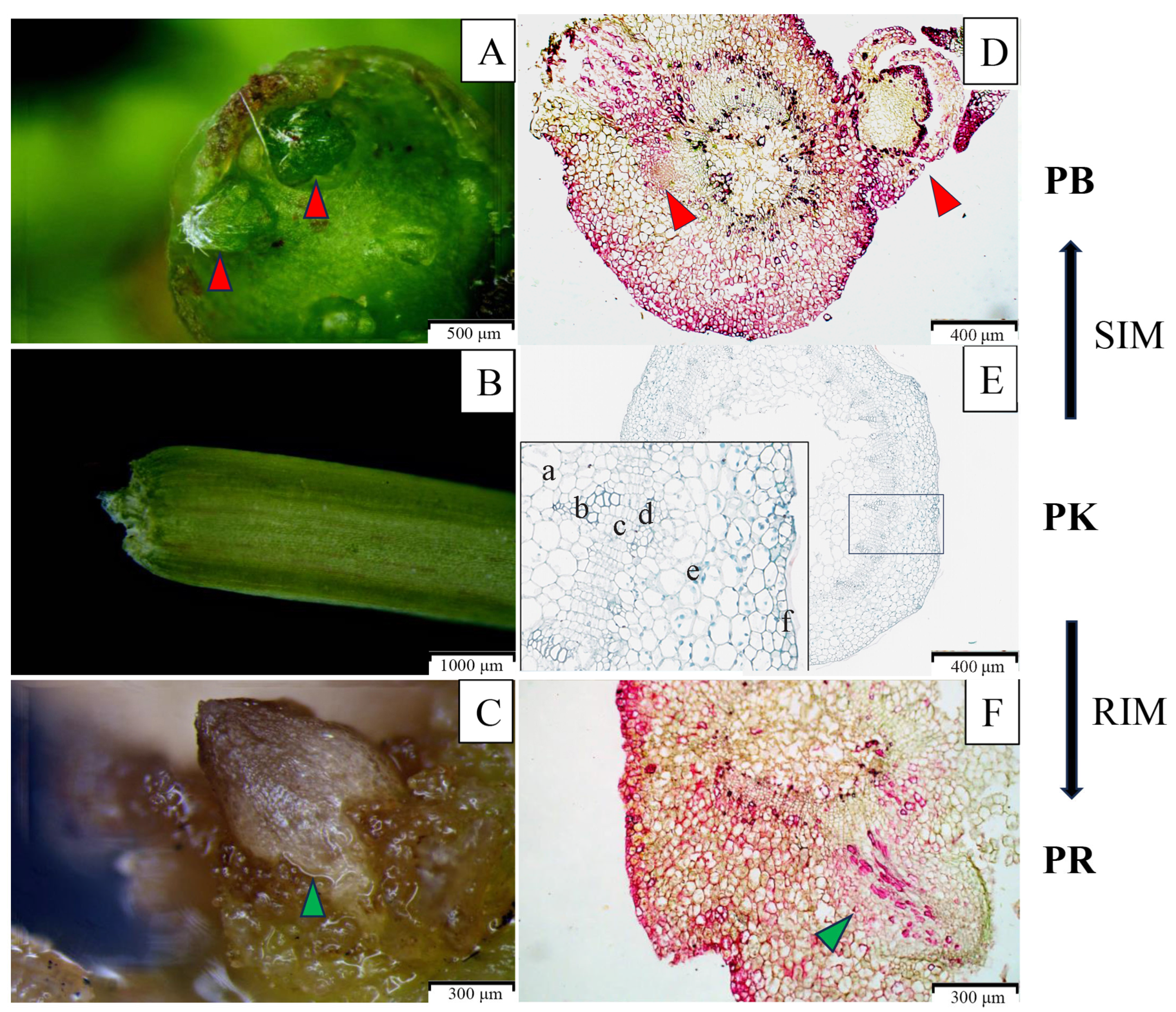
3.1. Analysis of Phenotype Characteristics of Explants at the Early Stage of In Vitro Regeneration
3.2. Changes in Nutrient Content and Enzyme Activity
3.3. Changes in Endogenous Hormone Levels
3.4. Transcriptome and Differentially Expressed Genes Analysis
3.5. Functional Enrichment Analysis of DEGs
3.6. Analysis of DEGs in Major Enriched Pathways
3.6.1. Plant Hormone Signal Transduction Pathway
3.6.2. Phenylpropanoid Biosynthesis Pathway
3.6.3. Starch and Sugar Metabolism Pathway
3.7. Gene Expression Involved in the Metabolism of Related Substances
3.8. Validation of Gene Expression
4. Discussion
4.1. Physiological and Biochemical Changes During the Differentiation of the Young Stems
4.2. Gene Expression During the Differentiation of the Young Stems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABs | Adventitious buds |
| ARs | Adventitious roots |
| PB | 7 days of ABs induction |
| PK | Pre-induction |
| PR | 9 days of ARs induction |
| DEGs | Differentially expressed genes |
References
- Qiu, F.; Yang, H.; Fu, C.; Zhou, S.; Sheng, Y.; Zhang, T. Essential oil diversity of Cinnamomum parthenoxylon (Jack) Meisner from China. S. Afr. J. Bot. 2023, 158, 452–460. [Google Scholar] [CrossRef]
- Alencar-Luciano, W.; Magnani, M.; Martín-Belloso, O.; Salvia-Trujillo, L. Effect of digestible versus non-digestible citral nanoemulsions on human gut microorganisms: An in vitro digestion study. Food Res. Int. 2023, 173, 113313. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Su, B.; Jiang, H.; Cui, N.; Yu, Z.; Yang, Y.; Sun, Y. Traditional uses, phytochemistry and pharmacological activities of the genus Cinnamomum (Lauraceae): A review. Fitoterapia 2020, 146, 104675. [Google Scholar] [CrossRef]
- Dallay, C.; Malhiac, C.; Duchemin, B.; Savary, G.; Picard, C. Effect of linalool on lamellar-structured emulsions: From molecular organization to organoleptic properties. Food Hydrocoll. 2024, 149, 109575. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, W. Conservation and applications of camphor tree (Cinnamomum camphora) in China: Ethnobotany and genetic resources. Genet. Resour. Crop Evol. 2016, 63, 1049–1061. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, T.; Sheng, Y.; Wang, W.; Wen, S.; Qiu, F. Changes in essential oil contents and principal components of Cinnamomum parthenoxylon at different ages. South China For. Sci. 2023, 51, 5–10. (In Chinese) [Google Scholar]
- Costa Junior, E.D.S.; Barbosa, M.S.d.M.; Silva, C.M.d.; Silva, R.V.C.d.; Kiill, L.H.P.; Beckmann-Cavalcante, M.Z. Vegetative propagation of Rhaphiodon echinus Schauer (Lamiaceae): Effects of the period of cutting in rooting, cuttings arrangement and IBA concentrations for seedlings production. Ornam. Hortic. 2018, 3, 238–247. [Google Scholar] [CrossRef]
- Qiu, F.; Zhong, Y.; Zhang, T.; Liu, X.; Li, J. Effect of scion types and leaves number on cutting propagation of Cinnamomum porrectum. South China For. Sci. 2018, 46, 25–28. (In Chinese) [Google Scholar]
- Tate, H.; Page, T. Cutting propagation of Santalum austrocaledonicum: The effect of genotype, cutting source, cutting size, propagation medium, IBA and irradiance. New For. 2018, 49, 551–570. [Google Scholar] [CrossRef]
- Gautam, R.D.; Kumar, A.M.; Kumar, R.; Chauhan, R.; Singh, S.; Kumar, M.; Kumar, D.; Kumar, A.; Singh, S. Clonal propagation of Valeriana jatamansi retains the essential oil profile of mother plants: An approach toward generating homogenous grade of essential oil for industrial use. Front. Plant Sci. 2021, 12, 438247. [Google Scholar] [CrossRef]
- Sang, Y.L.; Cheng, Z.J.; Zhang, X.S. Plant stem cells and de novo organogenesis. New Phytol. 2018, 218, 1334–1339. [Google Scholar] [CrossRef]
- Verdeil, J.-L.; Alemanno, L.; Niemenak, N.; Tranbarger, T.J. Pluripotent versus totipotent plant stem cells: Dependence versus autonomy? Trends Plant Sci. 2007, 12, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Pokimica, N.; Ćosić, T.; Uzelac, B.; Ninković, S.; Raspor, M. Dissecting the roles of the cytokinin signaling network: The case of de novo shoot apical meristem formation. Biomolecules 2024, 14, 318. [Google Scholar] [CrossRef] [PubMed]
- Moreno-García, I.; Rodríguez, V.; García-Sogo, B.; Ventura, C.; Moreno, V.; Pineda, B. The first protocol for in vitro propagation of kalanchoe beharensis through adventitious shoots, a preliminary study. Horticulturae 2024, 10, 1379. [Google Scholar] [CrossRef]
- Zhai, N.; Xu, L. Pluripotency acquisition in the middle cell layer of callus is required for organ regeneration. Nat. Plants 2021, 7, 1453–1460. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, L.; Xu, Y.; Li, J.; Yang, Z.; Huang, H.; Xu, L. WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell 2014, 26, 1081–1093. [Google Scholar] [CrossRef]
- Pan, R.; Liu, Y.; Buitrago, S.; Jiang, W.; Gao, H.; Han, H.; Wu, C.; Wang, Y.; Zhang, W.; Yang, X. Adventitious root formation is dynamically regulated by various hormones in leaf-vegetable sweetpotato cuttings. J. Plant Physiol. 2020, 253, 153267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, J. Cell polarity: Compassing cell division and differentiation in plants. Curr. Opin. Plant Biol. 2018, 45, 127–135. [Google Scholar] [CrossRef]
- Ruan, J.; Lai, L.; Ou, H.; Yi, P. Two subtypes of GTPase-activating proteins coordinate tip growth and cell size regulation in Physcomitrium patens. Nat. Commun. 2023, 32, 2271. [Google Scholar] [CrossRef]
- Dai, X.; Liu, X.; Qiu, F.; Zhang, T.; Li, J. Establishment of regeneration system for stems of Cinnamomum porrectum. J. Cent. South Univ. For. Technol. 2018, 38, 20–25+33. (In Chinese) [Google Scholar]
- Xiao, Z.; Zheng, L.; Zhang, B.; Wang, Y.; Li, F.; Jiang, R.; Peng, Y.; Jin, Z. Study on tissue culture technology of stem segment of Cinnamomum camphora ‘Ganfang1’. J. Nanchang Inst. Technol. 2022, 41, 71–77. (In Chinese) [Google Scholar]
- Xiang, Z.; Wang, L.; Cao, L.; Liao, W.; Jin, Z.; Li, F.; Lv, X.; Zhang, H.; Zhao, J. Tissue Culture Technology of Stem Segment of Cinnamomum bodinieri var. citralifera. Bull. Bot. Res. 2020, 40, 196–201. (In Chinese) [Google Scholar]
- Su, Y.-H.; Liu, Y.-B.; Zhang, X.-S. Auxin–Cytokinin Interaction Regulates Meristem Development. Mol. Plant 2011, 4, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F.K. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Zhanmu, O.; Yang, X.; Gong, H.; Li, X. Paraffin-embedding for large volume bio-tissue. Sci. Rep. 2020, 10, 12639. [Google Scholar] [CrossRef]
- Alagbe, E.; Amlabu, Y.; Daniel, E.; Ojewumi, M. Effect of varying drying temperature on the soluble sugar and nutritional content of banana. Open Chem. Eng. J. 2020, 14, 11–16. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, C.; Sun, H.; Wang, W.; Liu, L.; Zhang, Y. Effects of drought on soluble protein content and protective enzyme system in cotton leaves. Front. Agric. China 2010, 4, 56–62. [Google Scholar] [CrossRef]
- Pierce, J.; Suelter, C. An evaluation of the Coomassie brilliant blue G-250 dye-binding method for quantitative protein determination. Anal. Biochem. 1977, 81, 478–480. [Google Scholar] [CrossRef]
- Brumós, J.; Robles, L.; Yun, J.; Vu, T.-C.; Jackson, S.; Alonso, J.M.; Stepanova, A.N. Local auxin biosynthesis is a key regulator of plant development. Dev. Cell 2018, 47, 306–318.e305. [Google Scholar] [CrossRef]
- Hedden, P.; Sponsel, V. A century of gibberellin research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [PubMed]
- Finkelstein, R. Abscisic acid synthesis and response. Arab. Book/Am. Soc. Plant Biol. 2013, 11, e0166. [Google Scholar] [CrossRef]
- Li, S.-M.; Zheng, H.-X.; Zhang, X.-S.; Sui, N. Cytokinins as central regulators during plant growth and stress response. Plant Cell Rep. 2021, 40, 271–282. [Google Scholar] [CrossRef]
- Niu, Q.; Zong, Y.; Qian, M.; Yang, F.; Teng, Y. Simultaneous quantitative determination of major plant hormones in pear flowers and fruit by UPLC/ESI-MS/MS. Anal. Methods 2014, 6, 1766–1773. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.; Squires, E.; Mahmoodi, H.; Wu, J.; Agarwal, S.; Hadley, M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic. Biol. Med. 1993, 15, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, K. Activation of indole-3-acetic acid oxidase from horseradish and Prunus by phenols and H2O2. Plant Growth Regul. 1982, 1, 73–84. [Google Scholar] [CrossRef]
- Bafana, A.; Dutt, S.; Kumar, A.; Kumar, S.; Ahuja, P.S. The basic and applied aspects of superoxide dismutase. J. Mol. Catal. B Enzym. 2011, 68, 129–138. [Google Scholar] [CrossRef]
- Yoruk, R.; Marshall, M.R. Physicochemical properties and function of plant polyphenol oxidase: A review 1. J. Food Biochem. 2003, 27, 361–422. [Google Scholar] [CrossRef]
- Tang, W.; Newton, R.J. Peroxidase and catalase activities are involved in direct adventitious shoot formation induced by thidiazuron in eastern white pine (Pinus strobus L.) zygotic embryos. Plant Physiol. Biochem. 2005, 43, 760–769. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Fki, L.; Bouaziz, N.; Kriaâ, W.; Benjemaa-Masmoudi, R.; Gargouri-Bouzid, R.; Rival, A.; Drira, N. Multiple bud cultures of ‘Barhee’ date palm (Phoenix dactylifera) and physiological status of regenerated plants. J. Plant Physiol. 2011, 168, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Yang, H.; Ma, S.; Shen, Q.; Liu, L.; Hou, C.; Cao, X.; Cheng, J. Physiological and transcriptomic changes during the early phases of adventitious root formation in mulberry stem hardwood cuttings. Int. J. Mol. Sci. 2019, 20, 3707. [Google Scholar] [CrossRef]
- Li, C.; Lu, X.; Liu, Y.; Xu, J.; Yu, W. Trehalose alleviates the inhibition of adventitious root formation caused by drought stress in cucumber through regulating ROS metabolism and activating trehalose and plant hormone biosynthesis. Plant Physiol. Biochem. 2023, 205, 108159. [Google Scholar] [CrossRef]
- Miller, G.; Schlauch, K.A.; Tam, R.C.Y.; Cortes, D.F.; Torres, M.A.; Shulaev, V.; Dangl, J.L.; Mittler, R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2009, 2, ra45. [Google Scholar] [CrossRef]
- Shin, S.Y.; Park, S.-J.; Kim, H.-S.; Jeon, J.-H.; Lee, H.-J. Wound-induced signals regulate root organogenesis in Arabidopsis explants. BMC Plant Biol. 2022, 22, 133. [Google Scholar] [CrossRef]
- Libik, M.; Konieczny, R.; Pater, B.; Ślesak, I.; Miszalski, Z. Differences in the activities of some antioxidant enzymes and in H2O2 content during rhizogenesis and somatic embryogenesis in callus cultures of the ice plant. Plant Cell Rep. 2005, 23, 834–841. [Google Scholar] [CrossRef]
- Bashri, G.; Prasad, S.M. Exogenous IAA differentially affects growth, oxidative stress and antioxidants system in Cd stressed Trigonella foenum-graecum L. seedlings: Toxicity alleviation by up-regulation of ascorbate-glutathione cycle. Ecotoxicol. Environ. Saf. 2016, 132, 329–338. [Google Scholar] [CrossRef]
- Zhai, X.; Yan, X.; Zenda, T.; Wang, N.; Dong, A.; Yang, Q.; Zhong, Y.; Xing, Y.; Duan, H. Overexpression of the peroxidase gene ZmPRX1 increases maize seedling drought tolerance by promoting root development and lignification. Crop J. 2024, 12, 753–765. [Google Scholar] [CrossRef]
- Rosspopoff, O.; Chelysheva, L.; Saffar, J.; Lecorgne, L.; Gey, D.; Caillieux, E.; Colot, V.; Roudier, F.; Hilson, P.; Berthomé, R.; et al. Direct conversion of root primordium into shoot meristem relies on timing of stem cell niche development. Development 2017, 144, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, V.B.; Mühlenbock, P.; Van Breusegem, F. Stress homeostasis–the redox and auxin perspective. Plant Cell Environ. 2012, 35, 321–333. [Google Scholar] [CrossRef]
- Zhang, T.; Ge, Y.; Cai, G.; Pan, X.; Xu, L. WOX-ARF modules initiate different types of roots. Cell Rep. 2023, 42, 112966. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Bae, S.; Seo, P.J. De novo shoot organogenesis during plant regeneration. J. Exp. Bot. 2020, 71, 63–72. [Google Scholar] [CrossRef]
- Mao, J.; Ma, D.; Niu, C.; Ma, X.; Li, K.; Tahir, M.M.; Chen, S.; Liu, X.; Zhang, D. Transcriptome analysis reveals the regulatory mechanism by which MdWOX11 suppresses adventitious shoot formation in apple. Hortic. Res. 2022, 9, uhac080. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, H.; Song, J.; Xie, W.; Zhang, L.; Li, S.-f.; Cai, Y.; Zhao, Z.-x. Morphological and transcriptome analyses reveal mechanism for efficient regeneration of adventitious buds from in vitro leaves of Rhododendron delavayi regulated by exogenous TDZ. Vitr. Cell. Dev. Biol. Plant 2022, 58, 1025–1037. [Google Scholar] [CrossRef]
- Guo, B.; He, W.; Zhao, Y.; Wu, Y.; Fu, Y.; Guo, J.; Wei, Y. Changes in endogenous hormones and H2O2 burst during shoot organogenesis in TDZ-treated Saussurea involucrate explants. Plant Cell Tissue Organ Cult. (PCTOC) 2016, 128, 1–8. [Google Scholar] [CrossRef]
- Mäkilä, R.; Wybouw, B.; Smetana, O.; Vainio, L.; Solé-Gil, A.; Lyu, M.; Ye, L.; Wang, X.; Siligato, R.; Jenness, M.K.; et al. Gibberellins promote polar auxin transport to regulate stem cell fate decisions in cambium. Nat. Plants 2023, 9, 631–644. [Google Scholar] [CrossRef]
- Mauriat, M.; Petterle, A.; Bellini, C.; Moritz, T. Gibberellins inhibit adventitious rooting in hybrid aspen and Arabidopsis by affecting auxin transport. Plant J. Cell Mol. Biol. 2014, 78, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mushtaq, N.; Xing, N.; Wu, S.; Liu, J.; Wang, Z. Efficient In vitro regeneration system and comparative transcriptome analysis offer insight into the early development characteristics of explants from cotyledon with partial petiole in small-fruited pepper (Capsicum annuum). Int. J. Mol. Sci. 2024, 25, 7547. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hou, X.; Mou, K.; Liu, H.; Zhao, Z.; Liao, W. The involvement of abscisic acid in glucose promoted adventitious root development in cucumber. Sci. Hortic. 2022, 295, 110816. [Google Scholar] [CrossRef]
- Zhai, N.; Pan, X.; Zeng, M.; Xu, L. Developmental trajectory of pluripotent stem cell establishment in Arabidopsis callus guided by a quiescent center-related gene network. Development 2023, 150, 200879. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Li, Y.; Zhang, Y.; Luo, C.; Zheng, Y.; Zhang, T.; Fu, C.; Liu, X. Integrated Physiological and Transcriptomic Analyses Reveal Mechanisms Regulating Endogenous Phytohormones in Adventitious Root Formation During Cinnamomum bodinieri Cutting Propagation. Forests 2025, 16, 509. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, Y.; Zhong, Y.; Wu, Y.; Li, Z.; Xu, L.; Xu, M. Transcriptome analysis and identification of genes related to terpenoid biosynthesis in Cinnamomum camphora. BMC Genom. 2018, 19, 550. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Niu, C.; Li, K.; Fan, L.; Liu, Z.; Li, S.; Ma, D.; Tahir, M.M.; Xing, L.; Zhao, C.; et al. Cytokinin-responsive MdTCP17 interacts with MdWOX11 to repress adventitious root primordium formation in apple rootstocks. Plant Cell 2022, 35, 1202–1221. [Google Scholar] [CrossRef]
- Fox, S.; Southam, P.; Pantin, F.; Kennaway, R.; Robinson, S.; Castorina, G.; Sánchez-Corrales, Y.E.; Sablowski, R.; Chan, J.; Grieneisen, V. Spatiotemporal coordination of cell division and growth during organ morphogenesis. PLoS Biol. 2018, 16, e2005952. [Google Scholar] [CrossRef]
- Varapparambath, V.; Mathew, M.M.; Shanmukhan, A.P.; Radhakrishnan, D.; Kareem, A.; Verma, S.; Ramalho, J.J.; Manoj, B.; Vellandath, A.R.; Aiyaz, M.; et al. Mechanical conflict caused by a cell-wall-loosening enzyme activates de novo shoot regeneration. Dev. Cell 2022, 57, 2063–2080. [Google Scholar] [CrossRef]
- Hoermayer, L.; Montesinos, J.; Trozzi, N.; Spona, L.; Yoshida, S.; Marhava, P.; Caballero-Mancebo, S.; Benková, E.; Heisenberg, C.-P.; Dagdas, Y.; et al. Mechanical forces in plant tissue matrix orient cell divisions via microtubule stabilization. Dev. Cell 2024, 59, 1333–1344. [Google Scholar] [CrossRef]
- Lakehal, A.; Chaabouni, S.; Cavel, E.; Le Hir, R.; Ranjan, A.; Raneshan, Z.; Novák, O.; Păcurar, D.I.; Perrone, I.; Jobert, F.; et al. A molecular framework for the control of adventitious rooting by TIR1/AFB2-Aux/IAA-Dependent auxin signaling in Arabidopsis. Mol. Plant 2019, 12, 1499–1514. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, X.; Li, J.; Liu, N.; Liu, X.; Li, S.; Xiang, F. The Type-B cytokinin response regulator ARR1 inhibits shoot regeneration in an ARR12-dependent manner in Arabidopsis. Plant Cell 2020, 32, 2271–2291. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Munir, M.Z.; Larriba, E.; Pérez-Pérez, J.M.; Gull, S.; Pervaiz, T.; Mahmood, U.; Mahmood, Z.; Sun, Y.; Li, Y. Temporal profiling of physiological, histological, and transcriptomic dissection during auxin-induced adventitious root formation in tetraploid Robinia pseudoacacia micro-cuttings. Planta 2024, 259, 66. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Cui, X.; Wu, C.; Shi, S.; Yan, S. Salicylic acid inhibits gibberellin signaling through receptor interactions. Mol. Plant 2022, 15, 1759–1771. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, C.; Wang, N.; Wei, L.; Li, W.; Yao, Y.; Liao, W. Roles of small-molecule compounds in plant adventitious root development. Biomolecules 2019, 9, 420. [Google Scholar] [CrossRef]
- Park, S.-H.; Elhiti, M.; Wang, H.; Xu, A.; Brown, D.; Wang, A. Adventitious root formation of in vitro peach shoots is regulated by auxin and ethylene. Sci. Hortic. 2017, 226, 250–260. [Google Scholar] [CrossRef]
- Feng, K.; Hou, X.; Xing, G.; Liu, J.; Duan, A.; Xu, Z.; Li, M.; Zhuang, J.; Xiong, A. Advances in AP2/ERF super-family transcription factors in plant. Crit. Rev. Biotechnol. 2020, 40, 750–776. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, F.; Chen, L.; Pan, Y.; Sun, L.; Bao, N.; Zhang, T.; Cui, C.-X.; Qiu, Z.; Zhang, Y.; et al. Jasmonate-mediated wound signalling promotes plant regeneration. Nat. Plants 2019, 5, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Chen, Z.-f.; Liu, X.; Sun, J.; Zhang, F.; Zhang, M.; Dong, C. 24-epibrassinolide facilitates adventitious root formation by coordinating cell-wall polyamine oxidase- and plasma membrane respiratory burst oxidase homologue-derived reactive oxygen species in Capsicum annuum L. Antioxidants 2023, 12, 1425. [Google Scholar] [CrossRef]
- Xu, P.; Fang, S.; Chen, H.; Cai, W. The brassinosteroid (BR) responsive xyloglucan endotransglucosylase/hydrolase 19 (XTH19) and XTH23 genes are involved in lateral root development under salt stress in Arabidopsis. Plant J. Cell Mol. Biol. 2020, 104, 59–75. [Google Scholar] [CrossRef]
- Tan, H.; Man, C.; Xie, Y.; Yan, J.; Chu, J.; Huang, J. A crucial role of ga-regulated flavonol biosynthesis in root growth of Arabidopsis. Mol. Plant 2019, 12, 521–537. [Google Scholar] [CrossRef] [PubMed]
- Lavhale, S.G.; Kondhare, K.R.; Sinthadurai, V.S.; Barvkar, V.T.; Kale, R.S.; Joshi, R.S.; Giri, A.P. Ocimum kilimandscharicum 4CL11 negatively regulates adventitious root development via accumulation of flavonoid glycosides. Plant J. Cell Mol. Biol. 2024, 119, 176–196. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Guo, W.; Dong, Y.; Jia, Z.; Zhao, X.; Jiang, Z.; Zhang, L.; Zhang, J.; Liu, J. Metabolic profiling reveals key metabolites regulating adventitious root formation in ancient Platycladus orientalis cuttings. Front. Plant Sci. 2023, 14, 1192371. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Lin, H. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Li, W.; Mi, B.; Ma, Z.; Dawuda, M.M.; Zuo, C.; Zhang, Y.; Jiang, X.; Chen, B. Transcriptome analysis revealed glucose application affects plant hormone signal transduction pathway in “Red Globe” grape plantlets. Plant Growth Regul. 2018, 84, 45–56. [Google Scholar] [CrossRef]
- Agrawal, R.; Singh, A.; Giri, J.; Magyar, Z.; Thakur, J.K. MEDIATOR SUBUNIT17 is required for transcriptional optimization of root system architecture in Arabidopsis. Plant Physiol. 2023, 192, 1548–1568. [Google Scholar] [CrossRef]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, C.; Zhang, T.; Dai, X.; Zhang, Y.; Zheng, Y.; Liu, X.; Zhang, X. Physiological Characteristics and Transcriptomic Analysis of Young Stems Differentiation in Adventitious Bud and Root Formation in Cinnamomum parthenoxylon. Forests 2025, 16, 1049. https://doi.org/10.3390/f16071049
Luo C, Zhang T, Dai X, Zhang Y, Zheng Y, Liu X, Zhang X. Physiological Characteristics and Transcriptomic Analysis of Young Stems Differentiation in Adventitious Bud and Root Formation in Cinnamomum parthenoxylon. Forests. 2025; 16(7):1049. https://doi.org/10.3390/f16071049
Chicago/Turabian StyleLuo, Chenglin, Ting Zhang, Xiaoying Dai, Yueting Zhang, Yongjie Zheng, Xinliang Liu, and Xuhui Zhang. 2025. "Physiological Characteristics and Transcriptomic Analysis of Young Stems Differentiation in Adventitious Bud and Root Formation in Cinnamomum parthenoxylon" Forests 16, no. 7: 1049. https://doi.org/10.3390/f16071049
APA StyleLuo, C., Zhang, T., Dai, X., Zhang, Y., Zheng, Y., Liu, X., & Zhang, X. (2025). Physiological Characteristics and Transcriptomic Analysis of Young Stems Differentiation in Adventitious Bud and Root Formation in Cinnamomum parthenoxylon. Forests, 16(7), 1049. https://doi.org/10.3390/f16071049





