Mapping Commercial Forests Infected by the Novel Variant of Elsinoë masingae, Using Unmanned Aerial Technology in Southern Africa
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Site
2.2. Image Acquisition and Processing
2.3. Field Data Collection
2.4. Image Texture Analysis
2.5. Vegetation Indices
2.6. Statistical Analysis
2.6.1. Fast Large Margin
2.6.2. Random Forest
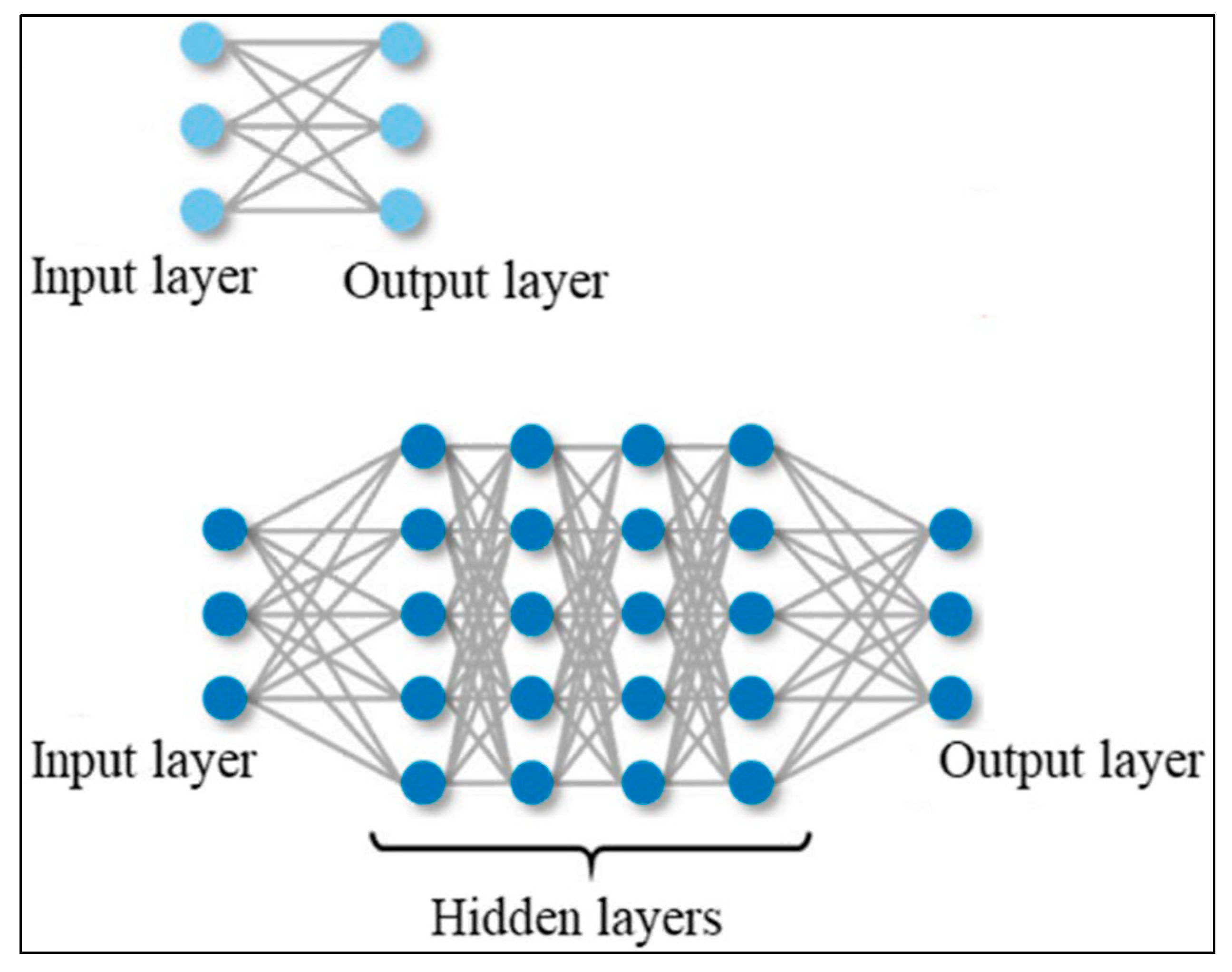
2.6.3. Deep Learning
2.7. Accuracy Assessment
3. Results
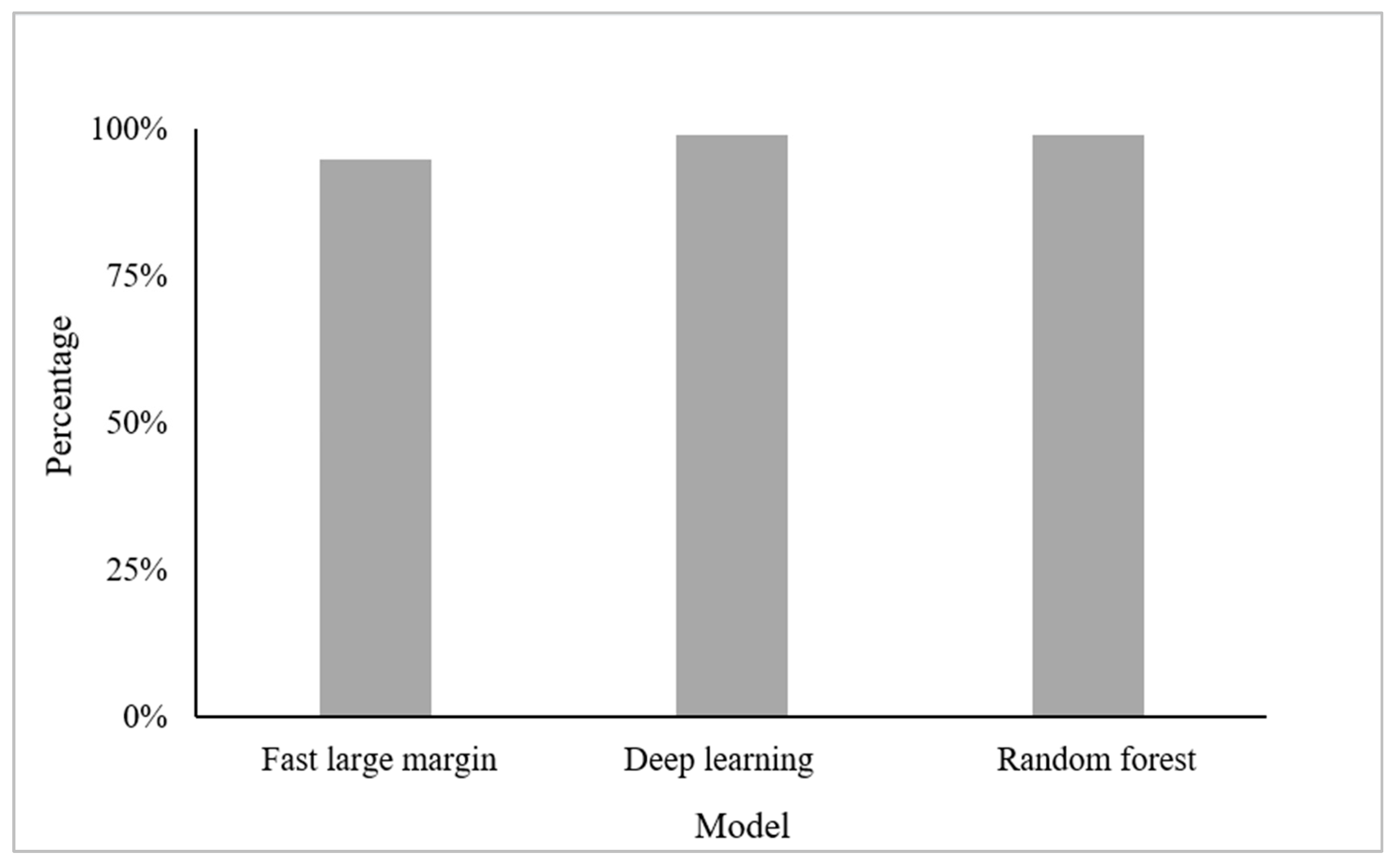
3.1. Classification Accuracies Using RGB Bands and Derived Visible Indices Only
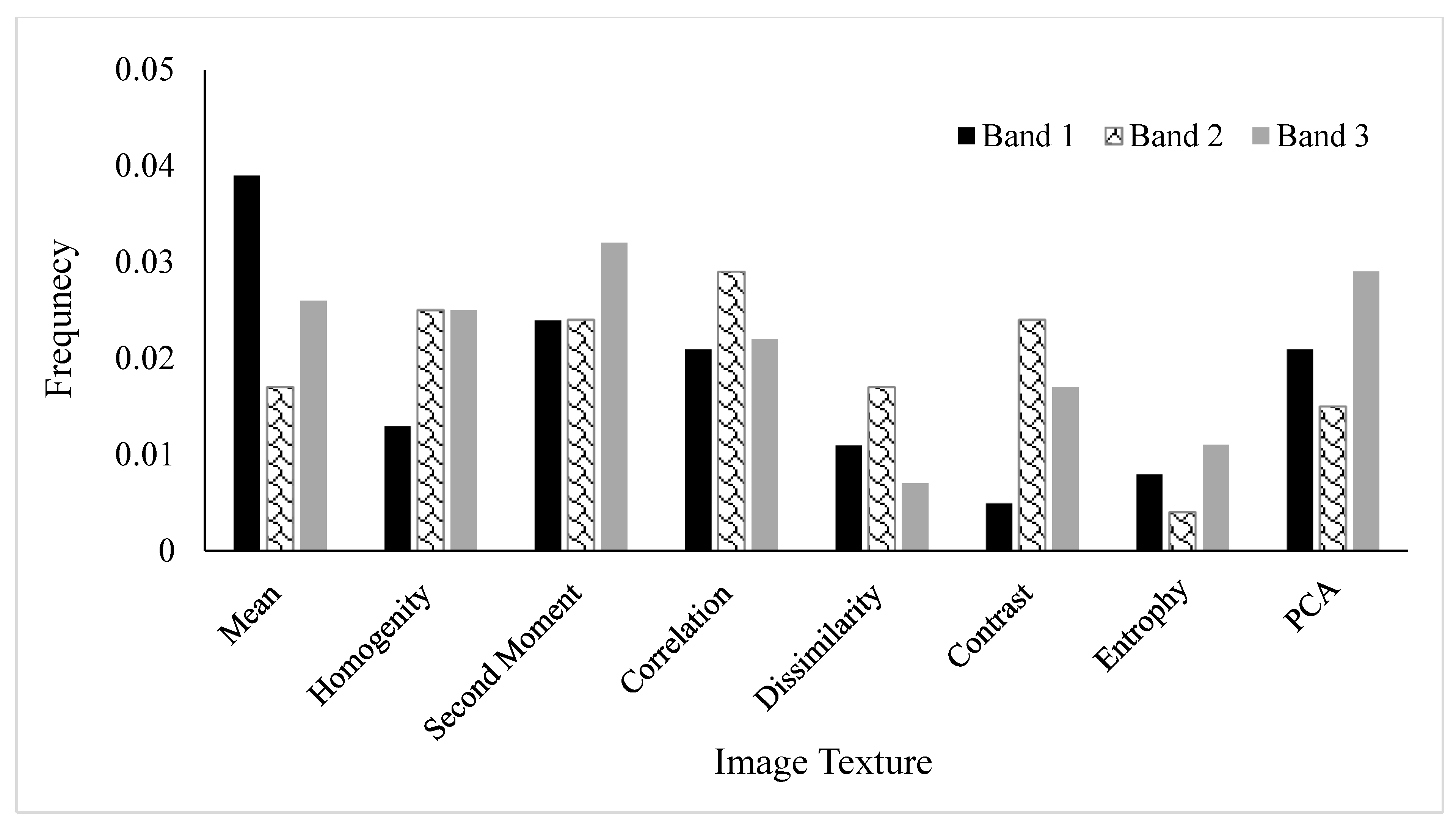
3.2. Frequency Analysis Showing the Most Contributing Variables Selected by FLM
3.3. Classification Accuracies When Combining RGB Bands and Indices with Texture
3.4. Frequency of Variables Selected by the DL Model
4. Discussion
4.1. Image Texture, Visible Indices, and Implications on Disease Mapping in Forestry
4.2. Model Comparisons and Variable Importance
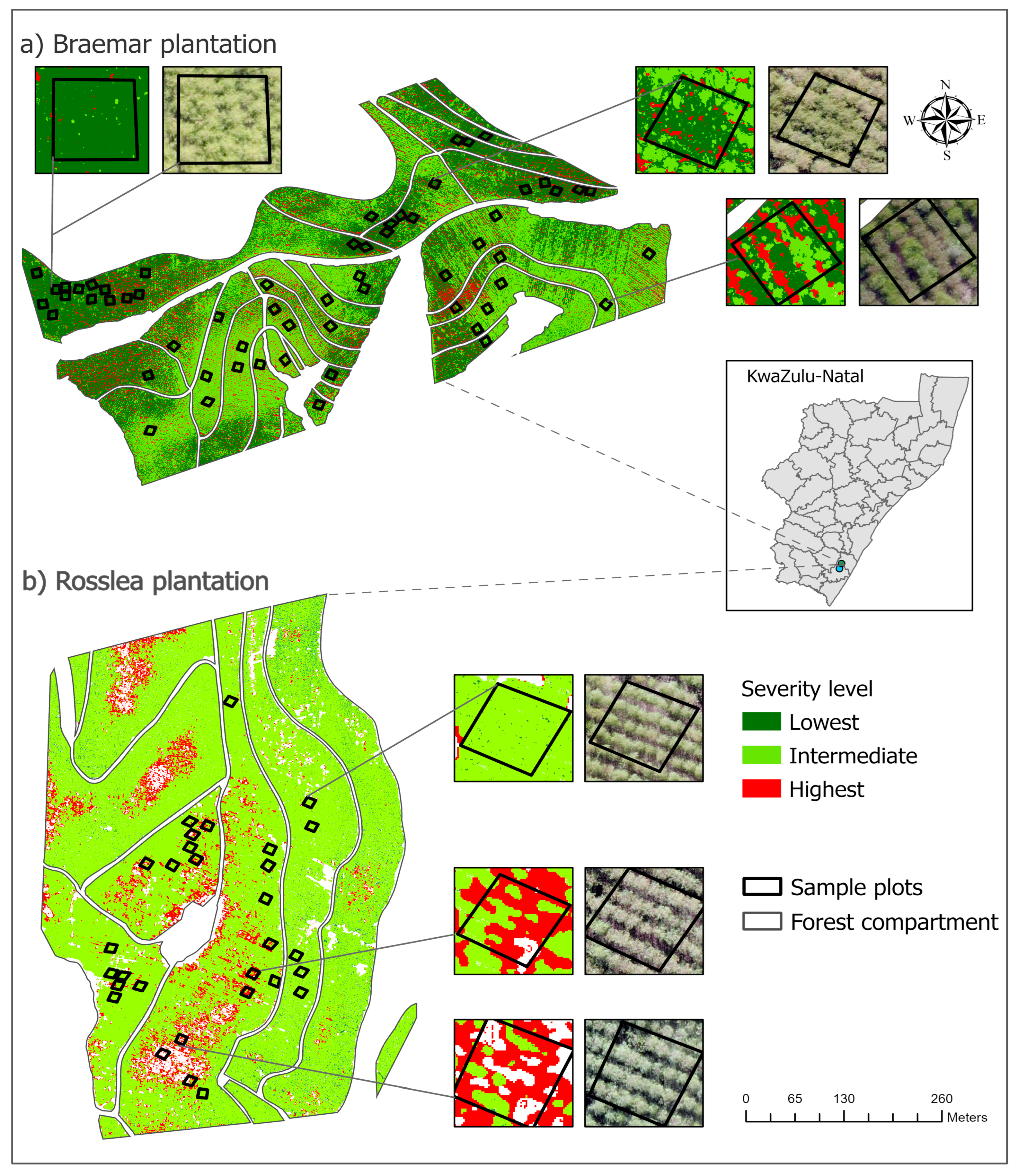
4.3. Spatial Distribution of E. masingae Within the Forest
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- DAFF. Report on Commercial Timber Resources and Primary Roundwood Processing in South Africa; DAFF: Gauteng, South Africa, 2008. [Google Scholar]
- Lottering, R.T.; Govender, M.; Peerbhay, K.; Lottering, S. Comparing partial least squares (PLS) discriminant analysis and sparse PLS discriminant analysis in detecting and mapping Solanum mauritianum in commercial forest plantations using image texture. ISPRS J. Photogramm. Remote Sens. 2020, 159, 271–280. [Google Scholar] [CrossRef]
- Uzu, J.; Bettinger, P.; Siry, J.; Mei, B. Timber business in West Africa: A review and outlook. Int. For. Rev. 2022, 24, 240–256. [Google Scholar] [CrossRef]
- Freer-Smith, P.H.; Webber, J.F. Tree pests and diseases: The threat to biodiversity and the delivery of ecosystem services. Biodivers. Conserv. 2017, 26, 3167–3181. [Google Scholar] [CrossRef]
- Sturrock, R.; Frankel, S.; Brown, A.; Hennon, P.; Kliejunas, J.; Lewis, K.; Worrall, J.; Woods, A. Climate change and forest diseases. Plant Pathol. 2011, 60, 133–149. [Google Scholar] [CrossRef]
- Ramsfield, T.; Bentz, B.; Faccoli, M.; Jactel, H.; Brockerhoff, E. Forest health in a changing world: Effects of globalization and climate change on forest insect and pathogen impacts. Forestry 2016, 89, 245–252. [Google Scholar] [CrossRef]
- Balla, A.; Silini, A.; Cherif-Silini, H.; Chenari Bouket, A.; Moser, W.K.; Nowakowska, J.A.; Oszako, T.; Benia, F.; Belbahri, L. The threat of pests and pathogens and the potential for biological control in forest ecosystems. Forests 2021, 12, 1579. [Google Scholar] [CrossRef]
- Fan, X.; Barreto, R.; Groenewald, J.; Bezerra, J.; Pereira, O.; Cheewangkoon, R.; Mostert, L.; Tian, C.; Crous, P. Phylogeny and taxonomy of the scab and spot anthracnose fungus Elsinoë (Myriangiales, Dothideomycetes). Stud. Mycol. 2017, 87, 1–41. [Google Scholar] [CrossRef]
- Swart, L.; Crous, P.W.; Kang, J.-C.; Mchau, G.R.; Pascoe, I.; Palm, M.E. Differentiation of species of Elsinoë associated with scab disease of Proteaceae based on morphology, symptomatology, and ITS sequence phylogeny. Mycologia 2001, 93, 366–379. [Google Scholar] [CrossRef]
- Chung, K.R. Elsinoë fawcettii and Elsinoë australis: The fungal pathogens causing citrus scab. Mol. Plant Pathol. 2011, 12, 123–135. [Google Scholar] [CrossRef]
- Roux, J.; Wingfield, M.J.; Marincowitz, S.; Solís, M.; Phungula, S.; Pham, N.Q. Eucalyptus scab and shoot malformation: A new disease in South Africa caused by a novel species, Elsinoe masingae. For. Int. J. For. Res. 2024, 97, 327–338. [Google Scholar] [CrossRef]
- Bulman, L.; Bradshaw, R.; Fraser, S.; Martín-García, J.; Barnes, I.; Musolin, D.L.; La Porta, N.; Woods, A.; Diez, J.J.; Koltay, A. A worldwide perspective on the management and control of Dothistroma needle blight. For. Pathol. 2016, 46, 472–488. [Google Scholar] [CrossRef]
- Pham, N.Q.; Marincowitz, S.; Solís, M.; Duong, T.A.; Wingfield, B.D.; Barnes, I.; Slippers, B.; Muro Abad, J.I.; Durán, A.; Wingfield, M.J. Eucalyptus scab and shoot malformation: A new and serious foliar disease of Eucalyptus caused by Elsinoe necatrix sp. nov. Plant Pathol. 2021, 70, 1230–1242. [Google Scholar] [CrossRef]
- Prospero, S.; Botella, L.; Santini, A.; Robin, C. Biological control of emerging forest diseases: How can we move from dreams to reality? For. Ecol. Manag. 2021, 496, 119377. [Google Scholar] [CrossRef]
- Coops, N.; Stanford, M.; Old, K.; Dudzinski, M.; Culvenor, D.; Stone, C. Assessment of Dothistroma needle blight of Pinus radiata using airborne hyperspectral imagery. Phytopathology 2003, 93, 1524–1532. [Google Scholar] [CrossRef]
- Dash, J.P.; Watt, M.S.; Pearse, G.D.; Heaphy, M.; Dungey, H.S. Assessing very high resolution UAV imagery for monitoring forest health during a simulated disease outbreak. ISPRS J. Photogramm. Remote Sens. 2017, 131, 1–14. [Google Scholar] [CrossRef]
- Nazir, M.M.; Najib, M.; Razak, T.; Ahmad, N.; Sheriza, M.R.; Roger, M. Early monitoring of health status of plantation-grown eucalyptus pellita at large spatial scale via visible spectrum imaging of canopy foliage using unmanned aerial vehicles. Forests 2021, 12, 1393. [Google Scholar] [CrossRef]
- Lausch, A.; Erasmi, S.; King, D.J.; Magdon, P.; Heurich, M. Understanding forest health with remote sensing-part I—A review of spectral traits, processes and remote-sensing characteristics. Remote Sens. 2016, 8, 1029. [Google Scholar] [CrossRef]
- Chemura, A.; Mutanga, O.; Dube, T. Separability of coffee leaf rust infection levels with machine learning methods at Sentinel-2 MSI spectral resolutions. Precis. Agric. 2017, 18, 859–881. [Google Scholar] [CrossRef]
- Oumar, M.S.; Peerbhay, K.; Germishuizen, I.; Mutanga, O.; Oumar, Z. Detecting canopy damage caused by Uromycladium acaciae on South African Black Wattle forest compartments using moderate resolution satellite imagery. South Afr. J. Geomat. 2019, 8, 69–83. [Google Scholar] [CrossRef]
- Ecke, S.; Dempewolf, J.; Frey, J.; Schwaller, A.; Endres, E.; Klemmt, H.-J.; Tiede, D.; Seifert, T. UAV-based forest health monitoring: A systematic review. Remote Sens. 2022, 14, 3205. [Google Scholar] [CrossRef]
- Allen, B.; Dalponte, M.; Ørka, H.O.; Næsset, E.; Puliti, S.; Astrup, R.; Gobakken, T. UAV-based hyperspectral imagery for detection of root, butt, and stem rot in Norway Spruce. Remote Sens. 2022, 14, 3830. [Google Scholar] [CrossRef]
- Shamaoma, H.; Chirwa, P.W.; Ramoelo, A.; Hudak, A.T.; Syampungani, S. The application of UASs in forest management and monitoring: Challenges and opportunities for use in the Miombo woodland. Forests 2022, 13, 1812. [Google Scholar] [CrossRef]
- Abdulridha, J.; Batuman, O.; Ampatzidis, Y. UAV-based remote sensing technique to detect citrus canker disease utilizing hyperspectral imaging and machine learning. Remote Sens. 2019, 11, 1373. [Google Scholar] [CrossRef]
- Marin, S.; Santos, L.; Barbosa, B.D.S.; Barata, R.A.P.; Osco, L.P.; Ramos, A.P.M.; Guimarães, P.H.S. Detecting coffee leaf rust with UAV-based vegetation indices and decision tree machine learning models. Comput. Electron. Agric. 2021, 190, 106476. [Google Scholar] [CrossRef]
- Mutanga, O.; Skidmore, A. Integrating imaging spectroscopy and neural networks to map grass quality in the Kruger National Park, South Africa. Remote Sens. Environ. 2004, 90, 104–115. [Google Scholar] [CrossRef]
- Purevdorj, T.; Tateishi, R.; Ishiyama, T.; Honda, Y. Relationships between percent vegetation cover and vegetation indices. Int. J. Remote Sens. 1998, 19, 3519–3535. [Google Scholar] [CrossRef]
- Sibiya, B.; Lottering, R.; Odindi, J. Discriminating commercial forest species using image texture computed from a worldview-2 pan-sharpened image and partial least squares discriminant analysis. Remote Sens. Appl. Soc. Environ. 2021, 23, 100605. [Google Scholar] [CrossRef]
- Moskal, L.; Franklin, S. Multi-layer forest stand discrimination with spatial co-occurrence texture analysis of high spatial detail airborne imagery. Geocarto Int. 2002, 17, 55–68. [Google Scholar] [CrossRef]
- Champion, I.; Germain, C.; Da Costa, J.P.; Alborini, A.; Dubois-Fernandez, P. Retrieval of forest stand age from SAR image texture for varying distance and orientation values of the gray level co-occurrence matrix. IEEE Geosci. Remote Sens. Lett. 2013, 11, 5–9. [Google Scholar] [CrossRef]
- Li, J.; Rich, W.; Buhl-Brown, D. Texture analysis of remote sensing imagery with clustering and Bayesian inference. Int. J. Image Graph. Signal Process. 2015, 7, 1–10. [Google Scholar] [CrossRef]
- Journaux, L.; Simon, J.-C.; Destain, M.-F.; Cointault, F.; Miteran, J.; Piron, A. Plant leaf roughness analysis by texture classification with generalized Fourier descriptors in a dimensionality reduction context. Precis. Agric. 2011, 12, 345–360. [Google Scholar] [CrossRef]
- Chetty, S.; Mutanga, O.; Lottering, R. Detecting and mapping invasive Parthenium hysterophorus L. along the northern coastal belt of KwaZulu-Natal, South Africa using image texture. Sci. Afr. 2021, 13, e00966. [Google Scholar] [CrossRef]
- Yuan, X.; King, D.; Vlcek, J. Sugar maple decline assessment based on spectral and textural analysis of multispectral aerial videography. Remote Sens. Environ. 1991, 37, 47–54. [Google Scholar] [CrossRef]
- Alvarenga, A.V.; Pereira, W.C.; Infantosi, A.F.C.; Azevedo, C.M. Complexity curve and grey level co-occurrence matrix in the texture evaluation of breast tumor on ultrasound images. Med. Phys. 2007, 34, 379–387. [Google Scholar] [CrossRef]
- Gebejes, A.; Huertas, R.; Tremeau, A.; Tomic, I.; Biswas, P.R.; Fraza, C.; Hauta-Kasari, M. Texture characterization by grey-level co-occurrence matrix from a perceptual approach. In Proceedings of the Color and Imaging Conference, San Diego, CA, USA, 7–11 November 2016; pp. 271–277. [Google Scholar]
- Lottering, R.; Mutanga, O.; Peerbhay, K.; Ismail, R. Detecting and mapping Gonipterus scutellatus induced vegetation defoliation using WorldView-2 pan-sharpened image texture combinations and an artificial neural network. J. Appl. Remote Sens. 2019, 13, 014513. [Google Scholar] [CrossRef]
- Materka, A.; Strzelecki, M. Texture analysis methods–a review. Tech. Univ. Lodz Inst. Electron. COST B11 Rep. Bruss. 1998, 10, 4968. [Google Scholar]
- Yuan, Y.; Hu, X. Random forest and objected-based classification for forest pest extraction from UAV aerial imagery. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2016, 41, 1093–1098. [Google Scholar] [CrossRef]
- Jackson, R.D.; Huete, A.R. Interpreting vegetation indices. Prev. Vet. Med. 1991, 11, 185–200. [Google Scholar] [CrossRef]
- Eng, L.S.; Ismail, R.; Hashim, W.; Baharum, A. The use of VARI, GLI, and VIgreen formulas in detecting vegetation in aerial images. Int. J. Technol. 2019, 10, 1385–1394. [Google Scholar] [CrossRef]
- Elazab, A.; Ordóñez, R.A.; Savin, R.; Slafer, G.A.; Araus, J.L. Detecting interactive effects of N fertilization and heat stress on maize productivity by remote sensing techniques. Eur. J. Agron. 2016, 73, 11–24. [Google Scholar] [CrossRef]
- Pretorius, M.; Van Huyssteen, C.; Brown, L. Soil color indicates carbon and wetlands: Developing a color-proxy for soil organic carbon and wetland boundaries on sandy coastal plains in South Africa. Environ. Monit. Assess. 2017, 189, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pandesenda, A.I.; Yana, R.R.; Sukma, E.A.; Yahya, A.; Widharto, P.; Hidayanto, A.N. Sentiment analysis of service quality of online healthcare platform using fast large-margin. In Proceedings of the 2020 International Conference on Informatics, Multimedia, Cyber and Information System (ICIMCIS), online, 19–20 November 2020; pp. 121–125. [Google Scholar]
- Mountrakis, G.; Im, J.; Ogole, C. Support vector machines in remote sensing: A review. ISPRS J. Photogramm. Remote Sens. 2011, 66, 247–259. [Google Scholar] [CrossRef]
- Suthaharan, S. Support vector machine. In Machine Learning Models and Algorithms for Big Data Classification: Thinking with Examples for Effective Learning; Springer: Berlin/Heidelberg, Germany, 2016; pp. 207–235. [Google Scholar]
- Noble, W.S. What is a support vector machine? Nat. Biotechnol. 2006, 24, 1565–1567. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Adam, E.; Mutanga, O.; Odindi, J.; Abdel-Rahman, E.M. Land-use/cover classification in a heterogeneous coastal landscape using RapidEye imagery: Evaluating the performance of random forest and support vector machines classifiers. Int. J. Remote Sens. 2014, 35, 3440–3458. [Google Scholar] [CrossRef]
- Liao, K.; Yang, F.; Dang, H.; Wu, Y.; Luo, K.; Li, G. Detection of Eucalyptus leaf disease with UAV multispectral imagery. Forests 2022, 13, 1322. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Alam, M.S.B.; Hassan, M.; Rozbu, M.R.; Ishtiak, T.; Rafa, N.; Mofijur, M.; Shawkat Ali, A.; Gandomi, A.H. Deep learning modelling techniques: Current progress, applications, advantages, and challenges. Artif. Intell. Rev. 2023, 56, 13521–13617. [Google Scholar] [CrossRef]
- Janiesch, C.; Zschech, P.; Heinrich, K. Machine learning and deep learning. Electron. Mark. 2021, 31, 685–695. [Google Scholar] [CrossRef]
- Onishi, M.; Ise, T. Explainable identification and mapping of trees using UAV RGB image and deep learning. Sci. Rep. 2021, 11, 903. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Pu, R.; Gonzalez-Moreno, P.; Yuan, L.; Wu, K.; Huang, W. Monitoring plant diseases and pests through remote sensing technology: A review. Comput. Electron. Agric. 2019, 165, 104943. [Google Scholar] [CrossRef]
- Peerbhay, K.Y.; Mutanga, O.; Ismail, R. Commercial tree species discrimination using airborne AISA Eagle hyperspectral imagery and partial least squares discriminant analysis (PLS-DA) in KwaZulu–Natal, South Africa. ISPRS J. Photogramm. Remote Sens. 2013, 79, 19–28. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, J.; Gong, J. UAV remote sensing for urban vegetation mapping using random forest and texture analysis. Remote Sens. 2015, 7, 1074–1094. [Google Scholar] [CrossRef]
- Sarker, L.R.; Nichol, J.E. Improved forest biomass estimates using ALOS AVNIR-2 texture indices. Remote Sens. Environ. 2011, 115, 968–977. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, J.; Wang, W.; Lin, C. A study for texture feature extraction of high-resolution satellite images based on a direction measure and gray level co-occurrence matrix fusion algorithm. Sensors 2017, 17, 1474. [Google Scholar] [CrossRef]
- Feriansyah, A.; Safe’i, R.; Darmawan, A.; Kaskoyo, H. Comparison of crown health assessment using forest health monitoring and remote sensing techniques (Case study: KTH Lestari Jaya 8, KPHL Kota Agung Utara, Lampung). IOP Conf. Ser. Earth Environ. Sci. 2022, 1115, 012055. [Google Scholar] [CrossRef]
- Hartling, S.; Sagan, V.; Sidike, P.; Maimaitijiang, M.; Carron, J. Urban tree species classification using a WorldView-2/3 and LiDAR data fusion approach and deep learning. Sensors 2019, 19, 1284. [Google Scholar] [CrossRef]
- Nezami, S.; Khoramshahi, E.; Nevalainen, O.; Pölönen, I.; Honkavaara, E. Tree species classification of drone hyperspectral and RGB imagery with deep learning convolutional neural networks. Remote Sens. 2020, 12, 1070. [Google Scholar] [CrossRef]
- Alam, A.; Reza, R.; Abrar, A.; Ahmed, T.; Ahmed, S.; Sharar, S.; Rasel, A.A. Patients’ Severity States Classification based on Electronic Health Record (EHR) Data using Multiple Machine Learning and Deep Learning Approaches. arXiv 2022, arXiv:2209.14907. [Google Scholar]
- Ghelardini, L.; Pepori, A.L.; Luchi, N.; Capretti, P.; Santini, A. Drivers of emerging fungal diseases of forest trees. For. Ecol. Manag. 2016, 381, 235–246. [Google Scholar] [CrossRef]
- La Porta, N.; Capretti, P.; Thomsen, I.; Kasanen, R.; Hietala, A.; Von Weissenberg, K. Forest pathogens with higher damage potential due to climate change in Europe. Can. J. Plant Pathol. 2008, 30, 177–195. [Google Scholar] [CrossRef]
- Chungu, D.; Muimba-Kankolongo, A.; Wingfield, M.J.; Roux, J. Plantation forestry diseases in Zambia: Contributing factors and management options. Ann. For. Sci. 2010, 67, 802. [Google Scholar] [CrossRef][Green Version]






| Parameter | Formula | Description | Texture Sample |
|---|---|---|---|
| Contrast | Views the local variation concerning image texture [34]. |  | |
| Correlation | Determines the local grey level that is evident on a textured image [35]. |  | |
| Dissimilarity | Measures the different grey level pairs that are evident on an image [36]. |  | |
| Homogeneity | Views how smooth the texture is [37]. |  | |
| Mean | Examines texture by looking at the average intensity level [38]. |  | |
| Second Moment | Views local homogeneity [34]. |  | |
| Variance | Calculates the pixels using their unique spectral characteristics [38]. |  | |
| Entropy | Calculates uncertainty using statistics [39]. |  |
| Vegetation Index | Abbreviation | Equation | Reference | Image Sample | |
|---|---|---|---|---|---|
| 1. | Visible Atmospheric Resistance Index | VARI | [41] |  | |
| 2. | Normalized Green Red Difference Index | NGRDI | [42] |  | |
| 3. | Green Leaf Index | GLI | [41] |  | |
| 4. | Soil Colour Index | SCI | (R − G)/(R + G) | [43] |  |
| High Infection | Med-High Infection | Low Infection | User Accuracy | |
|---|---|---|---|---|
| High infection | 10,600 | 0 | 0 | 100% |
| Med-high infection | 0 | 5000 | 0 | 100% |
| Low infection | 0 | 200 | 2800 | 93.33% |
| Producer accuracy | 100% | 96.15% | 100% | 98.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peerbhay, K.; Devsaran, N.; Lottering, R.; Agjee, N.; Parag, M. Mapping Commercial Forests Infected by the Novel Variant of Elsinoë masingae, Using Unmanned Aerial Technology in Southern Africa. Forests 2025, 16, 966. https://doi.org/10.3390/f16060966
Peerbhay K, Devsaran N, Lottering R, Agjee N, Parag M. Mapping Commercial Forests Infected by the Novel Variant of Elsinoë masingae, Using Unmanned Aerial Technology in Southern Africa. Forests. 2025; 16(6):966. https://doi.org/10.3390/f16060966
Chicago/Turabian StylePeerbhay, Kabir, Nishka Devsaran, Romano Lottering, Naeem Agjee, and Mikka Parag. 2025. "Mapping Commercial Forests Infected by the Novel Variant of Elsinoë masingae, Using Unmanned Aerial Technology in Southern Africa" Forests 16, no. 6: 966. https://doi.org/10.3390/f16060966
APA StylePeerbhay, K., Devsaran, N., Lottering, R., Agjee, N., & Parag, M. (2025). Mapping Commercial Forests Infected by the Novel Variant of Elsinoë masingae, Using Unmanned Aerial Technology in Southern Africa. Forests, 16(6), 966. https://doi.org/10.3390/f16060966









