Thermal Modulation of Leaf Nitrogen Forms in Chinese Fir Under Soil-Warming Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sites Description
2.2. Experimental Design
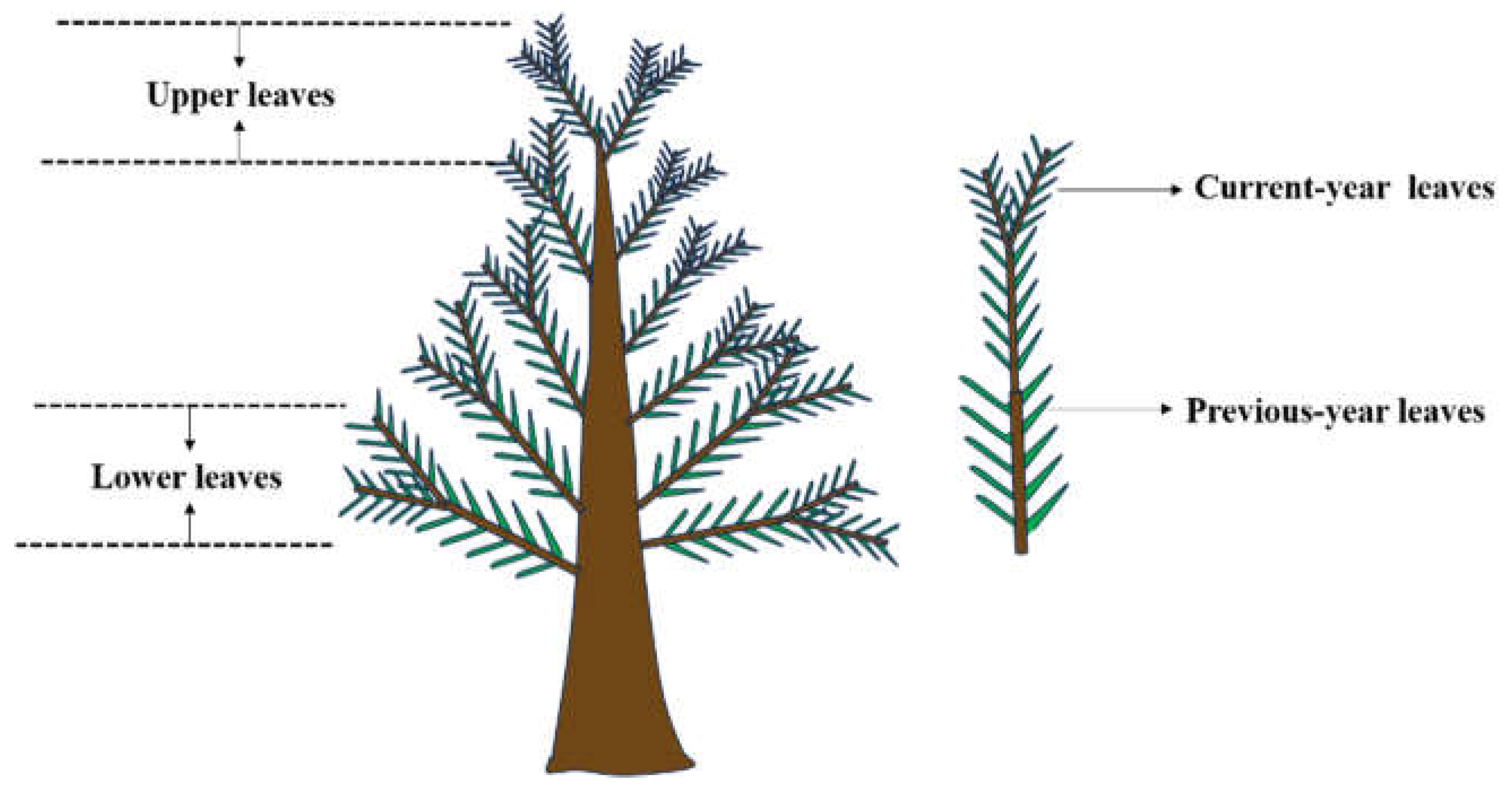
2.3. Sample Collection and Processing
2.4. Detection of Moisture and Available Nitrogen in Leaves
2.5. Statistical Analysis
3. Results
3.1. Leaf Moisture
3.2. Leaf-Available Nitrogen
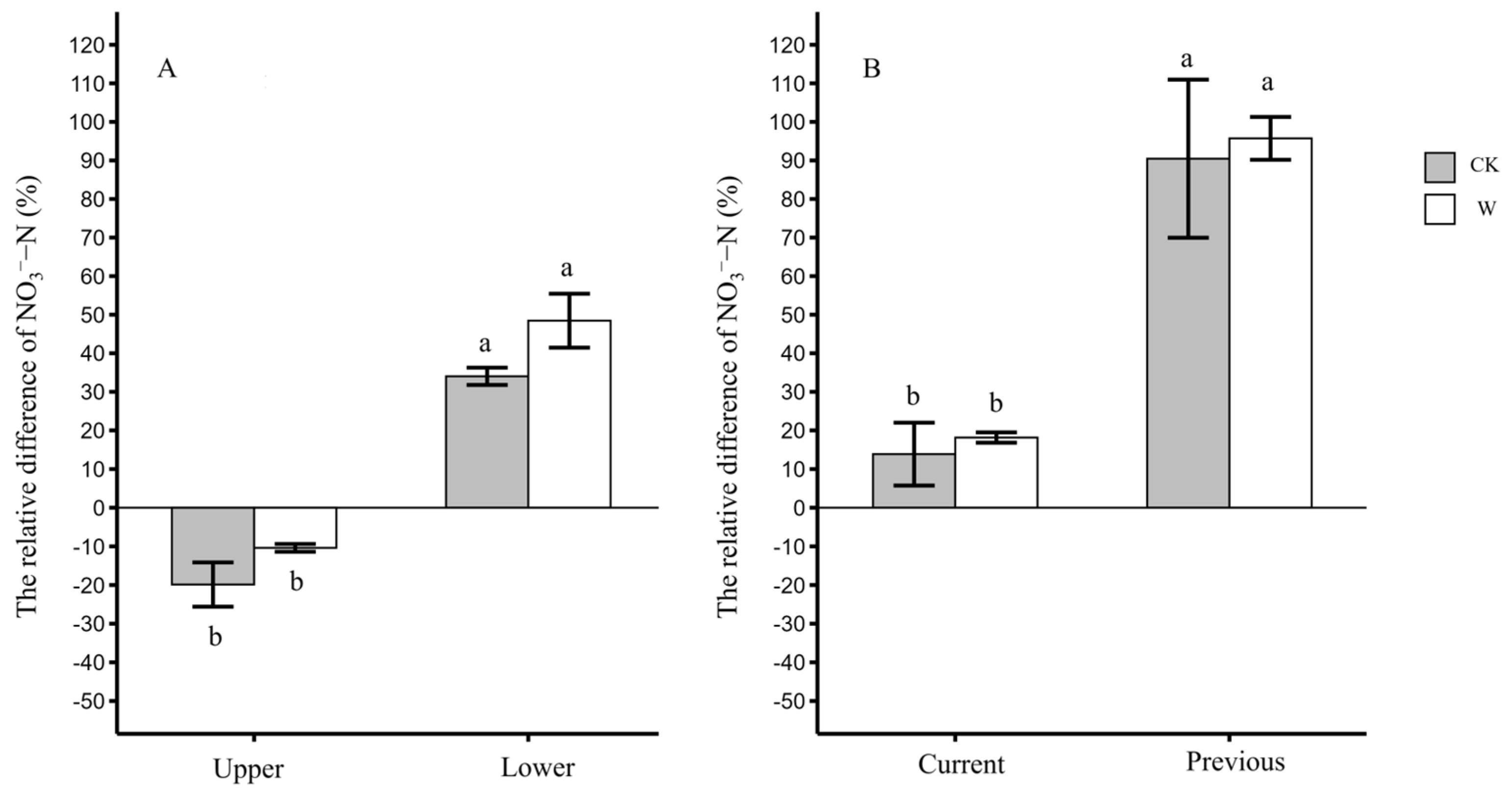
3.3. The Distribution Differences in Leaf NO3−-N
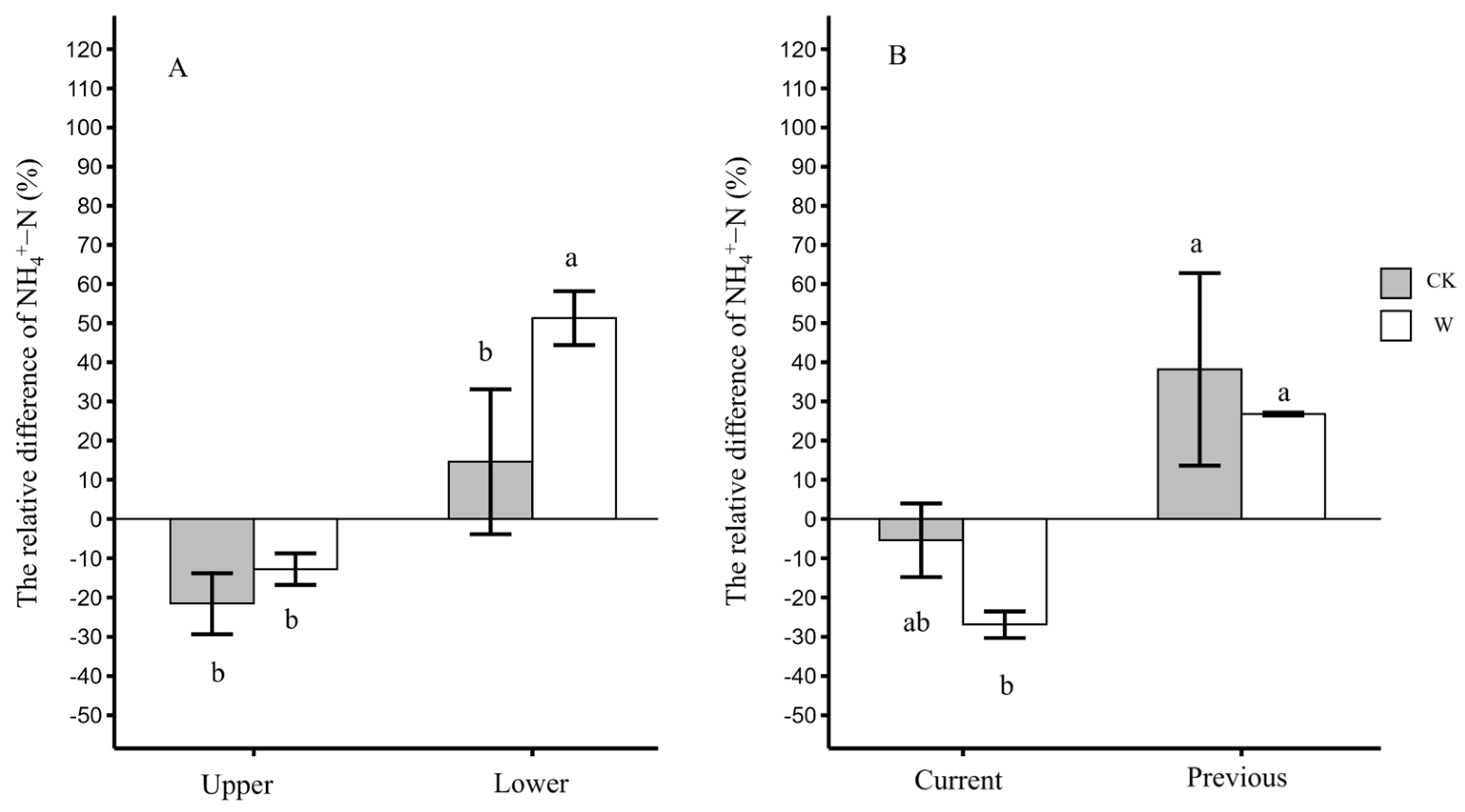
3.4. The Distribution Differences in Leaf NH4+-N
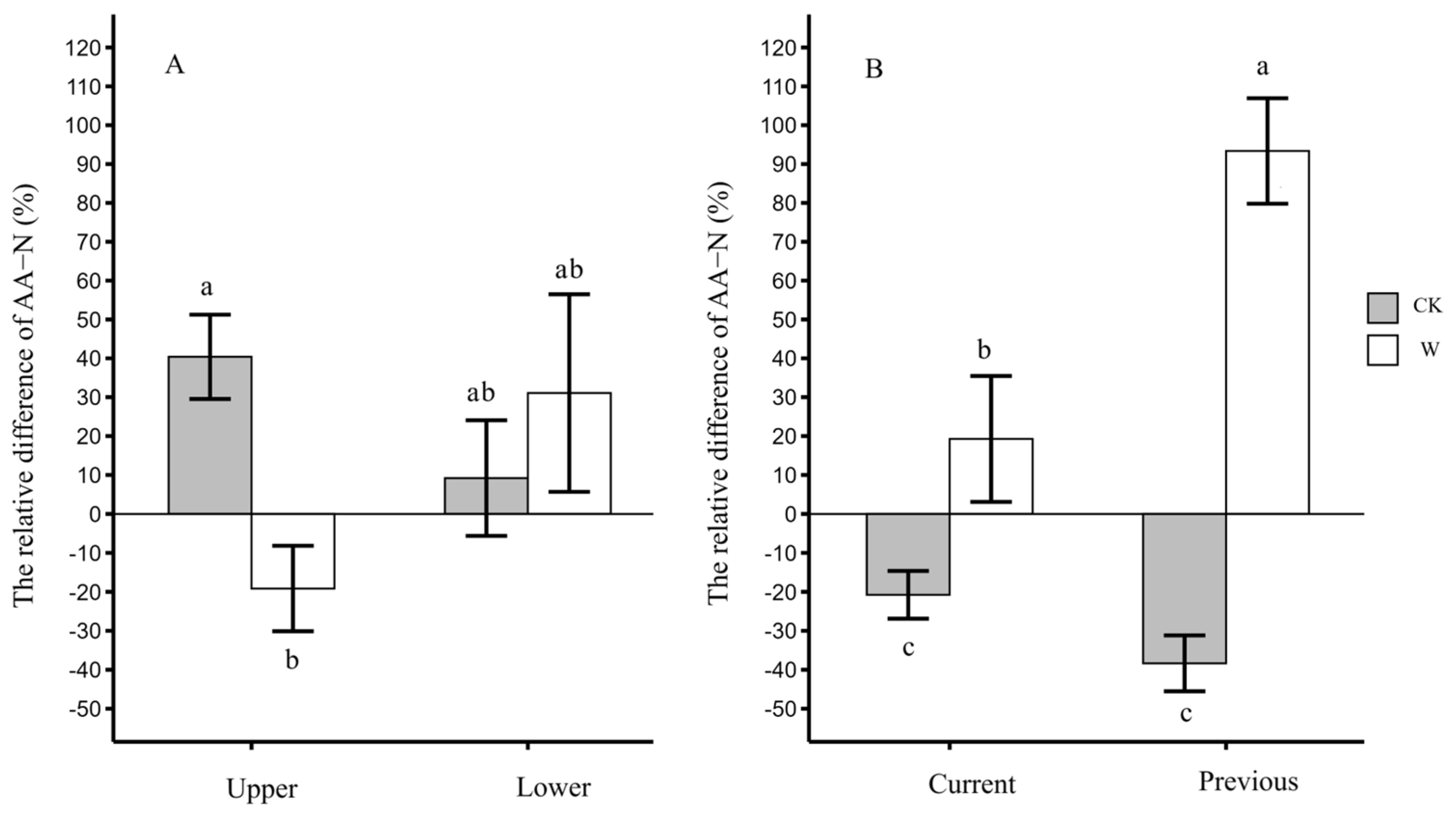
3.5. The Distribution Differences in Leaf AA-N
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar]
- Ren, T.; Smreczak, B.; Ukalska-Jaruga, A.; Hassan, W.; Cai, A. Asymmetric responses of soil dissolved organic carbon and dissolved organic nitrogen to warming: A meta-analysis. Catena 2025, 252, 108871. [Google Scholar] [CrossRef]
- Hu, W.; Zhao, P. Soil warming affects sap flow and stomatal gas exchange through altering functional traits in a subtropical forest. Sci. Total Environ. 2024, 918, 170581. [Google Scholar] [CrossRef] [PubMed]
- Crous, K.Y.; Uddling, J.; De Kauwe, M.G. Temperature responses of photosynthesis and respiration in evergreen trees from boreal to tropical latitudes. New Phytol. 2022, 234, 353–374. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Jian, S.F.; Huang, J.; Jin, Q.Y.; Cao, X.C. Trade-off of within-leaf nitrogen allocation between photosynthetic nitrogen-use efficiency and water deficit stress acclimation in rice (Oryza sativa L.). Plant Physiol. Biochem. 2019, 135, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Zuccarini, P.; Asensio, D.; Ogaya, R.; Sardans, J.; Peñuelas, J. Effects of seasonal and decadal warming on soil enzymatic activity in a P-deficient Mediterranean shrubland. Glob. Change Biol. 2020, 26, 3698–3714. [Google Scholar] [CrossRef]
- Butler, S.M.; Melillo, J.M.; Johnson, J.E.; Mohan, J.; Steudler, P.A.; Lux, H.; Burrows, E.; Smith, R.M.; Vario, C.L.; Scott, L.; et al. Soil warming alters nitrogen cycling in a New England Forest: Implications for ecosystem function and structure. Oecologia 2012, 168, 819–828. [Google Scholar] [CrossRef]
- Melillo, J.M.; Butler, S.; Johnson, J.; Mohan, J.; Steudler, P.; Lux, H.; Burrows, E.; Bowles, F.; Smith, R.; Scott, L.; et al. Soil warming, carbon-nitrogen interactions, and forest carbon budgets. Proc. Natl. Acad. Sci. USA 2011, 108, 9508–9512. [Google Scholar] [CrossRef]
- Bai, E.; Li, S.; Xu, W.; Li, W.; Dai, W.; Ping, J. A meta-analysis of experimental warming effects on terrestrial nitrogen pools and dynamics. New Phytol. 2013, 199, 441–451. [Google Scholar] [CrossRef]
- Miller, B.D.; Carter, K.R.; Reed, S.C.; Wood, T.E.; Cavaleri, M.A. Only sun-lit leaves of the uppermost canopy exceed both air temperature and photosynthetic thermal optima in a wet tropical forest. Agric. For. Meteorol. 2021, 301–302, 108347. [Google Scholar] [CrossRef]
- Girardin, M.P.; Hogg, E.H.; Bernier, P.Y.; Kurz, W.A.; Guo, X.J.; Cyr, G. Negative impacts of high temperatures on growth of black spruce forests intensify with the anticipated climate warming. Glob. Change Biol. 2016, 22, 627–643. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, E.; Zhou, D.; Luo, Z.; Zhang, Z. Rising soil temperature in China and its potential ecological impact. Sci. Rep. 2016, 6, 35530. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Tian, Y.; Heinzle, J.; Salas, E.; Kwatcho-Kengdo, S.; Borken, W.; Schindlbacher, A.; Wanek, W. Long-term soil warming decreases soil microbial necromass carbon by adversely affecting its production and decomposition. Glob. Change Biol. 2024, 30, e17379. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Schindlbacher, A.; Malo, C.U.; Shi, C.P.; Heinzle, J.; Kengdo, S.K.; Inselsbacher, E.; Borken, W.; Wanek, W. Long-term warming of a forest soil reduces microbial biomass and its carbon and nitrogen use efficiencies. Soil Biol. Biochem. 2023, 184, 109109. [Google Scholar] [CrossRef]
- Yoneyama, T.; Suzuki, A. Light-independent nitrogen assimilation in plant leaves: Nitrate incorporation into glutamine, glutamate, aspartate, and asparagine traced by 15N. Plants 2020, 9, 1303. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant Nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.C. Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef]
- Mason, R.E.; Craine, J.M.; Lany, N.K.; Jonard, M.; Ollinger, S.V.; Groffman, P.M.; Fulweiler, R.W.; Angerer, J.; Read, Q.D.; Reich, P.B.; et al. Evidence, causes, and consequences of declining nitrogen availability in terrestrial ecosystems. Science 2022, 376, eabh3767. [Google Scholar] [CrossRef]
- Shi, Z.M.; Tang, J.C.; Cheng, R.M.; Luo, D.; Liu, S.R. A review of nitrogen allocation in leaves and factors in its effects. Acta Ecol. Sin. 2015, 35, 5909–5919. [Google Scholar] [CrossRef]
- National Forestry and Grassland Administration. China Forest Resources Inventory Report (2014–2018); China Forestry Publishing House: Beijing, China, 2019. [Google Scholar]
- Lv, Z.; Duan, A.; Zhang, J. Influence of forest age, tree size, and climate factors on biomass and carbon storage allocation in Chinese fir forests. Ecol. Indic. 2024, 163, 112096. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, M.; Su, J.; Xu, J.; Zhang, X.; Shang, J.; Gao, J. Leaf nutrient traits of planted forests demonstrate a heightened sensitivity to environmental changes compared to natural forests. Front. Plant Sci. 2024, 15, 1372530. [Google Scholar] [CrossRef]
- Hikosaka, K.; Anten, N.P.; Borjigidai, A.; Kamiyama, C.; Sakai, H.; Hasegawa, T.; Oikawa, S.; Iio, A.; Watanabe, M.; Koike, T.; et al. A meta-analysis of leaf nitrogen distribution within plant canopies. Ann. Bot. 2016, 118, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Gong, Y.; Lyu, X.; Lin, J.; Yang, Y.; Lu, H. Split nitrogen application increases maize root growth, yield, and nitrogen use efficiency under soil warming conditions. Crop J. 2025, 13, 565–575. [Google Scholar] [CrossRef]
- Osada, N. Crown development in a pioneer tree, Rhus trichocarpa, in relation to the structure and growth of individual branches. New Phytol. 2006, 172, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, A.; Hobbie, E.A.; Zhu, F.; Qu, Y.; Dai, L.; Li, D.; Liu, X.; Zhu, W.; Koba, K.; et al. Mature conifers assimilate nitrate as efficiently as ammonium from soils in four forest plantations. New Phytol. 2021, 229, 3184–3194. [Google Scholar] [CrossRef]
- Wang, P. Improving the method for determining the total dissociative amino acid in fresh plat tissue. J. Beijing Agric. Coll. 1998, 13, 9–13. [Google Scholar] [CrossRef]
- Chen, X.; Liu, C.; Zhu, B.; Wei, W.; Sheng, R. The contribution of nitrate dissimilation to nitrate consumption in narG- and napA-Containing nitrate reducers with various oxygen and nitrate supplies. Microbiol. Spectr. 2022, 10, e00695-22. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Dietze, M.C.; Jackson, R.B.; Rhoades, C.C.; Rustad, L.E.; Vose, J.M. Forest biogeochemistry in response to drought. Glob. Change Biol. 2016, 22, 2318–2328. [Google Scholar] [CrossRef]
- Couvreur, V.; Vanderborght, J.; Draye, X.; Javaux, M. Dynamic aspects of soil water availability for isohydric plants: Focus on root hydraulic resistances. Water Resour. Res. 2014, 50, 8891–8906. [Google Scholar] [CrossRef]
- Doughty, C.E. An In Situ Leaf and Branch Warming Experiment in the Amazon. Biotropica 2011, 43, 658–665. [Google Scholar] [CrossRef]
- O’sullivan, O.S.; Heskel, M.A.; Reich, P.B.; Tjoelker, M.G.; Weerasinghe, K.W.L.K.; Penillard, A.; Zhu, L.; Egerton, J.J.G.; Bloomfield, K.J.; Creek, D.; et al. Thermal limits of leaf metabolism across biomes. Glob. Change Biol. 2017, 23, 209–223. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, D.; Yang, L.; Xie, J.; Yang, Z.; Zhou, J.; Li, X.; Xiong, D.; Chen, Y.; Yang, Y. Variations in rainfall affect the responses of foliar chemical properties of Cunninghamia lanceolata seedlings to soil warming. Front. Plant Sci. 2021, 12, 705861. [Google Scholar] [CrossRef] [PubMed]
- Boussadia, O.; Steppe, K.; Zgallai, H.; Ben, E.H.S.; Braham, M.; Lemeur, R.; Van Labeke, M.C. Effects of nitrogen deficiency on leaf photosynthesis carbohydrate status and biomass production in two olive cultivars ‘Meski’ and ‘Koroneik’. Sci. Hortic. 2010, 123, 336–342. [Google Scholar] [CrossRef]
- Xiong, D.; Yang, Z.; Chen, G.; Liu, X.; Lin, W.; Huang, J.; Bowles, F.P.; Lin, C.; Xie, J.; Li, Y.; et al. Interactive effects of warming and nitrogen addition on fine root dynamics of a young subtropical plantation. Soil. Boil. Biochem. 2018, 123, 180–189. [Google Scholar] [CrossRef]
- Lyu, M.; Chen, S.; Zhang, Q.; Yang, Z.; Xie, J.; Wang, C.; Wang, X.; Liu, X.; Xiong, D.; Xu, C.; et al. Rapid positive response of young trees growth to warming reverses nitrogen loss from subtropical soil. Funct. Ecol. 2024, 8, 1222–1235. [Google Scholar] [CrossRef]
- Tang, B.; Yang, H.; Yin, C.Y.; Sun, Y.Y.; Zheng, D.H.; Liu, Q. Effects of night warming on the uptake of inorganic nitrogen by two dominant species in subalpine coniferous forests. Chin. J. Plant Ecol. 2016, 40, 543–553. [Google Scholar] [CrossRef]
- Camenzind, T.; Hempel, S.; Homeier, J.; Horn, S.; Velescu, A.; Wilcke, W.; Rillig, M.C. Nitrogen and phosphorus additions impact arbuscular mycorrhizal abundance and molecular diversity in a tropical montane forest. Glob. Change Biol. 2014, 20, 3646–3659. [Google Scholar] [CrossRef]
- Okumoto, S.; Pilot, G. Amino acid export in plants: A missing link in nitrogen cycling. Mol. Plant 2011, 4, 453–463. [Google Scholar] [CrossRef]
- Wang, W.; Shang, J.; Ræbild, A.; Gao, T.; Xie, Q. Effects of Nitrate Assimilation in Leaves and Roots on Biomass Allocation and Drought Stress Responses in Poplar Seedlings. Forests 2024, 15, 779. [Google Scholar] [CrossRef]
- Andrews, M.; Raven, J.A. Root or shoot nitrate assimilation in terrestrial vascular plants—Does it matter? Plant Soil 2022, 476, 31–62. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Yang, Z.J.; Chen, T.T.; Gong, X.Y.; Xiong, D.C.; Ye, W.M.; Chen, Y.; Yang, Y. Warming alters plant chemical and nutrient compositions by affecting metabolites in Cunninghamia lanceolata (Lamb.) hook. Forests 2019, 10, 553. [Google Scholar] [CrossRef]
- Takashima, T.; Hikosaka, K.; Hirose, T. Photosynthesis or persistence: Nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ. 2004, 27, 1047–1054. [Google Scholar] [CrossRef]
- Yates, M.J.; Verboom, G.A.; Rebelo, A.G.; Cramer, M.D. Ecophysiological significance of leaf size variation in Proteaceae from the Cape Floristic Region. Funct. Ecol. 2010, 24, 485–492. [Google Scholar] [CrossRef]
- Wilson, P.J.; Thompson, K.E.N.; Hodgson, J.G. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol. 1999, 143, 155–162. [Google Scholar] [CrossRef]
- Cavaleri, M.A.; Oberbauer, S.F.; Clark, D.B.; Clark, D.A.; Ryan, M.G. Height is more important than light in determining leaf morphology in a tropical forest. Ecology 2010, 91, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, J.C.; Van Bodegom, P.M.; Witte, J.P.M.; Wright, I.J.; Reichet, P.B.; Aerts, R. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob. Ecol. Biogeogr. 2009, 18, 137–149. [Google Scholar] [CrossRef]
- Shangguan, Z.P.; Shao, M.A.; Dyckmans, J. Nitrogen nutrition and water stress effects on leaf photosynthetic gas exchange and water use efficiency in winter wheat. Environ. Exp. Bot. 2000, 44, 141–149. [Google Scholar] [CrossRef]
- Yu, C.; Leung, S.K.P.; Zhang, W.; Lai, L.T.F.; Chan, Y.K.; Wong, M.C.; Benlekbir, S.; Cui, Y.; Jiang, L.; Lau, W.C.Y. Structural basis of substrate recognition and thermal protection by a small heat shock protein. Nat. Commun. 2021, 12, 3007. [Google Scholar] [CrossRef]
- Hikosaka, K. Interspecific difference in the photosynthesis-nitrogen relationship: Patterns, physiological causes, and ecological importance. J. Plant Res. 2004, 117, 481–494. [Google Scholar] [CrossRef]
- Hikosaka, K.; Osone, Y. A paradox of leaf-trait convergence: Why is leaf nitrogen concentration higher in species with higher photosynthetic capacity? J. Plant Res. 2009, 122, 245–251. [Google Scholar] [CrossRef]
- Onoda, Y.; Hikosaka, K.; Hirose, T. Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Funct. Ecol. 2004, 18, 419–425. [Google Scholar] [CrossRef]
- Burns, A.E.; Gleadow, R.M.; Woodrow, I.E. Light alters the allocation of nitrogen to cyanogenic glycosides in Eucalyptus cladocalyx. Oecologia 2002, 133, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.L.; Lei, Y.B.; Wang, R.F.; Callaway, R.M.; Valiente, B.A.; Inderjit; Li, Y.P.; Zheng, Y.L. Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proc. Natl. Acad. Sci. USA 2009, 106, 1853–1856. [Google Scholar] [CrossRef] [PubMed]

| Situation | CK 1 (%) | W 1 (%) | |
|---|---|---|---|
| Upper | Current | 61.64 ± 5.43 | 59.13 ± 1.40 |
| Previous | 61.75 ± 2.51 | 59.23 ± 1.14 | |
| Lower | Current | 59.69 ± 5.14 | 58.53 ± 1.37 |
| Previous | 57.80 ± 2.75 | 55.31 ± 3.51 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhu, L.; Yang, Z.; Shen, C.; Li, Y.; Tang, Z.; Zhu, Y. Thermal Modulation of Leaf Nitrogen Forms in Chinese Fir Under Soil-Warming Conditions. Forests 2025, 16, 942. https://doi.org/10.3390/f16060942
Chen X, Zhu L, Yang Z, Shen C, Li Y, Tang Z, Zhu Y. Thermal Modulation of Leaf Nitrogen Forms in Chinese Fir Under Soil-Warming Conditions. Forests. 2025; 16(6):942. https://doi.org/10.3390/f16060942
Chicago/Turabian StyleChen, Xing, Lijuan Zhu, Zhijie Yang, Caixia Shen, Yin Li, Zexuan Tang, and Yankun Zhu. 2025. "Thermal Modulation of Leaf Nitrogen Forms in Chinese Fir Under Soil-Warming Conditions" Forests 16, no. 6: 942. https://doi.org/10.3390/f16060942
APA StyleChen, X., Zhu, L., Yang, Z., Shen, C., Li, Y., Tang, Z., & Zhu, Y. (2025). Thermal Modulation of Leaf Nitrogen Forms in Chinese Fir Under Soil-Warming Conditions. Forests, 16(6), 942. https://doi.org/10.3390/f16060942






